Influence of Reclaimed Water on the Visual Quality of Automotive Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Washing Water
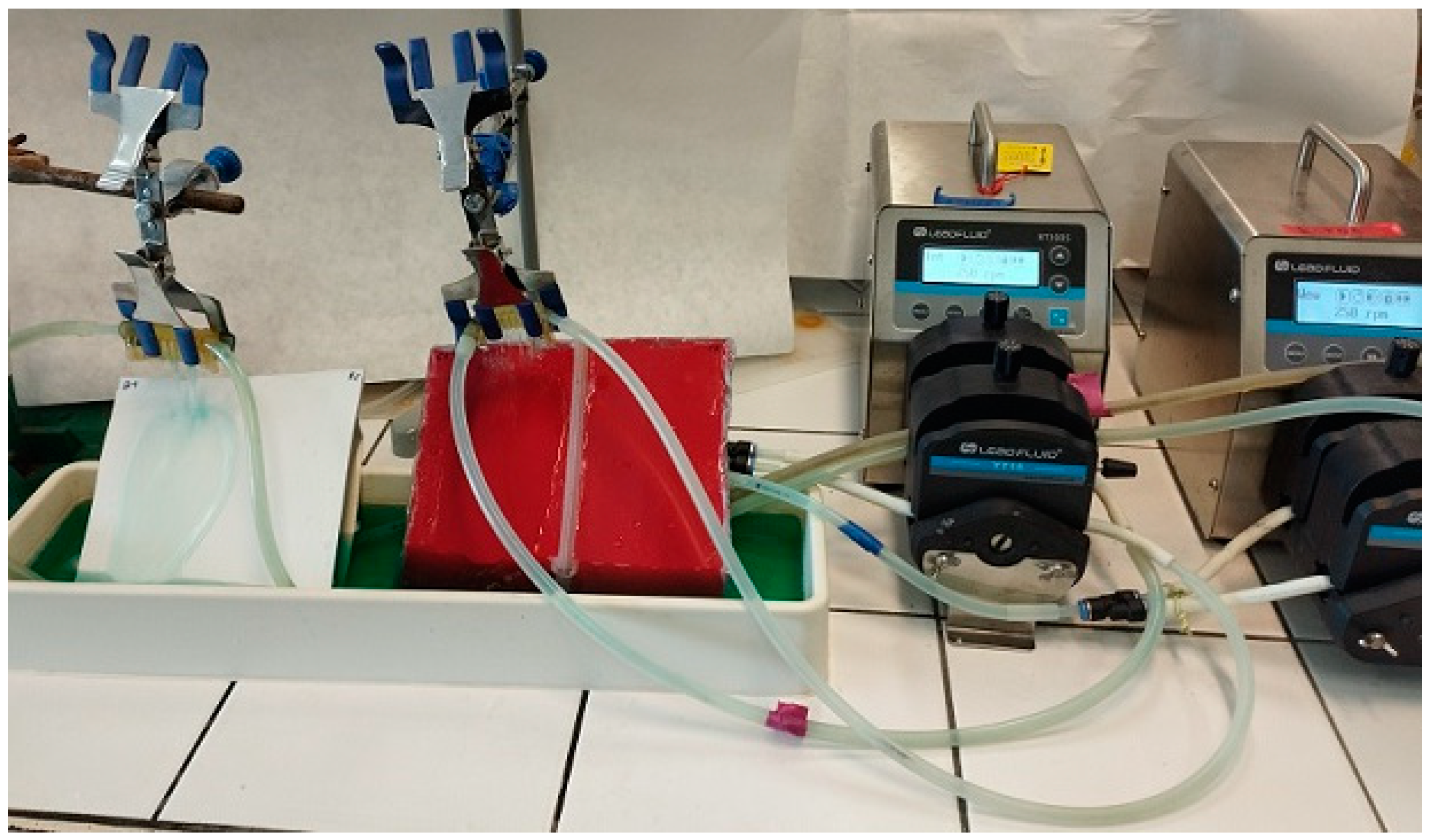
2.2. Experimental Installation
2.3. Measuring Equipment
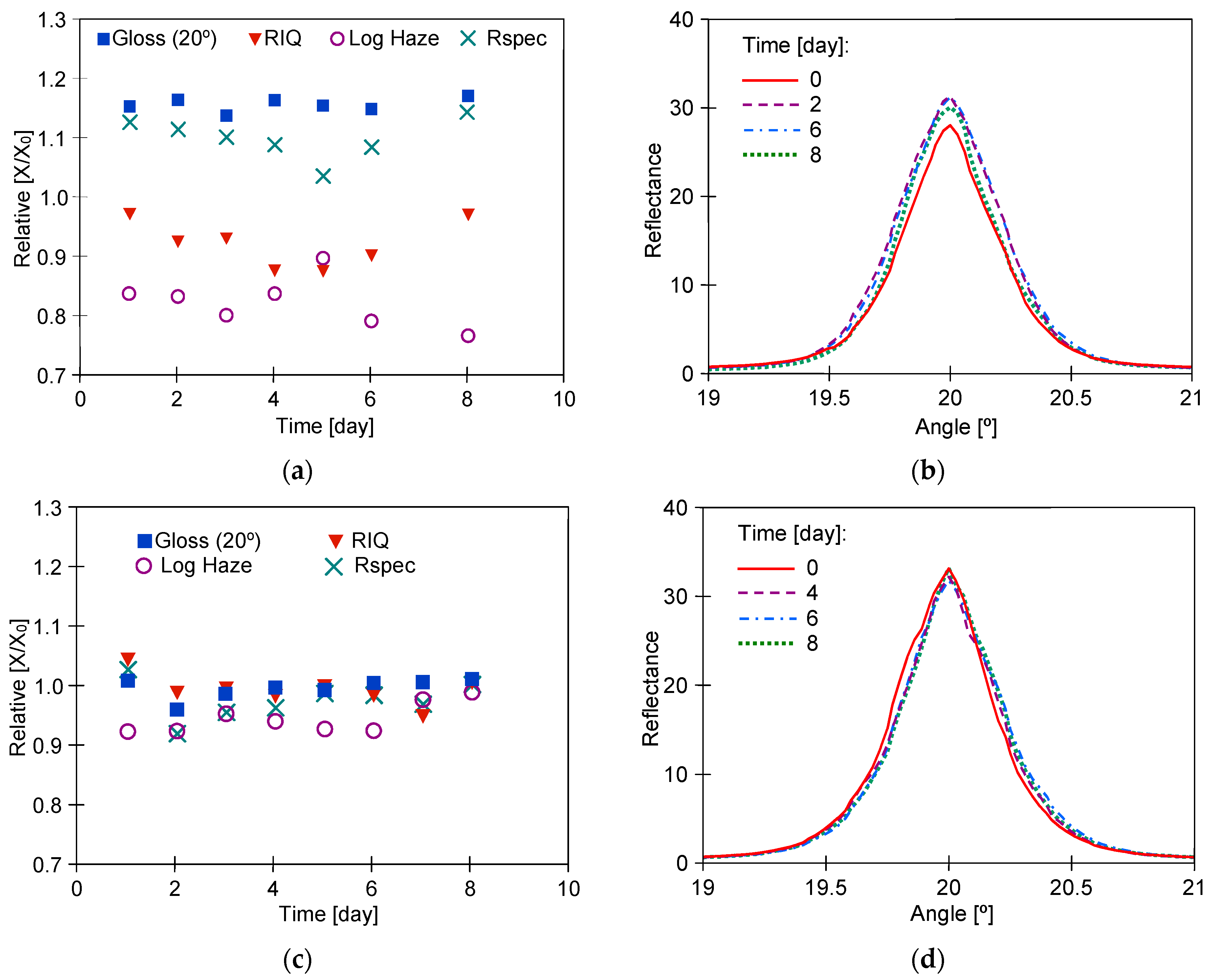
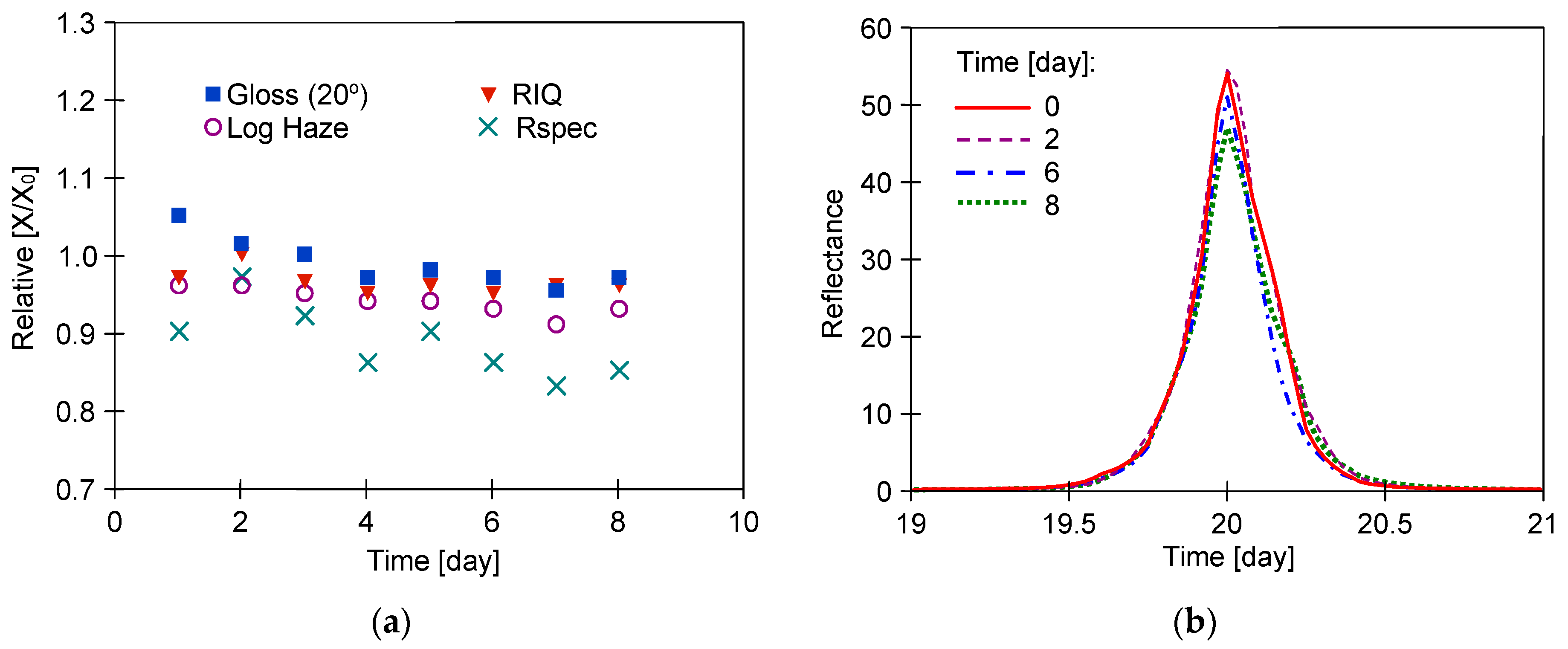
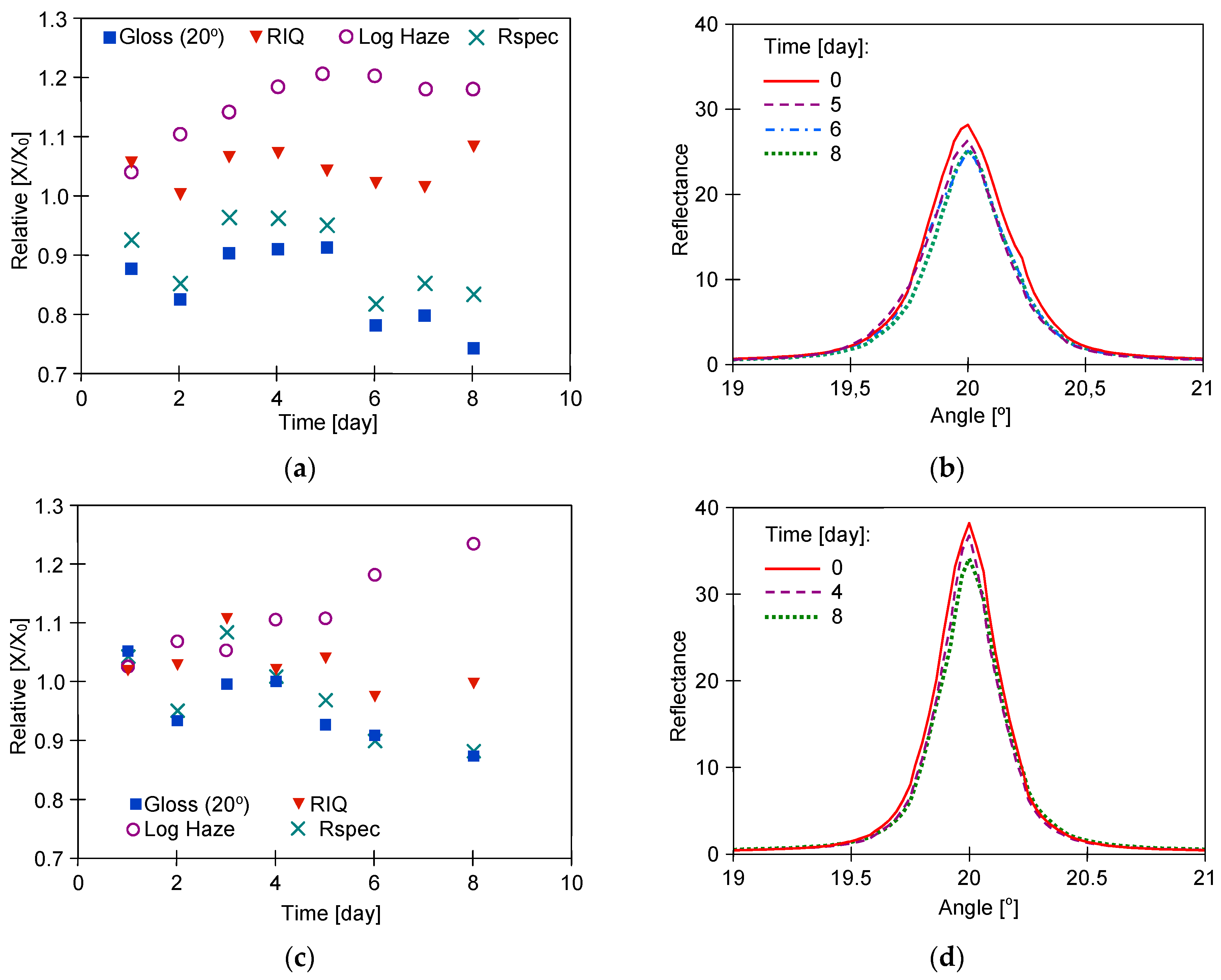
3. Results
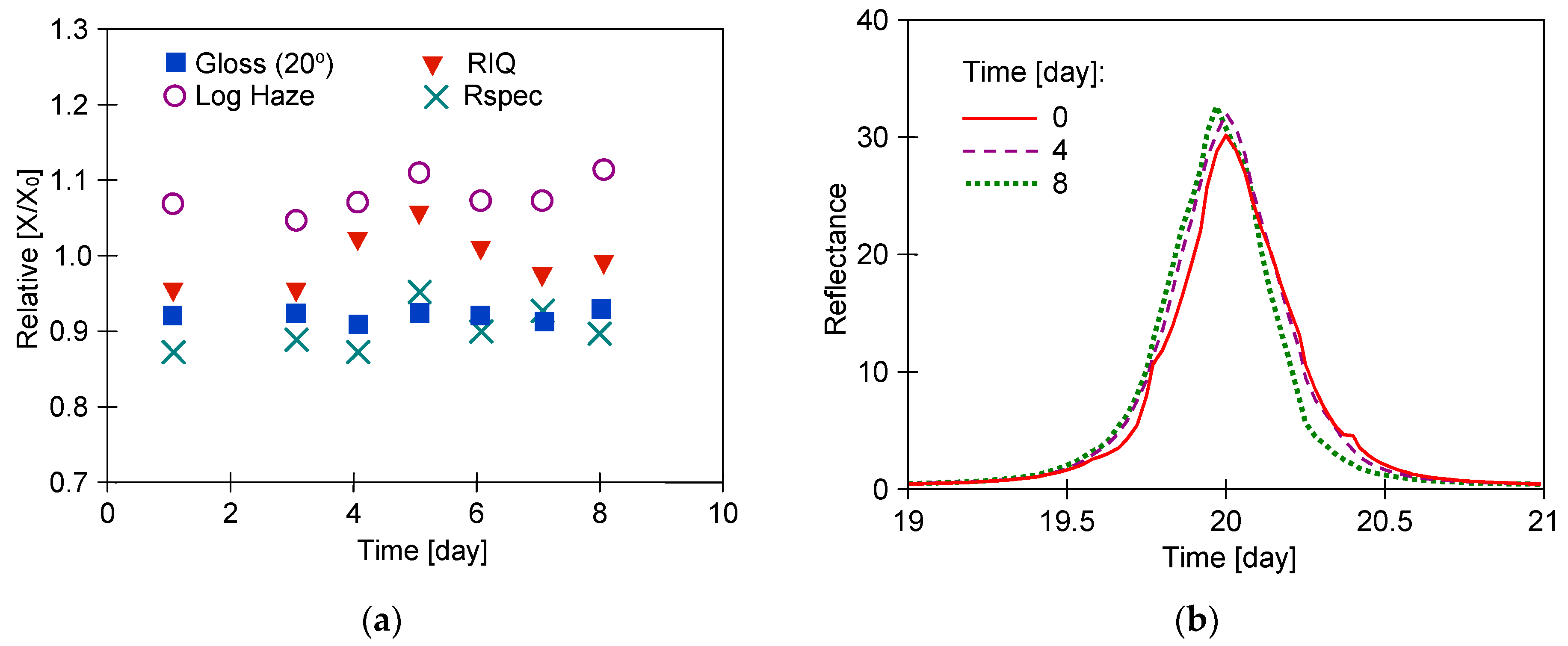
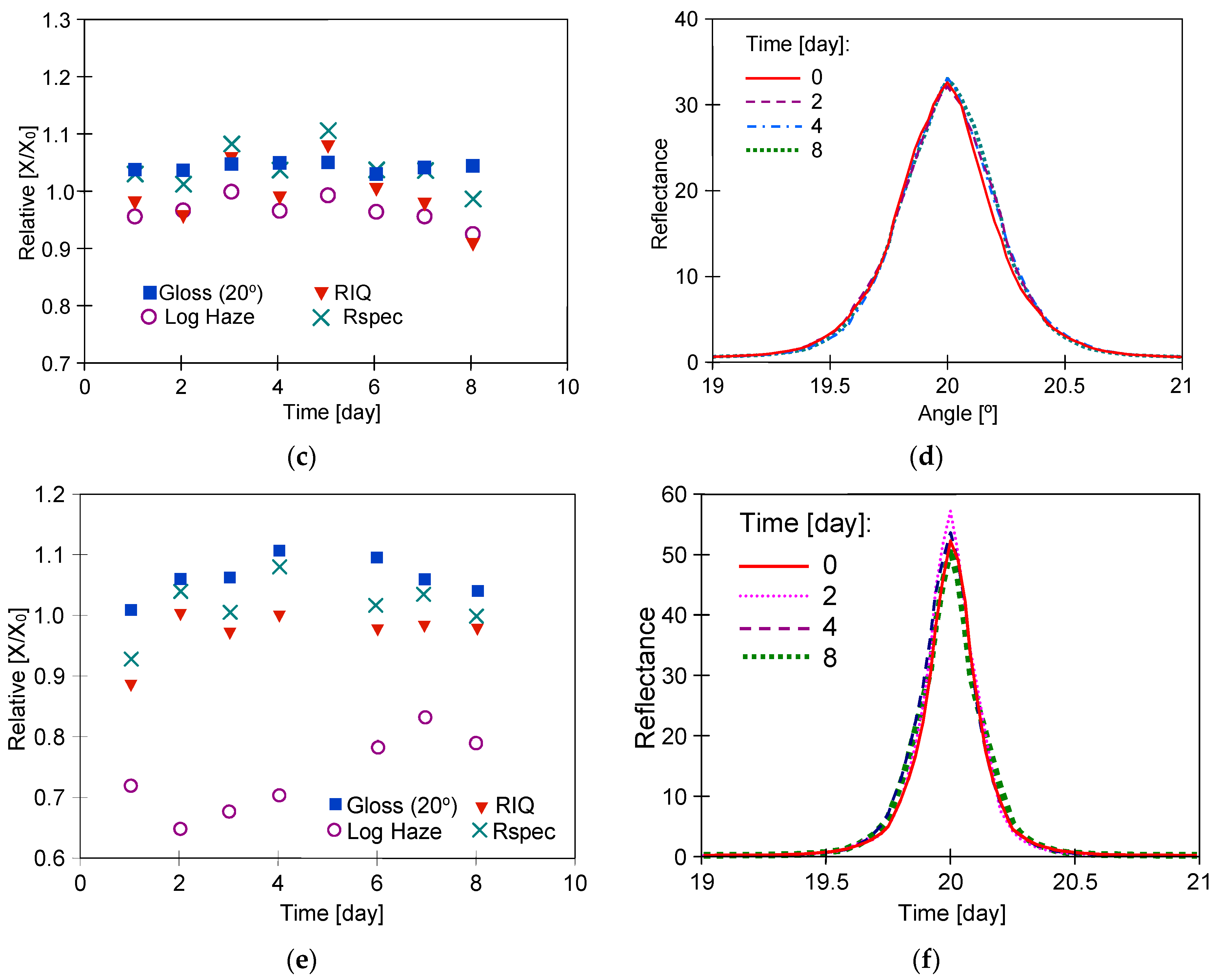
3.1. Long-Term Washing
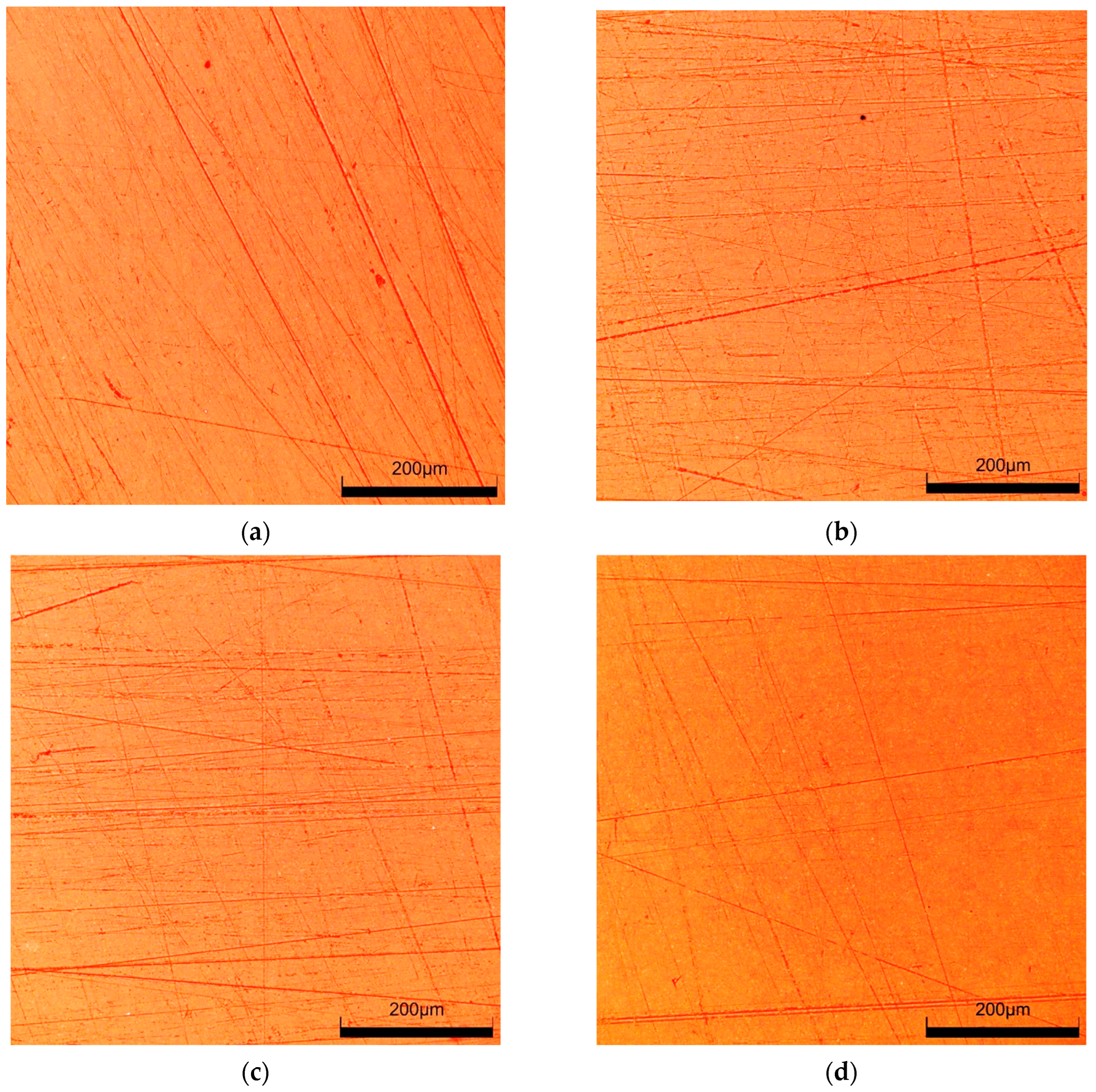
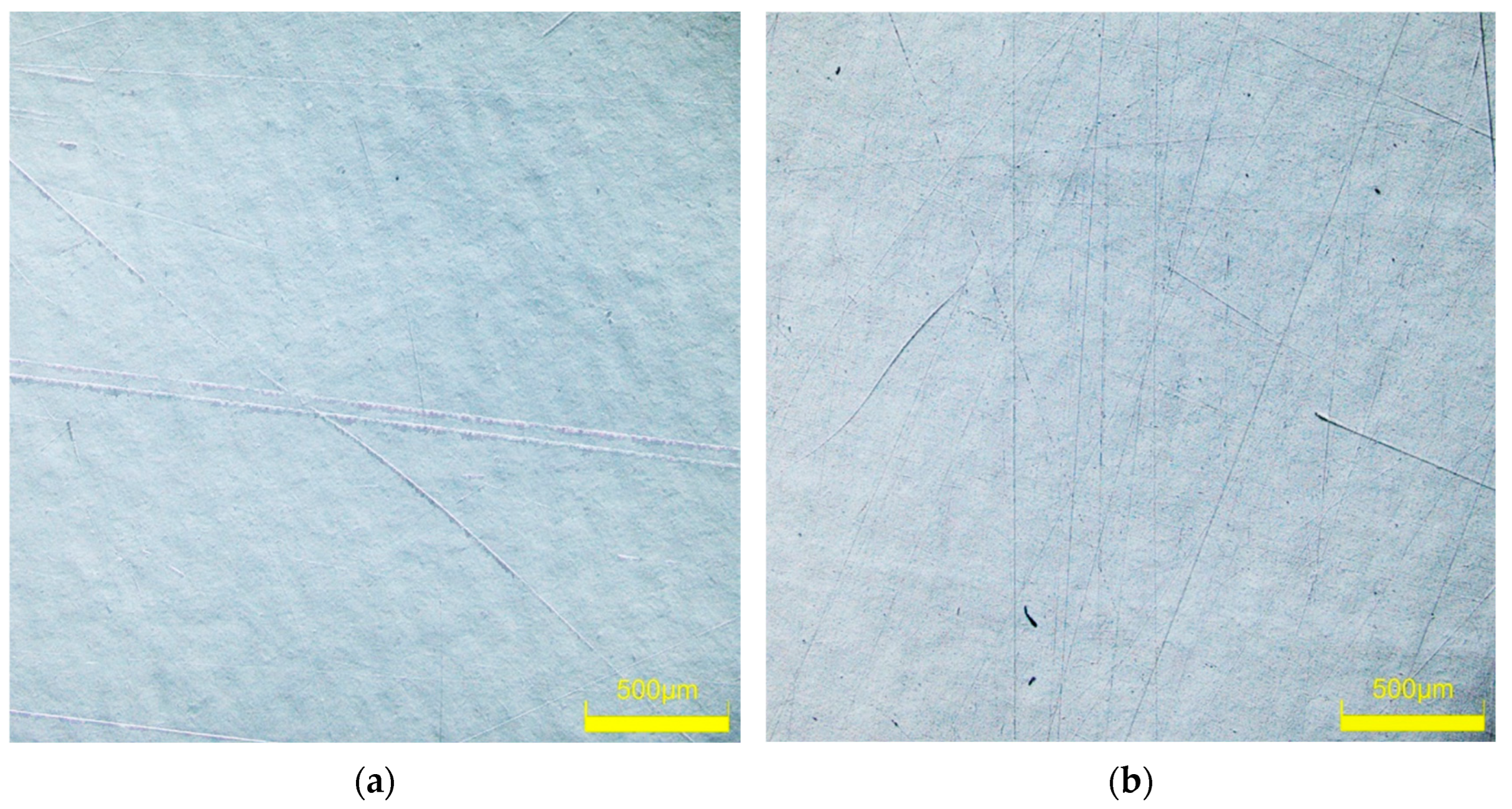
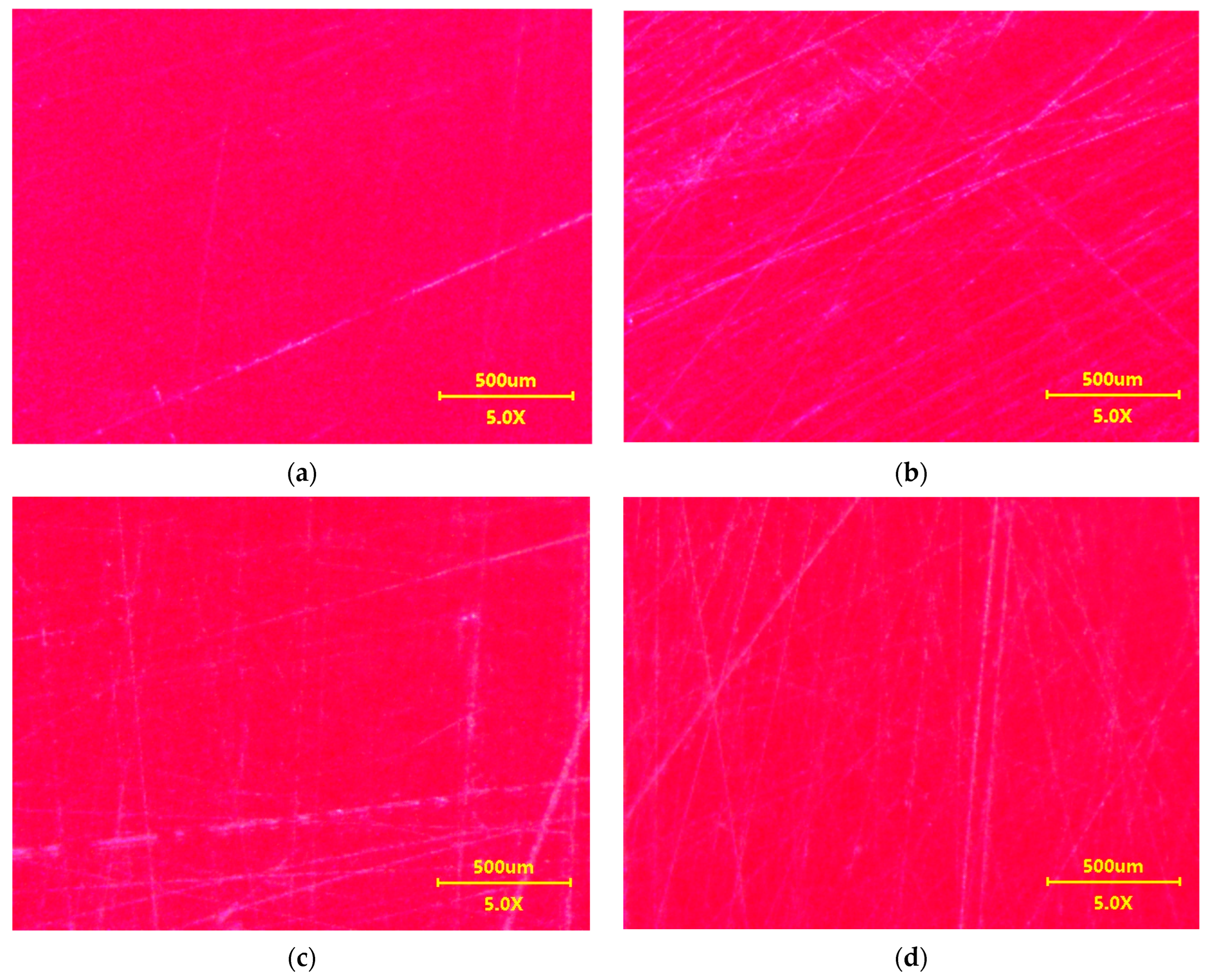
3.2. Scratch Resistance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef] [PubMed]
- Monney, I.; Donkor, E.A.; Buamah, R. Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, A.N.; Gikas, P. Water reuse: Overview of current practices and trends in the world with emphasis on EU states. Water Util. J. 2014, 8, 67–78. [Google Scholar]
- Elgaali, E.; Akram, M. Recycling and Reuse of Wastewater Generated in Car-Washing Facilities. Adv. Sci. Technol. Eng. Syst. J. 2021, 6, 521–525. [Google Scholar] [CrossRef]
- Mujumdar, M.M.; Rajagolkar, S.P.; Jadhav, P. Treatment of vehicle washing waste water for maximum reuse of treated water and reduce fresh water consumption. Int. J. Recent Res. Asp. 2020, 7, 1–5. Available online: https://www.academia.edu/43768187 (accessed on 17 September 2024).
- Hu, C.-Y.; Kuan, W.-H.; Ke, L.-W.; Wu, J.-M. A study of car wash wastewater treatment by cyclo-flow filtration. Water 2022, 14, 1476. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. Car wash wastewater reclamation. Full-scale application and upcoming features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Uçar, D. Membrane processes for the reuse of car washing wastewater. J. Water Reuse Desalin. 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Li, T.; Xue-Jun, T.; Fu-Yi, C.; Qi, Z.; Jun, Y. Reuse of carwash wastewater with hollow fiber membrane aided by enhanced coagulation and activated carbon treatments. Water Sci. Technol. 2007, 56, 111–118. [Google Scholar] [CrossRef]
- Pinto, A.C.S.; de Barros Grossi, L.; de Melo, R.A.C.; de Assis, T.M.; Ribeiro, V.M.; Amaral, M.C.S.; de Souza Figueiredo, K.C. Carwash Wastewater Treatment by Micro and Ultrafiltration Membranes: Effects of Geometry, Pore Size, Pressure Difference and Feed Flow Rate in Transport Properties. J. Water Process Eng. 2017, 17, 143–148. [Google Scholar] [CrossRef]
- Veit, M.T.; Novais, Í.G.V.; Juchen, P.T.; Palácio, S.M.; Da Cunha Gonçalves, G.; Zanette, J.C. Automotive Wash Effluent Treatment Using Combined Process of Coagulation/Flocculation/Sedimentation–Adsorption. Water Air Soil Pollut. 2020, 231, 494. [Google Scholar] [CrossRef]
- Fayed, M.; Shewitah, M.A.; Dupont, R.R.; Fayed, M.; Badr, M.M. Treatability Study of Car Wash Wastewater Using Upgraded Physical Technique with Sustainable Flocculant. Sustainability 2023, 15, 8581. [Google Scholar] [CrossRef]
- El-Ashtoukhy, E.-S.Z.; Amin, N.K.; Fouad, Y.O. Treatment of real wastewater produced from Mobil car wash station using electrocoagulation technique. Environ. Monit. Assess. 2015, 187, 628. [Google Scholar] [CrossRef] [PubMed]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Do, K.-U.; Kim, J.-H.; Chu, X.-Q. Sludge Characteristics and Performance of a Membrane Bioreactor for Treating Oily Wastewater from a Car Wash Service Station. Desalin. Water Treat. 2018, 120, 166–172. [Google Scholar] [CrossRef]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Woźniak, P.; Dubicki, M.; Gryta, M. Microbiological Hazard Analysis of Car Wash Wastewater. Pol. J. Environ. Stud. 2023, 32, 3871–3882. [Google Scholar] [CrossRef]
- Tomczak, W.; Woźniak, P.; Gryta, M.; Grzechulska-Damszel, J.; Daniluk, M. Cleaning of Ultrafiltration Membranes: Long-Term Treatment of Car Wash Wastewater as a Case Study. Membranes 2024, 14, 159. [Google Scholar] [CrossRef]
- Woźniak, P.; Gryta, M. Application of Polymeric Tubular Ultrafiltration Membranes for Separation of Car Wash Wastewater. Membranes 2024, 14, 210. [Google Scholar] [CrossRef]
- Kotnarowska, D. The Influence of Battery Acid on the Destruction of Acrylic Coatings of Car Bodies. Coatings 2021, 11, 967. [Google Scholar] [CrossRef]
- Dahatonde, B.; Kadam, M.; Gupta, S. Review: Degradation of automotive clear coat caused by bird droppings. Paintindia 2020, 70, 78–90. [Google Scholar]
- Zaneti, R.N.; Etchepare, R.; Rubio, J. Car Wash Wastewater Treatment and Water Reuse—A Case Study. Water Sci. Technol. 2013, 67, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Seubert, C.; Nietering, K.; Nichols, M.; Wykoff, R.; Bollin, S. An Overview of the Scratch Resistance of Automotive Coatings: Exterior Clearcoats and Polycarbonate Hardcoats. Coatings 2012, 2, 221–234. [Google Scholar] [CrossRef]
- Sawyer-Beaulieu, S.; Tam, E.; Hussein, A. Measuring Corrosion on Vehicles, in Real-Time, Using Digital Imaging and Analysis Techniques. Materials 2022, 15, 3053. [Google Scholar] [CrossRef]
- Kotnarowska, D.; Wojtyniak, M. Influence of Ageing on Mechanical Properties of Epoxy Coatings. Sol. St. Phen. 2009, 147–149, 825–830. [Google Scholar] [CrossRef]
- Gruber, D.P.; Buder-Stroisznigg, M.; Wallner, G.; Strauss, B.; Jandel, L.; Lang, R.W. A novel methodology for the evaluation of distinctness of image of glossy surfaces. Prog. Org. Coat. 2008, 63, 377–381. [Google Scholar] [CrossRef]
- Perrin, F.X.; Irigoyen, M.; Aragon, E.; Vernet, J.L. Evaluation of accelerated weathering tests for three paint systems: A comparative study of their aging behaviour. Polym. Degrad. Stab. 2001, 72, 115–124. [Google Scholar] [CrossRef]
- The Rhopoint IQ. Available online: https://www.rhopointamericas.com/download/rhopoint-iq-goniophotometer-datasheet-english/ (accessed on 17 September 2024).
- Redford, J.; Mullany, B. Classification of Visual Smoothness Standards Using Multi-Scale Areal Texture Parameters and Low-Magnification Coherence Scanning Interferometry. Materials 2024, 17, 1653. [Google Scholar] [CrossRef]
- Frankhuizen, N. Measuring Gloss? Paint Coat. Ind. 2015, 31, 70–73. [Google Scholar]
- Yari, H.; Moradian, S.; Tahmasebi, N. The weathering performance of acrylic melamine automotive clearcoats containing hydrophobic nanosilica. J. Coat. Technol. Res. 2014, 11, 351–360. [Google Scholar] [CrossRef]
- Edited by Streitberger, H.-J.; Dössel, K.-F. Automotive Paints and Coatings, 2nd ed.; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2008. [Google Scholar]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Permeate 50% | Permeate 75% | T. Active Green 1 | Wastewater 2 |
|---|---|---|---|---|
| COD [mg/L] | 535 ± 2.5 | 565 ± 0.5 | 2298 ± 13 | 1018 ± 10 |
| BOD [mg/L] | 237 ± 2 | 243 ± 2 | 690 ± 1 | 369 ± 1 |
| total N [mg/L] | 6.64 ± 0.02 | 8.27 ± 0.03 | 8.48 ± 0.02 | 14.4 ± 0.04 |
| total P [mg/L] | 5.26 ± 0.1 | 6.13 ± 0.07 | 0 | 12.5 ± 0.09 |
| anionic [mg/L] | 75.7 ± 0.7 | 79.6 ± 0.2 | 691 ± 11 | 135 ± 10 |
| nonionic [mg/L] | 3.46 ± 0.1 | 4.17 ± 0.06 | 30 ± 1.6 | 14.3 ± 1.6 |
| pH [-] | 8.5 ± 0.1 | 8.6 ± 0.1 | 8.7 ± 0.1 | 8.6 ± 0.1 |
| Sample | Gloss 20° | Gloss 60° | Gloss 85° | RIQ | Log Haze | Rspec |
|---|---|---|---|---|---|---|
| R#1 | 71.8 ± 12.2 | 86.3 ± 1.6 | 96.6 ± 2.8 | 57.8 ± 13.4 | 48.7 ± 5.8 | 36.5 ± 7.8 |
| 8d–T.A.G. 1 | 65.9 ± 3.9 | 82.5 ± 0.4 | 96.8 ± 0.3 | 54.3 ± 5.4 | 51.5 ± 3.8 | 31.3 ± 3.1 |
| R#2 | 66.2 ± 2.5 | 82.4 ± 1.6 | 88.8 ± 7.3 | 55.9 ± 5.3 | 47.3 ± 7.6 | 32.7 ± 2.3 |
| 8d–P.50% 2 | 64.0 ± 8.3 | 82.6 ± 1.3 | 87.9 ± 1.9 | 51.8 ± 8.5 | 42.9 ± 2.9 | 30.8 ± 4.7 |
| R#3 | 74.1 ± 0.7 | 87.1 ± 0.3 | 96.2 ± 0.4 | 58.9 ± 6.6 | 49.0 ± 2.9 | 38.2 ± 3.8 |
| 8d-P.75% 3 | 69.6 ± 3.3 | 82.1 ± 1.5 | 96.7 ± 0.5 | 60.1 ± 5.5 | 60.8 ± 4.0 | 36.2 ± 3.4 |
| R#4 | 70.1 ± 1.4 | 83.8 ± 0.7 | 96.3 ± 0.4 | 49.1 ± 3.5 | 69.7 ± 7.4 | 30.6 ± 1.4 |
| 8d-scratch | 55.7 ± 6.4 | 75.9 ± 2.2 | 95.8 ± 0.5 | 49.7 ± 4.8 | 79.5 ± 12.6 | 26.1 ± 3.5 |
| R#5 | 74.0 ± 2.3 | 87.2 ± 0.9 | 96.2 ± 2.5 | 58.9 ± 3.9 | 49.0 ± 3.5 | 38.2 ± 2.6 |
| 8d–scratch | 60.4 ± 2.6 | 79.9 ± 1.2 | 97.1 ± 0.5 | 62.9 ± 7.0 | 74.2 ± 7.7 | 33.6 ± 3.6 |
| R#6 | 64.3 ± 3.6 | 84.0 ± 1.4 | 95.8 ± 0.4 | 55.8 ± 3.6 | 77.0 ± 6.1 | 31.7 ± 2.1 |
| only scratch 4 | 63.1 ± 6.7 | 79.4 ± 2.4 | 96.4 ± 0.6 | 52.4 ± 6.4 | 79.4 ± 9.8 | 29.4 ± 3.4 |
| W#1 | 78.4 ± 1.4 | 93.1 ± 0.9 | 96.2 ± 0.4 | 44.6 ± 4.3 | 48.8 ± 4.8 | 32.0 ± 1.9 |
| 8d–T.A.G. | 81.3 ± 2.1 | 91.6 ± 1.0 | 97.5 ± 0.6 | 44.5 ± 6.6 | 46.5 ± 4.7 | 33.1 ± 2.8 |
| W#2 | 77.8 ± 4.6 | 85.7 ± 1.9 | 93.6 ± 0.5 | 45.9 ± 6.2 | 64.9 ± 8.6 | 27.9 ± 1.4 |
| 8d–P.50% | 81.6 ± 1.6 | 94.1 ± 0.4 | 96.8 ± 0.4 | 44.4 ± 4.2 | 49.5 ± 6.1 | 33.4 ± 2.6 |
| W#3 | 82.3 ± 0.8 | 93.1 ± 0.5 | 96.2 ± 0.7 | 45.8 ± 3.8 | 57.4 ± 2.2 | 33.4 ± 1.7 |
| 8d-P.75% | 82.8 ± 1.7 | 92.1 ± 0.4 | 95.8 ± 1.3 | 45.9 ± 5.9 | 56.5 ± 6.1 | 33.3 ± 2.7 |
| W#4 | 71.7 ± 3.8 | 87.9 ± 1.7 | 96.9 ± 0.2 | 31.2 ± 5.8 | 75.2 ± 13.1 | 22.7 ± 3.1 |
| CH#1 | 76.6 ± 16.4 | 86.9 ± 1.6 | 96.5 ± 2.5 | 74.4 ± 13.5 | 13.1 ± 4.5 | 51.9 ± 11.7 |
| 8d–T.A.G. | 81.4 ± 1.1 | 91.7 ± 0.9 | 91.6 ± 2.5 | 72.3 ± 4.5 | 10.1 ± 1.4 | 52.1 ± 3.9 |
| CH#2 | 83.1 ± 0.8 | 91.1 ± 0.7 | 93.2 ± 0.8 | 70.1 ± 7.4 | 11.5 ± 1.9 | 54.4 ± 6.6 |
| 8d–P.50% | 79.6 ± 8.3 | 88.9 ± 1.1 | 88.2 ± 4.6 | 62.9 ± 9.8 | 10.1 ± 0.7 | 45.5 ± 4.7 |
| Parameter | Non washed | Permeate 50% | Permeate 75% | T. Active Green |
|---|---|---|---|---|
| R-Sq | 3.07 ± 0.33 | 2.81 ± 0.22 | 1.63 ± 0.47 | 2.93 ± 0.41 |
| R-Sa | 2.33 ± 0.27 | 2.12 ± 0.14 | 1.41 ± 0.32 | 1.99 ± 0.25 |
| W-Sq | 4.94 ± 0.15 | 3.51 ± 0.50 | 3.42 ± 0.17 | 3.24 ± 0.11 |
| W-Sa | 3.81 ± 0.19 | 2.49 ± 0.48 | 2.41 ± 0.12 | 2.08 ± 0.09 |
| CH-Sq | 8.51 ± 2.74 | 2.81 ± 0.22 | - | 3.16 ± 0.03 |
| CH-Sa | 5.27 ± 1.34 | 2.12 ± 0.14 | - | 1.74 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, P.; Gryta, M. Influence of Reclaimed Water on the Visual Quality of Automotive Coating. Materials 2024, 17, 5382. https://doi.org/10.3390/ma17215382
Woźniak P, Gryta M. Influence of Reclaimed Water on the Visual Quality of Automotive Coating. Materials. 2024; 17(21):5382. https://doi.org/10.3390/ma17215382
Chicago/Turabian StyleWoźniak, Piotr, and Marek Gryta. 2024. "Influence of Reclaimed Water on the Visual Quality of Automotive Coating" Materials 17, no. 21: 5382. https://doi.org/10.3390/ma17215382
APA StyleWoźniak, P., & Gryta, M. (2024). Influence of Reclaimed Water on the Visual Quality of Automotive Coating. Materials, 17(21), 5382. https://doi.org/10.3390/ma17215382








