Ultrasound-Assisted Community Bureau of Reference (BCR) Procedure for Heavy Metal Removal in Sewage Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Equipment
2.3. Exchangeable and Acid Solution
2.4. Reducible Solution
2.5. Oxidable Solution
2.6. Collected SS and Leachate Samples
2.7. Application of Chemical Extraction Steps
- Step 1
- Over 1 g of dried SS with a particle size of 63 μm, 20 mL of CH3COOH 0.11M (pH = 2.8) was added, and the resulting solution was sonicated in the ultrasonic bath for 20 min at 50 kHz and 25 ± 2 °C, and centrifuged at 5000 rpm for 30 min, and the decanted supernatant was analyzed by ICP-OES, F1. The samples were washed with 20 mL of ultrapure water between each extraction step.
- Step 2
- The residue obtained from the first step of F1 was agitated for 20 min at 50 kHz at 25 ± 2 °C using 20 mL of NH2OH-HCl (pH = 2). After 30 min of centrifugation at 5000 rpm, the decanted supernatant was analyzed by ICP-OES, F2.
- Step 3
- The residue obtained from the second step was treated in an ultrasonic bath with 30% H2O2 until evaporation was done in ≈30 min. Subsequently, 20 mL of 1M CH3COONH4, pH = 2 was added over the dry residue, and the samples were stirred for 20 min, at 50 kHz and 25 ± 2 °C. The supernatant obtained after 30 min of centrifugation at 5000rpm was analyzed by ICP-OES, F3.
- Step 4
- The residue obtained from the F3 was washed with 20 mL of ultrapure water and treated for 30 min in a microwave oven with aqua regia (HCl: HNO3 in a 3:1 ratio 21 mL HCl: 7 mL HNO3) added to determine the residual fraction, F4.
2.8. Metal Analysis
2.9. Determination of Total Metal Content in SS Samples
2.10. Leaching Test Applied to SS Samples
2.11. Methodology Used to Retain Metal Ions on Maize Stalks from Leachate Samples
3. Results and Discussion
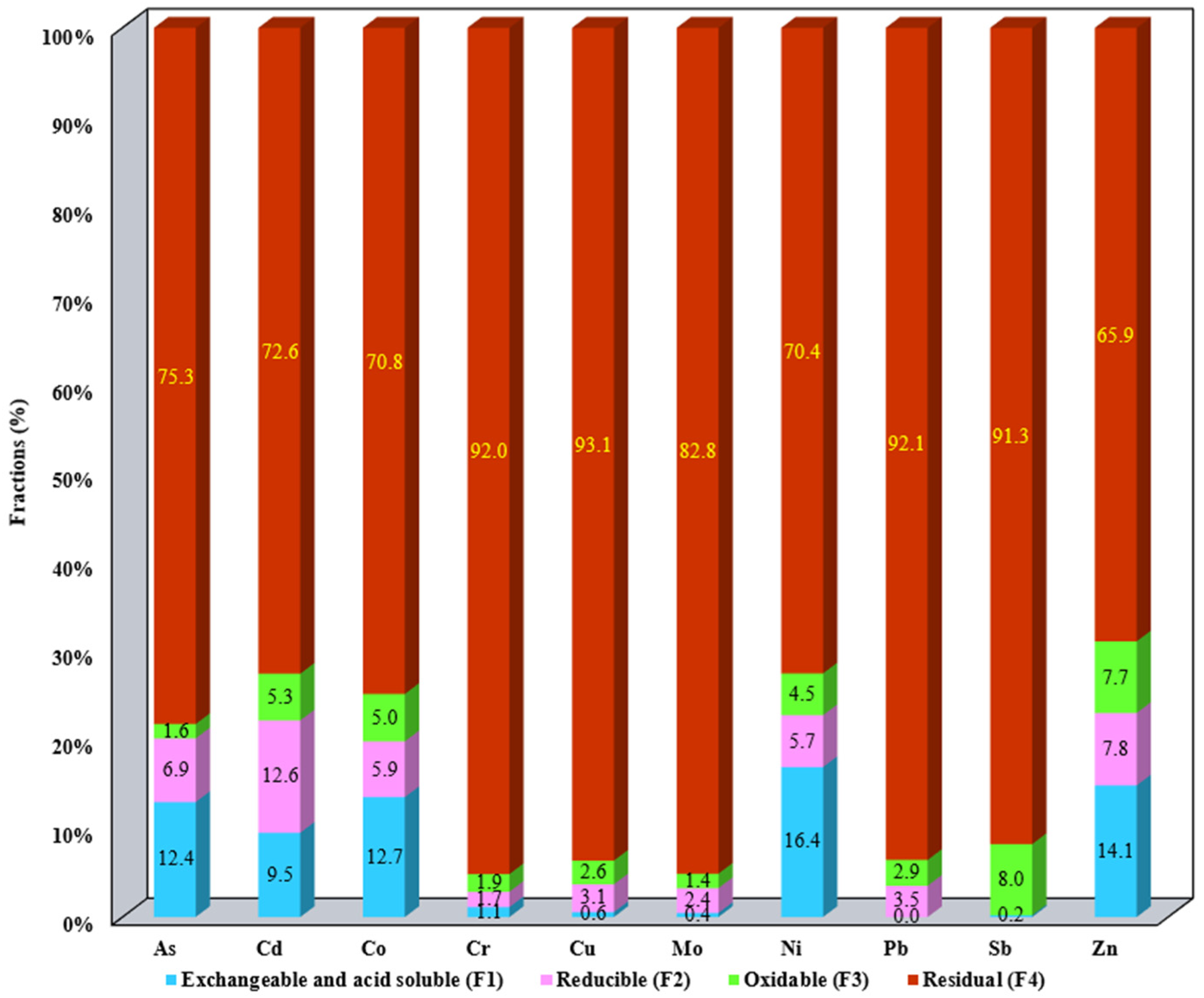
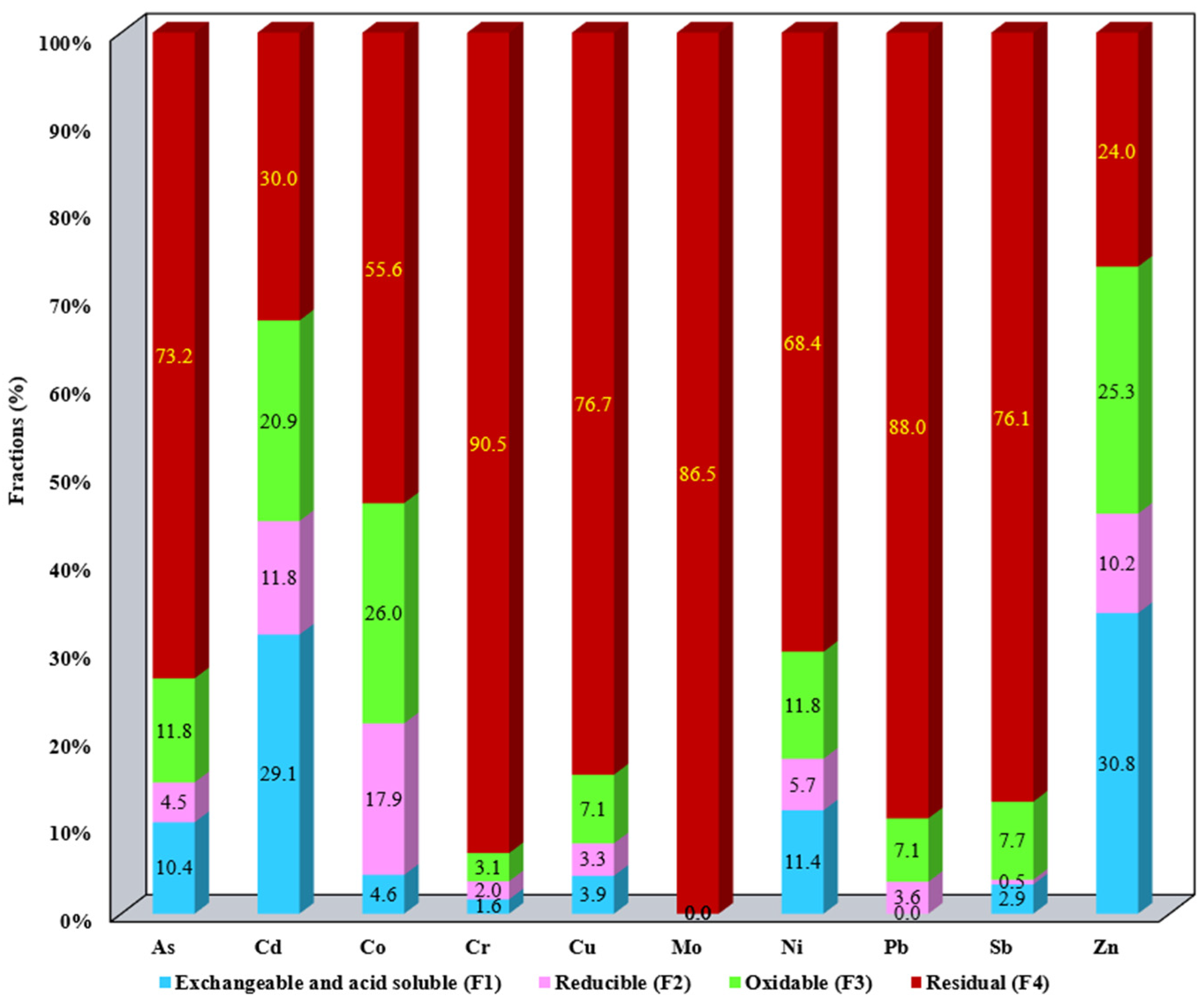
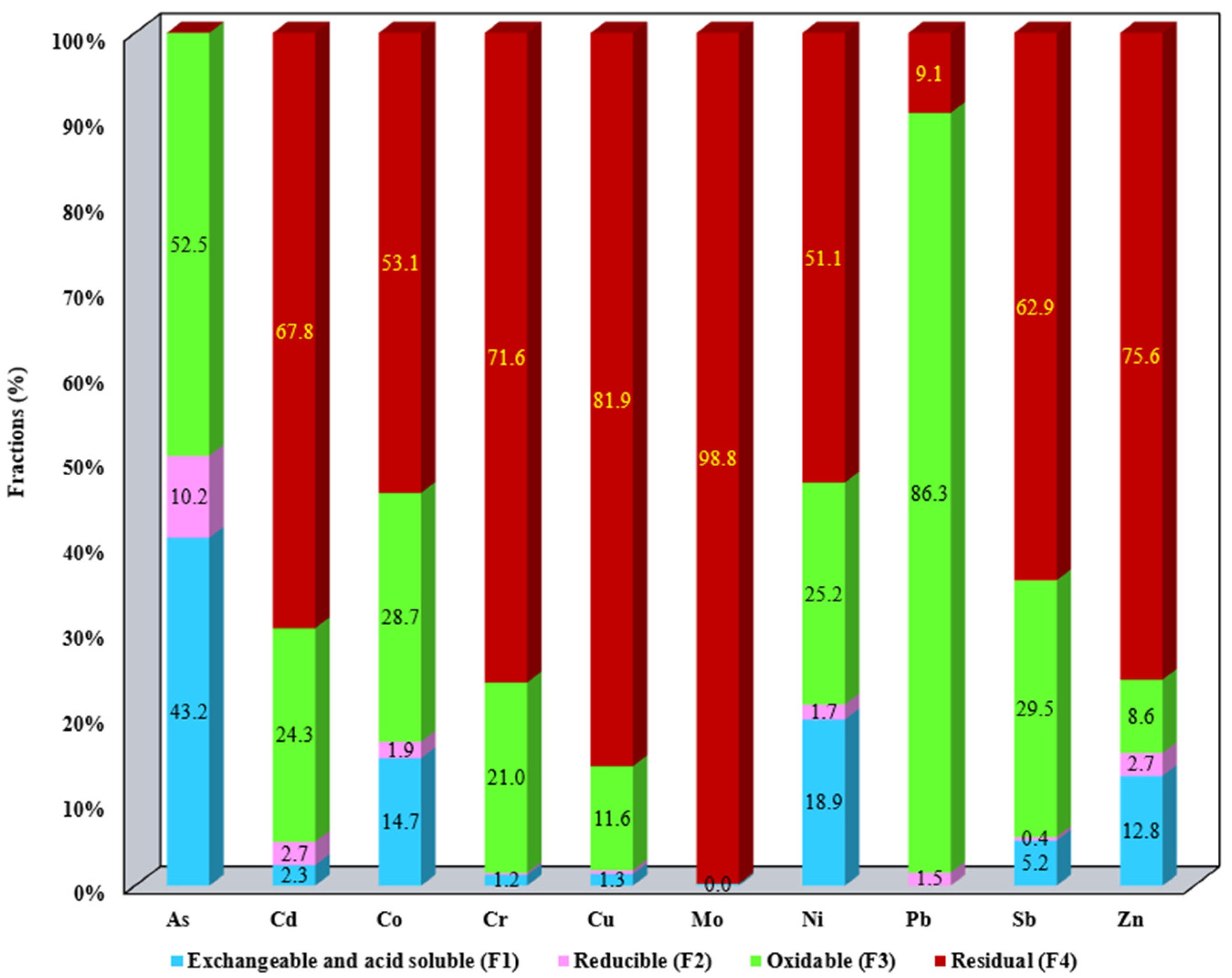
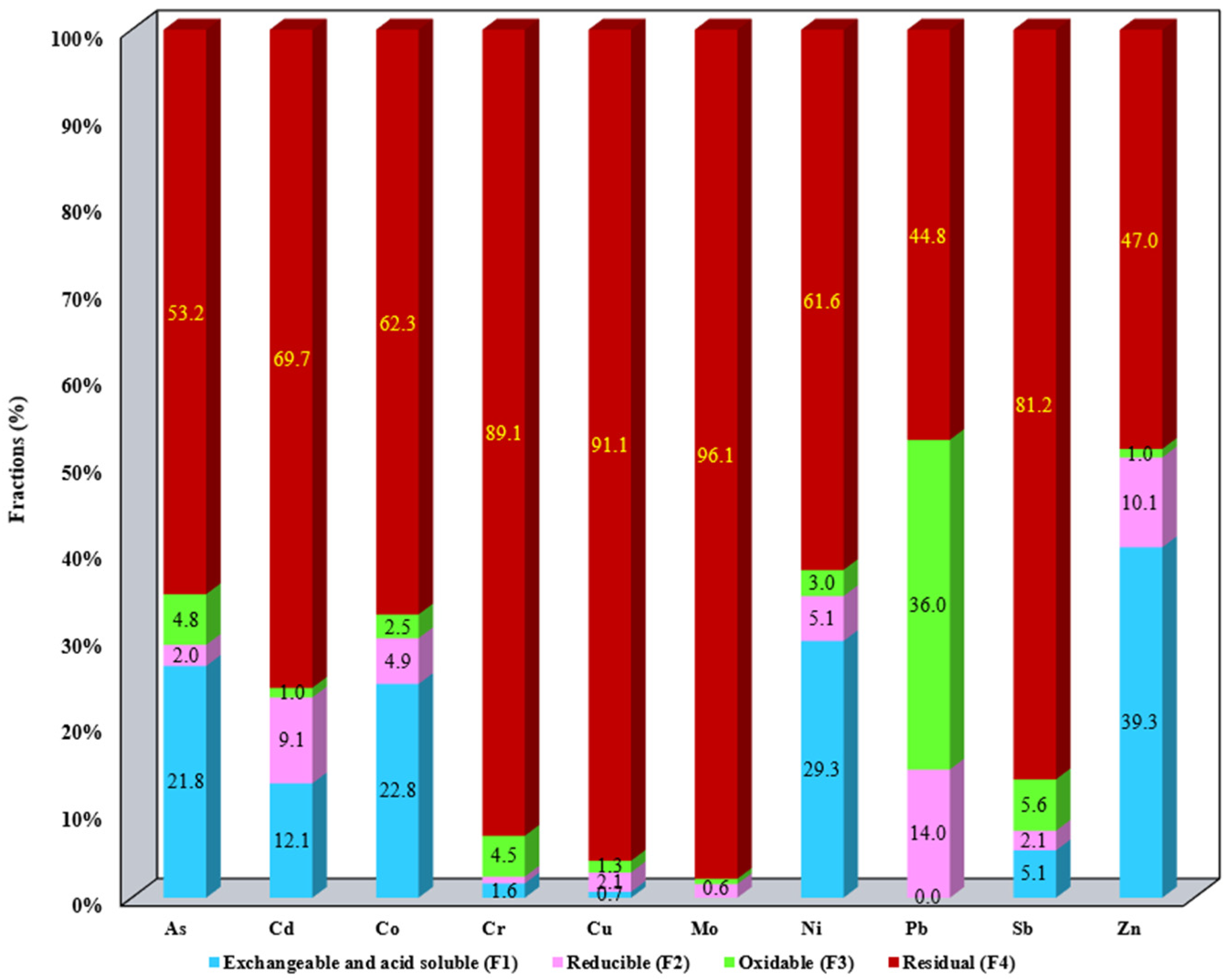
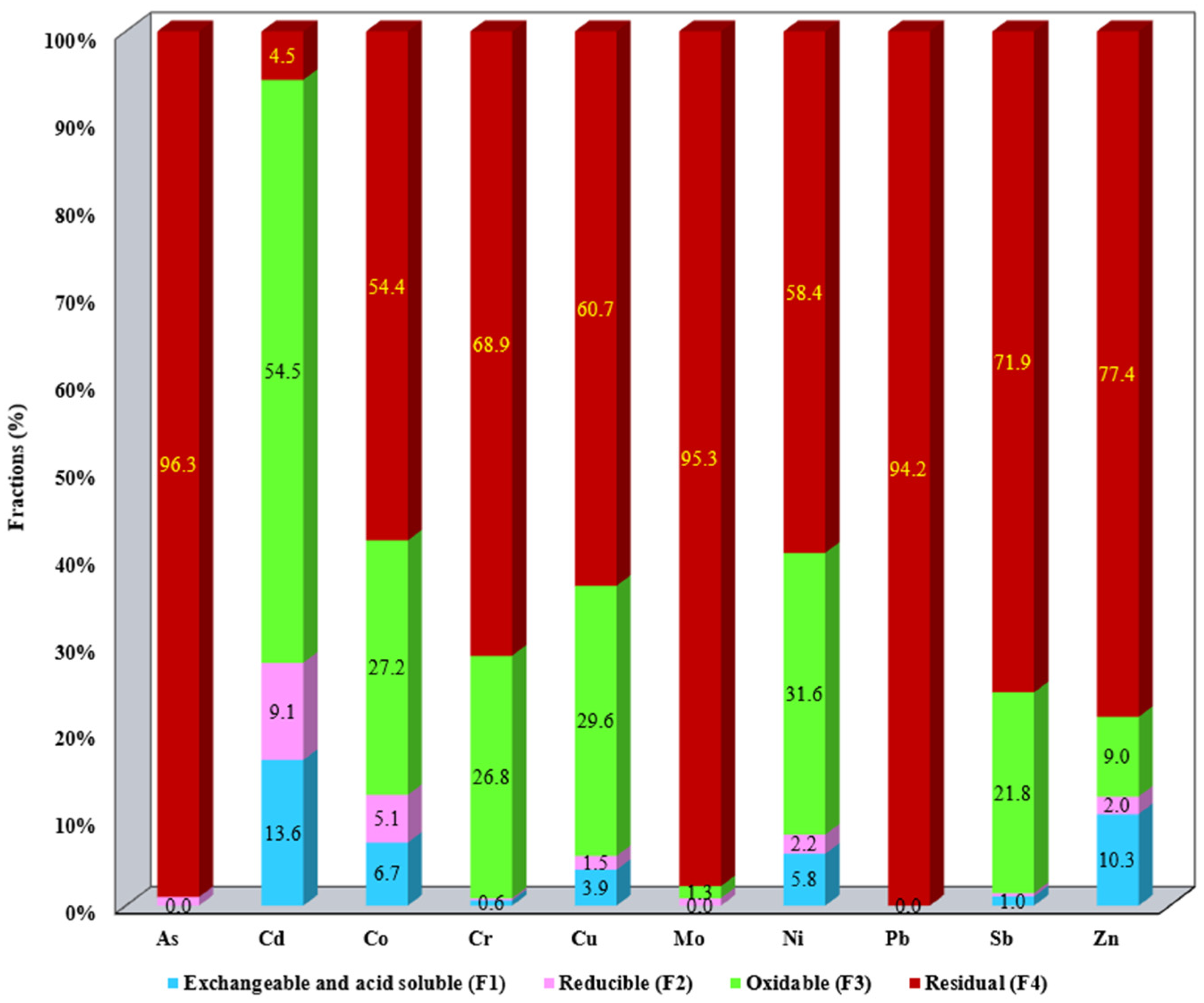
3.1. Evaluation of the Metallic Mobile Fraction from SS Samples
3.2. Evaluation of the BCR Extraction Procedure by the Certified Reference Material
3.3. Application of Maize Stalk for Metal Ions Removal from Leachate Concentrated Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusiatin, M.; Kulikowska, D.; Bernat, K. Municipal Sewage Sludge as a Resource in the Circular Economy. Energies 2024, 17, 2474. [Google Scholar] [CrossRef]
- Woo, D.C.Y.; Goh, Q.H.; Poh, P.E.; Chew, I.M.L. A technoeconomic analysis of sewage sludge valorization for carbon emission reduction. Biomass Convers. Biorefinery 2023, 13, 13591–13604. [Google Scholar] [CrossRef]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture–the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic solid waste biorefinery: Sustainable strategy for emerging circular bioeconomy in China. Ind. Crops Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Hernández, T.; López Aragón, R.F.; Garcia, C. The Use of Aerobic Urban Sewage Sludge in Agriculture: Potential Benefits and Contaminating Effects in Semi-Arid Zones. Agriculture 2024, 14, 983. [Google Scholar] [CrossRef]
- Vráblová, M.; Smutná, K.; Chamrádová, K.; Vrábl, D.; Koutník, I.; Rusín, J.; Bouchalová, M.; Gavlová, A.; Sezimová, H.; Navrátil, M. Co-composting of sewage sludge as an effective technology for the production of substrates with reduced content of pharmaceutical residues. Sci. Total Environ. 2024, 915, 169818. [Google Scholar] [CrossRef]
- Ignatowicz, K. The impact of sewage sludge treatment on the content of selected heavy metals and their fractions. Environ. Res. 2017, 156, 19–22. [Google Scholar] [CrossRef]
- Świnder, H.; Lejwoda, P. Obtaining nickel concentrates from sludge produced in the process of electrochemical metal surface treatment. Water Air Soil Pollut. 2021, 232, 505. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Gunasekara, C.; Karunarathna, A.; Law, D.; Pramanik, B.K. Improvement of heavy metal removal from urban runoff using modified pervious concrete. Sci. Total Environ. 2022, 815, 152936. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Janas, M.; Zawadzka, A.; Cichowicz, R. The influence of selected factors on leaching of metals from sewage sludge. Environ. Sci. Pollut. Res. 2018, 25, 33240–33248. [Google Scholar] [CrossRef] [PubMed]
- Nartowska, E.; Podlasek, A.; Vaverková, M.D.; Koda, E.; Jakimiuk, A.; Kowalik, R.; Kozłowski, T. Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation. Materials 2024, 17, 2957. [Google Scholar] [CrossRef] [PubMed]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Bayero, M.T.; Fedorenko, A.; Mahmoodi, N.M.; Sillanpää, M.; Bauer, T.; Soldatov, A. Metal-organic frameworks (MIL-101) decorated biochar as a highly efficient bio-based composite for immobilization of polycyclic aromatic hydrocarbons and copper in real contaminated soil. J. Environ. Chem. Eng. 2022, 10, 108821. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Marin, N.M.; Dinu, L.; Stanculescu, I.; Cristea, N.I.; Ionescu, A.I. Maize stalk material for on-site treatment of highly polluted leachate and mine wastewater. Materials 2021, 14, 956. [Google Scholar] [CrossRef]
- Marin, N.M. Maize stalk obtained after acid treatment and its use for simultaneous removal of Cu2+, Pb2+, Ni2+, Cd2+, Cr3+ and Fe3+. Polymers 2022, 14, 3141. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 1–50. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- SR EN 12457-1/2003; Characterization of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 L·kg–1 for Materials with Particle Size Below 10 mm (without or with Size Reduction). UNE: Madrid, Spain, 2003.
- Romanian Order no. 95/2005 Establishing Acceptance Criteria and Preliminary Procedures for Acceptances of Waste Storage and National List of Waste Accepted in Each Class of Landfill. Available online: https://legislatie.just.ro/Public/DetaliiDocument/59751 (accessed on 1 September 2024).
- Romanian Order no. 344/2004 Regarding Technical Rules on Environmental and in Particular, Soils When Are Used Amended Sewage Sludge in Agriculture. Available online: https://legislatie.just.ro/Public/DetaliiDocument/55968 (accessed on 1 September 2024).
- USEPA. Standards for the Use or Disposal of Sewage Sludge, 40 C.F.R. Sect. 503. US Environmental Office of Wastewater Management. 1993. Available online: https://p2infohouse.org/ref/48/47618.pdf (accessed on 1 September 2024).
- Marin, N.M. A New Approach of Complexing Polymers Used for the Removal of Cu2+ Ions. Polymers 2024, 16, 920. [Google Scholar] [CrossRef] [PubMed]
- Nazaripour, M.; Reshadi, M.A.M.; Mirbagheri, S.A.; Nazaripour, M.; Bazargan, A. Research trends of heavy metal removal from aqueous environments. J. Environ. Manage. 2021, 287, 112322. [Google Scholar] [CrossRef] [PubMed]
- Younas, F.; Younas, S.; Bibi, I.; Farooqi, Z.U.R.; Hameed, M.A.; Mohy-Ud-Din, W.; Shehzad, M.T.; Hussain, M.M.; Shakil, Q.; Shahid, M. A critical review on the separation of heavy metal (loid) s from the contaminated water using various agricultural wastes. Int. J. Phytoremediat. 2024, 26, 349–368. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O. Green sorbents from agriculture al wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Dong, L.; Yanyan, L.; Junxia, Y.; Yigang, D. Removal of copper (II) from aqueous solution with rape stalk modified by citric acid. J. Dispers. Sci. Technol. 2017, 38, 180–186. [Google Scholar] [CrossRef]
- Bekchanov, D.; Mukhamediev, M.; Yarmanov, S.; Lieberzeit, P.; Mujahid, A. Functionalizing natural polymers to develop green adsorbents for wastewater treatment applications. Carbohydr. Polym. 2024, 323, 121397. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]

| Method | Extracting Solutions | pH | Conditions | |
|---|---|---|---|---|
| BCR | 0.11 M CH3COOH | 2.8 | Report (m/V) = 1:40, 20 min, 50 kHz, 25 ± 2 °C | Our laboratory conditions |
| method | 0.43 M CH3COOH | 2.5 | Report (m/V) = 1:40, 16 h, 30 rpm, 25 ± 2 °C | BCR conditions from certified |
| Metal | Determined Value | Certified Value | Recovery | Bias (%) | |
|---|---|---|---|---|---|
| mg/Kg DM | Uncertainty (±%) | mg/Kg DM | (%) | ||
| Cd | 17.5 | 10 | 18.3 | 95.6 | 4.4 |
| Cr | 18.9 | 9 | 18.7 | 101.1 | −1.1 |
| Cu | 33.9 | 9 | 33.5 | 101.2 | −1.2 |
| Ni | 24.7 | 10 | 25.8 | 95.7 | 4.3 |
| Pb | 1.92 | 9.5 | 2.10 | 91.4 | 8.6 |
| Zn | 611 | 9.5 | 620 | 98.5 | 1.5 |
| Metal (mg/L) | S1L | S2L | S3L | S4L | S5L |
|---|---|---|---|---|---|
| As | 0.685 ± 0.027 | 0.945 ± 0.034 | 0.620 ± 0.036 | 0.156 ± 0.006 | 0.626 ± 0.020 |
| Cd | 0.190 ± 0.024 | 0.174 ± 0.006 | 0.207 ± 0.015 | 0.350 ± 0.022 | 0.083 ± 0.004 |
| Co | 0.427 ± 0.095 | 1.406 ± 0.015 | 0.579 ± 0.013 | 0.390 ± 0.010 | 0.266 ± 0.012 |
| Cr | 0.603 ± 0.069 | 1.048 ± 0.013 | 3.185 ± 0.051 | 0.732 ± 0.012 | 0.265 ± 0.008 |
| Cu | 3.895 ± 0.006 | 1.760 ± 0.021 | 2.191 ± 0.104 | 6.000 ± 0.160 | 1.790 ± 0.0174 |
| Mo | 0.099 ± 0.024 | 0.106 ± 0.003 | 0.133 ± 0.003 | 0.038 ± 0.008 | 0.061 ± 0.004 |
| Ni | 2.413 ± 0.077 | 9.718 ± 0.021 | 4.061 ± 0.031 | 0.902 ± 0.004 | 1.300 ± 0.062 |
| Pb | 0.129 ± 0.041 | 0.209 ± 0.003 | 0.025 ± 0.003 | 0.550 ± 0.021 | 0.182 ± 0.005 |
| Sb | 0.928 ± 0.122 | 1.880 ± 0.039 | 1.220 ± 0.070 | 0.975 ± 0.006 | 0.926 ± 0.006 |
| Zn | 103 ± 0.850 | 271 ± 3.266 | 210 ± 3.742 | 1.730 ± 0.049 | 350 ± 1.247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, N.M.; Galaon, T.; Pascu, L.F. Ultrasound-Assisted Community Bureau of Reference (BCR) Procedure for Heavy Metal Removal in Sewage Sludge. Materials 2024, 17, 5452. https://doi.org/10.3390/ma17225452
Marin NM, Galaon T, Pascu LF. Ultrasound-Assisted Community Bureau of Reference (BCR) Procedure for Heavy Metal Removal in Sewage Sludge. Materials. 2024; 17(22):5452. https://doi.org/10.3390/ma17225452
Chicago/Turabian StyleMarin, Nicoleta Mirela, Toma Galaon, and Luoana Florentina Pascu. 2024. "Ultrasound-Assisted Community Bureau of Reference (BCR) Procedure for Heavy Metal Removal in Sewage Sludge" Materials 17, no. 22: 5452. https://doi.org/10.3390/ma17225452
APA StyleMarin, N. M., Galaon, T., & Pascu, L. F. (2024). Ultrasound-Assisted Community Bureau of Reference (BCR) Procedure for Heavy Metal Removal in Sewage Sludge. Materials, 17(22), 5452. https://doi.org/10.3390/ma17225452






