Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants
Highlights
- A novel Z-scheme ZIF67/NiMoO4 heterojunction photocatalyst is successfully designed;
- Effective removal of tetracycline (TCE) and norfloxacin (NF) antibiotics is realized;
- The strong interfacial contact between ZIF67 and NiMoO4 promotes carrier transport;
- The heterojunction composite can be reused for up to five times;
- Superoxide (•O2−) and hole (h+) radicals are mainly responsible for the photodegradation.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of ZIF67
2.3. Preparation of NiMoO4 Microflowers
2.4. Preparation of ZINM Heterojunctions
2.5. Characterization Methods
3. Results and Discussion
3.1. Crystal Structure
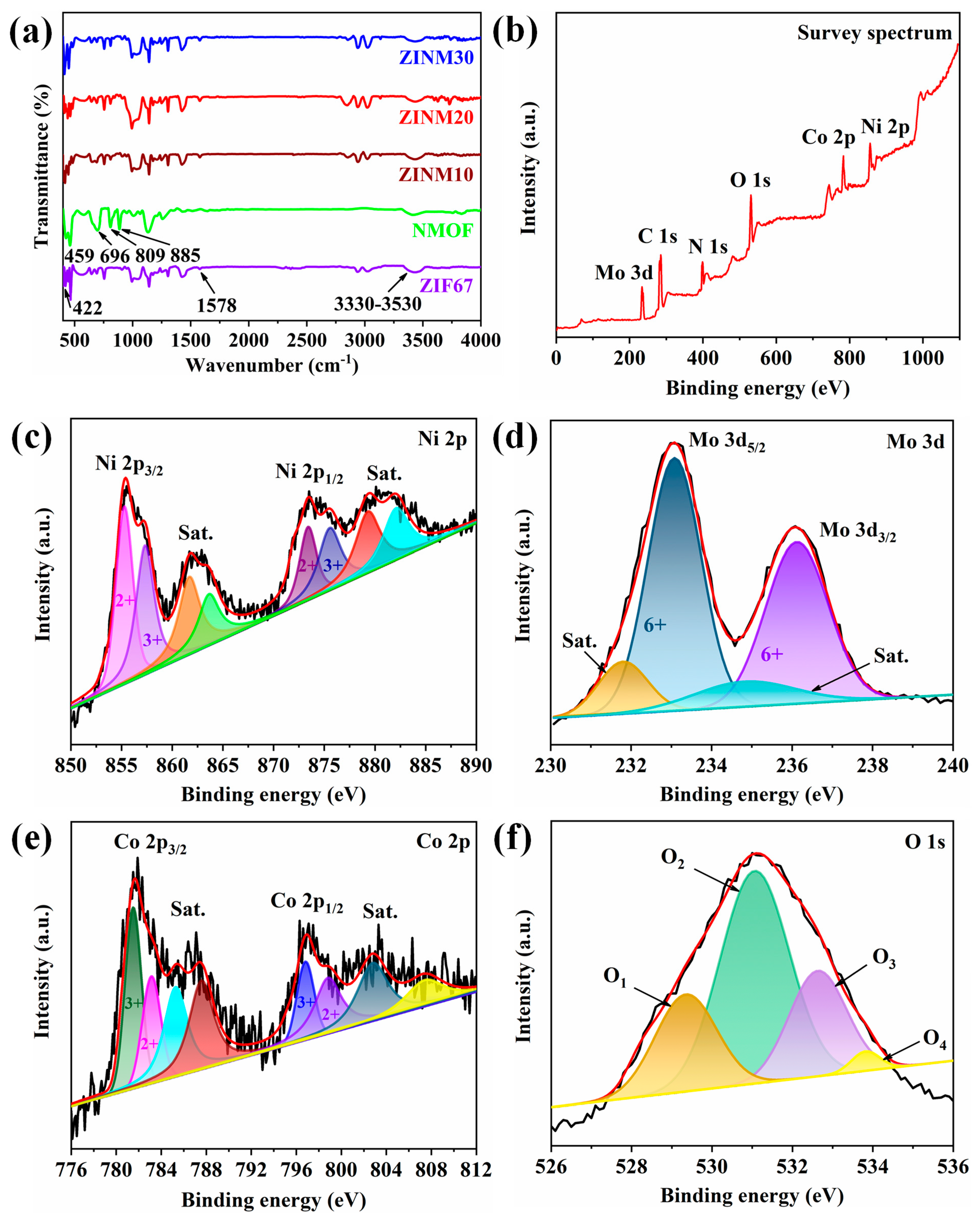
3.2. Functional Groups and Chemical States
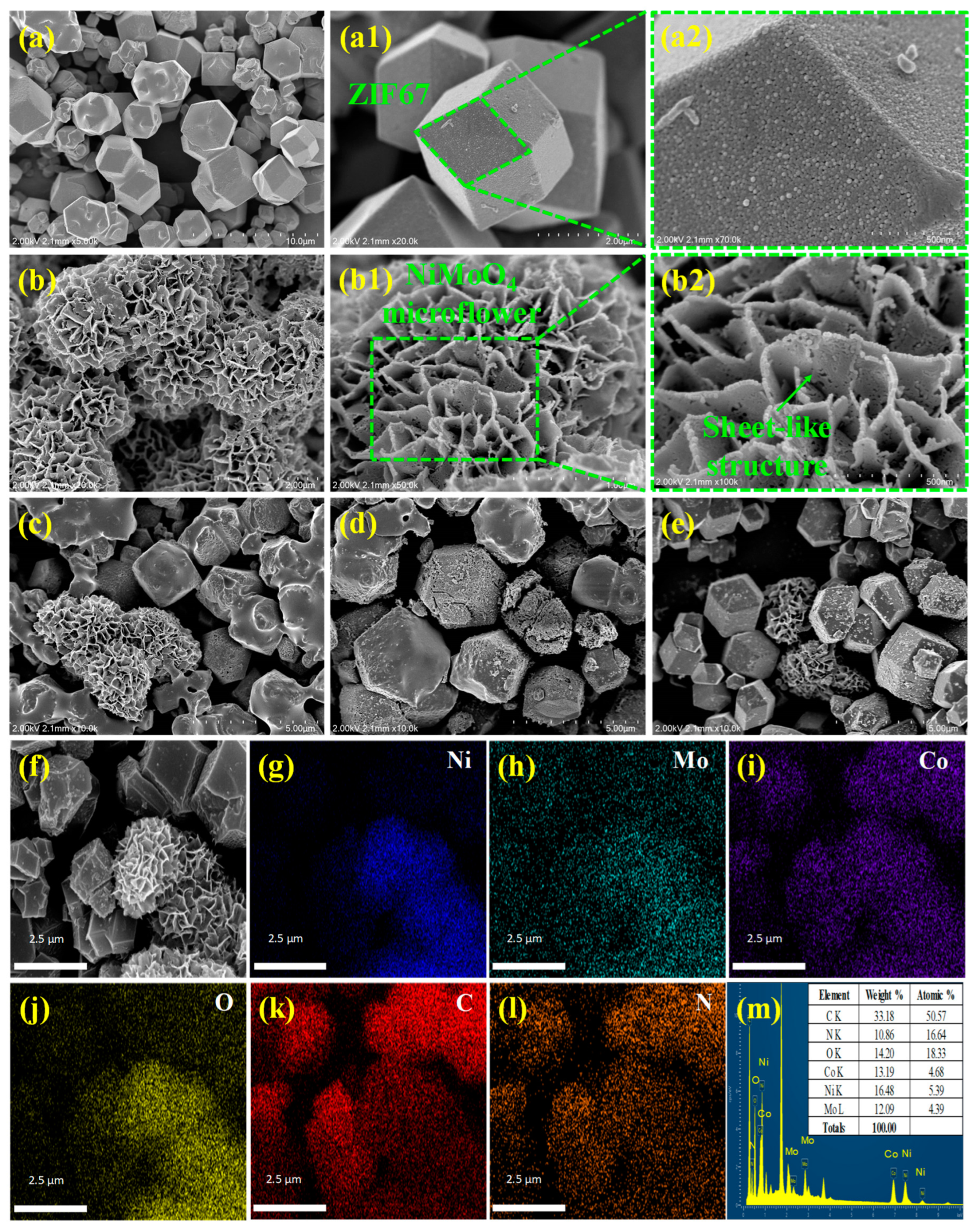
3.3. Morphological Properties
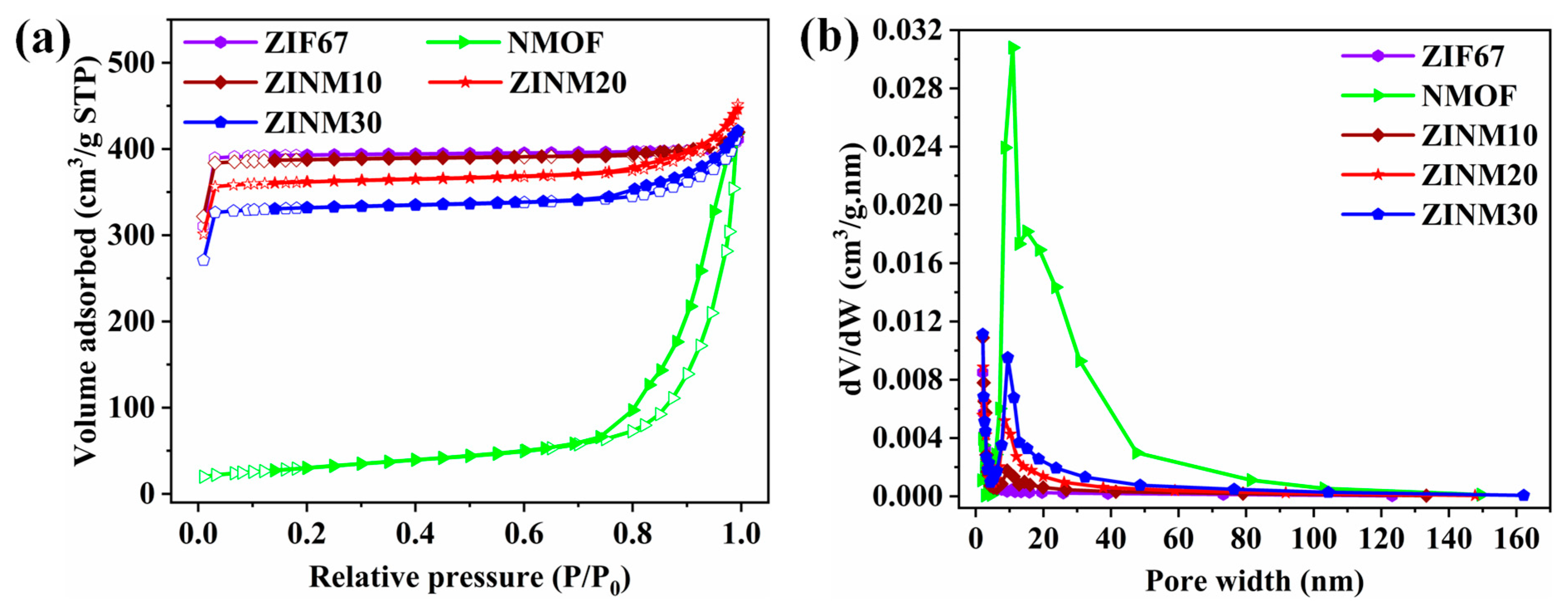
3.4. Specific Surface Area Measurements
3.5. Optical Absorption and Photoluminescence
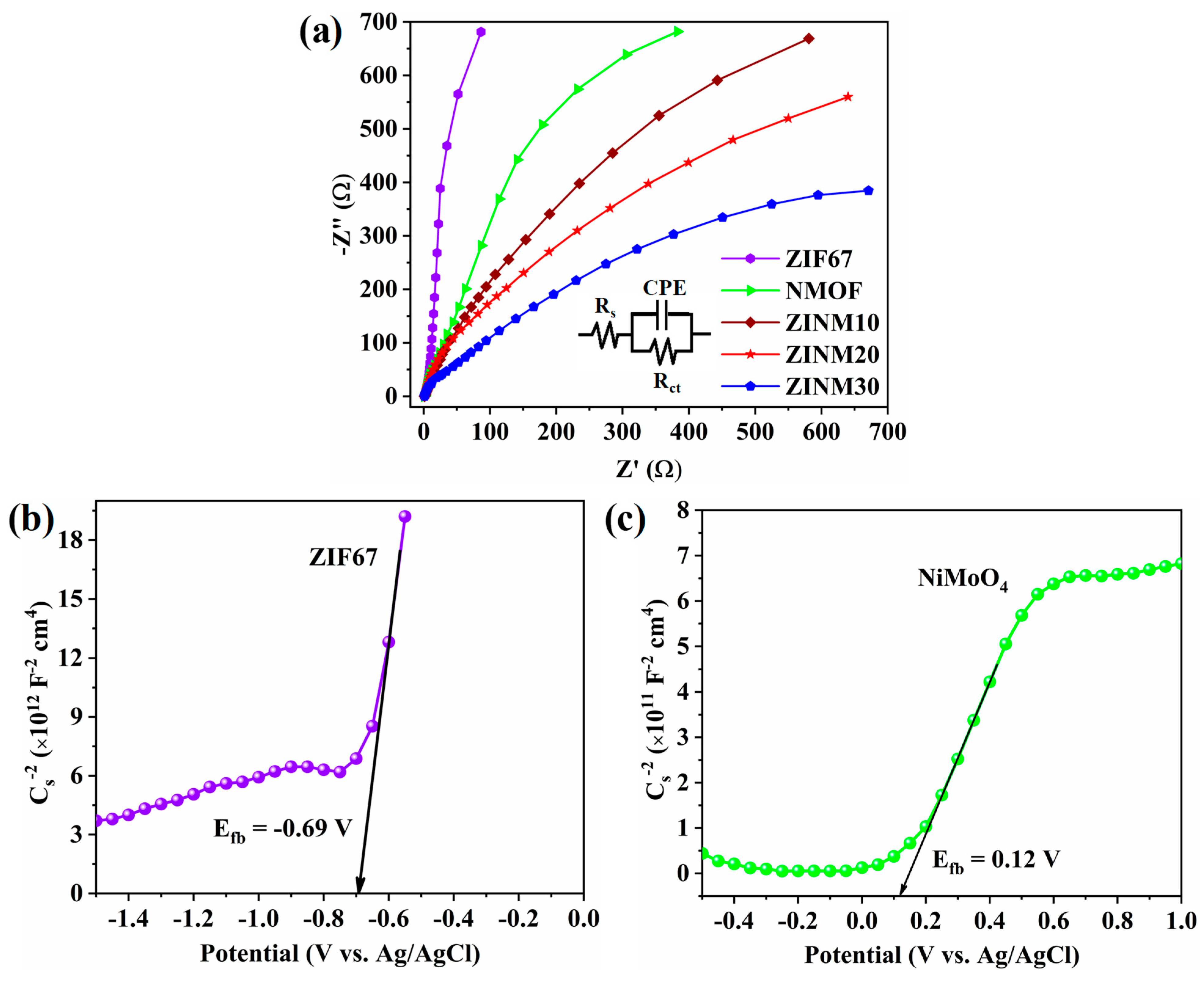
3.6. Photoelectrochemical Analysis
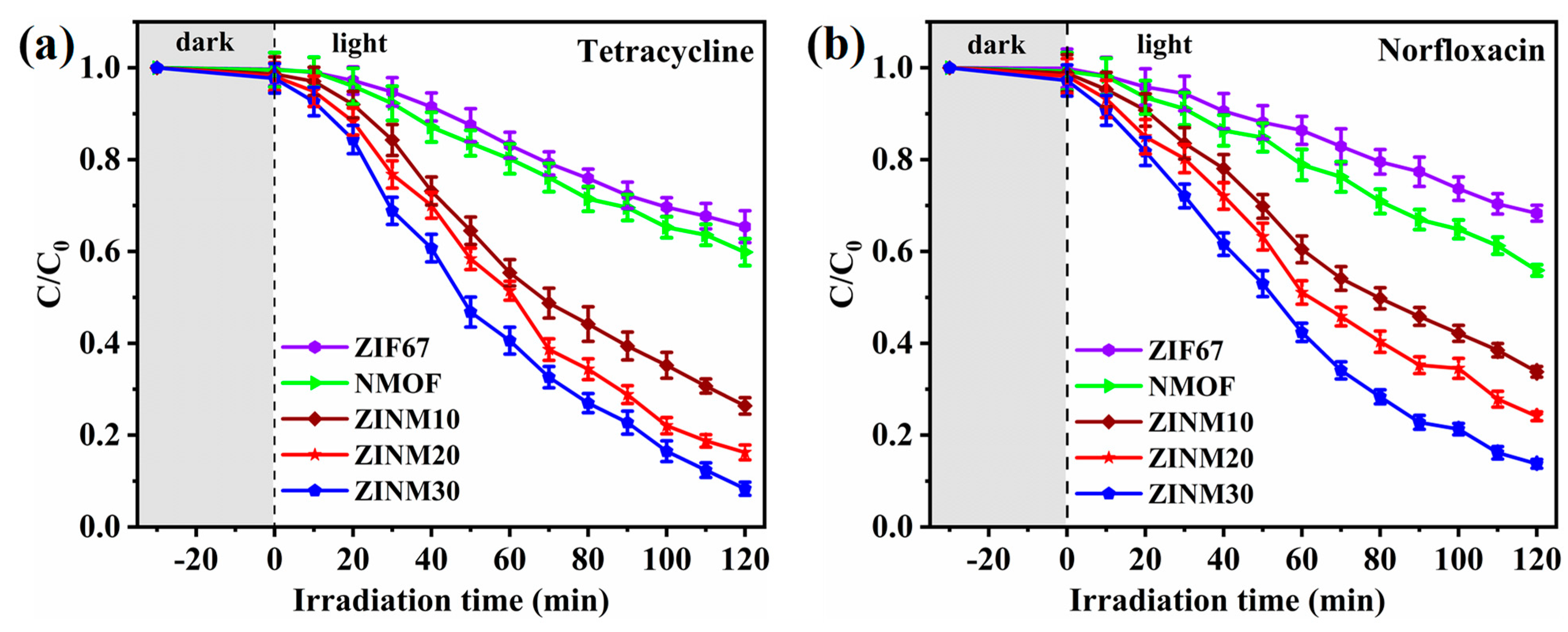
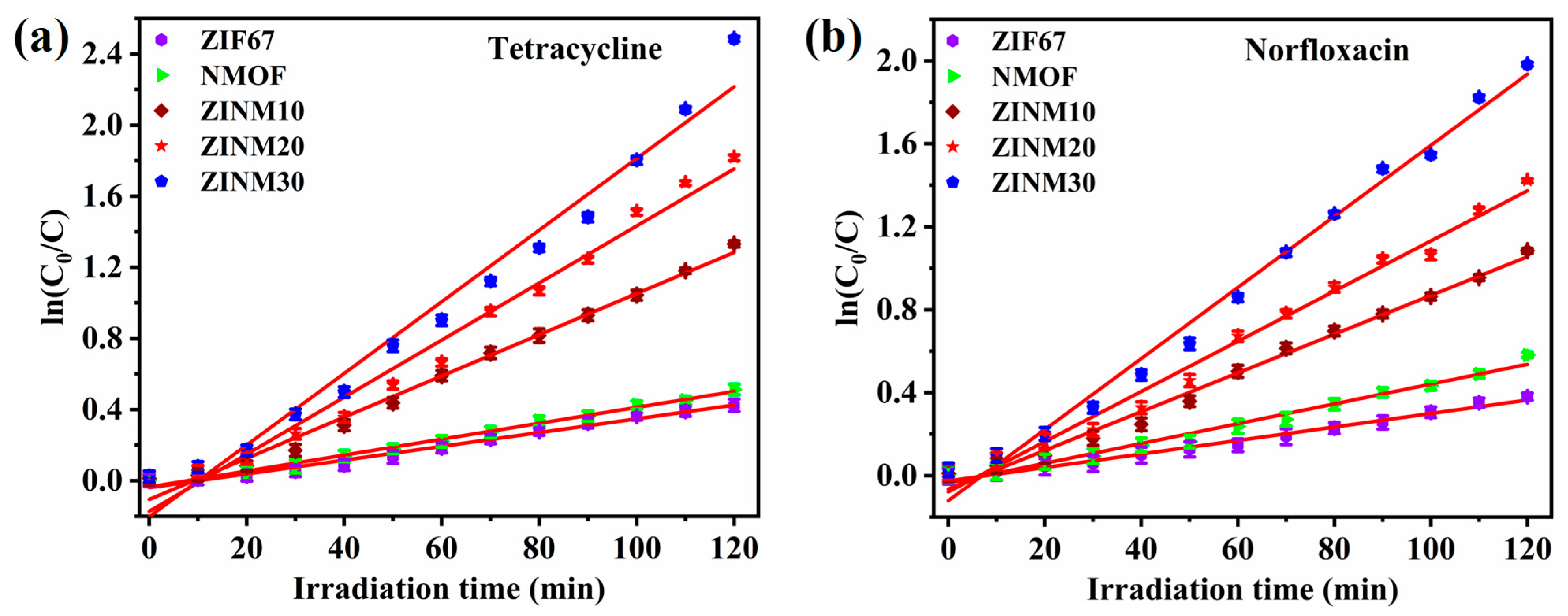
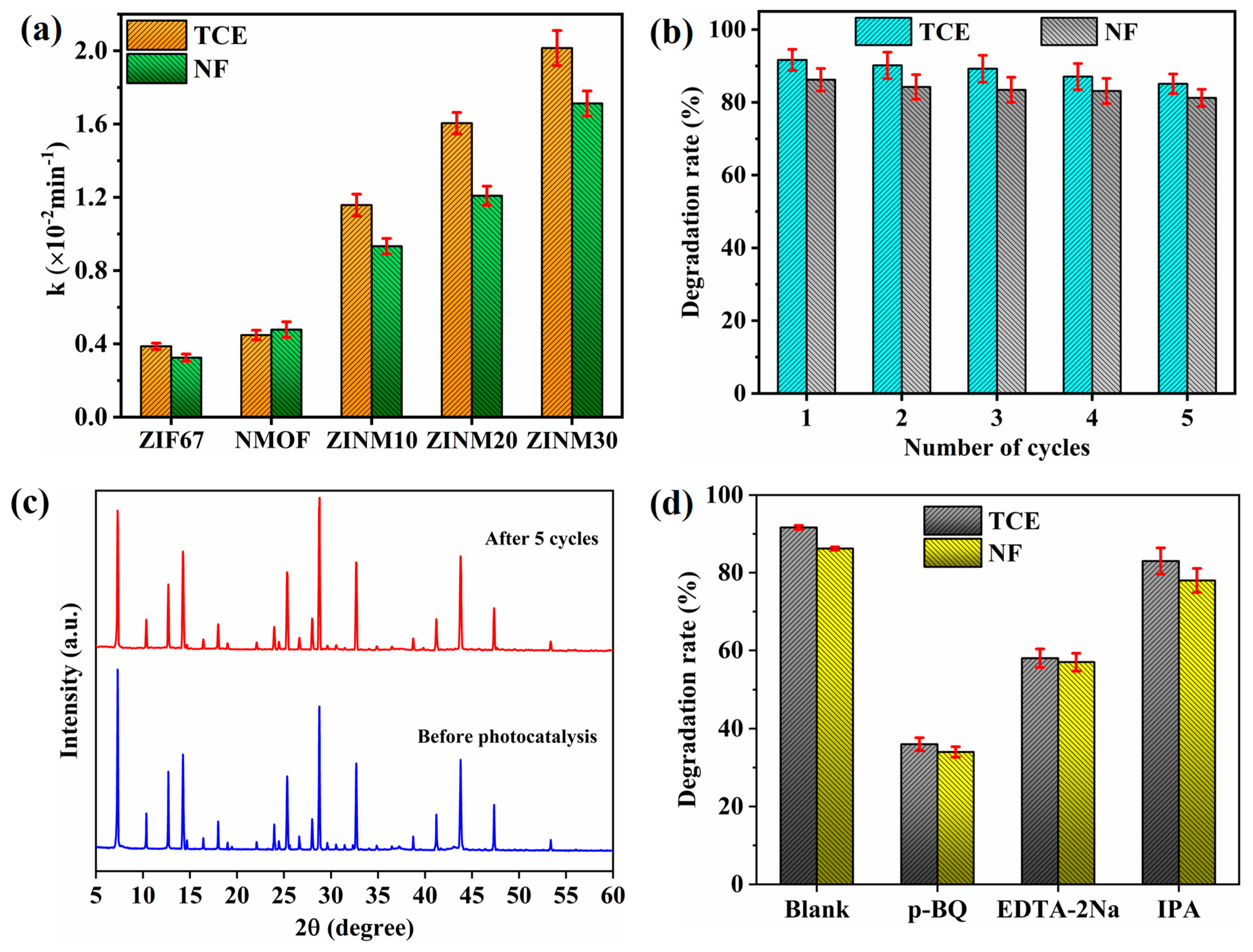
3.7. Photodegradation of Antibiotic Pollutants
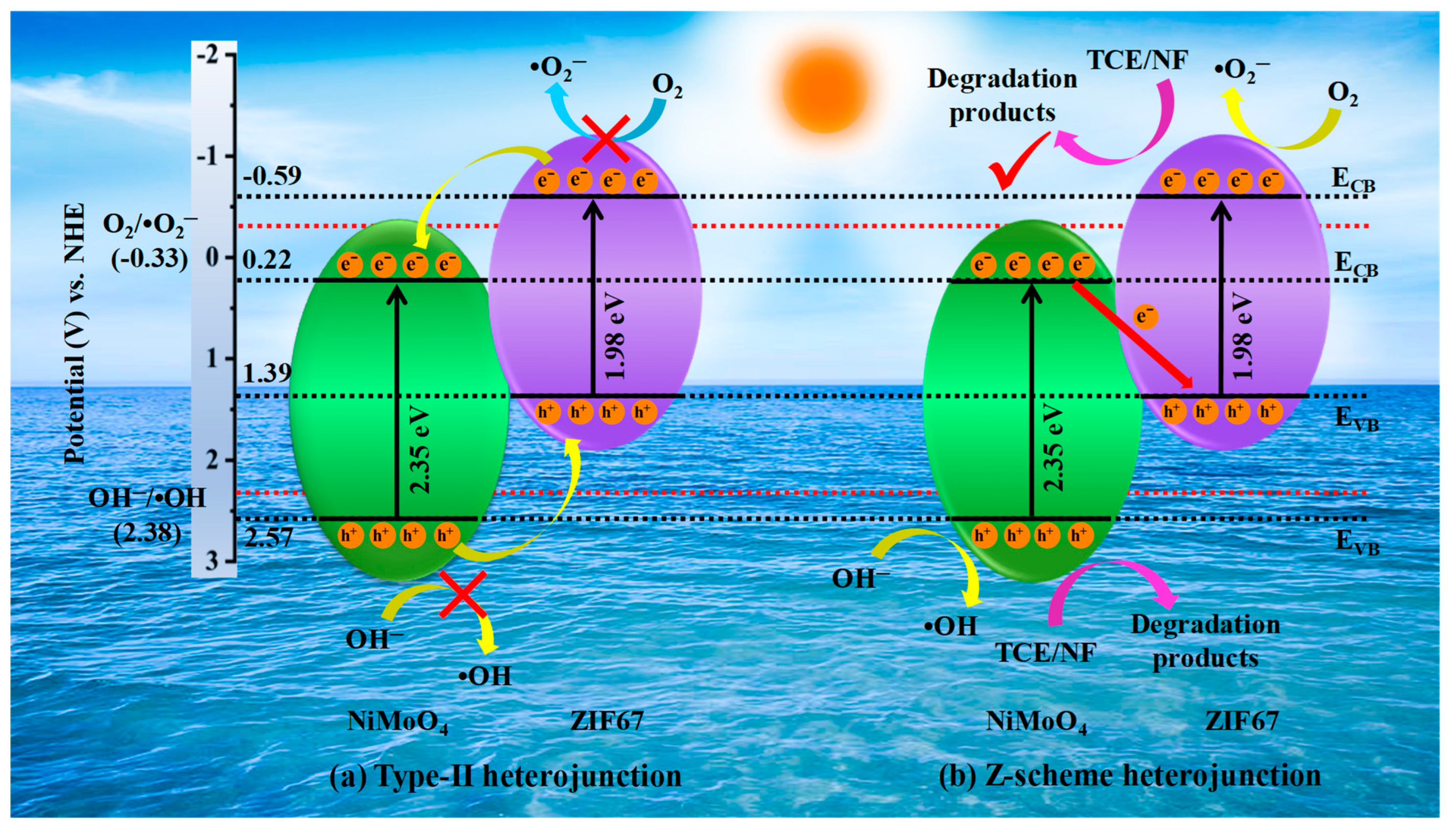
3.8. Description of Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajamanikandan, R.; Ilanchelian, M.; Ju, H. β-cyclodextrin functionalized gold nanoparticles as an effective nanocatalyst for reducing toxic nitroaromatics. Opt. Mater. 2023, 135, 113294. [Google Scholar] [CrossRef]
- Phuong, N.T.T.; Phung, V.D.; Le, T.B.N.; Chi, T.; Hien, B.T.T.; Tho, L.H.; Mai, N.X.D.; Phan, T.B.; Tran, N.H.T.; Ju, H. Ultrasensitive monitoring of cyanide concentrations in water using a Aucore-Agshell hybrid-coating-based fiber optical sensor. Langmuir 2023, 39, 15799–15807. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Prabakaran, D.S.; Sasikumar, K.; Seok, J.S.; Lee, G.; Ju, H. Biocompatible bright orange emissive carbon dots: Multifunctional nanoprobes for highly specific sensing toxic Cr (VI) ions and mitochondrial targeting cancer cell imaging. Talanta Open 2024, 10, 100353. [Google Scholar] [CrossRef]
- Chin, J.Y.; Ahmad, A.L.; Low, S.C. Evolution of photocatalytic membrane for antibiotics degradation: Perspectives and insights for sustainable environmental remediation. J. Water Process Eng. 2023, 51, 103342. [Google Scholar] [CrossRef]
- Sharma, M.; Mandal, M.K.; Pandey, S.; Kumar, R.; Dubey, K.K. Visible-light-driven photocatalytic degradation of tetracycline using heterostructured Cu2O-TiO2 nanotubes, kinetics, and toxicity evaluation of degraded products on cell lines. ACS Omega 2022, 7, 33572–33586. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, L.; Ban, Y.; Li, H. Hydrothermal synthesis of Step-scheme layer g-C3N4/columnar ZnZrO3 heterojunction photocatalyst toward efficient norfloxacin degradation under solar light. J. Solid State Chem. 2024, 337, 124815. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Zhu, P.; Liu, M.; Li, X.; Xin, X. A novel Bi12O17Cl2/GO/Co3O4 Z-type heterojunction photocatalyst with ZIF-67 derivative modified for highly efficient degradation of antibiotics under visible light. J. Colloid Interface Sci. 2025, 677, 1052–1068. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Liu, H.; Zhang, Y.; Ma, J.; Jiang, C.; Duan, T. High-efficiency photocatalytic degradation of tannic acid using TiO2 heterojunction catalysts. ACS Omega 2021, 6, 28538–28547. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Muneer, M.; Khalid, R.; Amin, H.M. ZnO-Bi2O3 heterostructured composite for the photocatalytic degradation of orange 16 reactive dye: Synergistic effect of UV irradiation and hydrogen peroxide. Catalysts 2023, 13, 1328. [Google Scholar] [CrossRef]
- Mafa, P.J.; Ntsendwana, B.; Mamba, B.B.; Kuvarega, A.T. Visible light driven ZnMoO4/BiFeWO6/rGO Z-scheme photocatalyst for the degradation of anthraquinonic dye. J. Phys. Chem. C 2019, 123, 20605–20616. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Acharya, L.; Biswal, L.; Priyadarshini, P.; Parida, K. Recent advancements in graphitic carbon nitride based direct Z- and S-scheme heterostructures for photocatalytic H2O2 production. Inorganic Chemistry Frontiers. Inorg. Chem. Front. 2024, 11, 4914–4973. [Google Scholar] [CrossRef]
- Khudhair, E.M.; Khudhair, W.N.; Ammar, S.H.; Mahdi, A.S. Assembling ZIF-67@Cd0.5Zn0.5S nanocomposites with an enhanced photocatalytic activity. Inorg. Chem. Commun. 2022, 142, 109639. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Wang, J.; Shi, H.; Wang, C.; Fan, Y.; Bai, Z.; Zhu, C.; Yan, X. Ni, Zn Co-doping ZIF-67-derived electrocatalyst based on CNT film for efficient overall water splitting. Int. J. Hydrogen Energy 2023, 48, 29189–29197. [Google Scholar] [CrossRef]
- Yu, Z.; Qian, L.; Zhong, T.; Ran, Q.; Huang, J.; Hou, Y.; Li, F.; Li, M.; Sun, Q.; Zhang, H. Enhanced visible light photocatalytic activity of CdS through controllable self-assembly compositing with ZIF-67. Mol. Catal. 2020, 485, 110797. [Google Scholar] [CrossRef]
- Gao, C.; Li, D.; Wen, Q.; Song, F.; Zhou, J. Synthesis of dual p-n heterojunction of NiWO4/CdS/ZIF-67 photocatalyst as a highly efficient photo-assisted fenton-like catalyst for removal of doxycycline hydrochloride. Appl. Surf. Sci. 2024, 645, 158872. [Google Scholar] [CrossRef]
- Firmansyah, R.; Bakri, R.; Yulizar, Y. Enhancement of photocatalytic activity of ZnO by ZnMoO4 compositing under visible light via hydrothermal green synthesis. Inorg. Chem. Commun. 2023, 155, 110893. [Google Scholar] [CrossRef]
- Ávila-López, M.A.; Luévano-Hipólito, E.; Torres-Martínez, L.M. Optimizing the CO2 reduction to produce CH3OH using flexible NiMoO4 coatings as a photocatalyst. J. Alloys Compd. 2022, 918, 165549. [Google Scholar] [CrossRef]
- Ashrafi, M.; Farhadian, M.; Nazar, A.R.S.; Hajiali, M.; Noorbaksh, A. The tetracycline degradation in a photocatalytic fuel cell microreactor using a ZnO nanorod/Bi2MoO6/ZIF-67 photocatalyst responsive to visible light. Energy Convers. Manag. 2024, 312, 118565. [Google Scholar] [CrossRef]
- Gandamalla, A.; Manchala, S.; Verma, A.; Fu, Y.P.; Shanker, V. Development of highly efficient Ce(MoO4)2/g-C3N4 composite for the photocatalytic degradation of methylene blue and ciprofloxacin under visible light. J. Mol. Struct. 2024, 1297, 136896. [Google Scholar] [CrossRef]
- Ray, S.K.; Dhakal, D.; Lee, S.W. Rapid degradation of naproxen by AgBr-α-NiMoO4 composite photocatalyst in visible light: Mechanism and pathways. Chem. Eng. J. 2018, 347, 836–848. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Pham, T.D.; Nguyen, M.V.; Van Thuan, D.; Trung, D.Q.; Thao, P.; Trang, H.T.; Nguyen, V.N.; Tran, D.T.; Minh, D.N.; et al. Advanced NiMoO4/g-C3N4 Z-scheme heterojunction photocatalyst for efficient conversion of CO2 to valuable products. J. Alloys Compd. 2020, 842, 155860. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Zhou, X.; Liu, Z.; Li, Y.; Zhao, S.; Mao, M. Amorphous CoS modified nanorod NiMoO4 photocatalysis for hydrogen production. J. Mater. Sci. Mater. Electron. 2020, 31, 182–195. [Google Scholar] [CrossRef]
- Sudha, S.; Sridhar, S.; Cholan, S. A simplified fabrication of α-NiMoO4 via greener approach: Properties and its photocatalytic activity. Phys. B 2022, 624, 413358. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, T.D.; Cuong, L.M.; Le, C.L.; Xuyen, H.T.H.; Van Thuan, D. Development of Nb-NiMoO4/g-C3N4 direct Z scheme heterojunctions for effective photocatalytic conversion of carbon dioxide to valuable products. Sustain. Chem. Pharm. 2022, 26, 100641. [Google Scholar] [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H. A high-performance NiMoO4/g-C3N4 direct Z-scheme heterojunction photocatalyst for the degradation of organic pollutants. Surf. Interfaces 2023, 42, 103389. [Google Scholar] [CrossRef]
- Hu, W.; Yu, J.; Jiang, X.; Liu, X.; Jin, R.; Lu, Y.; Zhao, L.; Wu, Y.; He, Y. Enhanced photocatalytic activity of g-C3N4 via modification of NiMoO4 nanorods. Colloids Surf. A 2017, 514, 98–106. [Google Scholar] [CrossRef]
- Shao, W.; Chen, Y.R.; Xie, F.; Zhang, H.; Wang, H.T.; Chang, N. Facile construction of a ZIF-67/AgCl/Ag heterojunction via chemical etching and surface ion exchange strategy for enhanced visible light driven photocatalysis. RSC Adv. 2020, 10, 38174–38183. [Google Scholar] [CrossRef]
- Aloui, T.; Guermazi, H.; Fourati, N.; Zerrouki, C.; Guermazi, S. Synthesis and characterization of nanosheet NiMoO4 powder as a highly efficient and reusable catalyst for environmental remediation. J. Nanopart. Res. 2022, 24, 35. [Google Scholar] [CrossRef]
- Lu, S.; You, S.; Hu, J.; Li, X.; Li, L. Magnetic MnFe2O4/ZIF-67 nanocomposites with high activation of peroxymonosulfate for the degradation of tetracycline hydrochloride in wastewater. RSC Adv. 2024, 14, 7528–7539. [Google Scholar] [CrossRef] [PubMed]
- Shameem, A.; Devendran, P.; Siva, V.; Packiaraj, R.; Nallamuthu, N.; Asath Bahadur, S. Electrochemical performance and optimization of α-NiMoO4 by different facile synthetic approach for supercapacitor application. J. Mater. Sci. Mater. Electron. 2019, 30, 3305–3315. [Google Scholar] [CrossRef]
- Paul, M.J.; Suresh, R.; Sibu, G.A.; Balasubramani, V.; Muthusamy, S. CuO nanoflowers: Multifaceted implications of various precipitating agents on rectification behaviour. Opt. Mater. 2024, 152, 115517. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, L.; Ruan, M.; Liu, Y.; Peng, C.; Cui, W.; Liang, W.; Shan, S.; Hu, T. HKUST-1/ZIF-67 nanocomposites as heterogeneous Cu–Co-bimetallic Fenton-like catalysts for efficient removal of methylene blue. ACS Appl. Nano Mater. 2024, 7, 2370–2381. [Google Scholar] [CrossRef]
- de Moura, A.P.; de Oliveira, L.H.; Rosa, I.L.V.; Xavier, C.S.; Lisboa-Filho, P.N.; Li, M.S.; La Porta, F.A.; Longo, E.; Varela, J.A. Structural, optical, and magnetic properties of NiMoO4 nanorods prepared by microwave sintering. Sci. World J. 2015, 2015, 315084. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, C.; Tai, G.; Ma, Y.; Yang, X.; Pan, Y.; Han, J.; Xing, W.; Wu, G. ZIF-67/BiOCl Z-scheme heterojunction photocatalyst for photodegradation of organic dyes and antibiotics. ACS Appl. Nano Mater. 2023, 6, 17814–17825. [Google Scholar] [CrossRef]
- Abbas, Y.; Yun, S.; Javed, M.S.; Chen, J.; Tahir, M.F.; Wang, Z.; Yang, C.; Arshad, A.; Hussain, S. Anchoring 2D NiMoO4 nano-plates on flexible carbon cloth as a binder-free electrode for efficient energy storage devices. Ceram. Int. 2020, 46, 4470–4476. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, W.; Zhang, G.; Yang, S.; Yu, L.; Cao, X.; Chen, H.; Liu, Y.; Song, L.; Qiu, Y. In-situ prepared NiMoO4 nanocrystals synergizes with nickel micro-tubes with hydrodynamic effects for energy-saving watersplitting. Chem. Eng. J. 2023, 471, 144657. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Li, L.; Cai, D.; Li, J.; Cao, J.; Han, W. Hierarchical core-shell structural NiMoO4@NiS2/MoS2 nanowires fabricated via an in situ sulfurization method for high performance asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 21759–21765. [Google Scholar] [CrossRef]
- Chen, S.; Xiang, Z.; Xiao, Z.; Wang, K.P.; Zhang, Q.; Wang, L. Dual-ion pre-inserted Mo glycerate template for constructing NiMo-OS core-shell structure with boosting performance in zinc ions hybrid supercapacitors. Nano Res. 2023, 16, 6922–6932. [Google Scholar] [CrossRef]
- Amin, H.M.; Attia, M.; Tetzlaff, D.; Apfel, U.P. Tailoring the electrocatalytic activity of pentlandite FexNi9-XS8 nanoparticles via variation of the Fe: Ni ratio for enhanced water oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, C.; Wang, T.; Chu, H.; Wang, C.C. Efficient ciprofloxacin removal over Z-scheme ZIF-67/V-BiOIO3 heterojunctions: Insight into synergistic effect between adsorption and photocatalysis. Sep. Purif. Technol. 2023, 313, 123511. [Google Scholar] [CrossRef]
- Gholinejad, M.; Naghshbandi, Z.; Sansano, J.M. Zeolitic imidazolate frameworks-67 (ZIF-67) supported PdCu nanoparticles for enhanced catalytic activity in Sonogashira-Hagihara and nitro group reduction under mild conditions. Mol. Catal. 2022, 518, 112093. [Google Scholar] [CrossRef]
- Huang, L.; Xiang, J.; Zhang, W.; Chen, C.; Xu, H.; Huang, Y. 3D interconnected porous NiMoO4 nanoplate arrays on Ni foam as high-performance binder-free electrode for supercapacitors. J. Mater. Chem. A 2015, 3, 22081–22087. [Google Scholar] [CrossRef]
- Liu, Z.; Amin, H.M.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-dependent intrinsic activity of single Co3O4 nanoparticles for oxygen evolution reaction. Adv. Funct. Mater. 2023, 33, 2210945. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Z.; Huang, L.; Guo, Y.; Zhai, Y.; Dong, S. Hydrothermal synthesis of polydopamine-functionalized cobalt-doped lanthanum nickelate perovskite nanorods for efficient water oxidation in alkaline solution. Nanoscale 2019, 11, 19579–19585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, H.; Li, Z.; Lou, X.W. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium–sulfur batteries. Angew. Chem. 2016, 128, 4050–4054. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z. Boosting photocatalytic hydrogen evolution achieved by NiSx coupled with g-C3N4@ZIF-67 heterojunction. J. Phys. Chem. C 2019, 123, 18248–18263. [Google Scholar] [CrossRef]
- Xuan, W.; Ramachandran, R.; Zhao, C.; Wang, F. Influence of synthesis temperature on cobalt metal-organic framework (Co-MOF) formation and its electrochemical performance towards supercapacitor electrodes. J. Solid State Electrochem. 2018, 22, 3873–3881. [Google Scholar] [CrossRef]
- Zhang, Y.; Ju, P.; Wang, N.; Nicholas, C.C.H.; Dou, K.; Zhai, X.; Duan, J.; Hou, B. Highly efficient photocatalytic antifouling activity of core-shell structural encapsulation Bi2MoO6 with visible-light responsive ZIF-67. Appl. Surf. Sci. 2024, 653, 159353. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Bagchi, D.; Katouah, H.A.; Hasan, M.N.; Altass, H.M.; Pal, S.K. Enhanced water stability and photoresponsivity in metal-organic framework (MOF): A potential tool to combat drug-resistant bacteria. Sci. Rep. 2019, 9, 19372. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Reddy, D.A.; Kim, Y.; Ma, R.; Choi, J.; Kim, T.K.; Lee, K.S. Zeolitic imidazolate framework-67 (ZIF-67) rhombic dodecahedrons as full-spectrum light harvesting photocatalyst for environmental remediation. Solid State Sci. 2016, 62, 82–89. [Google Scholar] [CrossRef]
- Pandey, P.K.; Bhave, N.S.; Kharat, R.B. Preparation and characterization of spray deposited NiMoO4 thin films for photovoltaic electrochemical studies. Mater. Res. Bull. 2006, 41, 1160–1169. [Google Scholar] [CrossRef]
- Dai, M.D.; Zhang, Q.; Dong, H.; Zhang, Y.W. Enhanced light driven CO2 conversion based on silver bismuth sulfide hollow octahedrons coated with amorphous metal-organic frameworks. Mater. Adv. 2024, 5, 1715–1725. [Google Scholar] [CrossRef]
- Lee, K.C.; Huang, J.H.; Wu, Y.J.; Wang, K.S.; Cho, E.C.; Hsu, S.C.; Liu, T.Y. Crystal structure-controlled synthesis of NiMoO4/NiO hierarchical microspheres for high-performance supercapacitors and photocatalysts. J. Energy Storage 2024, 97, 112639. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Qin, X.; Zhuang, J.; Yin, W.; Chen, T.; Yao, Y. Integrating photogenerated charge carriers for hydrogen production on noble-metal free dual-photocatalyst under visible light. Compos. Part B 2022, 241, 110012. [Google Scholar] [CrossRef]
- Salari, H.; Zahiri, Z. Design of S-scheme 3D nickel molybdate/AgBr nanocomposites: Tuning of the electronic band structure towards efficient interfacial photoinduced charge separation and remarkable photocatalytic activity. J. Photochem. Photobiol. A 2022, 426, 113751. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Mu, Z.; Zhang, X.; Dong, G.; Bai, L.; Guo, R.; Li, J.; Zhao, M.; Zhang, Z. Insight into the Novel Z-scheme ZIF67/WO3 Heterostructure for Improved Photocatalytic Degradation of Methylene Blue under visible light. J. Inorg. Organomet. Polym. Mater. 2023, 33, 90–104. [Google Scholar] [CrossRef]
- Chen, W.C.; Qi, X. An S-scheme heterojunction between Mn/Mg co-doped BiFeO3 and g-C3N4 nanosheets for photodegradation of organic pollutants. RSC Adv. 2023, 13, 27738–27745. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Wang, J.; Peng, T.; Li, R. Direct Z-scheme 2D/2D photocatalyst based on ultrathin g-C3N4 and WO3 nanosheets for efficient visible-light-driven H2 generation. ACS Appl. Mater. Interfaces 2019, 11, 27913–27923. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Tian, Y.; Tu, Y.; Liu, Y.; Jin, T.; Li, X.; Fang, D.; Wang, J. Design and construction of a novel immobilized Z-scheme-Type II-heterojunction ZnO/ZnCo2O4–Co3O4|Co photocatalyst composite film for efficient norfloxacin degradation with simultaneous hydrogen evolution. Mater. Sci. Semicond. Process. 2024, 171, 108041. [Google Scholar] [CrossRef]
- Ou, X.; Chen, P.; Zhao, K.; Xia, C. Fabrication of Z-scheme ZnO/g-C3N4 heterojunction modified by silver nanoparticles for photocatalytic removal of Norfloxacin and Rhodamine B. Opt. Mater. 2023, 144, 114305. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, K.; Xue, Z.; Hu, Z.; Yuan, N. Modulation of CdS nanoparticles decorated bimetallic Fe/Mn-MOFs Z-scheme heterojunctions for enhancing photocatalytic degradation of tetracycline. J. Alloys Compd. 2024, 992, 174462. [Google Scholar] [CrossRef]
- Zhang, J.; Dang, J.; Guan, W. Construction of Z-scheme Fe3O4/BiOBr/BiOI heterojunction with magnetically recyclable for enhanced photocatalytic reaction activity. Chem. Eng. Res. Des. 2024, 210, 382–392. [Google Scholar] [CrossRef]
- Zou, X.; Sun, B.; Wang, L.; Bai, H.; Meng, X.; Li, C.; Li, Z. Enhanced photocatalytic degradation of tetracycline by SnS2/Bi2MoO6-x heterojunction: Multi-electric field modulation through oxygen vacancies and Z-scheme charge transfer. Chem. Eng. J. 2024, 482, 148818. [Google Scholar] [CrossRef]
- Fu, S.; Chu, Z.; Huang, Z.; Dong, X.; Bie, J.; Yang, Z.; Zhu, H.; Pu, W.; Wu, W.; Liu, B. Construction of Z-scheme AgCl/BiOCl heterojunction with oxygen vacancies for improved pollutant degradation and bacterial inactivation. RSC Adv. 2024, 14, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Z.; Shang, Y.; Lv, C.; Zhang, X.; Li, F.; Jin, X. Boosting Carrier Separation on a BiOBr/Bi4O5Br2 Direct Z-Scheme Heterojunction for Superior Photocatalytic Nitrogen Fixation. ACS Catal. 2024, 14, 5779–5787. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasikumar, K.; Rajamanikandan, R.; Ju, H. Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. Materials 2024, 17, 6225. https://doi.org/10.3390/ma17246225
Sasikumar K, Rajamanikandan R, Ju H. Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. Materials. 2024; 17(24):6225. https://doi.org/10.3390/ma17246225
Chicago/Turabian StyleSasikumar, Kandasamy, Ramar Rajamanikandan, and Heongkyu Ju. 2024. "Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants" Materials 17, no. 24: 6225. https://doi.org/10.3390/ma17246225
APA StyleSasikumar, K., Rajamanikandan, R., & Ju, H. (2024). Construction of Z-Scheme ZIF67/NiMoO4 Heterojunction for Enhanced Photocatalytic Degradation of Antibiotic Pollutants. Materials, 17(24), 6225. https://doi.org/10.3390/ma17246225








