Water Vapor-Impermeable AlON/HfOx Bilayer Films Deposited by Hybrid High-Power Impulse Magnetron Sputtering/Radio-Frequency Magnetron Sputtering Processes
Abstract
1. Introduction
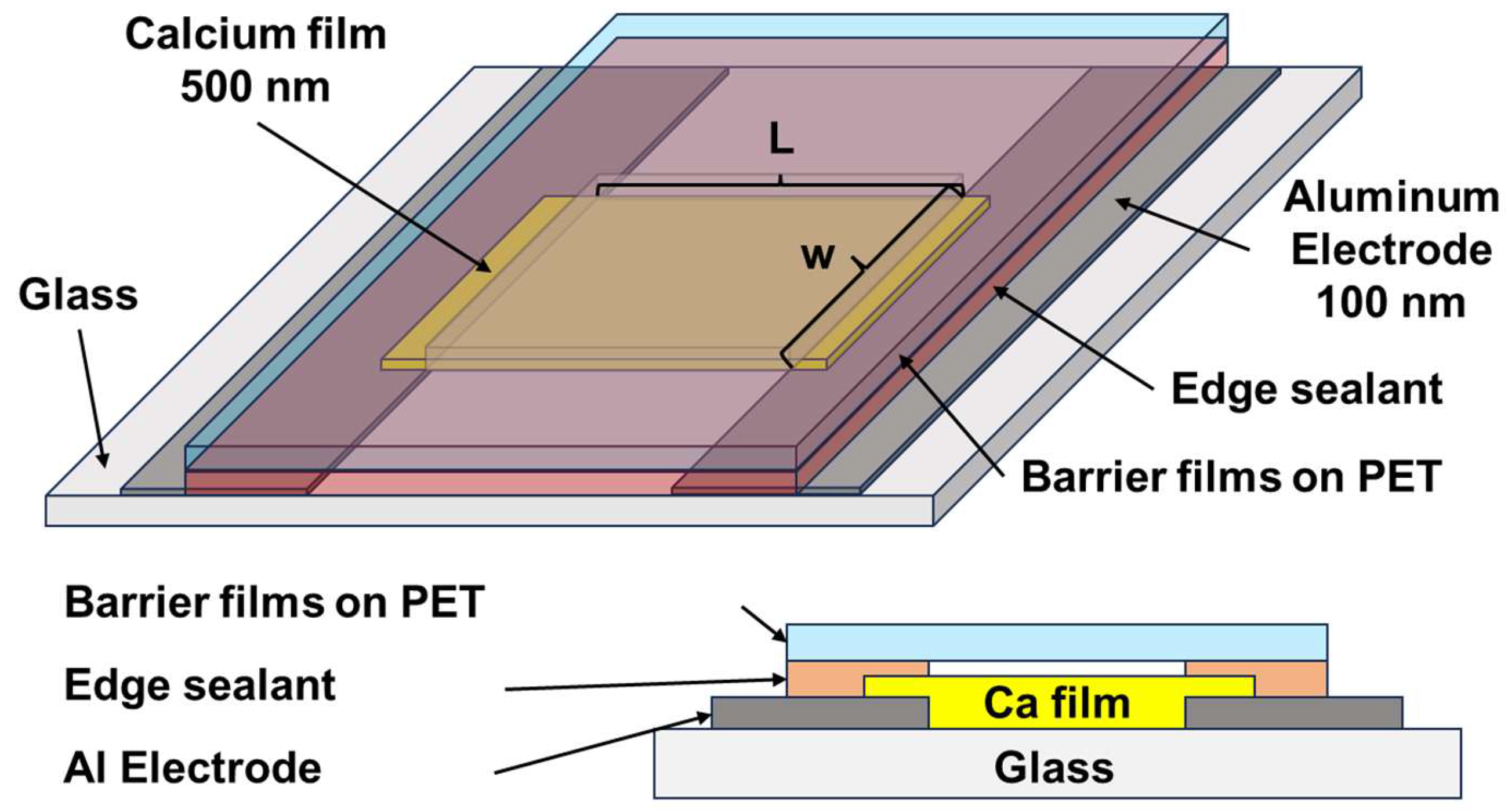
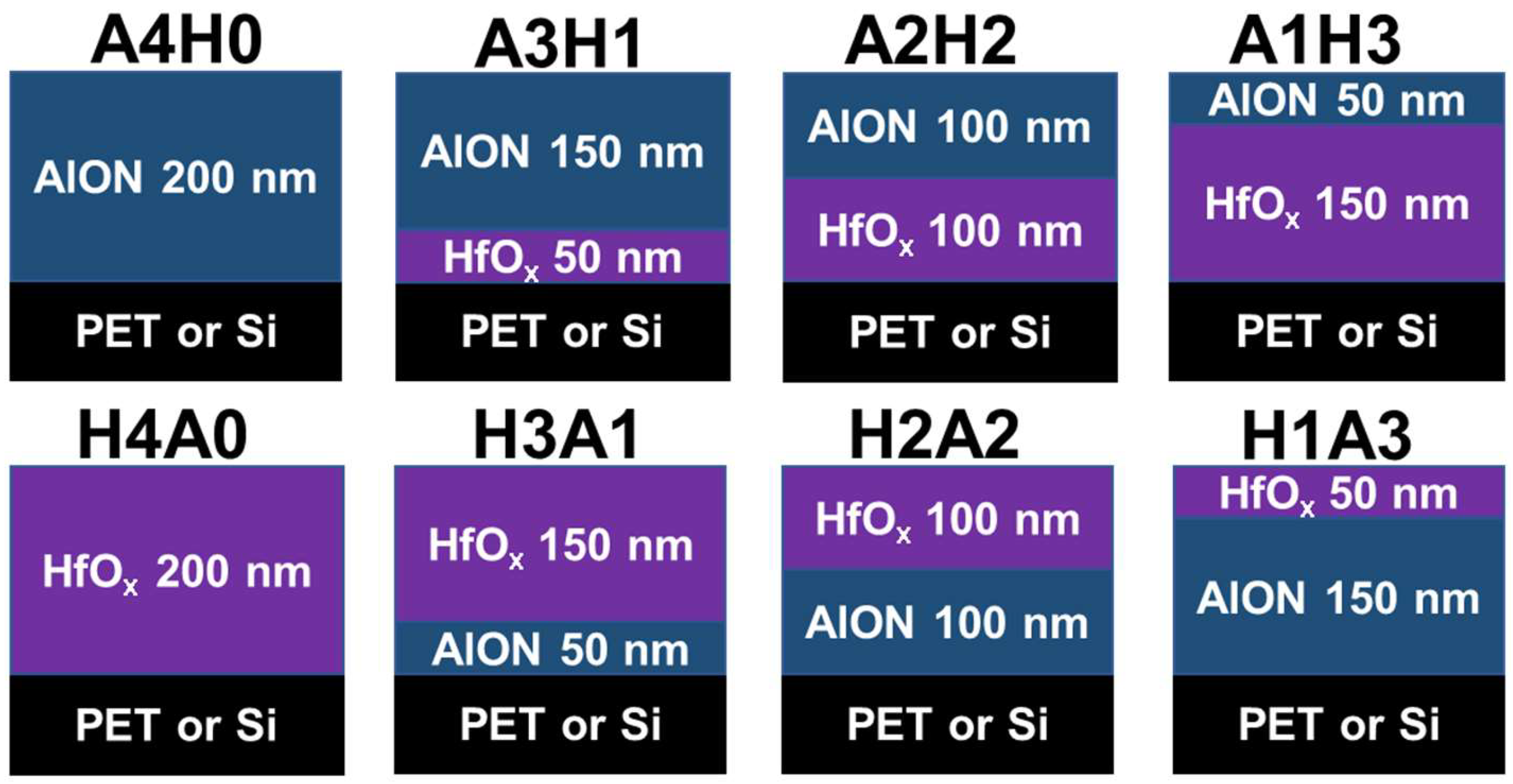
2. Materials and Methods
3. Results and Discussion
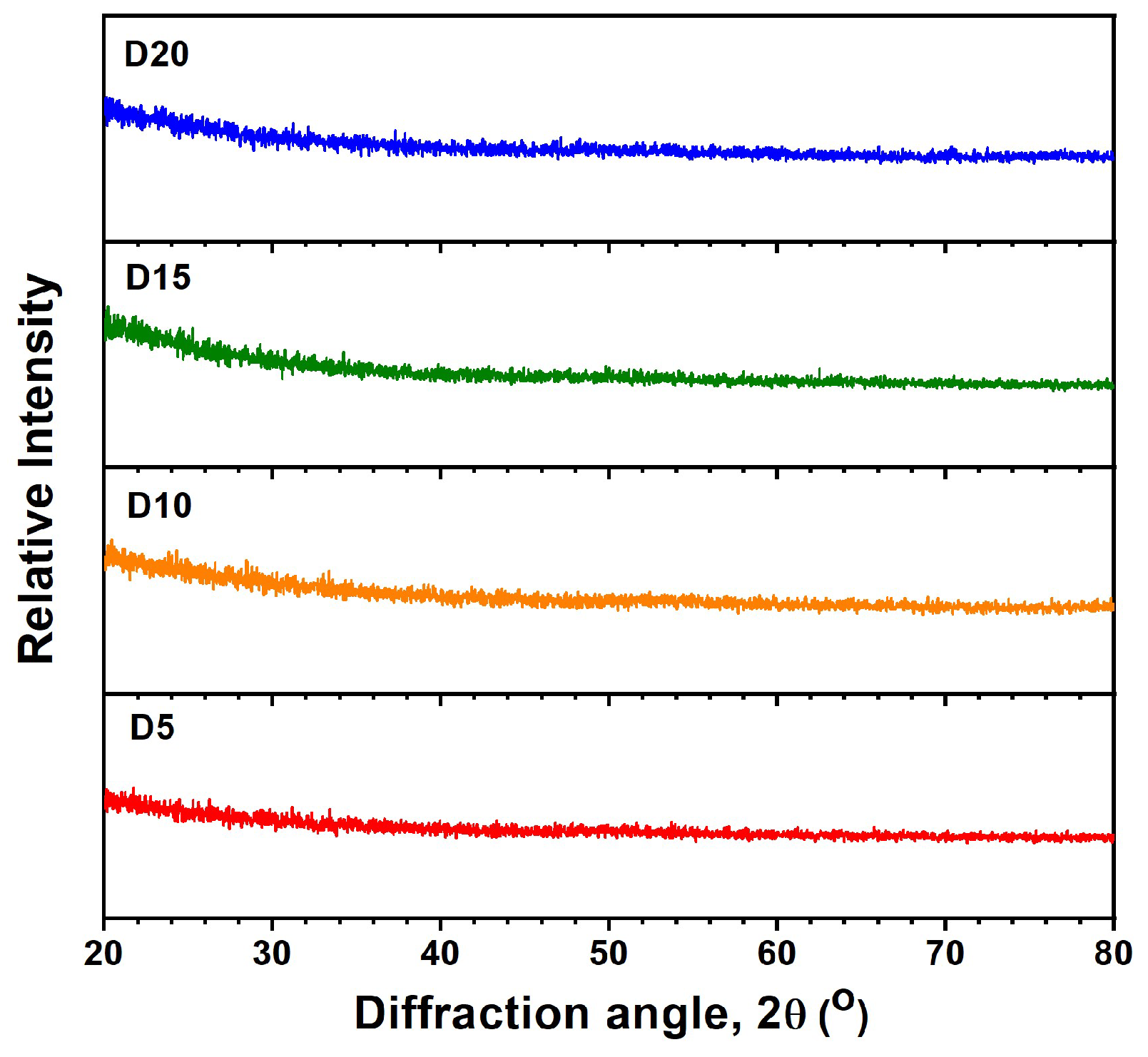
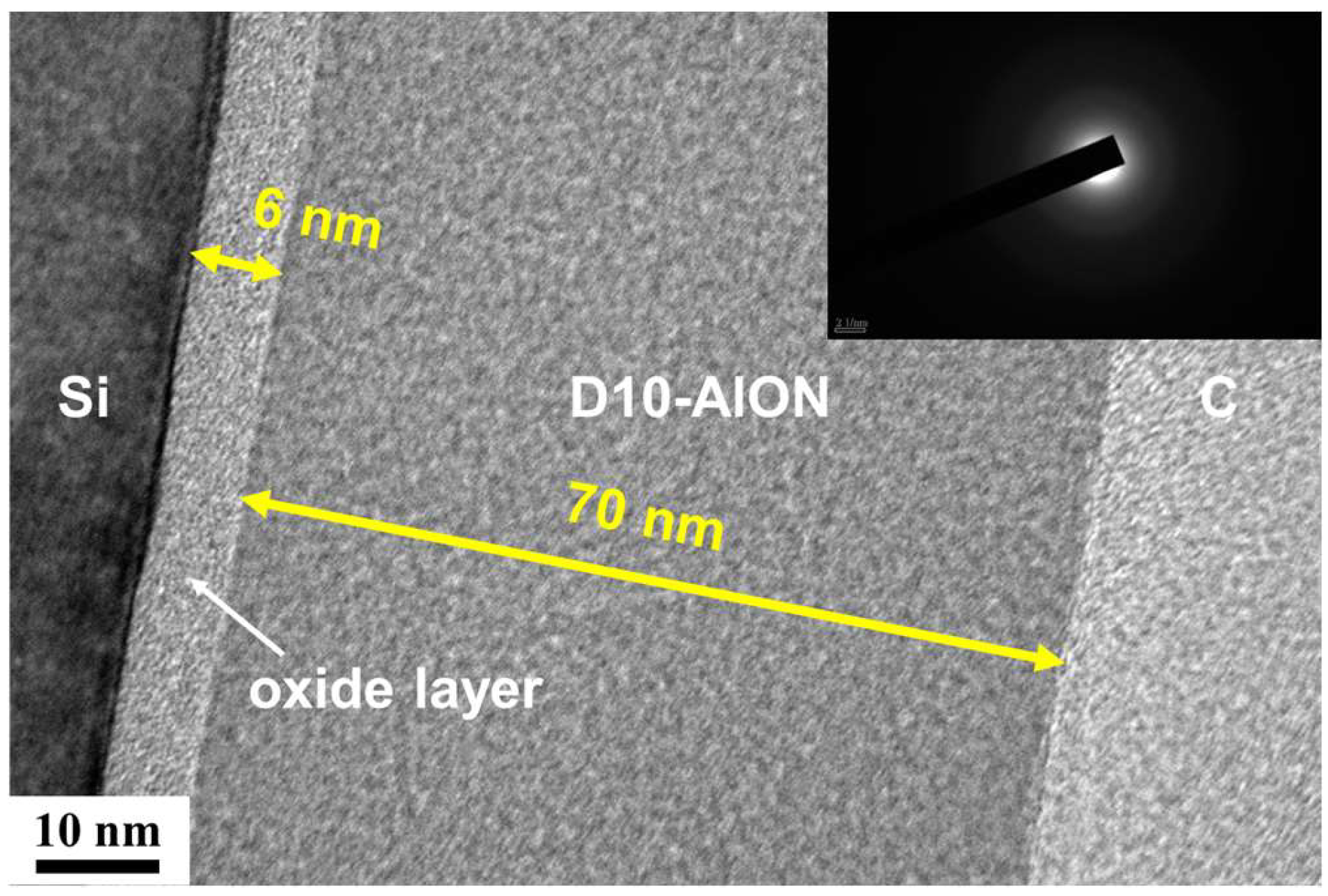
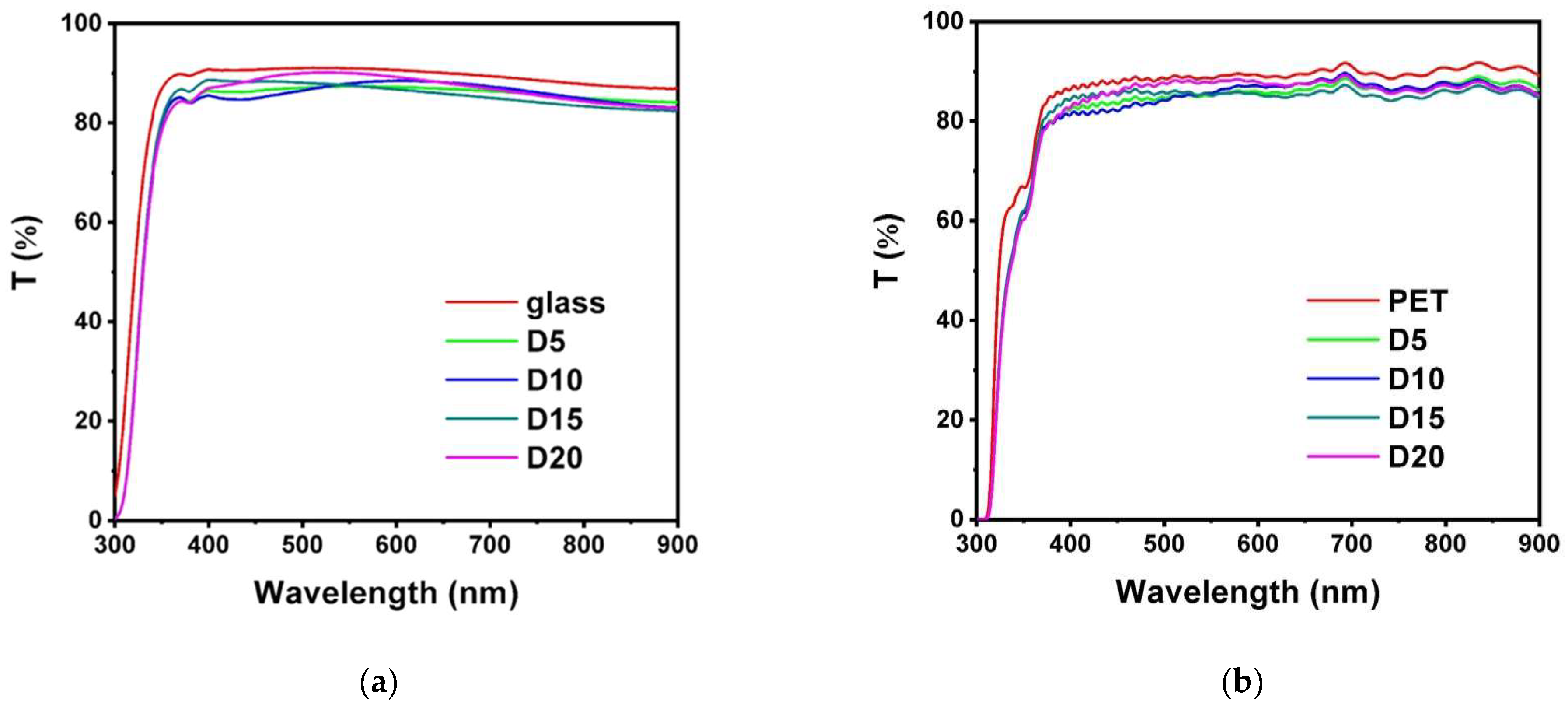
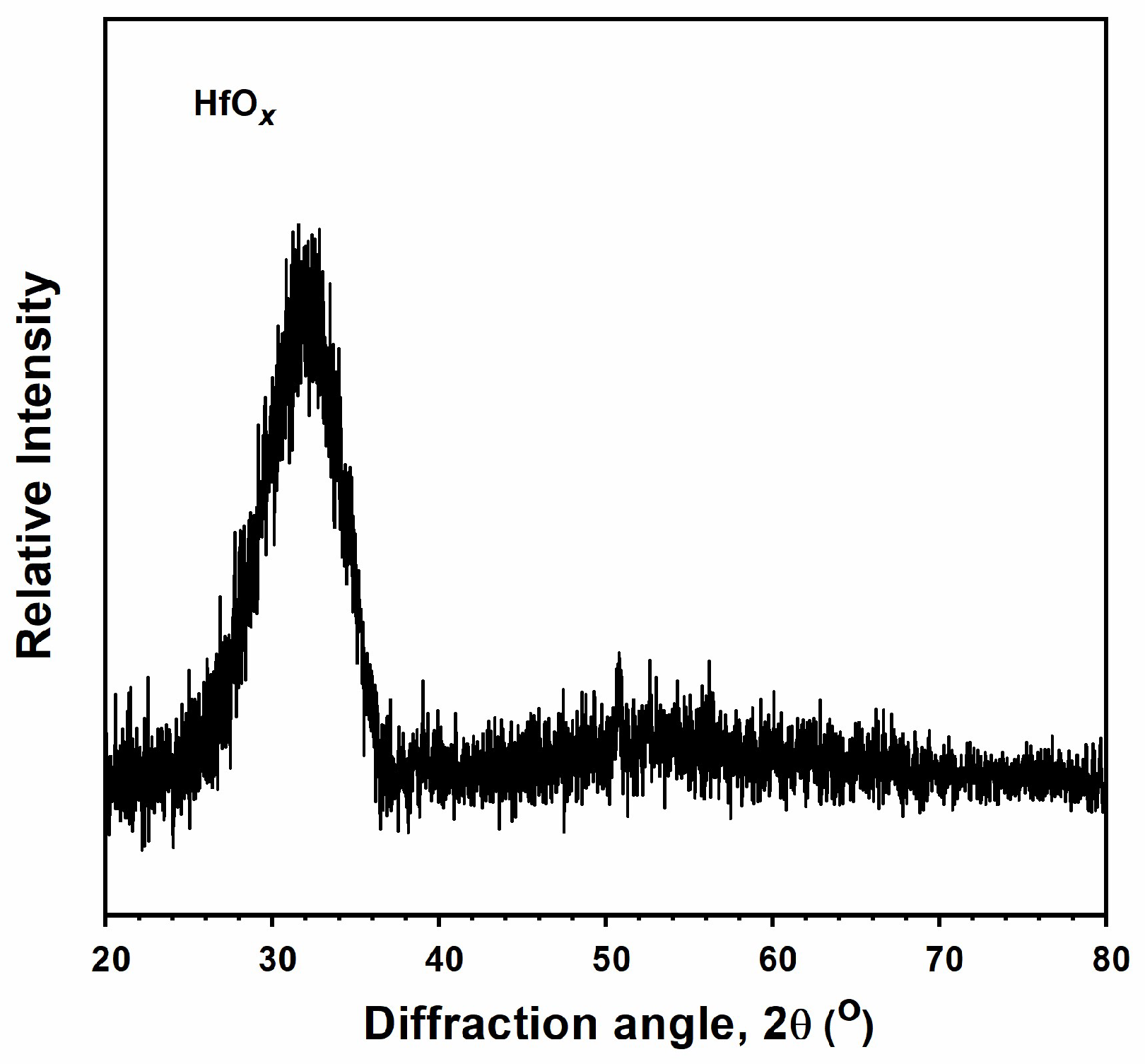
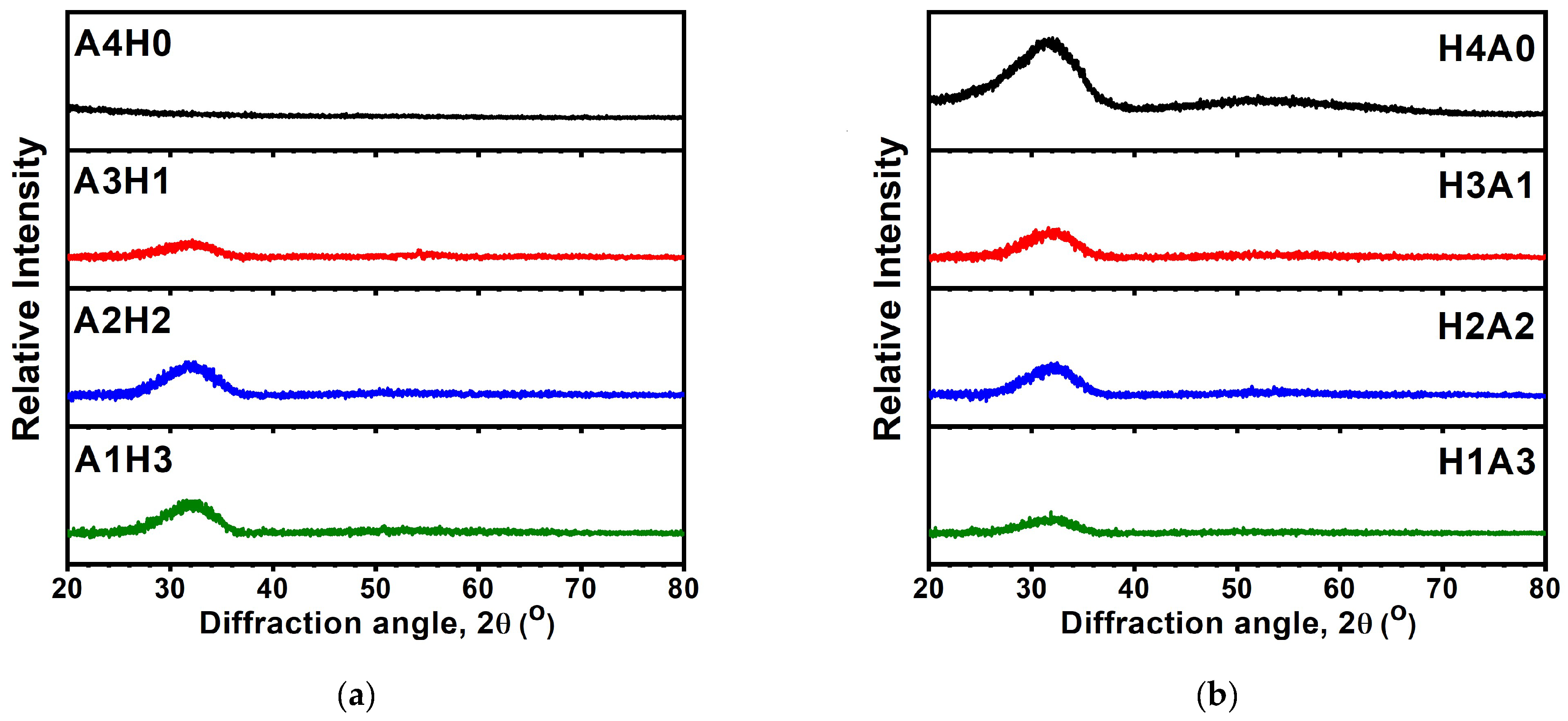
3.1. Monolithic AlON and HfOx Films
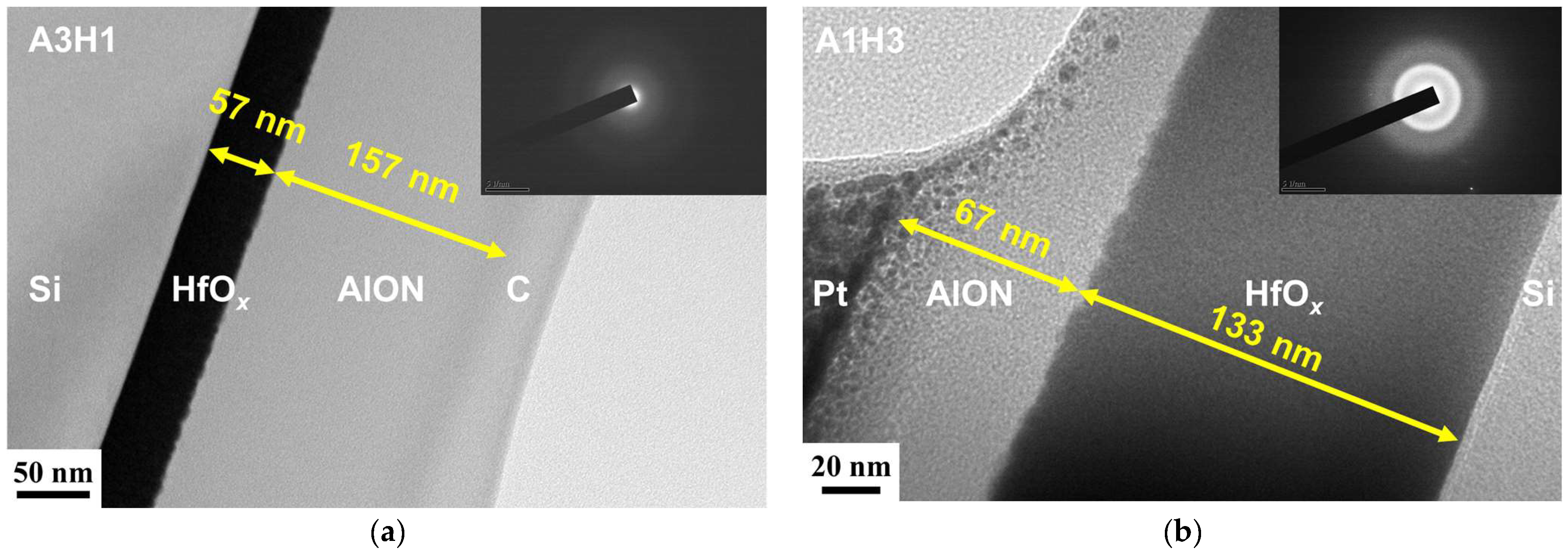
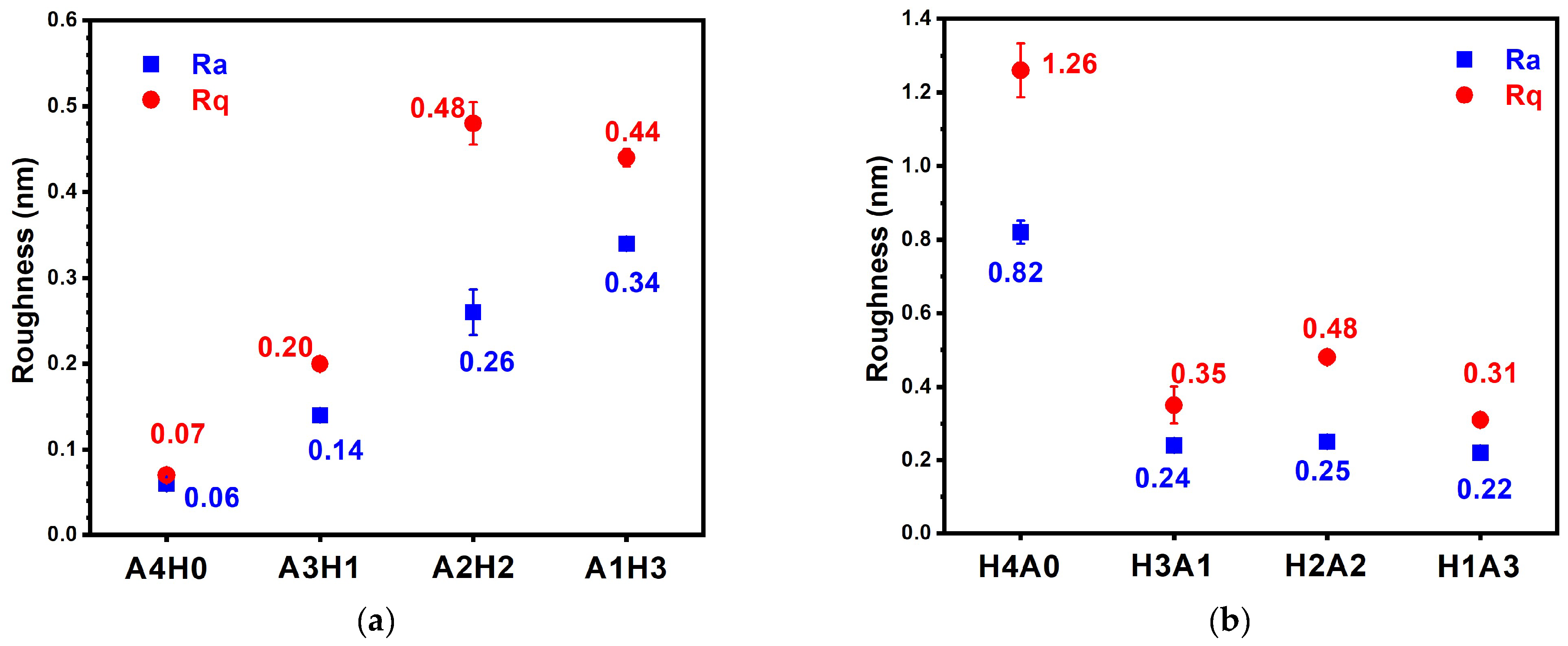
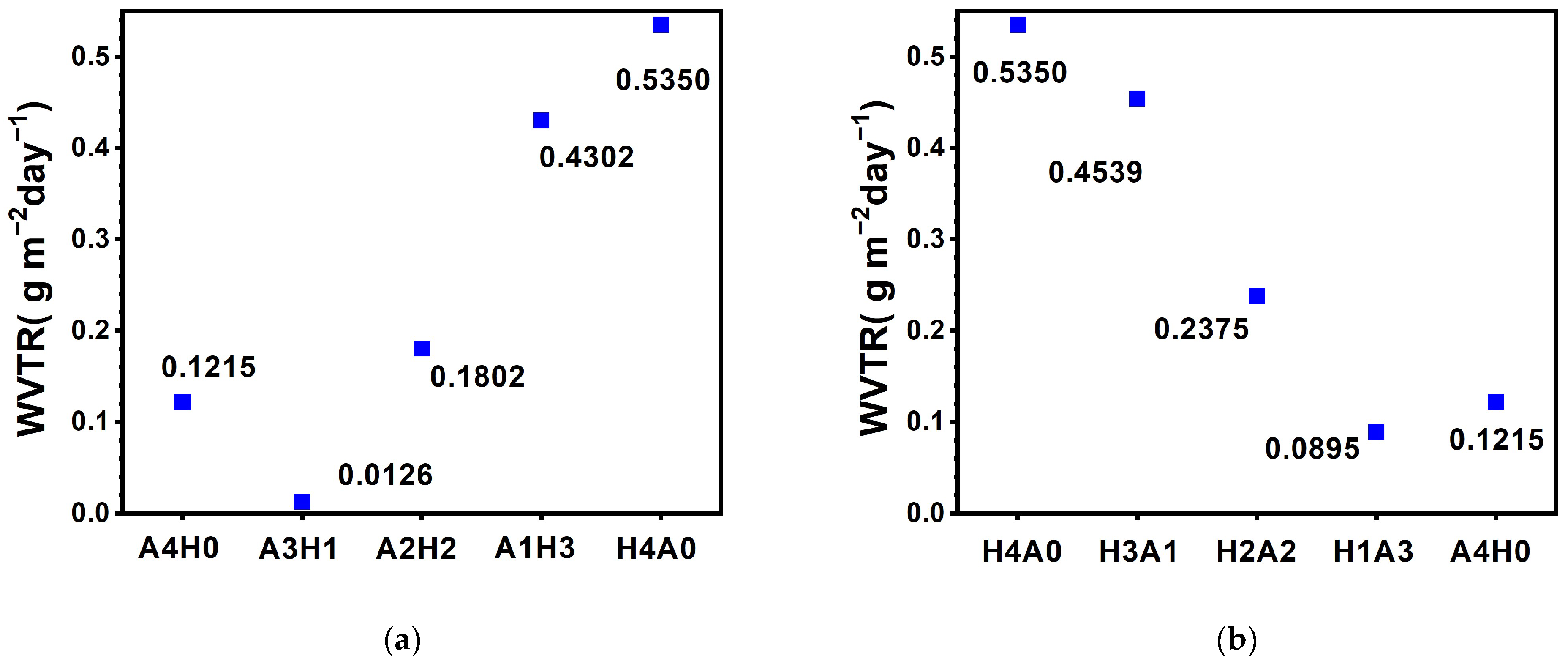
3.2. AlON/HfOx Bilayer Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, A.; Ahnood, A.; Cole, M.T.; Lee, S.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A.; et al. Flexible electronics: The next ubiquitous platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.Q.; Chen, Z.; Tao, Y.; Liu, Y.F. Recent progress on thin-film encapsulation technologies for organic electronic devices. Opt. Commun. 2016, 362, 43–49. [Google Scholar] [CrossRef]
- Yanaka, M.; Henry, B.M.; Roberts, A.P.; Grovenor, C.R.M.; Briggs, G.A.D.; Sutton, A.P.; Miyamoto, T.; Tsukahara, Y.; Takeda, N.; Chater, R.J. How cracks in SiOx-coated polyester films affect gas permeation. Thin Solid Films 2001, 397, 176–185. [Google Scholar] [CrossRef]
- Roberts, A.P.; Henry, B.M.; Sutton, A.P.; Grovenor, C.R.M.; Briggs, G.A.D.; Miyamoto, T.; Kano, M.; Tsukahara, Y.; Yanaka, M. Gas permeation in silicon-oxide/polymer (SiOx/PET) barrier films: Role of the oxide lattice, nano-defects and macro-defects. J. Memb. Sci. 2002, 208, 75–88. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation barriers on polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]
- Erlat, A.G.; Henry, B.M.; Grovenor, C.R.M.; Briggs, A.G.D.; Chater, R.J.; Tsukahara, Y. Mechanism of water vapor transport through PET/AlOxNy gas barrier films. J. Phys. Chem. B 2004, 108, 883–890. [Google Scholar] [CrossRef]
- Park, S.; Kim, L.H.; Jeong, Y.J.; Kim, K.; Park, M.; Baek, Y.; An, T.K.; Nam, S.; Jang, J.; Park, C.E. Reduced water vapor transmission rates of low-temperature processed and sol-gel-derived titanium oxide thin films on flexible substrates. Org. Electron. 2016, 36, 133–139. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Heeger, A.J. Extended lifetime of organic field-effect transistors encapsulated with titanium sub-oxide as an “active” passivation/barrier layer. Adv. Mater. 2009, 21, 1941–1944. [Google Scholar] [CrossRef]
- Yun, S.J.; Abidov, A.; Kim, S.; Choi, J.S.; Cho, B.S.; Chung, S.C. Water vapor transmission rate property of SiNx thin films prepared by low temperature (<100 °C) linear plasma enhanced chemical vapor deposition. Vacuum 2018, 48, 33–40. [Google Scholar]
- Kim, L.H.; Jang, J.H.; Jeong, Y.J.; Kim, K.; Baek, Y.; Kwon, H.J.; An, T.K.; Nam, S.; Kim, S.H.; Jang, J.; et al. Highly-impermeable Al2O3/HfO2 moisture barrier films grown by low-temperature plasma-enhanced atomic layer deposition. Org. Electron. 2017, 50, 296–303. [Google Scholar] [CrossRef]
- Chen, R.; Le, K.; Liu, Y.; Zheng, X.; Zhu, X.; Bao, L.; Yang, Z.; Xu, S.; Liu, W. Investigation of water vapor barrier performances of diamond-like carbon coatings on flexible PET fabricated at room temperature. Surf. Coat. Technol. 2024, 477, 130339. [Google Scholar] [CrossRef]
- Groner, M.D.; George, S.M.; McLean, R.S.; Carcia, P.F. Gas diffusion barriers on polymers using Al2O3 atomic layer deposition. Appl. Phys. Lett. 2006, 88, 051907. [Google Scholar] [CrossRef]
- Han, Y.C.; Jang, C.; Kim, K.J.; Choi, K.C.; Jung, K.H.; Bae, B.S. The encapsulation of an organic light-emitting diode using organic–inorganic hybrid materials and MgO. Org. Electron. 2011, 12, 609–613. [Google Scholar] [CrossRef]
- Bang, S.H.; Hwang, N.M.; Kim, H.L. Permeation barrier properties of silicon oxide films deposited on polyethylene terephthalate (PET) substrate using roll-to-roll reactive magnetron sputtering system. Microelectron. Eng. 2024, 166, 39–44. [Google Scholar] [CrossRef]
- Ehiasarian, A.P. High-power impulse magnetron sputtering and its applications. Pure Appl. Chem. 2010, 82, 1247–1258. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Chang, L.C.; Wu, C.E.; Ou, T.Y. Mechanical properties and diffusion barrier performance of CrWN coatings fabricated through hybrid HiPIMS/RFMS. Coatings 2021, 11, 690. [Google Scholar] [CrossRef]
- Jarvis, K.L.; Evans, P.J.; Cooling, N.A.; Vaughan, B.; Habsuda, J.; Belcher, W.J.; Bilen, C.; Griffiths, G.; Dastoor, P.C.; Triani, G. Comparing three techniques to determine the water vapour transmission rates of polymers and barrier films. Surf. Interfaces 2017, 9, 182–188. [Google Scholar] [CrossRef]
- Kempe, M.D.; Reese, M.O.; Dameron, A.A. Evaluation of the sensitivity limits of water vapor transmission rate measurements using electrical calcium test. Rev. Sci. Instrum. 2013, 84, 025109. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Cho, T.Y.; Choa, S.H.; Cho, S.K. Environmental reliability and moisture barrier properties of silicon nitride and silicon oxide films using roll-to-roll plasma enhanced chemical vapor deposition. Thin Solid Films 2021, 720, 138524. [Google Scholar] [CrossRef]


| Sample | Duty Cycle | Atomic Compositions (at.%) | ||
|---|---|---|---|---|
| Al | O | N | ||
| D5 | 5% | 38.54 ± 0.33 | 59.31 ± 0.30 | 2.15 ± 0.25 |
| D10 | 10% | 37.13 ± 0.55 | 61.10 ± 0.54 | 1.77 ± 0.15 |
| D15 | 15% | 37.51 ± 0.27 | 60.61 ± 0.27 | 1.88 ± 0.12 |
| D20 | 20% | 36.89 ± 0.41 | 61.26 ± 0.33 | 1.85 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-C.; Lin, S.-E. Water Vapor-Impermeable AlON/HfOx Bilayer Films Deposited by Hybrid High-Power Impulse Magnetron Sputtering/Radio-Frequency Magnetron Sputtering Processes. Materials 2024, 17, 5453. https://doi.org/10.3390/ma17225453
Chang L-C, Lin S-E. Water Vapor-Impermeable AlON/HfOx Bilayer Films Deposited by Hybrid High-Power Impulse Magnetron Sputtering/Radio-Frequency Magnetron Sputtering Processes. Materials. 2024; 17(22):5453. https://doi.org/10.3390/ma17225453
Chicago/Turabian StyleChang, Li-Chun, and Sheng-En Lin. 2024. "Water Vapor-Impermeable AlON/HfOx Bilayer Films Deposited by Hybrid High-Power Impulse Magnetron Sputtering/Radio-Frequency Magnetron Sputtering Processes" Materials 17, no. 22: 5453. https://doi.org/10.3390/ma17225453
APA StyleChang, L.-C., & Lin, S.-E. (2024). Water Vapor-Impermeable AlON/HfOx Bilayer Films Deposited by Hybrid High-Power Impulse Magnetron Sputtering/Radio-Frequency Magnetron Sputtering Processes. Materials, 17(22), 5453. https://doi.org/10.3390/ma17225453









