Changes in Corrosion Behaviour of Zinc and Aluminium Coatings with Increasing Seawater Acidification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Structural and Surface Analysis
2.3. Electrochemical Analysis
- E is SCE versus WE potential [V], and /Ipol/ is the modulus of polarisation current density [A × cm−2].
2.4. Statistical Analysis and Design of the Experiment
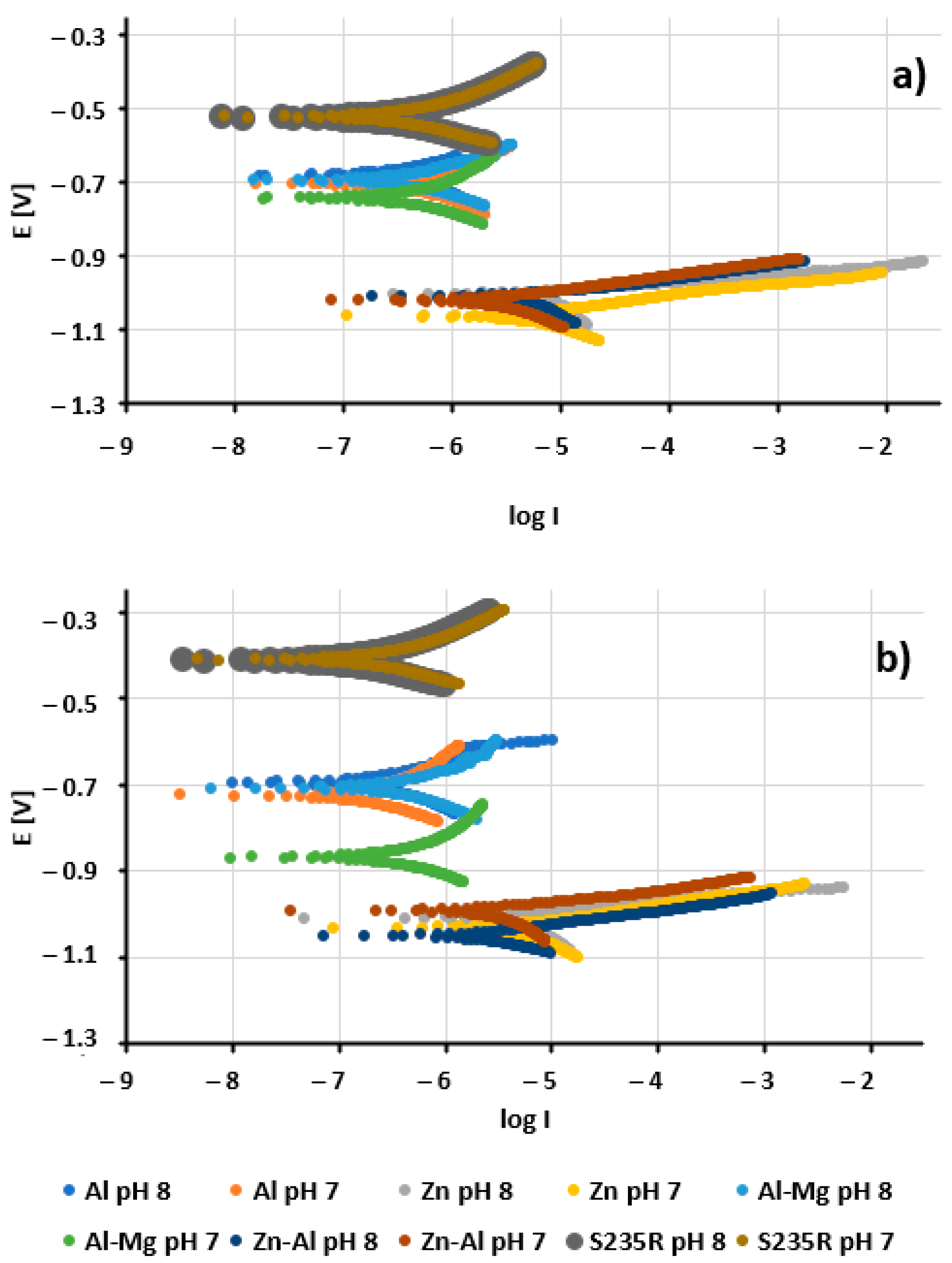
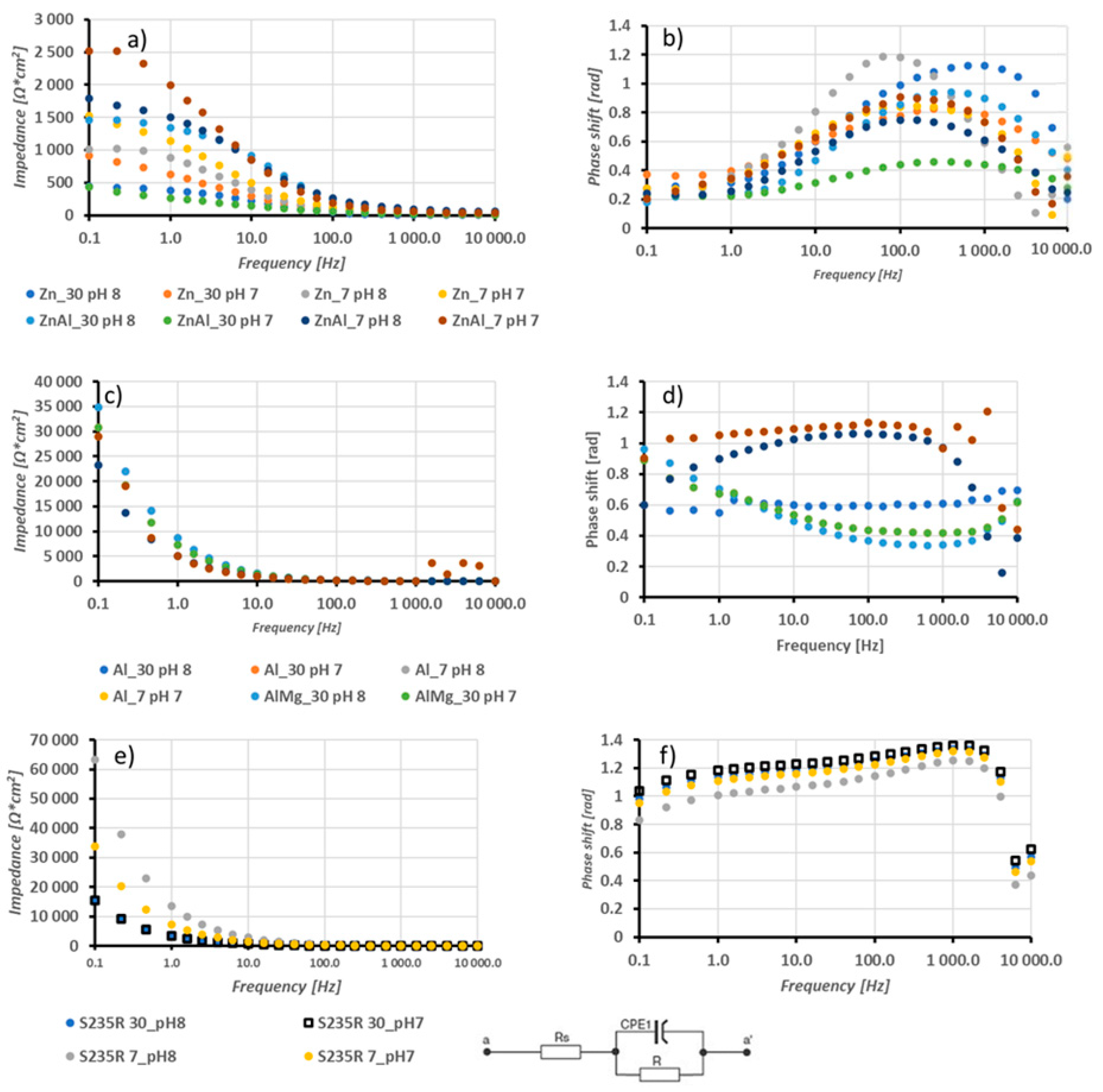
3. Results and Discussion
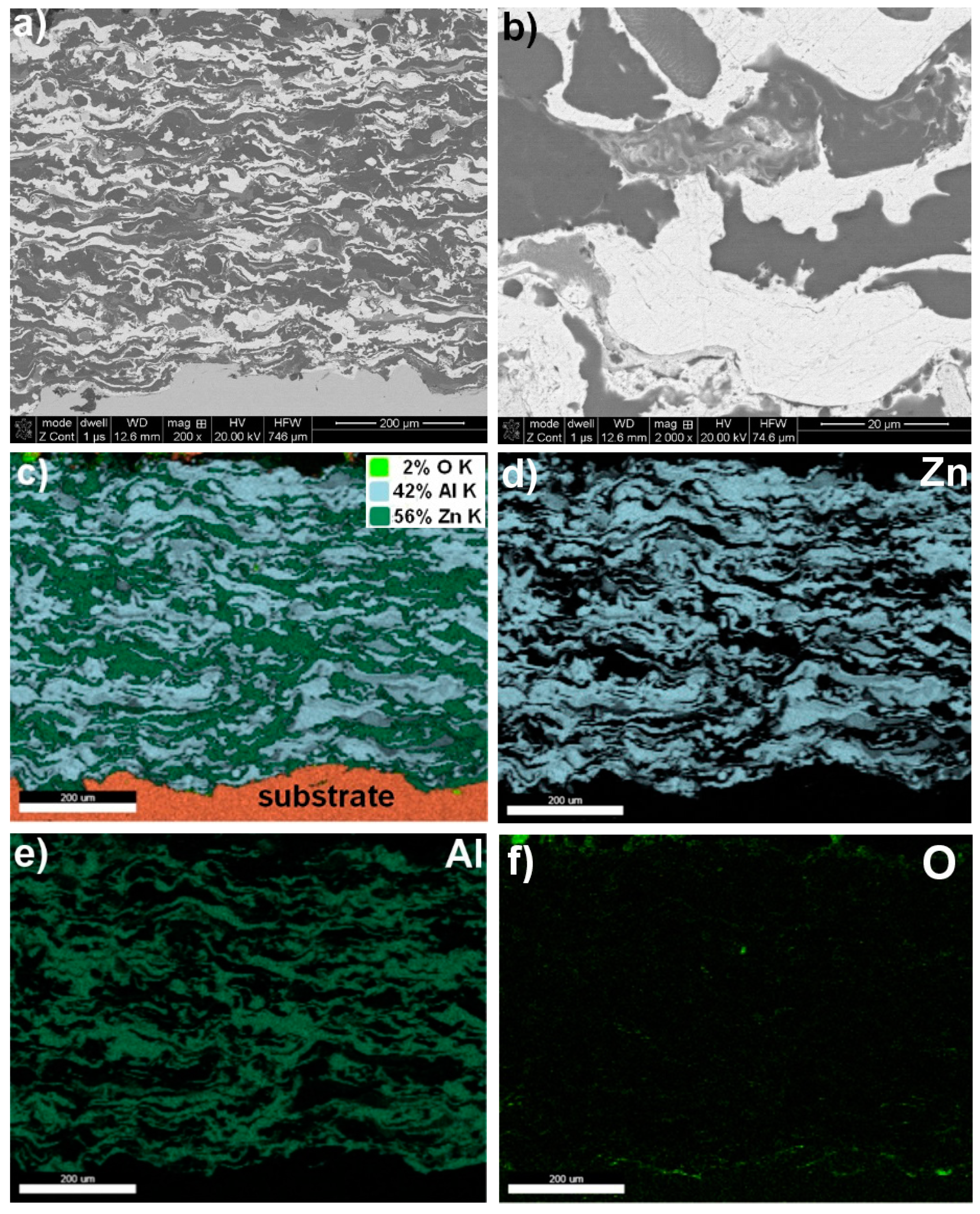
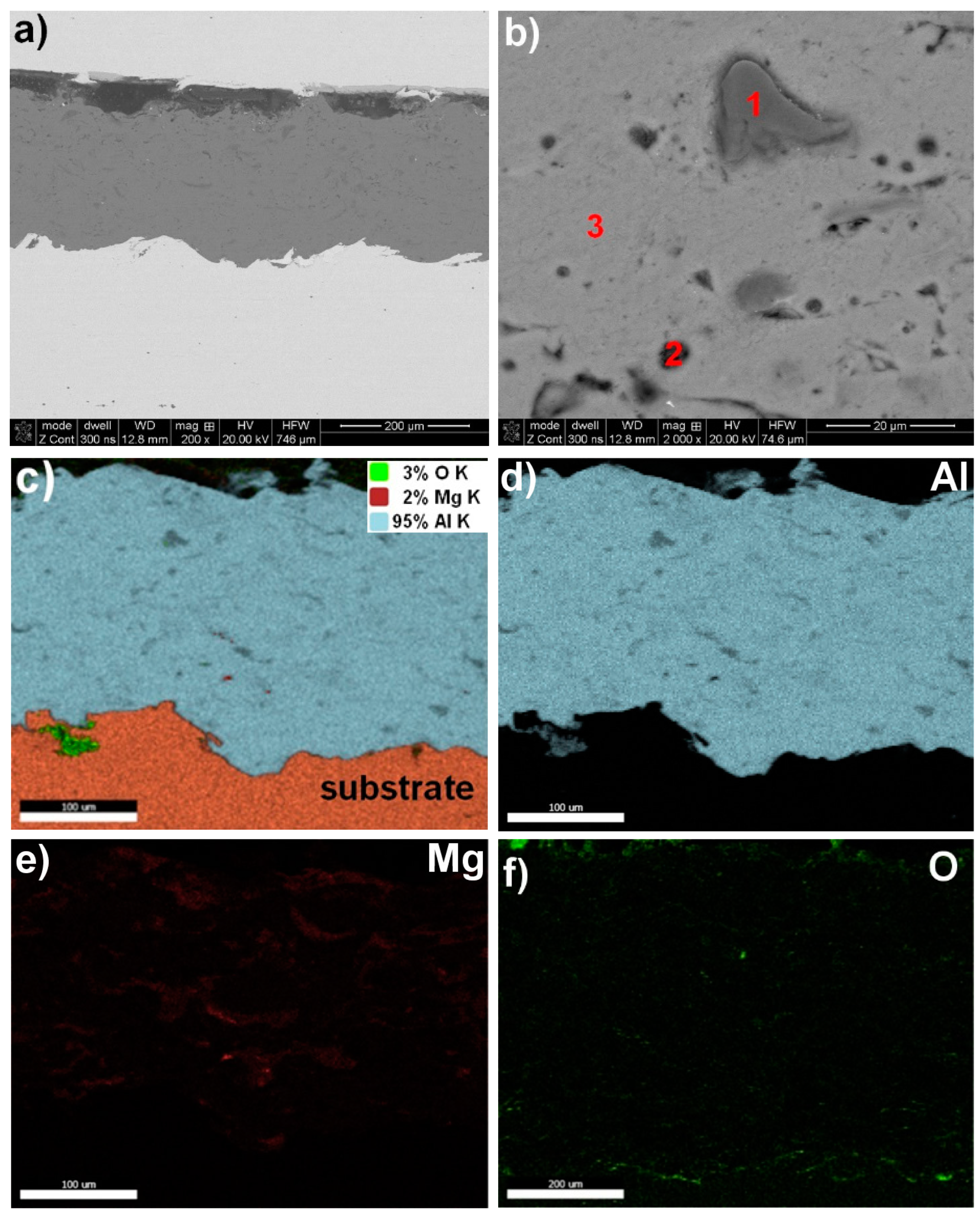
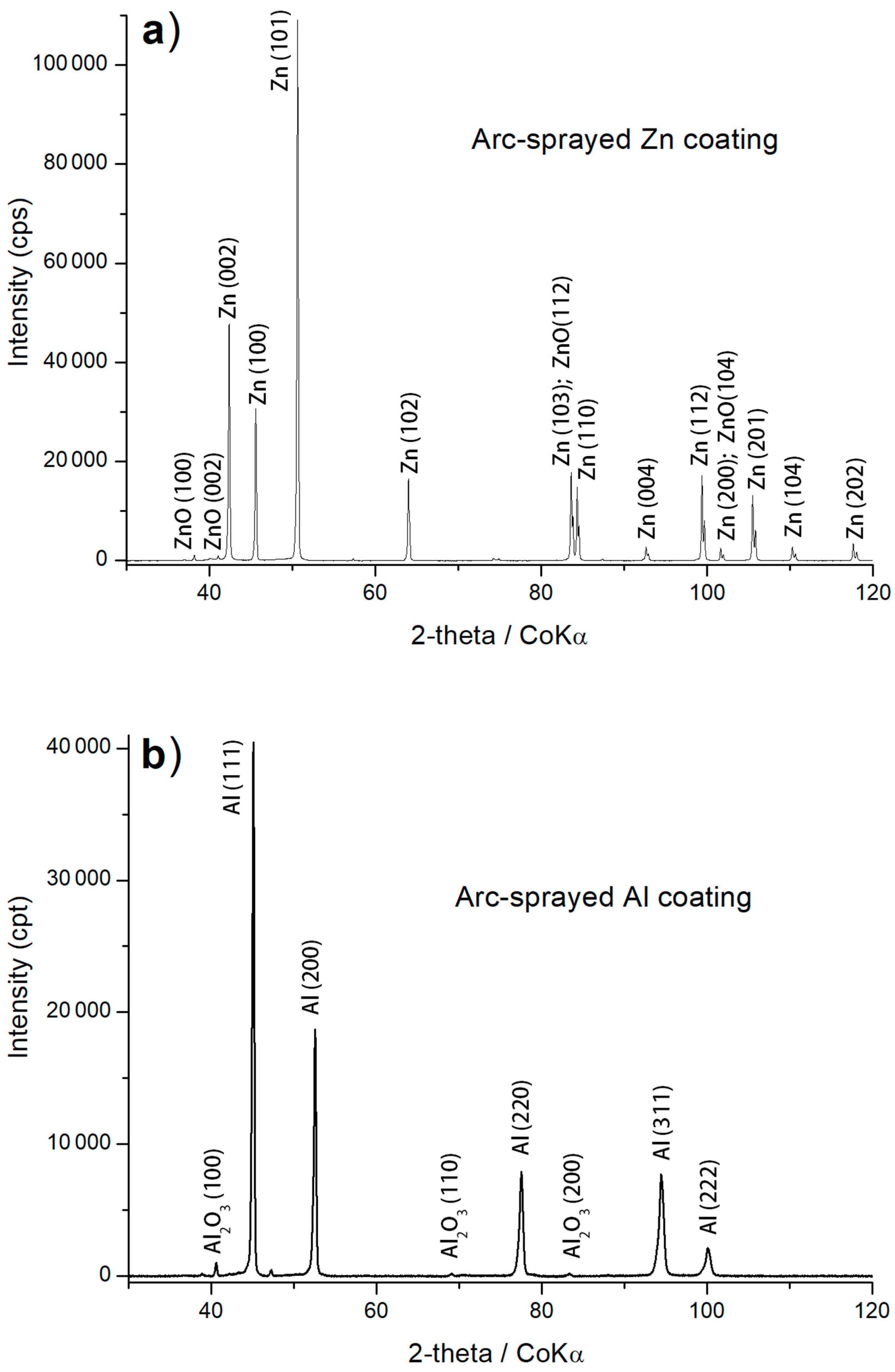
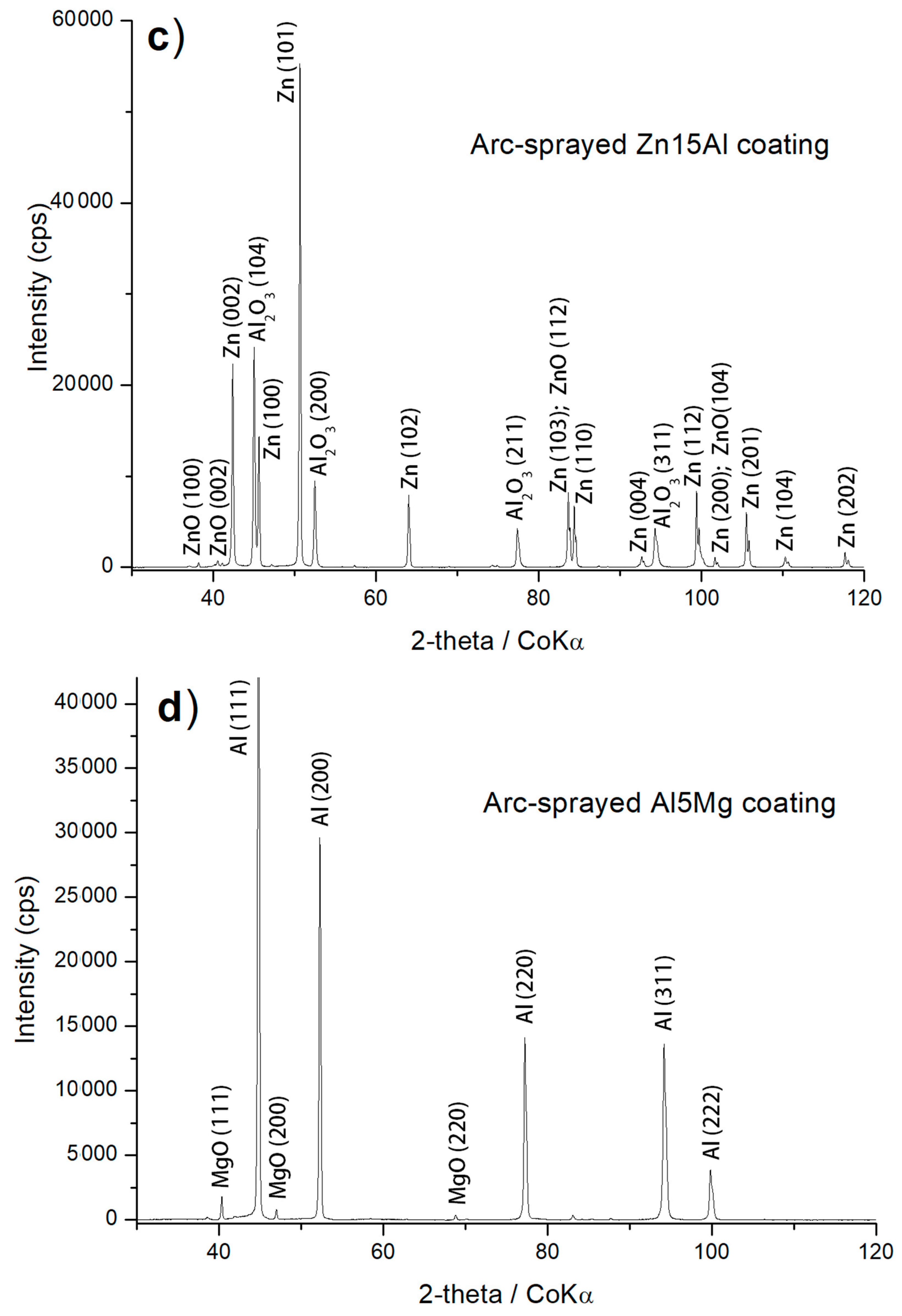
3.1. Structure Analysis
3.2. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Ruihong, Y.; Zhuangzhuang, Z.; Zhen, Q.; Xixi, L.; Tingxi, L.; Ruizhong, G. Greenhouse gas emissions from the water-air interface of a grassland river: A case study of the Xilin River. Sci. Rep. 2021, 11, 2659. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.A.; Lui, H.-K.; Hsieh, C.-H.; Yanagi, T.; Kosugi, N.; Ishii, M.; Gong, G.-C. Deep oceans may acidify faster than anticipated due to global warming. Nat. Clim. Chang. 2017, 7, 890–894. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Häder, D.-P. Individual and interactive effects of ocean acidification, global warming, and UV radiation on phytoplankton. J. Appl. Phycol. 2018, 30, 743–759. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kwon, S.-J.; Singh, J.K.; Ismail, M.A. Influence of Zn and Mg alloying on the corrosion resistance properties of Al coating applied by arc thermal spray process in simulated weather solution. Acta Metall. Sin. 2018, 31, 591–603. [Google Scholar] [CrossRef]
- Garcíaa, S.J.; Muster, T.H.; Özkanat, Ö.; Sherman, N.; Hughes, A.E.; Terryn, H.; de Wit, J.H.W.; Mol, J.M.C. The influence of pH on corrosion inhibitor selection for 2024-T3 aluminium alloy assessed by high-throughput multielectrode and potentiodynamic testing. Electrochim. Acta 2010, 55, 2457–2465. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, G.; Shang, T.; Qiu, W. Corrosion properties of steel sheet with zinc-base alloy coatings. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Society; Springer International Publishing: Cham, Switzerland, 2019; pp. 949–957. [Google Scholar]
- Dolgikh, O.; Simillion, H.; Lamaka, S.V.; Bastos, A.C.; Xue, H.B.; Taryba, M.G.; Oliveira, A.R.; Allély, C.; Van Den Bossche, B.; Van Den Bergh, K.; et al. Corrosion protection of steel cut-edges by hot-dip galvanized Al(Zn, Mg) coatings in 1 wt% NaCl: Part I. Experimental study. Mater. Corros. 2018, 70, 768–779. [Google Scholar] [CrossRef]
- Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising methods for corrosion protection of magnesium alloys in the case of Mg–Al, Mg–Mn–Ce and Mg–Zn–Zr: A recent progress review. Metals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Le, T.D.; Le, Q.T.; Pham, H.T.; Nguyen, A.T.; Pham, L.T.; Dao, T.B.; Ly, C.Q. Characterization and corrosion resistance of the twin-wire Arc spray Al-5Mg alloy coating applied on a carbon steel substrate. J. Therm. Spray Technol. 2023, 1–17. [Google Scholar] [CrossRef]
- Li, H.Y.; Duan, J.Y.; Wei, D.D. Comparison on corrosion behaviour of arc sprayed and zinc-rich coatings. Surf. Coat. Technol. 2013, 235, 259–266. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xu, G.; Song, W.; Xie, Y.; Zhao, L.; Li, W.; Xia, M.; Li, W. Friction and anti-corrosion characteristics of arc sprayed Al–Zn coatings on steel structures prepared in atmospheric environment. J. Mater. Res. Technol. 2021, 15, 6562–6573. [Google Scholar] [CrossRef]
- Thomas, S.; Birbilis, N.; Venkatraman, M.S.; Cole, I.S. Corrosion of zinc as a function of pH. Corrosion 2012, 68, 015009-1. [Google Scholar] [CrossRef]
- Liu, Y.W.; Wang, Z.Y.; Cao, G.W.; Lv, W.Y.; Su, W. Corrosion behavior of Zn in simulated acid rain atmospheric environment. Chin. J. Nonferrous Met. 2015, 25, 375–383. [Google Scholar]
- Qiao, L.; Wu, Y.; Duan, J.; Gao, W.; Hong, S. Corrosion behavior of arc-sprayed pore-sealed Zn and Al coatings in seawater containing sulfate-reducing bacteria (SRB). J. Therm. Spray Technol. 2021, 30, 1557–1565. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, F.; Zhou, H.; Cheng, S.; Xu, K.; Wang, Z.; Xie, S.; Tian, J. Effect of Al content on the long-term corrosion behavior of arc-sprayed ZnAl alloy coatings. Coatings 2023, 13, 1720. [Google Scholar] [CrossRef]
- Huang, I.-W.; Hurley, B.L.; Yang, F.; Buchheit, R.G. Dependence on temperature, pH, and Cl in the uniform corrosion of aluminum alloys 2024-T3, 6061-T6, and 7075-T6. Electrochem. Acta 2016, 1992, 242–253. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Gupta, R.K.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour of the b-phase intermetallic (Mg2Al3) as a function of pH as relevant to corrosion of aluminium–magnesium alloys. Corros. Sci. 2013, 70, 290–293. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peńa, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminum and zinc/aluminum alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Malek, M.H.A.; Saad, N.H.; Abas, S.K.; Shah, N.M. Thermal arc spray overview. IOP Conf. Ser. Mater. Sci. Eng. 2013, 46, 012028. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, D.D.; Duan, J.Y. Effect of sealing treatment on corrosion behaviours of arc sprayed zinc coatings. Corros. Eng. Sci. Technol. 2013, 48, 65–70. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The corrosion behavior of cold sprayed zinc coatings on mild steel substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Tariq, N.; Wang, J.; Cui, X.; Xiong, T. Microstructure and corrosion behavior of cold-sprayed Zn-Al composite coating. Coatings 2020, 10, 931. [Google Scholar] [CrossRef]
- Yong, W.; Timing, Z.; Weimin, Z. Sealing treatment of aluminum coating on S235 steel with thermal diffusion of zinc. J. Therm. Spray Technol. 2015, 24, 1052–1059. [Google Scholar]
- Esfahani, E.A.; Salimijazi, H.; Golozar, M.A.; Javad, M.; Pershin, L. Study of corrosion behavior of arc sprayed aluminum coating on mild steel. J. Therm. Spray Technol. 2012, 21, 1195–1202. [Google Scholar] [CrossRef]
- Bobzin, K.; Oete, M.; Linke, T.F.; Schulz, C. Corrosion of wire arc sprayed ZnMgAl. Mater. Corros. 2015, 66, 520–526. [Google Scholar] [CrossRef]
- Schuerz, S.; Fleischanderl, M.; Luckeneder, G.H.; Preis, K.; Haunschmied, T.; Mori, G.; Kneissl, A.C. Corrosion behaviour of Zn–Al–Mg coated steel sheet in sodium chloride-containing environment. Corros. Sci. 2009, 51, 2355–2363. [Google Scholar] [CrossRef]
- Adamiak, M.; Czupryński, A.; Kopyć, A.; Monica, Z.; Olender, M.; Gwiazda, A. The Properties of arc-sprayed aluminum coatings on armor-grade steel. Metals 2017, 8, 142. [Google Scholar] [CrossRef]
- Chmielewski, T.; Chmielewski, M.; Piatkowska, A.; Grabias, A.; Skowronska, B.; Siwek, P. Structure Evolution of the Fe-Al Arc-Sprayed Coating Stimulated by Annealing. Materials 2021, 14, 3210. [Google Scholar] [CrossRef]
- Senderowski, C.; Rejmer, W.; Bilko, P. Effect of low chloride and sulfate concentrations on corrosion behavior of aluminum and zinc arc thermal sprayed coatings. Coatings 2022, 12, 653. [Google Scholar] [CrossRef]
- Zakowski, K.; Narozny, M.; Szocinski, M.; Darowicki, K. Influence of water salinity on corrosion risk—The case of the southern Baltic Sea coast. Environ. Monit. Assess. 2014, 186, 4871–4879. [Google Scholar] [CrossRef]
- Senderowski, C.; Chodala, M.; Bojar, Z. Corrosion behavior of detonation gas sprayed Fe-Al type intermetallic coating. Materials 2015, 8, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Comp. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Xu, J.L.; Tao, S.C.; Bao, L.Z.; Luo, J.M.; Zheng, Y.F. Effects of Mo contents on the microstructure, properties and cyto-compatibility of the microwave sintered porous Ti–Mo alloys. Mat. Sci. Eng. C 2019, 97, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Gadomska-Gajadhur, A.; Jańczewski, D.; Różycki, C.; Synoradzki, L. Projektowanie Procesów Technologicznych. Matematyczne metody Planowania Eksperymentów, 1st ed.; Oficyna Wydawnicza Politechniki Warszawskiej: Warsaw, Poland, 2020; pp. 39–52. [Google Scholar]
- Lee, H.-S.; Singh, J.; Ismail, M.; Bhattacharya, C.; Seikh, A.; Alharthi, N.; Hussain, R. Corrosion mechanism and kinetics of Al-Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 3399. [Google Scholar] [CrossRef] [PubMed]
- Bonabi, S.F.; Ashrafizadeh, F.; Sanati, A.; Nahvi, S.M. Structure and corrosion behaviour of arc-sprayed Zn–Al coatings on ductile iron substrate. J. Therm. Spray Technol. 2018, 27, 524–537. [Google Scholar] [CrossRef]
- Panas, A.J.; Senderowski, C.; Fikus, B. Thermophysical properties of multiphase of Fe-Al intermetallic-oxide-ceramic coatings deposited by gas detonation spraying. Thermochim. Acta. 2019, 676, 164–171. [Google Scholar] [CrossRef]
- Wolczynski, W.; Senderowski, C.; Morgiel, J.; Garzel, G. D-gun sprayed Fe–Al single particle solidification. Arch. Metall. Mater. 2014, 59, 211–220. [Google Scholar] [CrossRef]
- Pawlowski, A.; Czeppe, T.; Major, L.; Senderowski, C. Structure morphology of FeAl coating detonation sprayed on to carbon steel substrate. Arch. Metall. Mater. 2009, 54, 783–788. [Google Scholar]
- Fikus, B.; Senderowski, C.; Panas, A.J. Modeling of dynamics and thermal history of Fe40Al intermetallic powder particles under gas detonation spraying using propane-air mixture. J. Therm. Spray Technol. 2019, 28, 346–358. [Google Scholar] [CrossRef]
- Senderowski, C.; Pawłowski, A.; Bojar, Z.; Wołczyński, W.; Faryna, M.; Morgiel, J. TEM microstructure of Fe–Al coatings detonation sprayed on to steel substrate. Arch. Metall. Mater. 2010, 55, 373–381. [Google Scholar]
- Samad, U.A.; Alam, M.A.; Anis, A.; Abdo, H.S.; Shaikh, H.; Al-Zahrani, S.M. Nanomechanical and Electrochemical Properties of ZnO–Nanoparticle–Filled Epoxy Coatings. Coatings 2022, 12, 282. [Google Scholar] [CrossRef]
- Ranjandish Laleh, R.; Savaloni, H.; Abdi, F.; Abdi, Y. Corrosion inhibition enhancement of Al alloy by graphene oxide coating in NaCl solution. Prog. Org. Coat. 2019, 127, 300–307. [Google Scholar] [CrossRef]
- Smith, F.; Brownlie, F.; Hodgkiess, T.; Toumpis, A.; Pearson, A.; Galloway, A.M. Effect of salinity on the corrosive wear behavior of engineering steels in aqueous solutions. Wear 2020, 462, 203515. [Google Scholar] [CrossRef]
- Wang, S.; Guo, T.; Xu, G.; Ding, F. Corrosion behavior and mechanism of electric arc-sprayed Al–Mg coating and Zn–Al–Mg pseudo-alloy coatings. Surf. Coat. Technol. 2023, 475, 130126. [Google Scholar] [CrossRef]
- Jiang, Q.; Miao, Q.; Liang, W.P.; Ying, F.; Tong, F.; Xu, Y.; Ren, B.L.; Yao, Z.J.; Zhang, P.Z. Corrosion behavior of arc sprayed Al–Zn–Si–Re coatings on mild steel in 3.5 wt% NaCl solution. Electrochim. Acta 2014, 115, 644–656. [Google Scholar] [CrossRef]
- Deslouis, C.; Festy, D.; Tribollet, B. Characterization of calcareous deposits in artificial seawater by impedance techniques: 1—Deposit of CaCO3 without Mg(OH)2. Electrochim. Acta 1998, 43, 1891–1901. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg, Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corros. Sci. 2015, 90, 472–481. [Google Scholar] [CrossRef]
- Park, I.C.; Kim, S.J. Cavitation damage behavior in seawater for Al–Mg alloy arc thermal spray coating with Mg content. Acta Phys. Pol. A 2016, 129, 572–577. [Google Scholar] [CrossRef]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of pH on the in vitro corrosion rate of magnesium degradable implant material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- McMahon, M.; Krojenic, A.; Scully, J.R.; Burns, J. Mechanistic insight into Al–Zn, Mg, and Al–Mg rich primer design for enhanced cathodic prevention on sensitized Al–Mg alloys. Corrosion 2023, 79, 647–664. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corr. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Salvago, G.; Maffi, S.; Magagnin, L.; Benedetti, A.; Pasqualin, S.; Olzi, E. Calcareous deposits, hydrogen evolution and pH on structures under cathodic polarization in seawater. Proc. Int. Offshore Polar Eng. Conf. 2003, 13, 353–359. [Google Scholar]
- Martin, A.; Texier-Mandoki, N.; Crusset, D.; Sabot, R.; Creus, J.; Refait, P. Corrosion behavior and sacrificial properties of Zn and Zn-Al coatings in conditions simulating deep geological disposal of radioactive waste at 80 °C. Coatings 2022, 12, 1044. [Google Scholar] [CrossRef]
- Singh, J.K.; Mandal, S.; Adnin, R.J.; Lee, H.-S.; Yang, H.-M. Role of coating processes on the corrosion kinetics and mechanism of zinc in artificial seawater. Materials 2021, 14, 7464. [Google Scholar] [CrossRef]


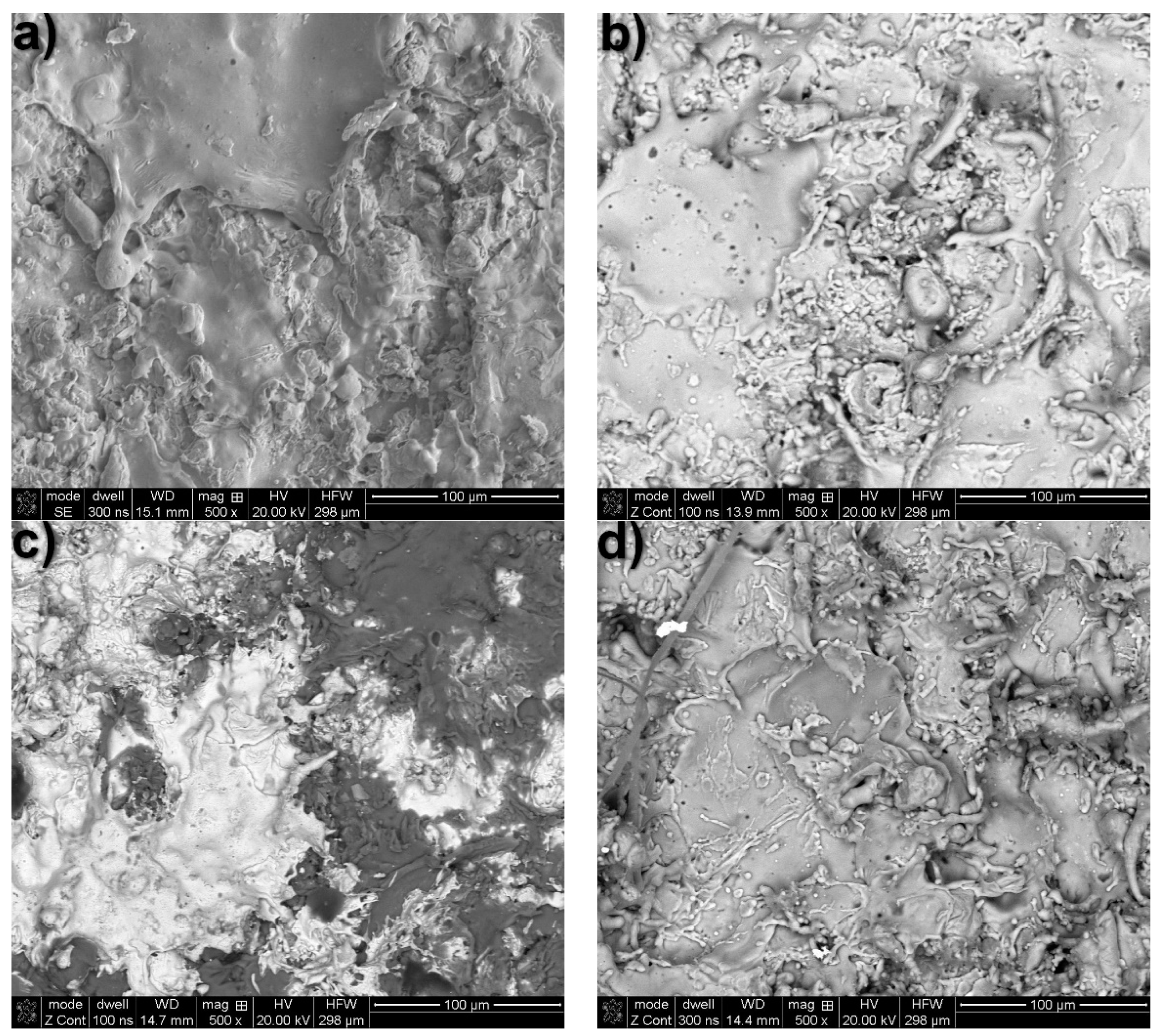
| Element | C | P | N | S | Fe |
|---|---|---|---|---|---|
| wt. % | 0.18 | 0.013 | 0.009 | 0.027 | rem. |
| Arc Spraying Parameters | Feedstock Wire Materials Used in Thermal Arc-Spraying Experiments | |||
|---|---|---|---|---|
| Zn | Al | Zn15Al | Al5Mg | |
| Atomising gas pressure [MPa] | 0.65 | 0.65 | 0.65 | 0.65 |
| Arc current [A] | 50 | 50 | 100 | 50 |
| Arc voltage [V] | 20 | 36 | 25 | 36 |
| Power input [kW] | 1 | 1.8 | 2.5 | 1.8 |
| Distance spraying [mm] | 150–250 (for all types of coatings) | |||
| Compound | Mass of Compound per Litre of Solution [g] (Salinity 30 g/L) | Mass of Compound per Litre of Solution [g] (Salinity 7 g/L) |
|---|---|---|
| NaCl | 20.5 | 4.78 |
| MgCl2 | 4.3 | 1 |
| Na2SO4 | 3.4 | 0.79 |
| CaCl2 | 1.0 | 0.23 |
| KCl | 0.6 | 0.14 |
| NaHCO3 | 0.2 | 0.05 |
| Solution Number | Salinity (g/L) | pH |
|---|---|---|
| 1 | 30 | 8 |
| 2 | 30 | 7 |
| 3 | 7 | 8 |
| 4 | 7 | 7 |
| Arc–Wire Sprayed Coatings | Thickness (μm) | Porosity (%) | Roughness Ra (µm) | Phase Composition (XRD Patterns) |
|---|---|---|---|---|
| Zn | 430 ± 23 | 0.3 ± 0.1 | 9.73 | zinc, zinc oxides |
| Al | 710 ± 19 | 2.5 ± 0.2 | 12.20 | aluminium, α–Al2O3 |
| Zn15Al | 592 ± 32 | 0.35 ± 0.1 | 12.59 | zinc, ZnO, α–Al2O3 |
| Al5Mg | 250 ± 19 | 0.4 ± 0.15 | 12.06 | aluminium {Mg(Al) α–phase}, MgO |
| Designation of Grain Area according to Figure 1, Figure 2, Figure 3 and Figure 4 | Zn | Al | Mg | O |
|---|---|---|---|---|
| Zn coating—Figure 1 | ||||
| mapping | ~96.0 | − | − | ~4.0 |
| Al coating—Figure 2 | ||||
| mapping | − | ~97.5 | − | ~2.5 |
| Zn15Al coating—Figure 3 | ||||
| mapping | 56.0 | 42.0 | − | ~2.0 |
| Al5Mg—Figure 4b | ||||
| 1—grey | − | 68.8 | 2.0 | ~29.2 |
| 2—dark grey | − | 94.3 | 1.5 | ~4.2 |
| 3—light grey | − | 96.7 | 1.0 | ~2.3 |
| mapping | − | 95.0 | 2.0 | ~3.0 |
| Zn | ||||
|---|---|---|---|---|
| Solution No | Eocp (V) | Ecorr (V) | Icorr (µA × cm−2) | r (g × m−2 × y−1) |
| 1 | –1 ± 0.01 | –1 ± 0.01 | 3.149 ± 0.923 | 334.5 ± 98 |
| 2 | –1.04 ± 0.02 | –1.06 ± 0.03 | 3.525 ± 0.940 | 374.5 ± 100 |
| 3 | –1 ± 0.01 | –1.01 ± 0.01 | 2.068 ± 0.731 | 220 ± 78 |
| 4 | –1.02 ± 0.02 | –1.03 ± 0.02 | 3.048 ± 0.701 | 324 ± 75 |
| Al | ||||
| Solution No | Eocp (V) | Ecorr (V) | Icorr (µA × cm−2) | r (g × m−2 × y−1) |
| 1 | –0.7 ± 0.02 | –0.68 ± 0.04 | 0.186 ± 0.028 | 5 ± 0.8 |
| 2 | –0.69 ± 0.02 | –0.7 ± 0.03 | 0.271 ± 0.037 | 29 ± 2.3 |
| 3 | –0.69 ± 0.04 | –0.7 ± 0.07 | 0.069 ± 0.006 | 2 ± 0.4 |
| 4 | –0.7 ± 0.04 | –0.73 ± 0.06 | 0.093 ± 0.020 | 2.73 ± 2 |
| Zn15Al wt.% | ||||
| Solution no | Eocp (V) | Ecorr (V) | Icorr (µA × cm−2) | r (g × m−2 × y−1) |
| 1 | –0.99 ± 0.01 | –0.99 ± 0.02 | 2.410 ± 0.125 | 211 ± 11 |
| 2 | –1.01 ± 0.01 | –1.02 ± 0.04 | 1.919 ± 0.041 | 227 ± 1.2 |
| 3 | –1.02 ± 0.01 | –1.05 ± 0.05 | 1.175 ± 0.17 | 108.5 ± 1.6 |
| 4 | –0.99 ± 0.01 | –0.99 ± 0.03 | 1.430 ± 0.37 | 125.6 ± 3.2 |
| Al5Mg wt.% | ||||
| Solution no | Eocp(V) | Ecorr (V) | Icorr (µA × cm−2) | r (g × m−2 × y−1) |
| 1 | –0.72 ± 0.01 | –0.73 ± 0.01 | 0.209 ± 0.013 | 6.1 ± 0.4 |
| 2 | –0.68 ± 0.07 | –0.66 ± 0.07 | 0.168 ± 0.007 | 5.3 ± 0.2 |
| 3 | –0.69 ± 0.02 | –0.7 ± 0.01 | 0.155 ± 0.011 | 5.1 ± 0.3 |
| 4 | –0.7 ± 0.01 | –0.7 ± 0.03 | 0.178 ± 0.016 | 5.2 ± 0.5 |
| S235R | ||||
| Solution no | Eocp(V) | Ecorr (V) | Icorr (µA × cm−2) | r (g × m−2 × y−1) |
| 1 | −0.51 ± 0.01 | −0.52 ± 0.01 | 0.225 ± 0.013 | 13.76 ± 0.81 |
| 2 | −0.52 ± 0.02 | −0.52 ± 0.01 | 0.246 ± 0.014 | 15.02 ± 0.89 |
| 3 | −0.40 ± 0.02 | −0.41 ± 0.02 | 0.102 ± 0.006 | 6.25 ± 0.37 |
| 4 | −0.41 ± 0.02 | −0.41 ± 0.02 | 0.143 ± 0.008 | 8.76 ± 0.52 |
| Zn | ||
|---|---|---|
| Solution No. | R1 [Ω × cm2] | R2 [Ω × cm2] |
| 1 | 1.5 ± 0.1 | 167.4 ± 27.9 |
| 2 | 1.3 ± 0.2 | 62.2 ± 0.8 |
| 3 | 1.8 ± 0.1 | 119.0 ± 5.7 |
| 4 | 0.3 ± 0.1 | 201.3 ± 1.3 |
| Al | ||
| Solution no. | R1 [Ω × cm2] | R2 [Ω × cm2] |
| 1 | 23.5 ± 1.2 | 31,140 ± 4 |
| 2 | 36.3 ± 0.2 | 21,820 ± 0.8 |
| 3 | 59.9 ± 0.1 | 35,714 ± 5.7 |
| 4 | 29 ± 0.1 | 51,994 ± 13 |
| Zn15Al | ||
| Solution no. | R1 [Ω × cm2] | R2 [Ω × cm2] |
| 1 | 4.1 ± 0.5 | 555.3 ± 78.5 |
| 2 | 0.1 ± 0.02 | 251.3 ± 16 |
| 3 | 40.1 ± 5.5 | 73.6 ± 12.5 |
| 4 | 3.7 ± 0.6 | 229.7 ± 7.3 |
| Al5Mg | ||
| Solution no. | R1 [Ω × cm2] | R2 [Ω × cm2] |
| 1 | 7.4 ± 1.3 | 71,775 ± 472 |
| 2 | 32.7 ± 5.6 | 83,236 ± 135 |
| 3 | 5.1 ± 0.05 | 42,468 ± 258 |
| 4 | 25.9 ± 4.4 | 247,097 ± 345 |
| S235R | ||
| Solution no. | R1 [Ω × cm2] | R2 [Ω × cm2] |
| 1 | 0 | 32,602 ± 136 |
| 2 | 0 | 32,158 ± 134 |
| 3 | 0 | 131,074 ± 547 |
| 4 | 0 | 69,980 ± 292 |
| Salinity | Electrochemical Parameter | |||||
|---|---|---|---|---|---|---|
| Coating | Estat | Ecorr | Icorr | r | R1 | R2 |
| Al | 0.0 N | 1.9 N | 158.9 S | 50.3 S | 0.8 S | 92.6 S |
| Zn | 3.2 S | 3.0 S | 776.7 S | 253.3 S | 0.5 S | 5.5 S |
| Zn15Al | 1.8 N | 2.2 N | 897.0 S | 266.2 S | 3.5 N | 12.9 S |
| Al5Mg | 5.7 S | 6.9 S | 131.4 S | 25.2 S | 2.1 N | 207.0 S |
| pH | Electrochemical parameter | |||||
| Coating | Estat | Ecorr | Icorr | r | R1 | R2 |
| Al | 1.1 N | 2.1 N | 96.7 S | 45.5 S | 1.0 N | 92.6 S |
| Zn | 4.6 S | 5.5 S | 724.7 S | 236.0 S | 0.7 N | 2.8 N |
| Zn15Al | 3.6 S | 3.6 S | 637.2 S | 203.2 S | 3.5 S | 7.1 S |
| Al5Mg | 5.2 S | 4.9 S | 80.5 S | 18.1 S | 4.1 S | 223.1 S |
| Salinity | ||
|---|---|---|
| Coating | Icorr | r |
| Al | 73.83 | 7.40 |
| Zn | 389.43 | 41.41 |
| Al5Mg | –17.07 | –0.63 |
| Zn15Al | 421.71 | 37.15 |
| pH | ||
| Coating | Icorr | r |
| Al | –27.34 | –3.04 |
| Zn | –339.23 | –35.99 |
| Al5Mg | 6.40 | 0.32 |
| Zn15Al | 212.68 | 21.67 |
| Analysis Area according to Figure 8a–d | Content, at.% | ||||||
|---|---|---|---|---|---|---|---|
| Zn | Al | Mg | O | Cl | K | Ca | |
| Zn coating—Figure 8a | |||||||
| mapping | ~94.7 | − | − | ~4.9 | ~0.18 | ~0.22 | − |
| Al coating—Figure 8b | |||||||
| mapping | − | ~89.4 | − | ~9.7 | ~0.33 | ~0.14 | ~0.43 |
| Zn15Al coating—Figure 8c | |||||||
| mapping | ~35 | ~55 | − | ~9 | ~0.37 | ~0.12 | ~0.5 |
| Al5Mg—Figure 8d | |||||||
| mapping | − | ~92.0 | ~1.8 | ~5.6 | ~0.54 | ~0.06 | ~0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senderowski, C.; Rejmer, W.; Vigilianska, N.; Jeznach, A. Changes in Corrosion Behaviour of Zinc and Aluminium Coatings with Increasing Seawater Acidification. Materials 2024, 17, 536. https://doi.org/10.3390/ma17030536
Senderowski C, Rejmer W, Vigilianska N, Jeznach A. Changes in Corrosion Behaviour of Zinc and Aluminium Coatings with Increasing Seawater Acidification. Materials. 2024; 17(3):536. https://doi.org/10.3390/ma17030536
Chicago/Turabian StyleSenderowski, Cezary, Wojciech Rejmer, Nataliia Vigilianska, and Arkadiusz Jeznach. 2024. "Changes in Corrosion Behaviour of Zinc and Aluminium Coatings with Increasing Seawater Acidification" Materials 17, no. 3: 536. https://doi.org/10.3390/ma17030536
APA StyleSenderowski, C., Rejmer, W., Vigilianska, N., & Jeznach, A. (2024). Changes in Corrosion Behaviour of Zinc and Aluminium Coatings with Increasing Seawater Acidification. Materials, 17(3), 536. https://doi.org/10.3390/ma17030536







