Assessment of Corrosion and Cavitation Resistance of Laser Remelted GX40CrNiSi25-20 Cast Stainless Steel
Abstract
1. Introduction
- An incubation period, during which there are no measurable material losses, and the material undergoes elastic or plastic deformations, potentially forming some microcracks;
- An accumulation zone associated with an increase in the rate of material loss due to the propagation of cracks in the mechanically worked material;
- A mitigation region where the rate of mass loss is decreasing;
- A stable equilibrium state where the erosion rate is constant.
2. Materials, Experimental Details
- -
- Ultrasonic Generator Settings: The ultrasonic generator was set to produce vibrations with a double amplitude of 50 μm and a frequency of 20.000 ± 2% Hz. An electrical power of 500 W was supplied to the generator to generate the required ultrasonic vibrations;
- -
- Cavitating Liquid: Water from the public network was used as the cavitating liquid. The temperature of the water was maintained at 22 ± 1 °C throughout the experiment;
- -
- Sample Preparation: Before subjecting the samples to cavitation attack, their surfaces were polished to achieve a specified roughness of Rz = 0.2 ÷ 0.8 µm. This ensured uniformity in surface conditions for all samples;
- -
- Measurement Procedure: Each sample was weighed at regular time intervals using a Zatklady analytical balance with a precision of 10–5 g. This allowed for the measurement of mass loss due to cavitation erosion over time;
- -
- Test Duration and Periods: The cavitation test duration was set to 165 min. This duration was divided into 12 periods (∆t), with each period consisting of intervals of 5 min, 10 min, and 15 min. This division allowed for monitoring the progression of cavitation erosion at different time intervals;
- -
- Computer-Controlled Testing: The entire testing program was conducted using specialized software developed for this purpose, which enabled precise control and monitoring of the experimental parameters and data acquisition.
- -
- Electrochemical Testing: A corrosion cell was utilized for the experimentation, consisting of the SP-150 galvanostat (Biologic, Seyssinet-Pariset, France). Three electrodes were used in the cell: the reference electrode (calomel), the auxiliary electrode (platinum electrode), and the working electrode (sample). For testing, 20 mm in diameter and 5 mm in thickness disc-shaped samples were made. These samples were ground and mirror-polished on the testing surface to provide a uniform and smooth surface finish. After preparation, the samples were degreased and ultrasonically cleaned in ethanol for 15 min to remove any contaminants or residues. The tested surface, which was in contact with the corrosive media, was 1 cm2. Using a rate of 0.166 mV/s, the applied potential was adjusted between −2500 and 2500 mV before the potentiodynamic polarisation tests were conducted. To guarantee a steady state, the samples were left at their open circuit potential for 30 min. All electrochemical measurements were performed at least three times to guarantee the results’ repeatability. Polarisation curves were drawn in a 3.5% NaCl and 0.5 M H2SO4 solution at ambient temperature. By using both corrosive media, researchers can compare how a material performs under different corrosive conditions: one simulating saltwater exposure and the other testing resistance to acid corrosion. This helps obtain a comprehensive understanding of the material’s corrosion behavior in various real-world environments;
- -
- Microstructural Analysis: Cross-sectional slices of the samples, which were subjected to both cavitation and corrosion testing, were prepared for microstructural studies. The microstructure of the samples was examined using optical microscopy (Leica DM 2700 M) and scanning electron microscopy (TESCAN VEGA 3 LMU) with Bruker EDX Quantax. These techniques allowed for the visualization of the internal structure and any changes induced by cavitation and corrosion;
- -
- X-ray diffraction analysis (Philips X’Pert Diffractometer, PANalytical, The Netherlands) was performed using Cu Kα radiation generated at 40 kV and 30 mA to identify the phases present in the structure;
- -
- Surface Hardness Measurement: Surface hardness was measured using the Vickers HVS-10A1 instrument (from ZwickRoell, Ulm, Germany) at a load of 5 daN and a dwell time of 15 s. This provided information about the mechanical properties of the samples and any changes resulting from the laser remelting process.
3. Discussing the Experimental Results
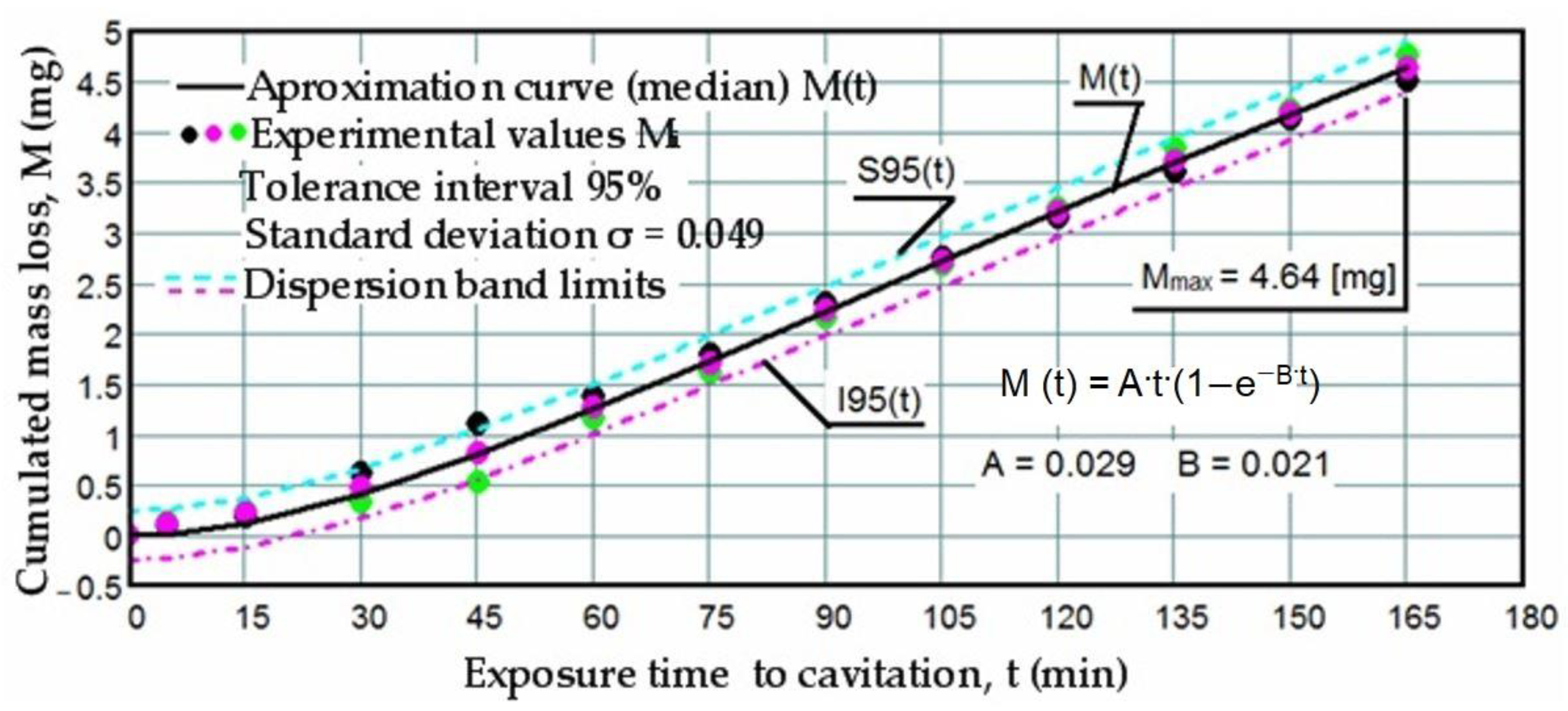
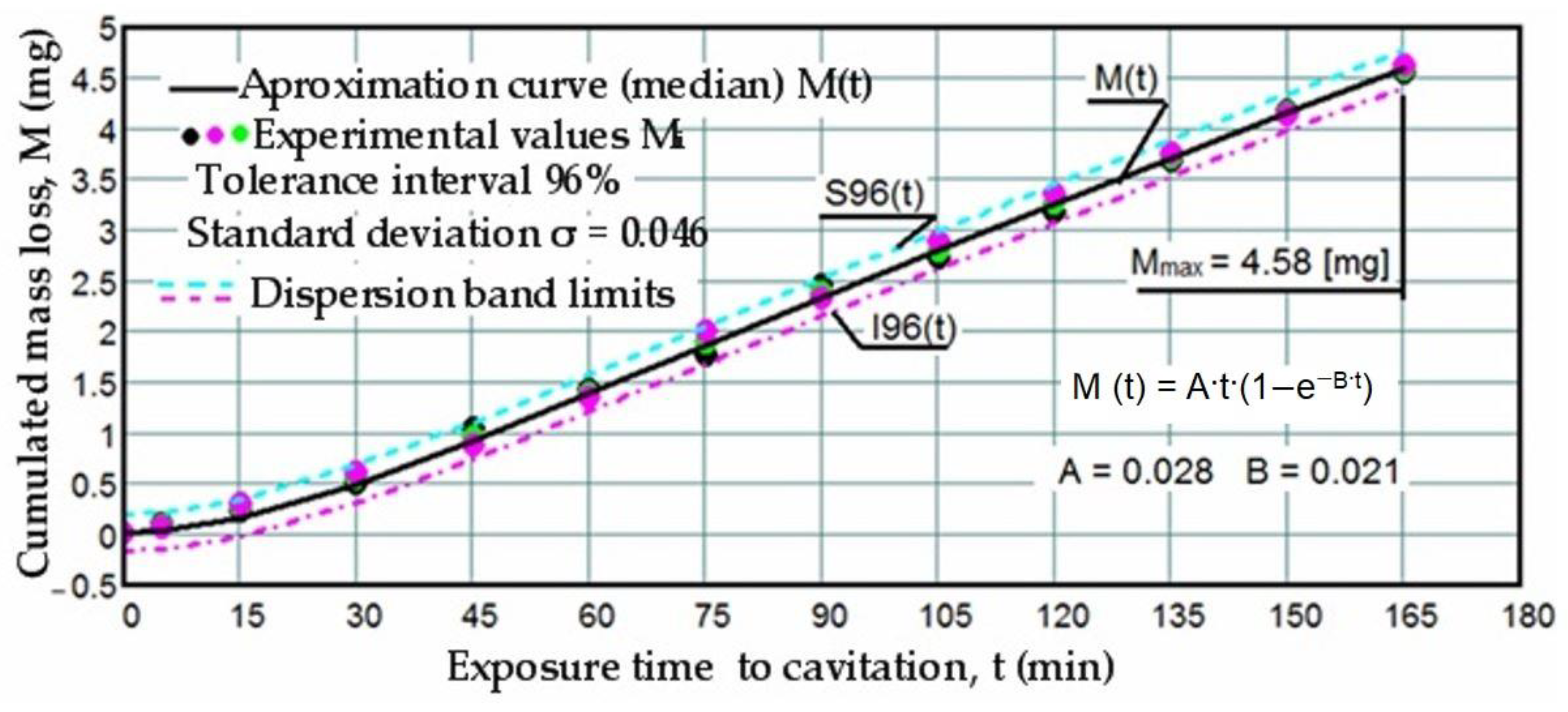
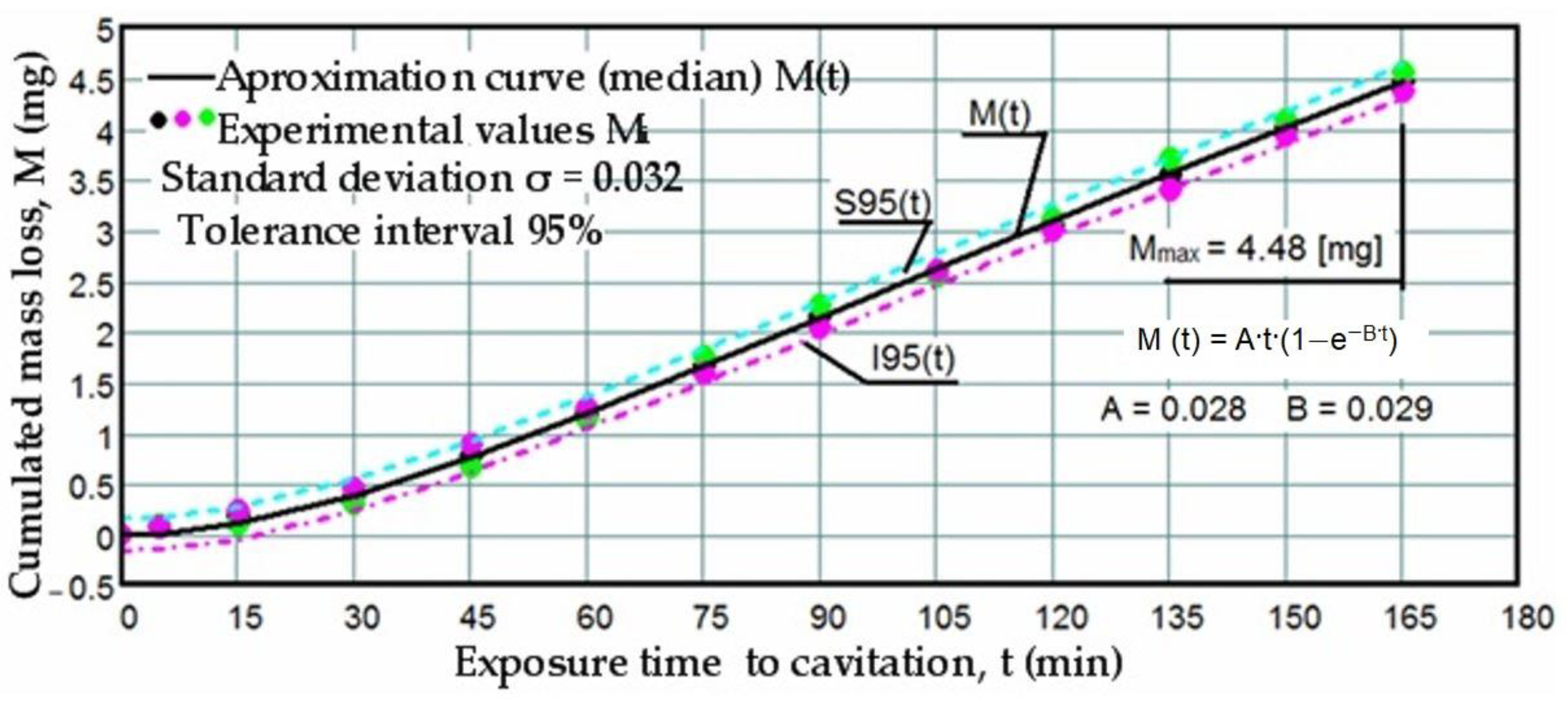
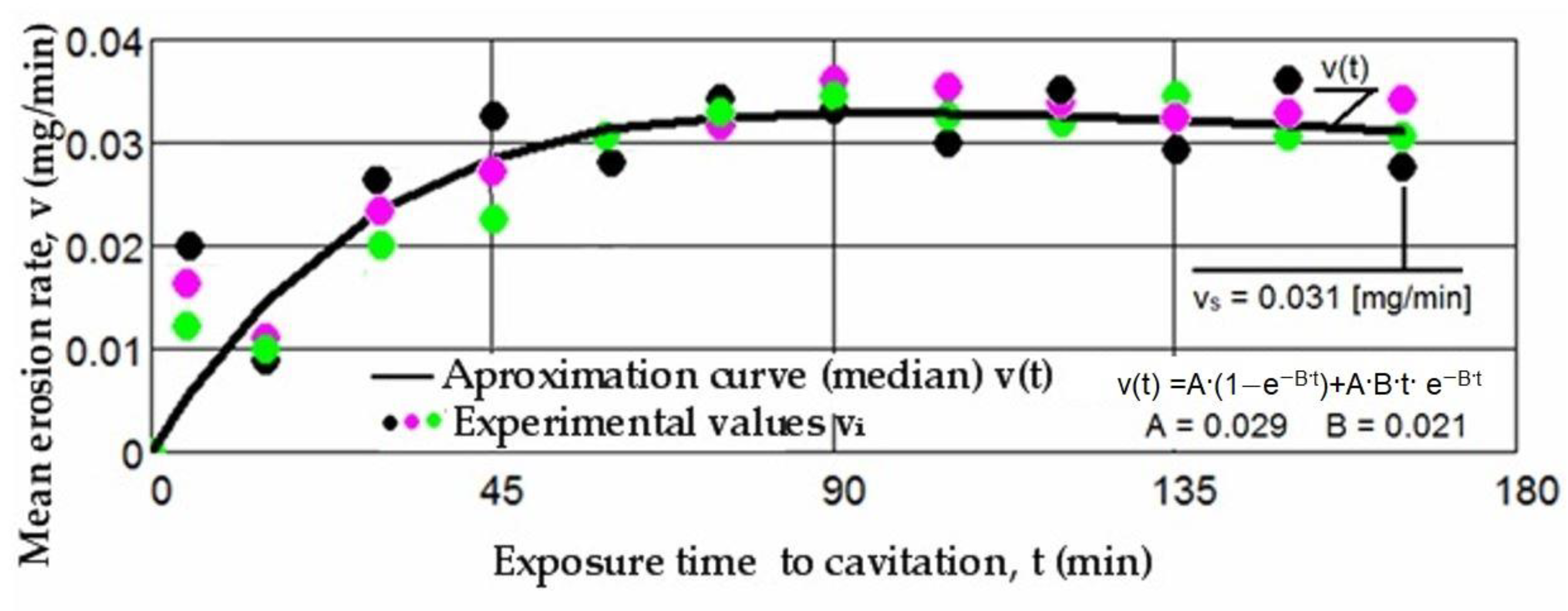
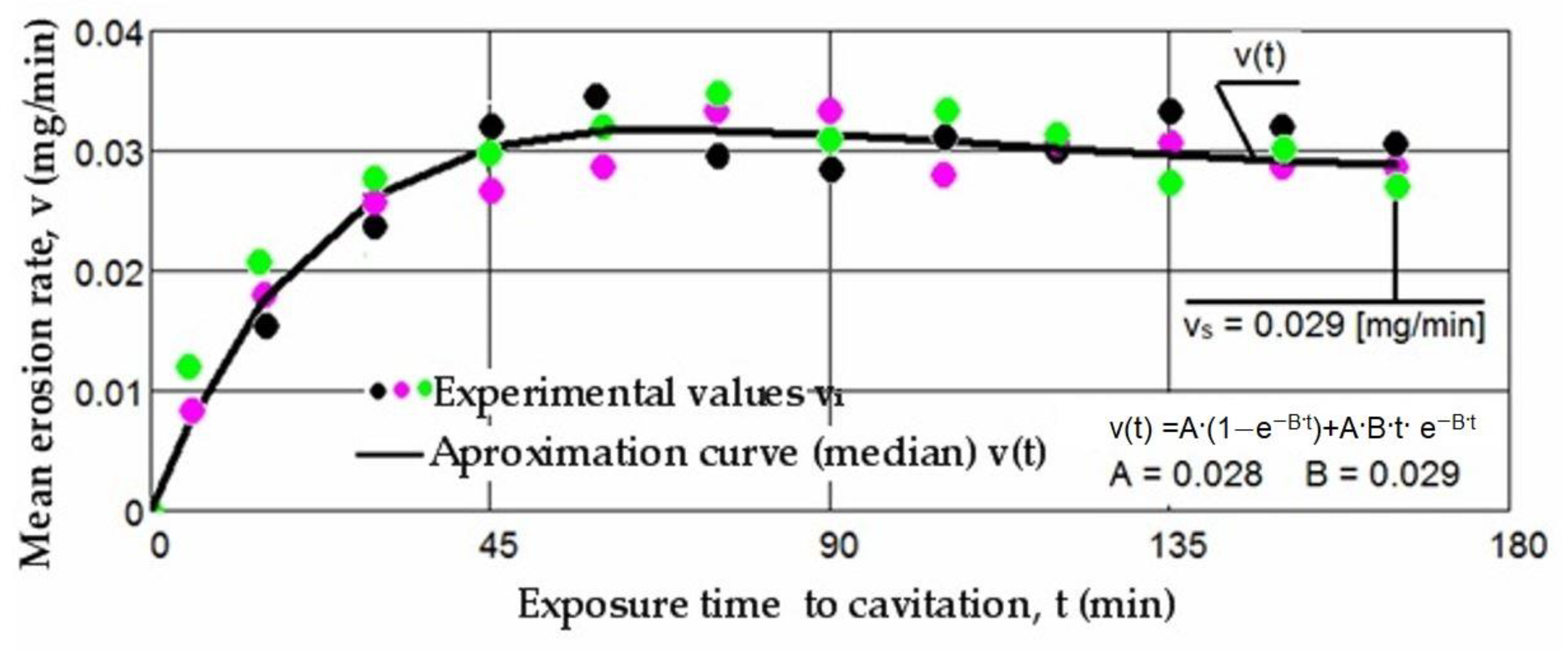
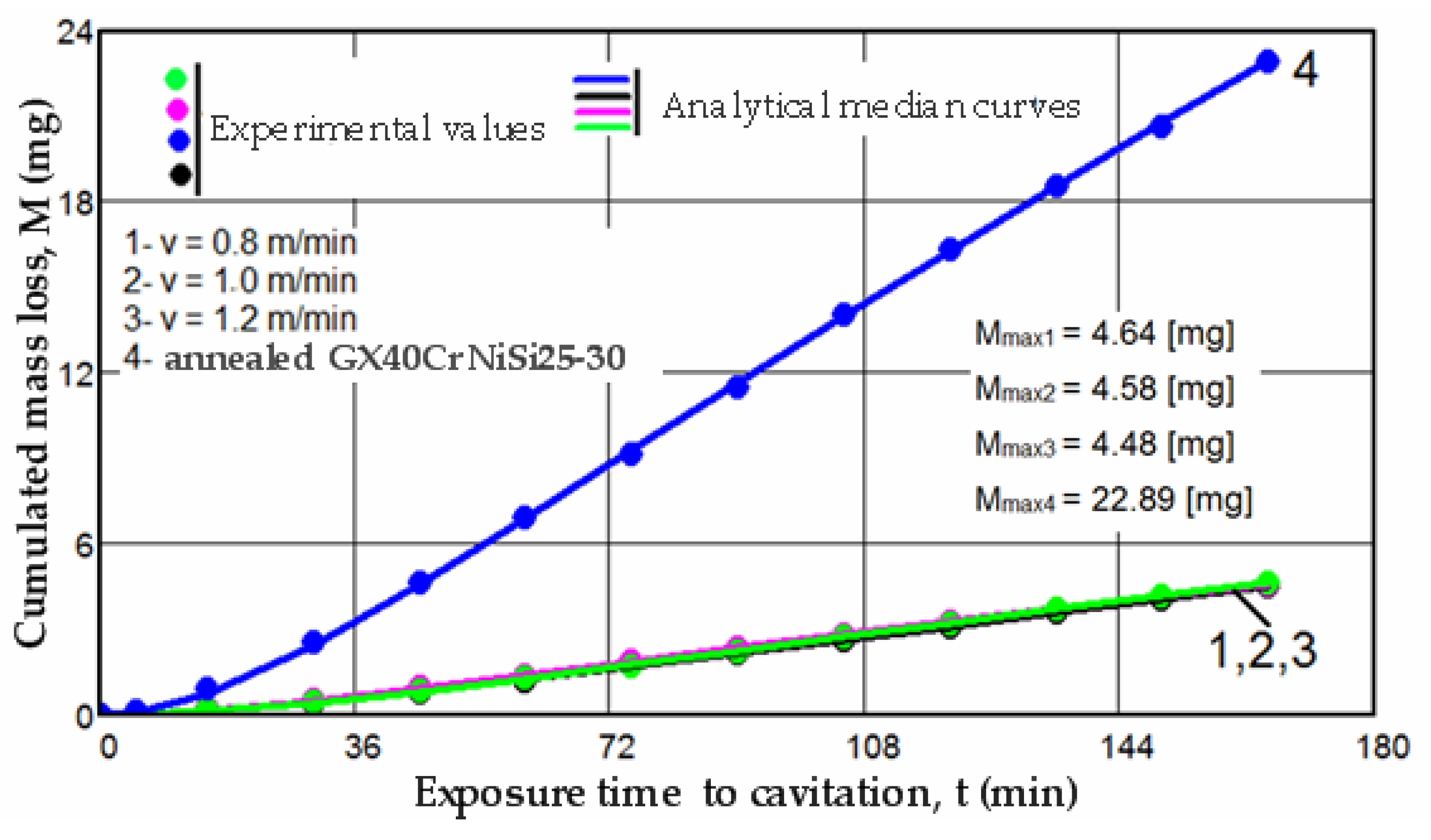
3.1. Cavitation Curves
- -
- Mi—is the experimental value of the cumulative eroded mass lost over the time ti = ∑Δti of the cavitation attack;
- -
- Δmi—is the mass of material lost through cavitation erosion during the intermediate period “i” of time, Δti (for i = 1 time is Δt1 = 5 min, for i = 2 times is Δt2 = 15 min, and from i = 3 to i = 12 times, Δti increases by 15 min each period).
- -
- vi—is the rate value corresponding to the mass loss during the period “i”.
- -
- The precision of measurements was conducted on sets of three samples, with stringent control of parameters determining the intensity of vibrational cavitation. This precision is evidenced by the standard deviations (σ) ranging from 0.032 to 0.049 and the dispersion range of values corresponding to tolerance intervals of 95–96%, irrespective of the structural condition of the samples;
- -
- Similar behaviors were exhibited by the three sets of samples, each corresponding to a distinct structural state, over the duration of cavitation exposure. This consistency indicates the accurate execution of the laser remelting technological process, with precise control of technological parameters such as scanning speed, laser beam diameter, and power. Consequently, similar hardness values are achieved on surfaces that are quickly remelted and solidified;
- -
- The comparable evolution of median curves M(t) suggests that surfaces remelted under the three regimes exhibit very similar cavitation resistance and behaviors, with minor discrepancies in hardness values;
- -
- Natural variations observed in the σ values imply that laser remelting at a speed of v = 1.2 m/min yields a microstructure that offers the most robust resistance to cavitation erosion.
- -
- The v(t) curves exhibit similar trends, initially increasing towards a maximum and then experiencing a slight decrease towards the stabilized value vs. this pattern, consistent with previous experiences, suggests that structures and mechanical properties resulting from remelting have acquired high resistance to the impact of cavitation microjets;
- -
- Very small differences between the experimental values, and in certain periods even identical values, reaffirm the conclusions drawn from the analysis of the data in Figure 5, Figure 6 and Figure 7. Specifically, they support the notion that each set of three samples from distinct structural states demonstrates approximately similar behaviors and resistances to vibrational cavitation solicitations throughout the attack duration.
- -
- The increase in resistance achieved by remelting at a scanning speed of 1.2 m/min, compared to samples processed at laser scanning speeds of 0.8 m/min and 1.0 m/min, is slightly higher, ranging from 2.2% to 3.5% when analyzed based on the maximum value of the cumulative mean mass (Mmax), and from 3.4% to 6.8% when analyzed based on the value vs. toward which the mean erosion rate tends to stabilize;
- -
- The increase in resistance achieved at a working speed of 1.0 m/min, compared to samples processed at a laser scanning speed of 0.8 m/min, is insignificant (only a 1.3% increase) when analyzed based on the maximum value of the cumulative mean mass (Mmax), and slightly higher (around 3.3%) when analyzed based on the value vs. toward which the mean erosion rate tends to stabilize.
- -
- Regardless of their type, whether M(t) or v(t), the trends of the mean curves and the level of experimental values, represented by the algebraic mean, indicate that surfaces hardened by laser remelting exhibit significantly superior behavior compared to those treated with bulk homogeneous annealing heat treatment;
- -
- The values of standard deviations (σ) show that the dispersions of the experimental values obtained on samples hardened by laser surface remelting are significantly smaller than those obtained with homogeneous annealing.
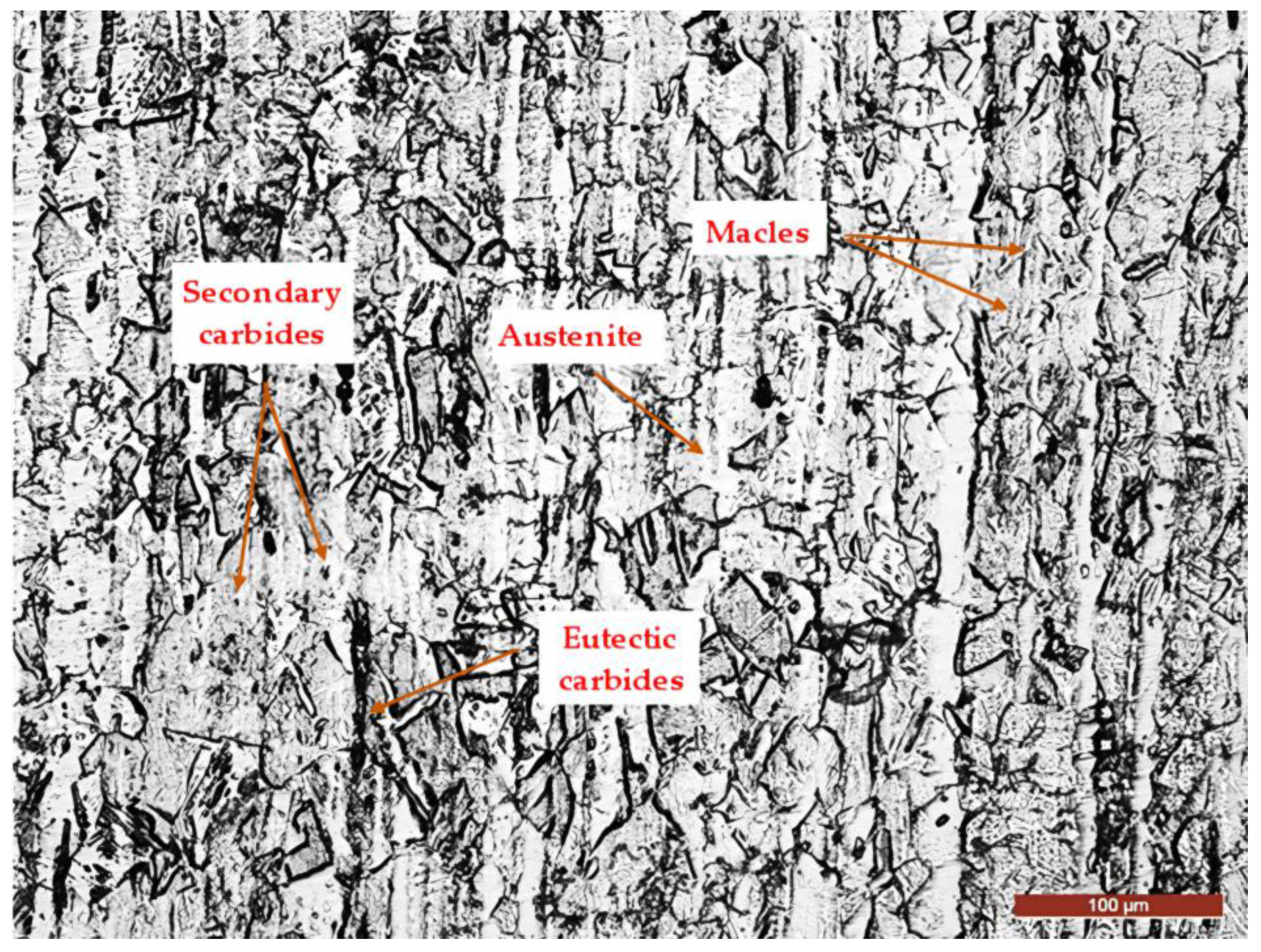
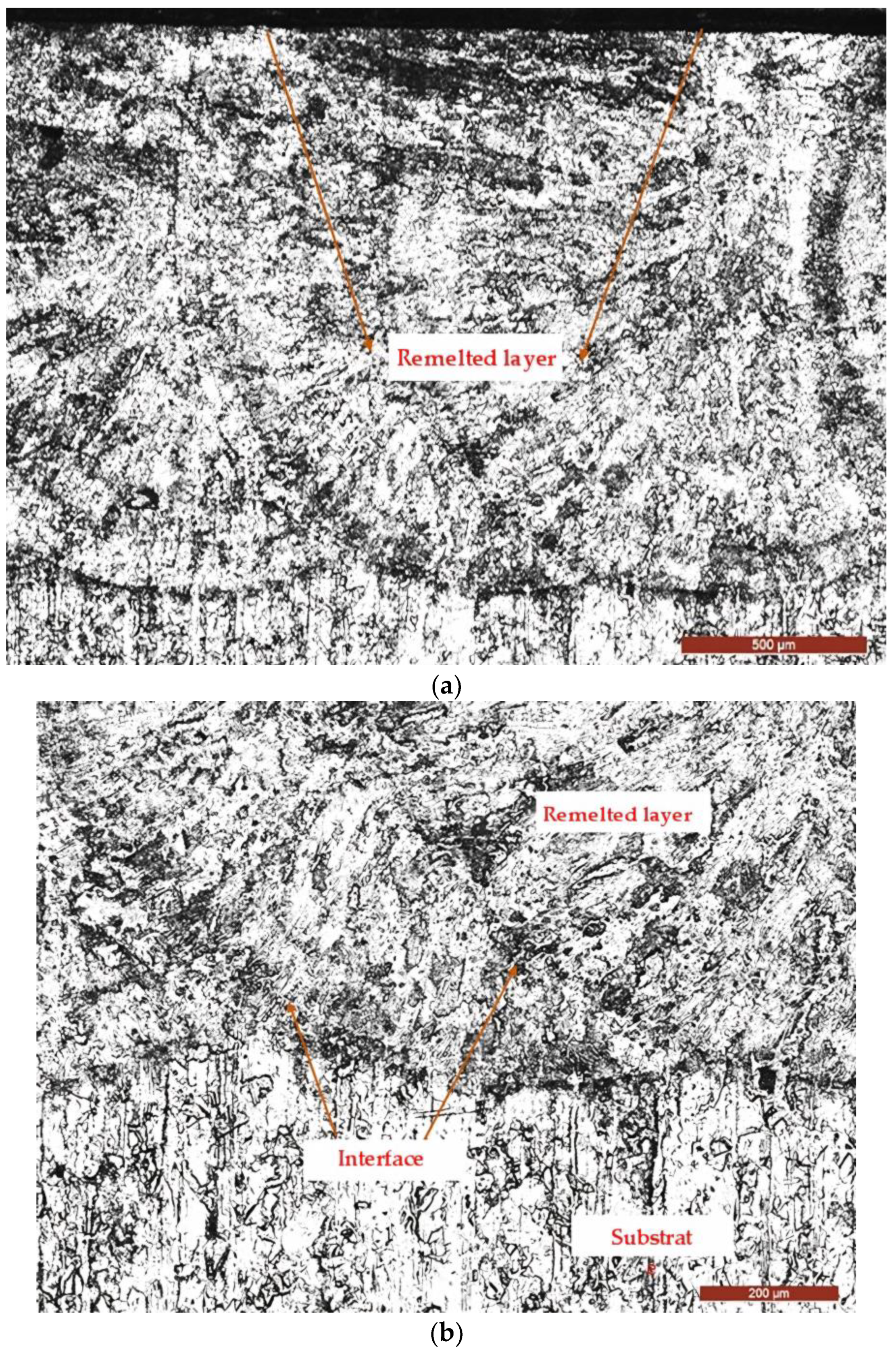
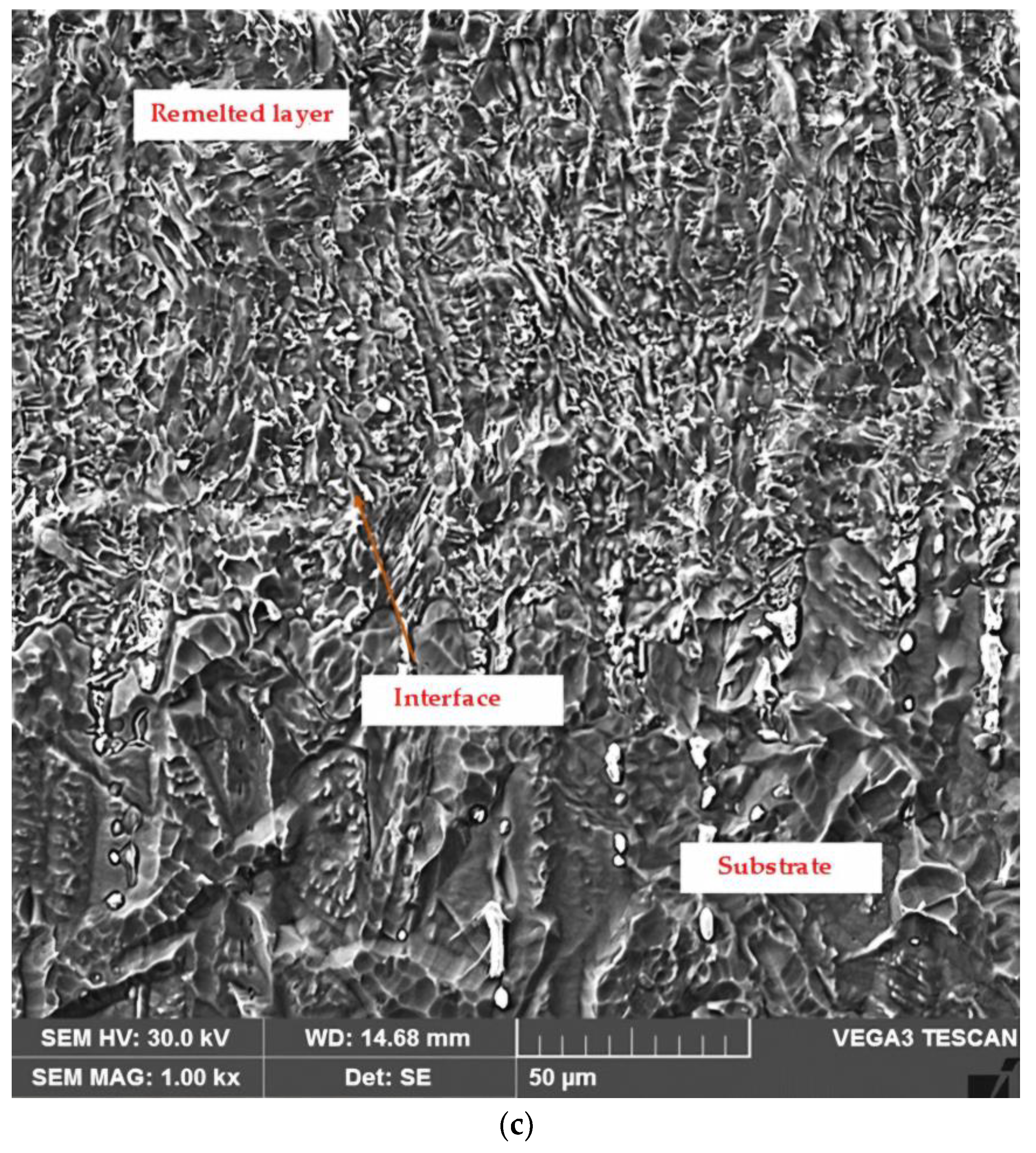
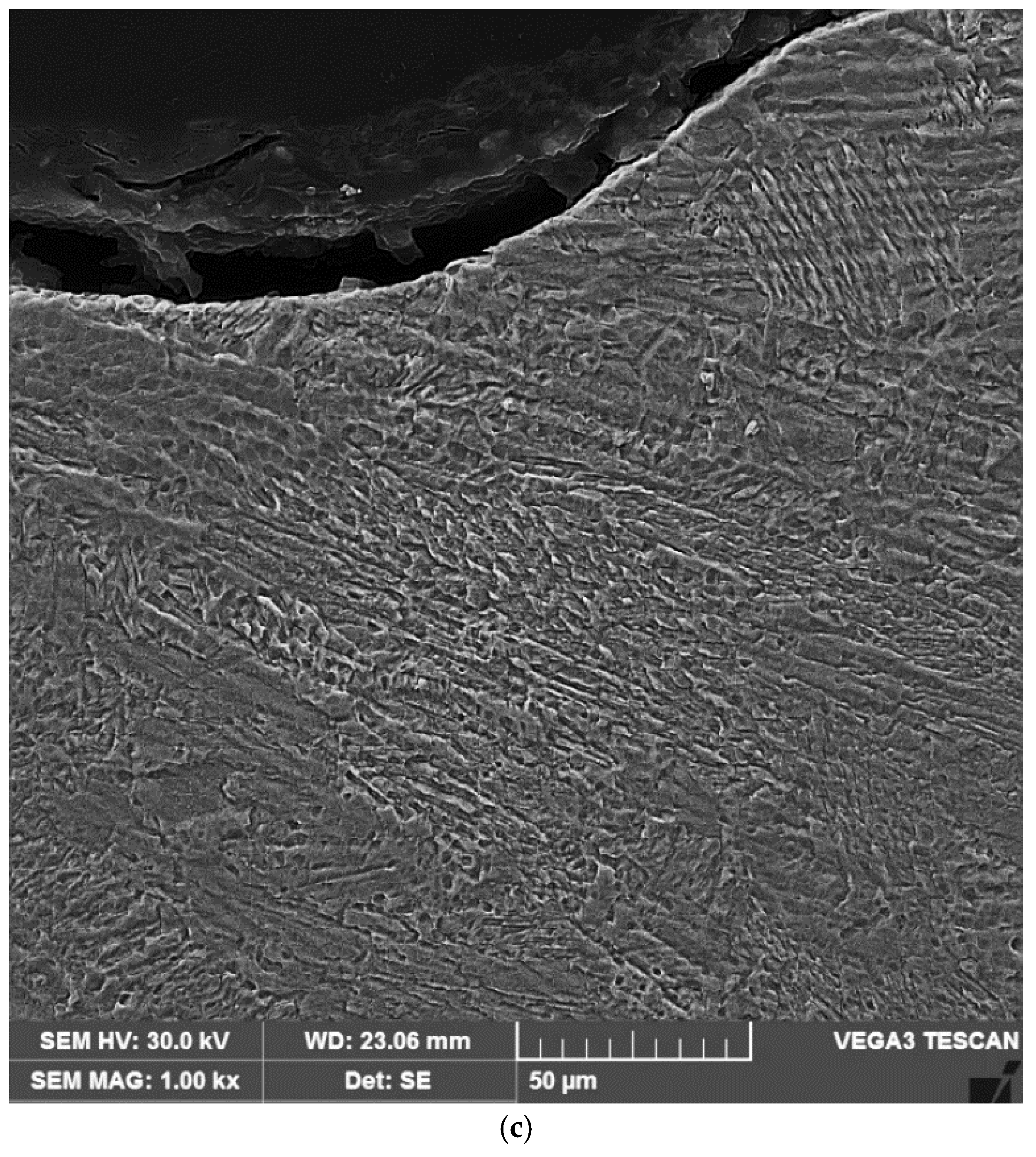
3.2. Microstructural Examinations
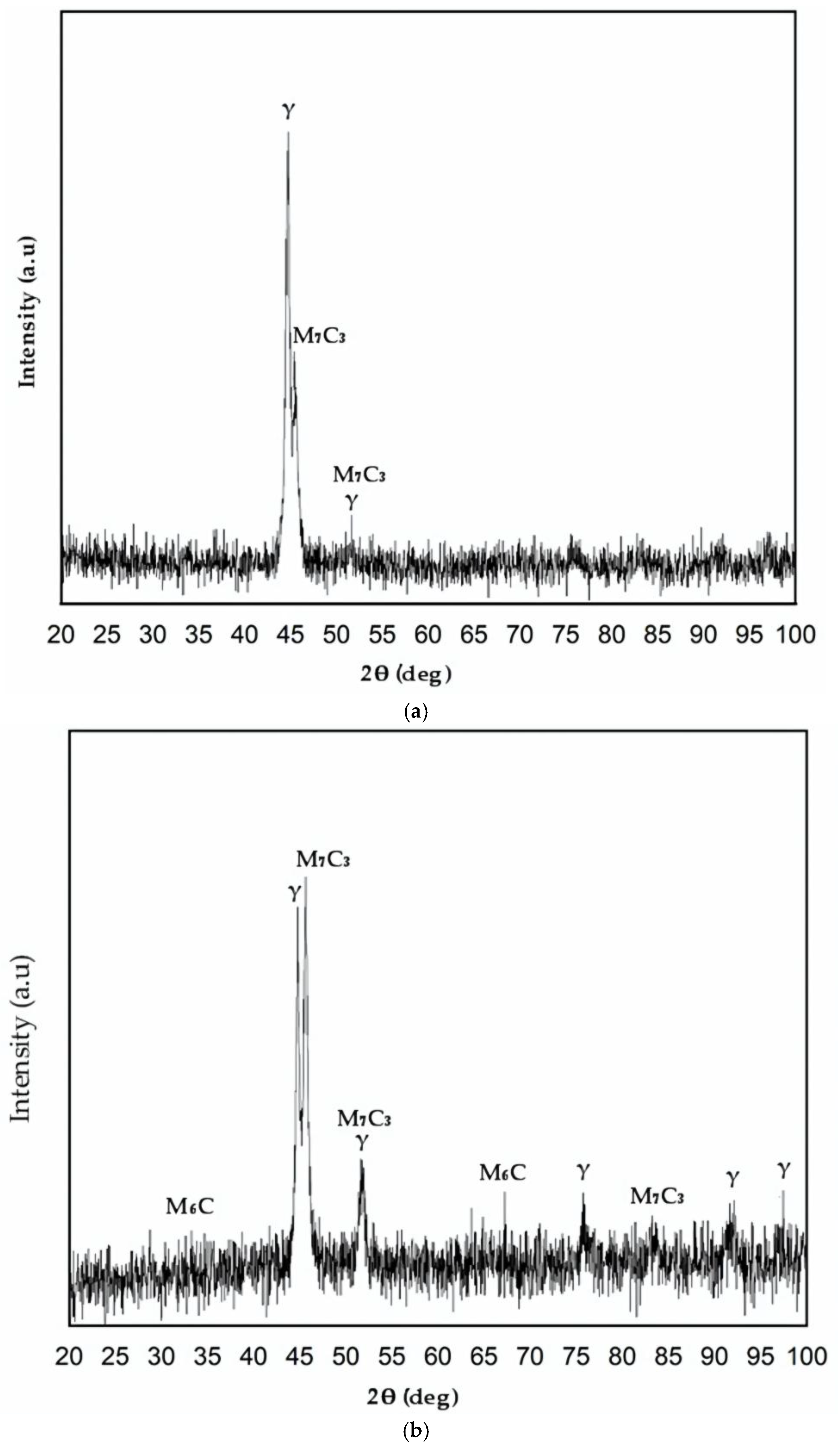
3.3. X-Ray Diffraction Analyses
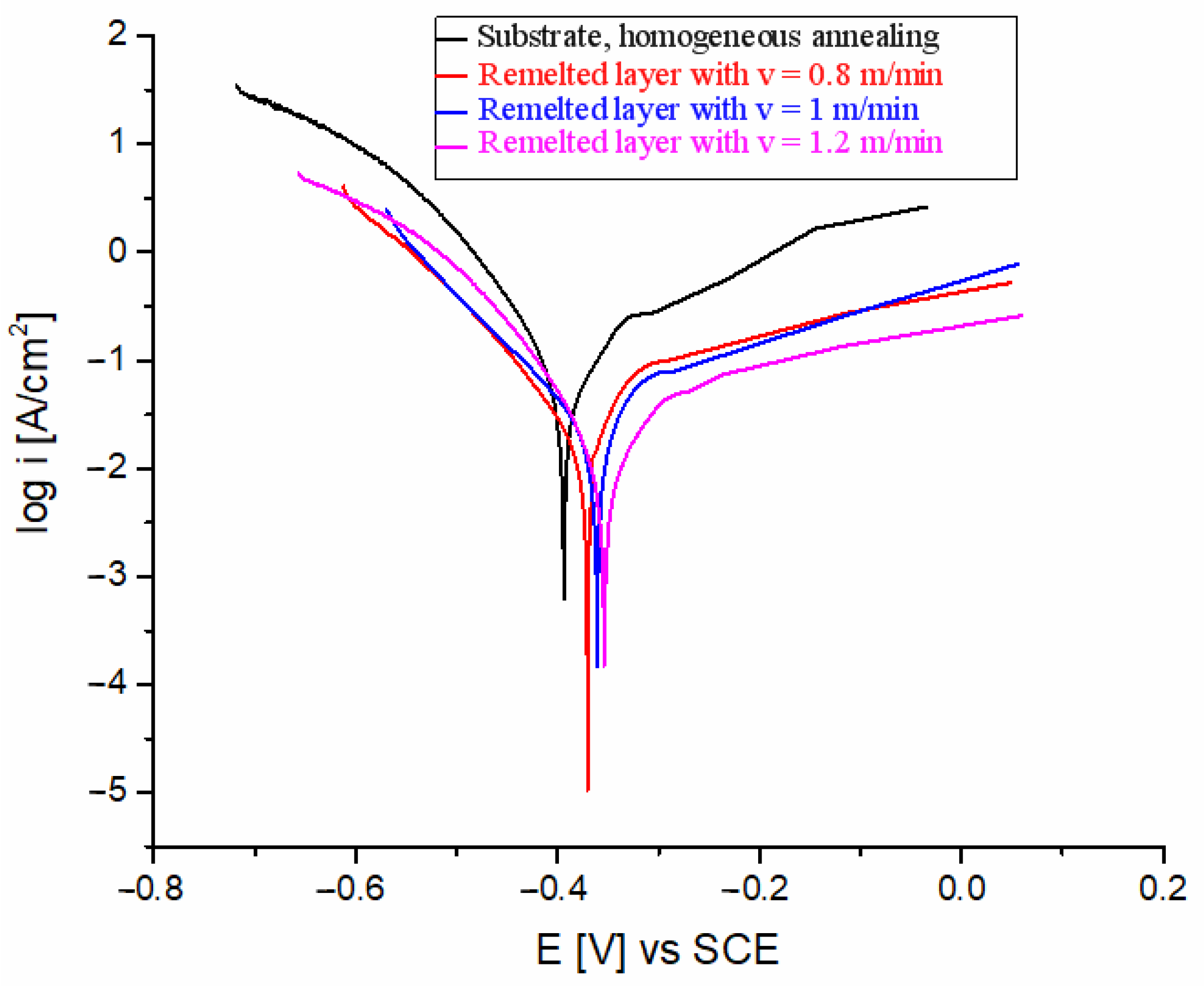
3.4. Corrosion Test Results
4. Conclusions
- Significantly improves resistance to cavitation erosion and electrochemical corrosion;
- Develops a metallurgical bond at the interface between the layer and the substrate, resulting in enhanced adhesion of the coating;
- Refines the microstructure and increases the hardness of the coating layer;
- Provides greater flexibility, such as shorter processing time, fewer thermal distortions, and minimal changes to the substrate’s microstructure.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, R.; Zhao, X.; Dong, L.; Liu, G.; Huang, Y.; Xu, Y. On the cavitation erosion-corrosion of pipeline steel at different locations of Venturi pipe. Eng. Fail. Anal. 2022, 138, 106333. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Cheng, F.T.; Man, H.C. Cavitation erosion resistance of AISI 316L stainless steel laser surface-modified with NiTi. Mater. Sci. Eng. A 2005, 392, 348–358. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Cavitation erosion and its effect on corrosion resistance of nuclear-grade Z3CN20–09M stainless steel. Mater. Des. 2022, 221, 110978. [Google Scholar] [CrossRef]
- Franc, J.-P.; Michel, J.-M. Fundamentals of Cavitation; Springer Netherlands: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Cottam, R.; Luzin, V.; Moody, H.; Edwards, D.; Majumdar, A.; Wong, Y.C.; Wang, J.; Brandt, M. The role of microstructural characteristics in the cavitation erosion behaviour of laser melted and laser processed Nickel–Aluminium Bronze. Wear 2014, 317, 56–63. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, C.; Zhang, T.; Yang, M.; Han, B.; Neville, A. Effects of laser surface melting on erosion–corrosion of X65 steel in liquid–solid jet impingement conditions. Wear 2016, 362–363, 39–52. [Google Scholar] [CrossRef]
- Hardes, C.; Pöhl, F.; Röttger, A.; Thiele, M.; Theisen, W.; Esen, C. Cavitation erosion resistance of 316L austenitic steel processed by selective laser melting (SLM). Addit. Manuf. 2019, 29, 100786. [Google Scholar] [CrossRef]
- Mesa, D.H.; Garzón, C.M.; Tschiptschin, A.P. Influence of cold-work on the cavitation erosion resistance and on the damage mechanisms in high-nitrogen austenitic stainless steels. Wear 2011, 271, 1372–1377. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Du, S.; He, Z.; Han, J.; Zhang, Y. Cavitation erosion behavior of GH 4738 nickel-based superalloy. Tribol. Int. 2021, 156, 106833. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B. Cavitation erosion mechanism and microstructural design of pure titanium. Wear 2022, 490–491, 204189. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B. In-situ study on cavitation erosion behavior of super ferritic stainless steel. Wear 2021, 482–483, 203986. [Google Scholar] [CrossRef]
- Rajput, A.; Ramkumar, J.; Mondal, K. Effect of pearlitic morphology with varying fineness on the cavitation erosion behavior of eutectoid rail steel. Ultrason. Sonochem. 2021, 71, 105399. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.H.; Cheng, F.T.; Kwok, C.T.; Man, H.C. Improvement of cavitation erosion resistance of AISI 316 stainless steel by laser surface alloying using fine WC powder. Surf. Coat. Technol. 2003, 165, 258–267. [Google Scholar] [CrossRef]
- He, Z.; Qin, Z.; Gao, Z.; Wu, Z.; Hu, W. Synergistic effect between cavitation erosion and corrosion of Monel K500 alloy in 3.5 wt% NaCl solution. Mater. Charact. 2023, 205, 113340. [Google Scholar] [CrossRef]
- Lo, K.H.; Kwok, C.T.; Wang, K.Y.; Ai, W. Implications of solution treatment on cavitation erosion and corrosion resistances and synergism of austenitic stainless steel. Wear 2017, 392–393, 159–166. [Google Scholar] [CrossRef]
- Abedini, M.; Reuter, F.; Hanke, S. Corrosion and material alterations of a CuZn38Pb3 brass under acoustic cavitation. Ultrason. Sonochem. 2019, 58, 104628. [Google Scholar] [CrossRef]
- Cao, B.S.; Wu, C.L.; Wang, L.; Zhang, S.; Zhang, C.H.; Sun, X.Y. Effect of residual stress and phase constituents on corrosion-cavitation erosion behavior of 304 stainless steel by iso-material manufacturing of laser surface melting. J. Mater. Res. Technol. 2023, 26, 6532–6551. [Google Scholar] [CrossRef]
- Al-Hashem, A.; Caceres, P.G.; Riad, W.T.; Shalaby, H.M. Cavitation Corrosion Behavior of Cast Nickel-Aluminum Bronze in Seawater. Corrosion 1995, 51, 331–342. [Google Scholar] [CrossRef]
- Bakhshandeh, H.R.; Allahkaram, S.R.; Zabihi, A.H. An investigation on cavitation-corrosion behavior of Ni/β-SiC nanocomposite coatings under ultrasonic field. Ultrason. Sonochem. 2019, 56, 229–239. [Google Scholar] [CrossRef]
- Pollet, B.G. Does power ultrasound affect heterogeneous electron transfer kinetics? Ultrason. Sonochem. 2019, 52, 6–12. [Google Scholar] [CrossRef]
- Silveira, L.L.; Pukasiewicz, A.G.M.; de Aguiar, D.J.M.; Zara, A.J.; Björklund, S. Study of the corrosion and cavitation resistance of HVOF and HVAF FeCrMnSiNi and FeCrMnSiB coatings. Surf. Coat. Technol. 2019, 374, 910–922. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Cavitation erosion and erosion-corrosion resistance of austenitic stainless steel by plasma transferred arc welding. Eng. Fail. Anal. 2017, 76, 115–124. [Google Scholar] [CrossRef]
- Thapliyal, S.; Dwivedi, D.K. On cavitation erosion behavior of friction stir processed surface of cast nickel aluminium bronze. Wear 2017, 376–377, 1030–1042. [Google Scholar] [CrossRef]
- Allenstein, A.N.; Lepienski, C.M.; Buschinelli, A.J.A.; Brunatto, S.F. Improvement of the cavitation erosion resistance for low-temperature plasma nitrided Ca-6NM martensitic stainless steel. Wear 2014, 309, 159–165. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.H.; Cheng, F.T.; Man, H.C. Effect of processing conditions on the corrosion performance of laser surface-melted AISI 440C martensitic stainless steel. Surf. Coat. Technol. 2003, 166, 221–230. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T. Cavitation erosion and pitting corrosion of laser surface melted stainless steels. Surf. Coat. Technol. 1998, 99, 295–304. [Google Scholar] [CrossRef]
- Kwok, C.T.; Man, H.C.; Cheng, F.T. Cavitation erosion and pitting corrosion behaviour of laser surface-melted martensitic stainless steel UNS S42000. Surf. Coat. Technol. 2000, 126, 238–255. [Google Scholar] [CrossRef]
- Tang, C.H.; Cheng, F.T.; Man, H.C. Effect of laser surface melting on the corrosion and cavitation erosion behaviors of a manganese–nickel–aluminium bronze. Mater. Sci. Eng. A 2004, 373, 195–203. [Google Scholar] [CrossRef]
- Tang, C.H.; Cheng, F.T.; Man, H.C. Improvement in cavitation erosion resistance of a copper-based propeller alloy by laser surface melting. Surf. Coat. Technol. 2004, 182, 300–307. [Google Scholar] [CrossRef]
- Abbasi, M.; Vahdatnia, M.; Navaei, A. Solidification Microstructure of HK Heat Resistant Steel. Int. J. Met. 2015, 9, 19–26. [Google Scholar] [CrossRef]
- Haro, S.; Lopez, D.; Wong, A.; Martinez, L.; Velasco, A.; Viramontes, R. Aging of a Heat Resistant Alloy. In 19th ASM Heat Treating Society Conference Proceedings Including Steel Heat Treating in the New Millennium; ASM International: Almere, The Netherlands, 2000; pp. 1–7. [Google Scholar]
- Cosma, D.; Mitelea, I.; Bordeașu, I.; Uțu, I.-D.; Crăciunescu, C.M. Ultrasonic Cavitation Erosion Behavior of GX40CrNiSi25-20. Cast Stainless Steel through Yb-YAG Surface Remelting. Materials 2024, 17, 4180. [Google Scholar] [CrossRef] [PubMed]
- ASTM G32—2016; Standard Test Method for Cavitation Erosion Using Vibratory Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- Bordeaşu, I. Monografia laboratorului de cercetare a eroziunii prin cavitaţie al Universităţii Politehnica Timişoara “Monograph of the Cavitation Erosion Research Laboratory of the Polytechnic University of Timisoara (1960–2020)”; Politehnica: Timişoara, Romania, 2020. [Google Scholar]
- Bordeaşu, I.; Patrascoiu, C.; Badarau, R.; Sucitu, L.; Popoviciu, M.O.; Balasoiu, V. New contributions to cavitation erosion curves modeling. FME Trans. 2006, 34, 39–43. [Google Scholar]
- ASTM G5—2014; Standard Test Method for Making Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- Sarmiento, A.M.; Rodriguez-Perez, A.M.; Davila, J.M.; Luisa de la Torre, M. Effect of acid mine drainage on the mechanical properties of AISI 1020 carbon steel and AW6060 aluminium. J. Mater. Res. Technol. 2024, 32, 541–549. [Google Scholar] [CrossRef]








| Carbon (C) | 0.3–0.5 |
| Silicon (Si) | 1–2.5 |
| Manganese (Mn) | max. 2 |
| Nickel (Ni) | 19–22 |
| Phosphorous (P) | max. 0.04 |
| Sulfur | max 0.03 |
| Chromium | 24–27 |
| Molybden (Mo) | max. 0.5 |
| Iron (Fe) | Balance |
| Sample | E [mV] | icorr [µA/cm2] |
|---|---|---|
| Substrate, homogeneous annealing | −219 | 0.181 |
| Remelted layer with v = 0.8 m/min | 5.69 | 0.023 |
| Remelted layer with v = 1.0 m/min | −14.4 | 0.096 |
| Remelted layer with v = 1.2 m/min | −7.62 | 0.041 |
| Sample | E [mV] | icorr [µA/cm2] |
|---|---|---|
| Substrate, homogeneous annealing | −403.7 | 39.52 |
| Remelted layer with v = 0.8 m/min | −370.23 | 9.12 |
| Remelted layer with v = 1.0 m/min | −360.15 | 8.76 |
| Remelted layer with v = 1.2 m/min | −352.47 | 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitelea, I.; Bordeașu, I.; Cosma, D.; Buzdugan, D.; Crăciunescu, C.M.; Uțu, I.D. Assessment of Corrosion and Cavitation Resistance of Laser Remelted GX40CrNiSi25-20 Cast Stainless Steel. Materials 2024, 17, 6278. https://doi.org/10.3390/ma17246278
Mitelea I, Bordeașu I, Cosma D, Buzdugan D, Crăciunescu CM, Uțu ID. Assessment of Corrosion and Cavitation Resistance of Laser Remelted GX40CrNiSi25-20 Cast Stainless Steel. Materials. 2024; 17(24):6278. https://doi.org/10.3390/ma17246278
Chicago/Turabian StyleMitelea, Ion, Ilare Bordeașu, Daniela Cosma, Dragoș Buzdugan, Corneliu Marius Crăciunescu, and Ion Dragoș Uțu. 2024. "Assessment of Corrosion and Cavitation Resistance of Laser Remelted GX40CrNiSi25-20 Cast Stainless Steel" Materials 17, no. 24: 6278. https://doi.org/10.3390/ma17246278
APA StyleMitelea, I., Bordeașu, I., Cosma, D., Buzdugan, D., Crăciunescu, C. M., & Uțu, I. D. (2024). Assessment of Corrosion and Cavitation Resistance of Laser Remelted GX40CrNiSi25-20 Cast Stainless Steel. Materials, 17(24), 6278. https://doi.org/10.3390/ma17246278








