Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Morphology
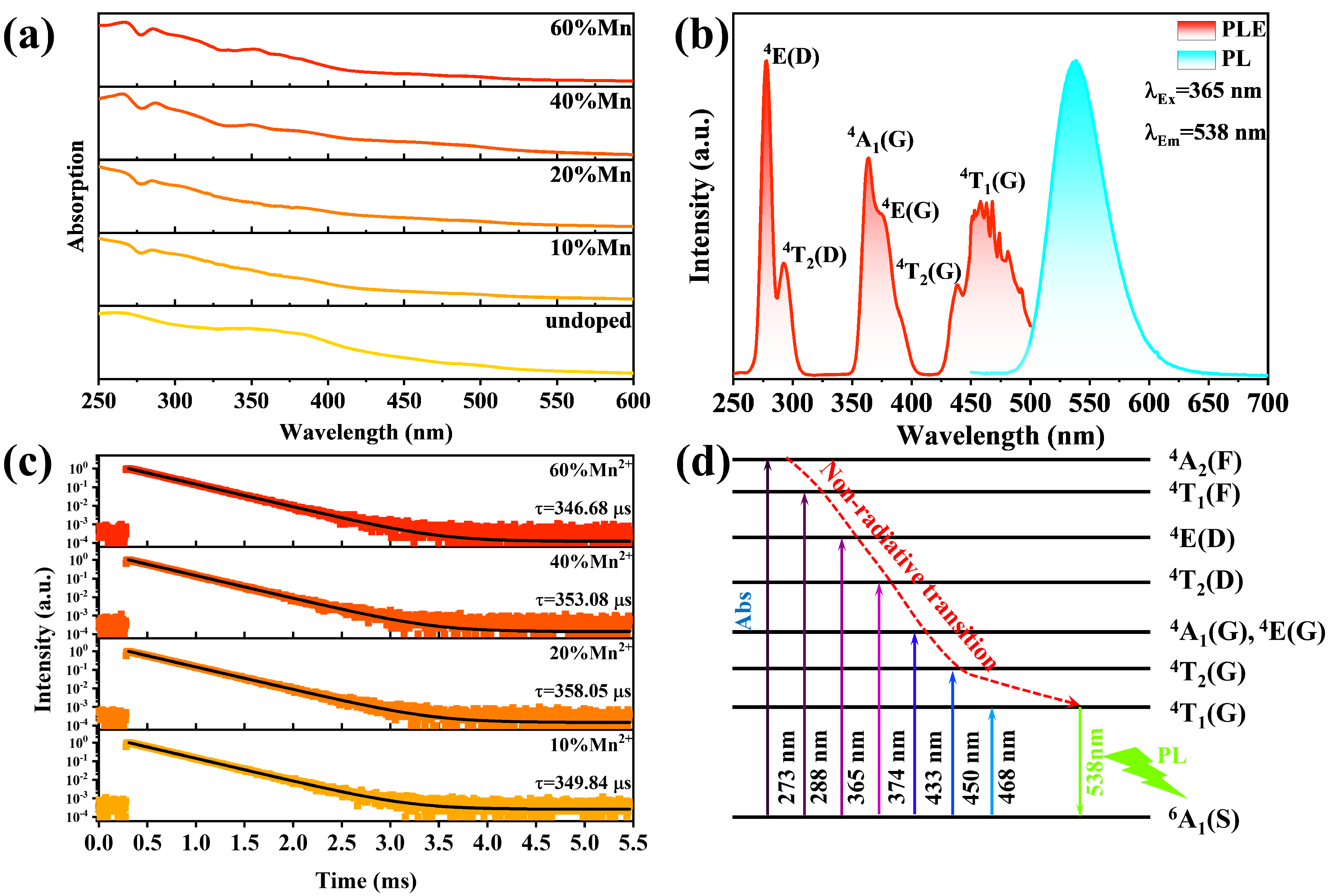
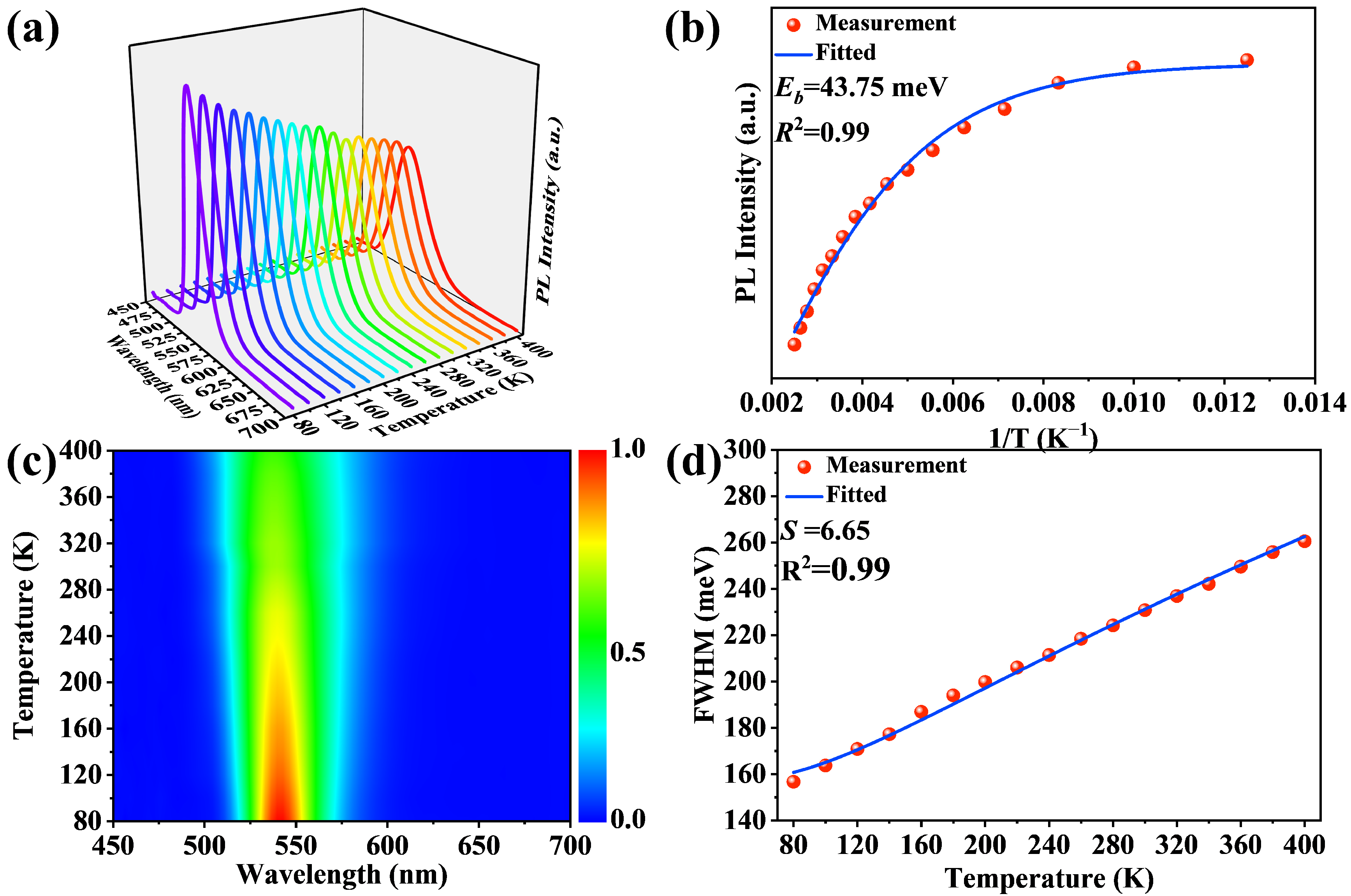
2.2. Optical Properties
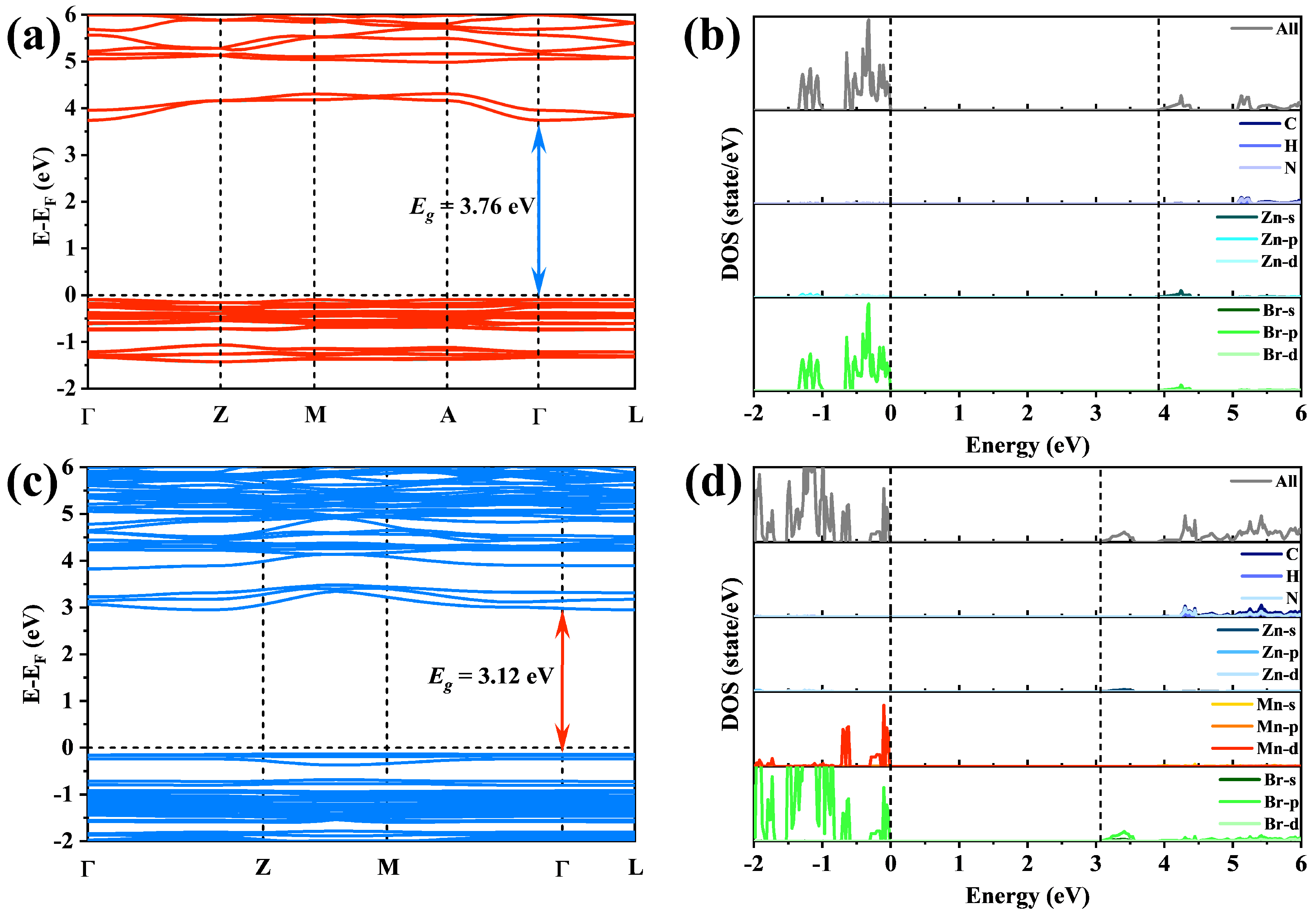
2.3. Theoretical Calculation
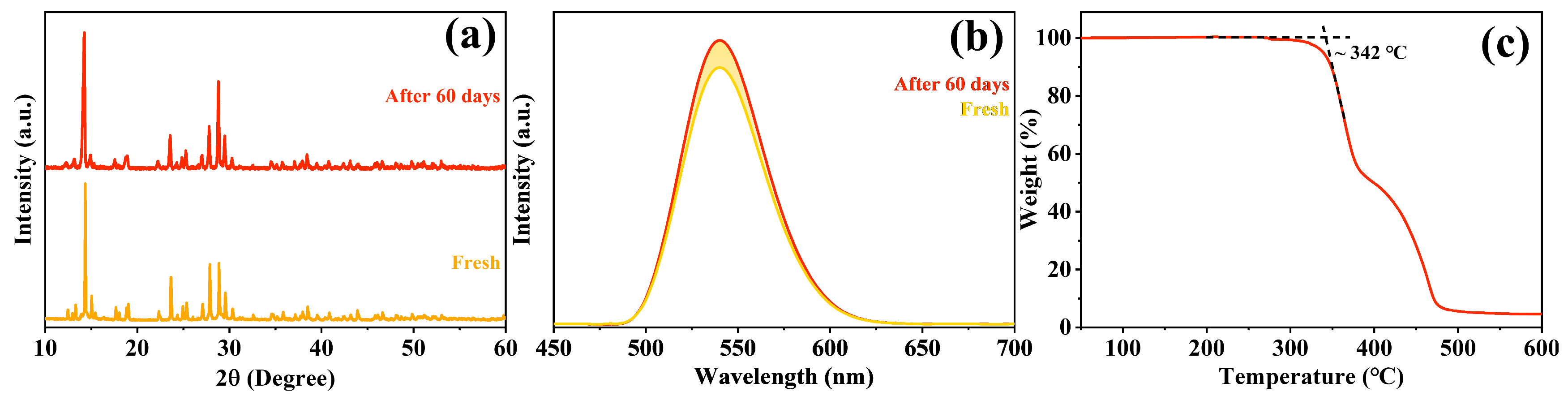
2.4. Stability
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, J.; Byranvand, M.M.; Martínez, C.O.; Hoye, R.L.; Saliba, M.; Polavarapu, L. Defect Passivation in Lead-Halide Perovskite Nanocrystals and Thin Films: Toward Efficient LEDs and Solar Cells. Angew. Chem. Int. Ed. 2021, 133, 21804–21828. [Google Scholar] [CrossRef]
- Safronov, K.R.; Popkova, A.A.; Markina, D.I.; Pushkarev, A.P.; Makarov, S.V.; Bessonov, V.O.; Fedyanin, A.A. Efficient Emission Outcoupling from Perovskite Lasers into Highly Directional and Long-Propagation-Length Bloch Surface Waves. Laser Photonics Rev. 2022, 16, 2100728. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jokar, E.; Cai, L.; Han, J.; Nacpil, E.J.C.; Jeon, I. Emerging Opportunities in Lead-Free and Lead–Tin Perovskites for Environmentally Viable Photodetector Applications. Chem. Mater. 2023, 35, 3404–3426. [Google Scholar] [CrossRef]
- Moseley, O.D.; Roose, B.; Zelewski, S.J.; Stranks, S.D. Identification and Mitigation of Transient Phenomena That Complicate the Characterization of Halide Perovskite Photodetectors. ACS Appl. Energy Mater. 2023, 6, 10233–10242. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Zhumagali, S.; Merino, L.V.T.; De Bastiani, M.; McCulloch, I.; De Wolf, S. Molecular Engineering of Contact Interfaces for High-Performance Perovskite Solar Cells. Nat. Rev. Mater. 2023, 8, 89–108. [Google Scholar] [CrossRef]
- Furasova, A.; Voroshilov, P.; Sapori, D.; Ladutenko, K.; Barettin, D.; Zakhidov, A.; Di Carlo, A.; Simovski, C.; Makarov, S. Nanophotonics for Perovskite Solar Cells. Adv. Photonics Res. 2022, 3, 2100326. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite Light-Emitting Diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Sánchez, R.S.; Villanueva-Antolí, A.; Bou, A.; Ruiz-Murillo, M.; Mora-Seró, I.; Bisquert, J. Radiative Recombination Processes in Halide Perovskites Observed by Light Emission Voltage Modulated Spectroscopy. Adv. Mater. 2023, 35, 2207993. [Google Scholar] [CrossRef]
- Liao, C.-H.; Mahmud, M.A.; Ho-Baillie, A.W. Recent Progress in Layered Metal Halide Perovskites for Solar Cells, Photodetectors, and Field-Effect Transistors. Nanoscale 2023, 15, 4219–4235. [Google Scholar] [CrossRef]
- Dyrvik, E.G.; Warby, J.H.; McCarthy, M.M.; Ramadan, A.J.; Zaininger, K.-A.; Lauritzen, A.E.; Mahesh, S.; Taylor, R.A.; Snaith, H.J. Reducing Nonradiative Losses in Perovskite LEDs Through Atomic Layer Deposition of Al2O3 on The Hole-Injection Contact. ACS Nano 2023, 17, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Martínez-Sarti, L.; Zanoni, K.P.; Sessolo, M.; Tordera, D.; Bolink, H.J. Semitransparent Near-Infrared Sn–Pb Hybrid Perovskite Photodetectors. J. Mater. Chem. C 2022, 10, 13878–13885. [Google Scholar] [CrossRef]
- Rahmany, S.; Etgar, L. Semitransparent Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 1519–1531. [Google Scholar] [CrossRef]
- Bisquert, J.; Gonzales, C.; Guerrero, A. Transient On/Off Photocurrent Response of Halide Perovskite Photodetectors. J. Phys. Chem. C 2023, 127, 21338–21350. [Google Scholar] [CrossRef]
- Hutter, E.M.; Sangster, R.; Testerink, C.; Ehrler, B.; Gommers, C.M. Metal Halide Perovskite Toxicity Effects on Arabidopsis Thaliana Plants are Caused by Iodide Ions. Iscience 2022, 25, 103583. [Google Scholar] [CrossRef]
- Ikram, M.; Malik, R.; Raees, R.; Imran, M.; Wang, F.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Recent advancements and future insight of lead-free non-toxic perovskite solar cells for sustainable and clean energy production: A review. Sustain. Energy Technol. Assess. 2022, 53, 102433. [Google Scholar] [CrossRef]
- Ray, A.; De Trizio, L.; Zito, J.; Infante, I.; Manna, L.; Abdelhady, A.L. Light Emission from Low-Dimensional Pb-Free Perovskite-Related Metal Halide Nanocrystals. Adv. Opt. Mater. 2023, 11, 2202005. [Google Scholar] [CrossRef]
- Morana, M.; Kaiser, W.; Chiara, R.; Albini, B.; Meggiolaro, D.; Mosconi, E.; Galinetto, P.; De Angelis, F.; Malavasi, L. Origin of Broad Emission Induced by Rigid Aromatic Ditopic Cations in Low-Dimensional Metal Halide Perovskites. J. Phys. Chem. Lett. 2023, 14, 7860–7868. [Google Scholar] [CrossRef]
- Schäfer, T.C.; Becker, J.; Heuler, D.; Seuffert, M.T.; Sedykh, A.E.; Müller-Buschbaum, K. Coordination Polymers Based on Aluminum and Indium Halides Together with Pyrazine. Aust. J. Chem. 2022, 75, 676–683. [Google Scholar] [CrossRef]
- Pinchetti, V.; Moro, F.; Zhang, B.; Fanciulli, M.; De Trizio, L.; Meinardi, F.; Manna, L.; Brovelli, S. Magnetic Transitions and Energy Transfer Processes in Sb-Based Zero-Dimensional Metal Halide Nanocrystals Doped with Manganese. ACS Energy Lett. 2022, 7, 1566–1573. [Google Scholar] [CrossRef]
- Abdelhadi, A.B.; Gutiérrez, M.; Cohen, B.; Lezama, L.; Lachkar, M.; Douhal, A. A New Eco-Friendly and Highly Emitting Mn-Based Hybrid Perovskite toward High-Performance Green Down-Converted LEDs. J. Mater. Chem. C 2024, 12, 286–295. [Google Scholar] [CrossRef]
- Kshirsagar, A.S.; Singh, Y.; Kuruppu, U.M.; Donnadieu, B.; Rai, N.; Gangishetty, M.K. Pivotal Role of A-Site Cations in Tailoring the Band-Edge States, Optical Properties, and Stability of 0D Hybrid Indium Chlorides. Chem. Mater. 2022, 34, 10928–10939. [Google Scholar] [CrossRef]
- Dutta, S.; Yoo, J.H.; Kwon, S.B.; Yoo, H.; Yoon, D.H. Simple Water-Assisted Synthesis of Ce-Doped Lead-Free Cesium Metal Halides for Visible-Blind UV Photodetectors. ACS Sustain. Chem. Eng. 2022, 11, 92–100. [Google Scholar] [CrossRef]
- Biswas, A.; Bakthavatsalam, R.; Das, D.K.; Sam, J.; Mali, B.P.; Biswas, C.; Maana, N.; Thomson, S.; Raavi, S.S.K.; Kurungot, S. Synergistic Electronic Coupling/Cross-Talk between the Isolated Metal Halide Units of Zero Dimensional Heterometallic (Sb, Mn) Halide Hybrid with Enhanced Emission. J. Mater. Chem. C 2022, 10, 360–370. [Google Scholar] [CrossRef]
- McWhorter, T.M.; Zhang, Z.; Creason, T.D.; Thomas, L.; Du, M.H.; Saparov, B. (C7H11N2)2MBr4 (M = Cu, Zn): X-ray Sensitive 0D Hybrid Metal Halides with Tunable Broadband Emission. Eur. J. Inorg. Chem. 2022, 2022, e202100954. [Google Scholar] [CrossRef]
- Marayathungal, J.H.; Das, D.K.; Bakthavatsalam, R.; Sam, J.; Hathwar, V.R.; Pallepogu, R.; Dutta, S.; Kundu, J. Mn2+-Activated Zero-Dimensional Metal (Cd, Zn) Halide Hybrids with Near-Unity PLQY and Zero Thermal Quenching. J. Phys. Chem. C 2023, 127, 8618–8630. [Google Scholar] [CrossRef]
- Popy, D.; Evans, B.; Jiang, J.; Creason, T.; Banerjee, D.; Loftus, L.; Pachter, R.; Glatzhofer, D.; Saparov, B. Intermolecular Arrangement Facilitated Broadband Blue Emission in Group-12 Metal (Zn, Cd) Hybrid Halides and Their Applications. Mater. Today Chem. 2023, 30, 101502. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Liu, Y.; Li, C.; Li, Y.; Li, Q.; Wei, Y.; Zhang, L.; Xu, B.; Chang, X. Integrated Afterglow and Self-Trapped Exciton Emissions in Hybrid Metal Halides for Anti-Counterfeiting Applications. Adv. Mater. 2022, 34, 2200607. [Google Scholar] [CrossRef]
- Creason, T.D.; Fattal, H.; Gilley, I.W.; Evans, B.N.; Jiang, J.; Pachter, R.; Glatzhofer, D.T.; Saparov, B. Stabilized Photoemission from Organic Molecules in Zero-Dimensional Hybrid Zn and Cd Halides. Inorg. Chem. Front. 2022, 9, 6202–6210. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wang, W.-Q.; Zhang, B.-L.; Wang, Y.-J.; Ren, M.-P.; Jing, Z.; Yue, C.-Y. Zero-Dimensional Organic–Inorganic Hybrid Znc Halide with Broadband Yellow Light Emission. Cryst. Eng. Comm. 2023, 25, 444–449. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Qi, Z.; Xiao, G.; Fang, X.; Yan, D. Metal-Halide Coordination Polymers with Excitation Wavelength-and Time-Dependent Ultralong Room-Temperature Phosphorescence. Inorg. Chem. 2022, 61, 16477–16483. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.H.; Ou, W.T.; Luo, J.B.; Kuang, D.B. Zero-Dimensional Zn-Based Halides with Ultra-Long Room-Temperature Phosphorescence for Time-Resolved Anti-Counterfeiting. Angew. Chem. Int. Ed. 2022, 134, e202207985. [Google Scholar] [CrossRef]
- Li, K.-J.; Zhao, Y.-Y.; Sun, M.-E.; Chen, G.-S.; Zhang, C.; Liu, H.-L.; Li, H.-Y.; Zang, S.-Q.; Mak, T.C. Zero-Dimensional Zinc Halide Organic Hybrids with Excellent Optical Waveguide Properties. Cryst. Growth. Des. 2022, 22, 3295–3302. [Google Scholar] [CrossRef]
- Marciniak, L.; Kniec, K.; Elżbieciak-Piecka, K.; Trejgis, K.; Stefanska, J.; Dramićanin, M. Luminescence Thermometry with Transition Metal Ions: A Review. Coordin. Chem. Rev. 2022, 469, 214671. [Google Scholar] [CrossRef]
- Adhikari, S.D.; Echeverría-Arrondo, C.; Sánchez, R.S.; Chirvony, V.S.; Martínez-Pastor, J.P.; Agouram, S.; Muñoz-Sanjosé, V.; Mora-Seró, I. White Light Emission from Lead-Free Mixed-Cation Doped Cs2SnCl6 Nanocrystals. Nanoscale 2022, 14, 1468–1479. [Google Scholar] [CrossRef]
- Babu, R.; López-Fernández, I.; Prasanthkumar, S.; Polavarapu, L. All-Inorganic Lead-Free Doped-Metal Halides for Bright Solid-State Emission from Primary Colors to White Light. ACS Appl. Mater. Interfaces 2023, 15, 35206–35215. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zhou, T.; Wang, Y.; Zheng, J.; Liu, R.; Hou, J.; Li, X.; Wang, L.; Jiang, W. Efficient Emission in Copper-Doped Cs3ZnX5 (X = Cl, I) for White LED and X-ray Scintillator. J. Mater. Chem. C 2023, 11, 9850–9859. [Google Scholar] [CrossRef]
- Geng, D.; Cai, B.; Ouyang, Z.; Cheng, P.; Li, B.; Zeng, H. Heterovalent Doping Enabled Efficient and Stable Extrinsic Self-Trapped Exciton Emission in Zero-Dimensional Cesium Zinc Halides. Adv. Opt. Mater. 2022, 10, 2201061. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Xu, R.; Tu, J.; Fang, G.; Pan, Y. Bright Tunable Luminescence of Sb3+ Doping in Zero-Dimensional Lead-Free Halide Cs3ZnCl5 Perovskite Crystals. Dalton Trans. 2022, 51, 10029–10035. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, L.; Luo, W.; Shen, W.; Li, M.; He, R. Highly Stable Metal Halides Cs2ZnX4 (X= Cl, Br) with Sn2+ as Dopants for Eefficient Deep-Red Photoluminescence. Chinese. Chem. Lett. 2023, 34, 107556. [Google Scholar] [CrossRef]
- Morad, V.; Cherniukh, I.; Pöttschacher, L.; Shynkarenko, Y.; Yakunin, S.; Kovalenko, M.V. Manganese (II) in Tetrahedral Halide Environment: Factors Governing Bright Green Luminescence. Chem. Mater. 2019, 31, 10161–10169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Q.; Chen, Y.; Ali, N.; Ren, Z.; Bi, G.; Wu, H. Self-Trapped Exciton Emission in an Sn(II)-Doped All-Inorganic Zero-Dimensional Zinc Halide Perovskite Variant. Nanoscale 2021, 13, 15285–15291. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, R.; Cheng, S.; Li, G.; Jia, M.; Chen, X.; Wu, D.; Li, X.; Shi, Z. Mn and Cu Codoped Cs2ZnBr4 Metal Halide with Multiexcitonic Emission Toward Anti-Counterfeiting. J. Phys-Condens. Mat. 2022, 34, 204009. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, J.; Chen, B.; Zheng, W.; Zhang, X.; Suo, H.; Chun, F.; Wei, X.; Wang, F. Sequential Thermochromic Switching in Zero-Dimensional Cs2ZnCl4 Metal Halides. Mater. Today Phys. 2023, 35, 101111. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, Y.; Sun, D.; Yu, Y.; Miao, C.; Fu, Y.; Yu, L. Pb2+/Mn2+ Co-Doped Cs2ZnBr4 Microcrystals with Dual-Band Tunable White Light Emission. Ceram. Int. 2023, 50, 3958–3966. [Google Scholar] [CrossRef]
- Gautier, R.; Paris, M.; Massuyeau, F. Templating Effect of Trans-2, 5-Dimethylpiperazine (TDMP) on the Structural Dimensionality of Hybrid Metal Halides. Dalton Trans. 2022, 51, 10758–10762. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Efective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Barreda-Argüeso, J.A.; Nataf, L.; Rodríguez-Lazcano, Y.; Aguado, F.; González, J.s.; Valiente, R.; Rodríguez, F.; Wilhelm, H.; Jephcoat, A.P. Bulk and Molecular Compressibilities of Organic–Inorganic Hybrids [(CH3)4N]2MnX4 (X= Cl, Br): Role of Intermolecular Interactions. Inorg. Chem. 2014, 53, 10708–10715. [Google Scholar] [CrossRef]
- Late, D.J.; Shirodkar, S.N.; Waghmare, U.V.; Dravid, V.P.; Rao, C.N. Thermal Expansion, Anharmonicity and Temperature-Dependent Raman Spectra of Single- and Few-Layer MoSe2 and WSe2. ChemPhysChem 2014, 15, 1592–1598. [Google Scholar] [CrossRef]
- Zhong, H.; Pan, F.; Yue, S.; Qin, C.; Hadjiev, V.; Tian, F.; Liu, X.; Lin, F.; Wang, Z.; Bao, J. Idealizing Tauc Plot for Accurate Bandgap Determination of Semiconductor with Ultraviolet–Visible Spectroscopy: A Case Study for Cubic Boron Arsenide. J. Phys. Chem. Lett. 2023, 14, 6702–6708. [Google Scholar] [CrossRef]
- Yadav, R.S.; Pandey, S.K.; Pandey, A.C. BaAl12O19:Mn2+ Green Emitting Nanophosphor for PDP Application Synthesized by Solution Combustion Method and its Vacuum Ultra-Violet Photoluminescence Characteristics. J. Lumin. 2011, 131, 1998–2003. [Google Scholar] [CrossRef]
- Samanta, T.; Viswanath, N.S.M.; Jang, S.W.; Min, J.W.; Cho, H.B.; Han, J.H.; Im, W.B. Thermally Stable Self-Trapped Assisted Single-Component White Light from Lead-Free Zero-Dimensional Metal Halide Nanocrystals. Adv. Opt. Mater. 2023, 11, 2202744. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Castro, J.; Ferraro, V. Dual Emission from Mn (II) Complexes with Carbazolyl-Substituted Phosphoramides. Inorg. Chim. Acta. 2022, 536, 120896. [Google Scholar] [CrossRef]
- Sreenan, B.; Lee, B.; Wan, L.; Zeng, R.; Zhao, J.; Zhu, X. Review of Mn-Doped Semiconductor Nanocrystals for Time-Resolved Luminescence Biosensing/Imaging. ACS Appl. Nano Mater. 2022, 5, 17413–17435. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-H.; Liao, J.-F.; Wang, X.-D.; Zhou, L.; Jiang, Y.; Kuang, D.-B. All-Inorganic Lead-Free Heterometallic Cs4MnBi2Cl12 Perovskite Single Crystal with Highly Efficient Orange Emission. Matter 2020, 3, 892–903. [Google Scholar] [CrossRef]
- Li, C.; Luo, Z.; Liu, Y.; Wei, Y.; He, X.; Chen, Z.; Zhang, L.; Chen, Y.; Wang, W.; Liu, Y. Self-Trapped Exciton Emission with High Thermal Stability in Antimony-Doped Hybrid Manganese Chloride. Adv. Opt. Mater. 2022, 10, 2102746. [Google Scholar] [CrossRef]
- Das, D.K.; Bakthavatsalam, R.; Hathwar, V.R.; Pallepogu, R.; Kundu, J. Intrinsic vs. Extrinsic STE Emission Enhancement in ns2 Ion Doped Metal (Cd, In) Halide Hybrids. J. Mater. Chem. C 2023, 11, 3855–3864. [Google Scholar] [CrossRef]
- Dakshinamurthy, A.C.; Sudakar, C. Sublattice Distortion Enabled Strong Interplay between Phonon Vibrations, Electron–Phonon Coupling, and Self-Trapped Excitonic Emissions in Cs2Ag1–xNaxBiCl6 Double Perovskites. J. Phys. Chem. Lett. 2022, 13, 433–439. [Google Scholar] [CrossRef]
- McCall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron–Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A= Cs, Rb; M= Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- Gong, X.; Voznyy, O.; Jain, A.; Liu, W.; Sabatini, R.; Piontkowski, Z.; Walters, G.; Bappi, G.; Nokhrin, S.; Bushuyev, O. Electron–Phonon Interaction in Efficient Perovskite Blue Emitters. Adv. Mater. 2018, 17, 550–556. [Google Scholar] [CrossRef]
- Lenferink, E.J.; LaMountain, T.; Stanev, T.K.; Garvey, E.; Watanabe, K.; Taniguchi, T.; Stern, N.P. Tunable Emission from Localized Excitons Deterministically Positioned in Monolayer p–n Junctions. ACS Photonics 2022, 9, 3067–3074. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Wei, J.; Duan, L.; Tian, Y.; Wei, Q. Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield. Materials 2024, 17, 562. https://doi.org/10.3390/ma17030562
Peng C, Wei J, Duan L, Tian Y, Wei Q. Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield. Materials. 2024; 17(3):562. https://doi.org/10.3390/ma17030562
Chicago/Turabian StylePeng, Chengyu, Jiazheng Wei, Lian Duan, Ye Tian, and Qilin Wei. 2024. "Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield" Materials 17, no. 3: 562. https://doi.org/10.3390/ma17030562
APA StylePeng, C., Wei, J., Duan, L., Tian, Y., & Wei, Q. (2024). Mn(II)-Activated Zero-Dimensional Zinc(II)-Based Metal Halide Hybrids with Near-Unity Photoluminescence Quantum Yield. Materials, 17(3), 562. https://doi.org/10.3390/ma17030562




