Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Surface Characterization
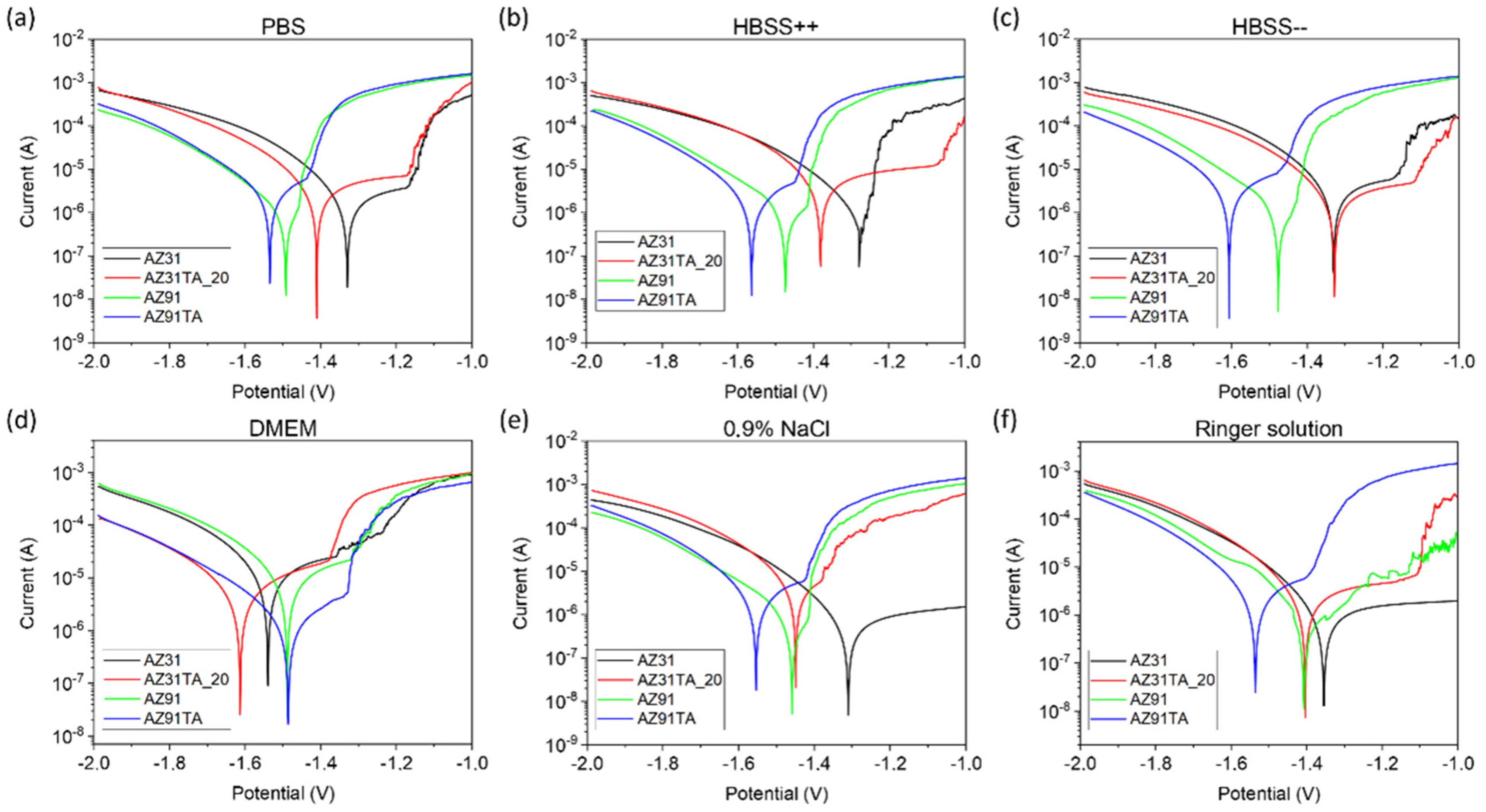
2.3. Degradation and Corrosion Studies
2.3.1. Electrochemical Cell
2.3.2. Electrochemical Corrosion Tests
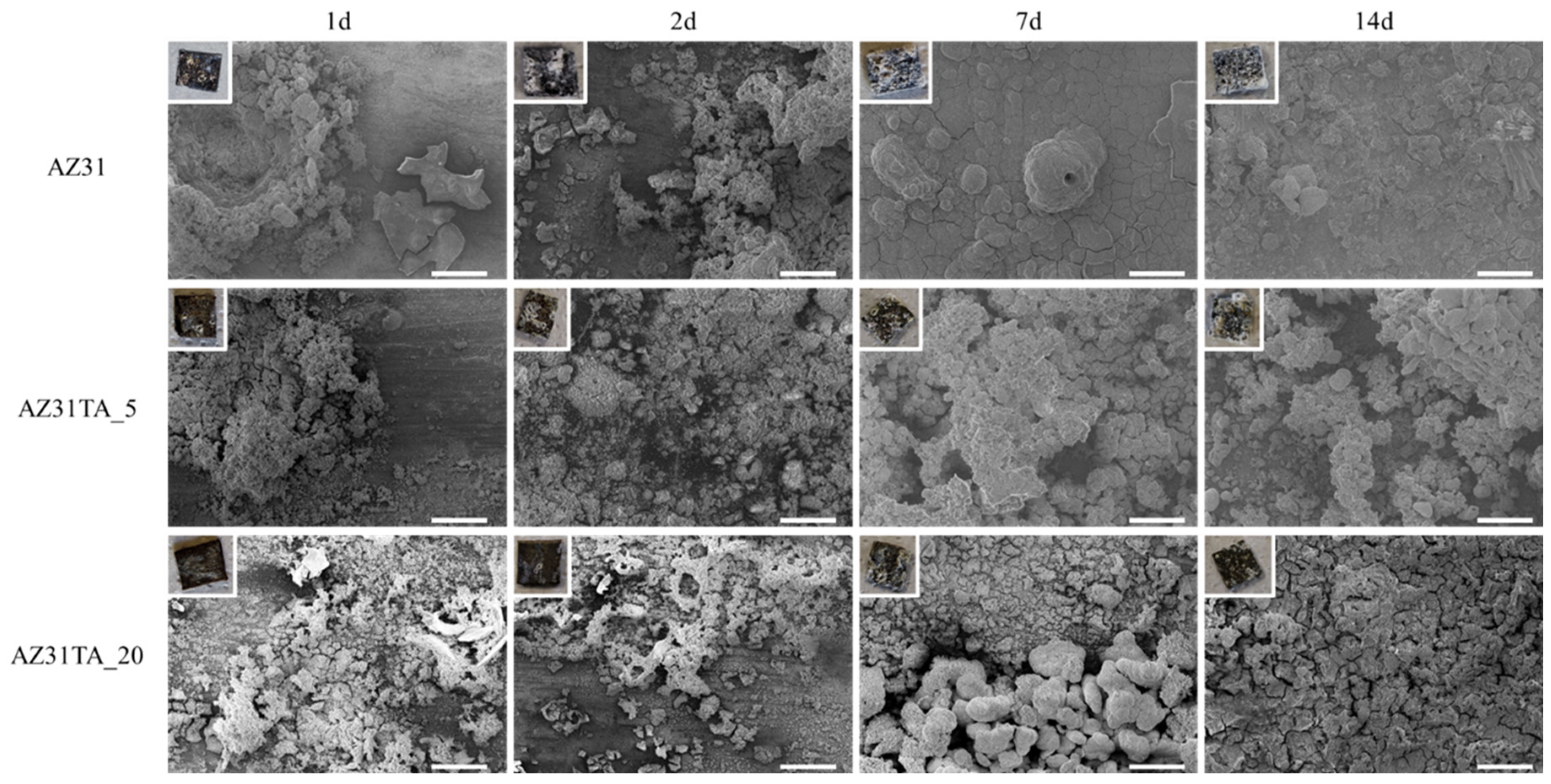
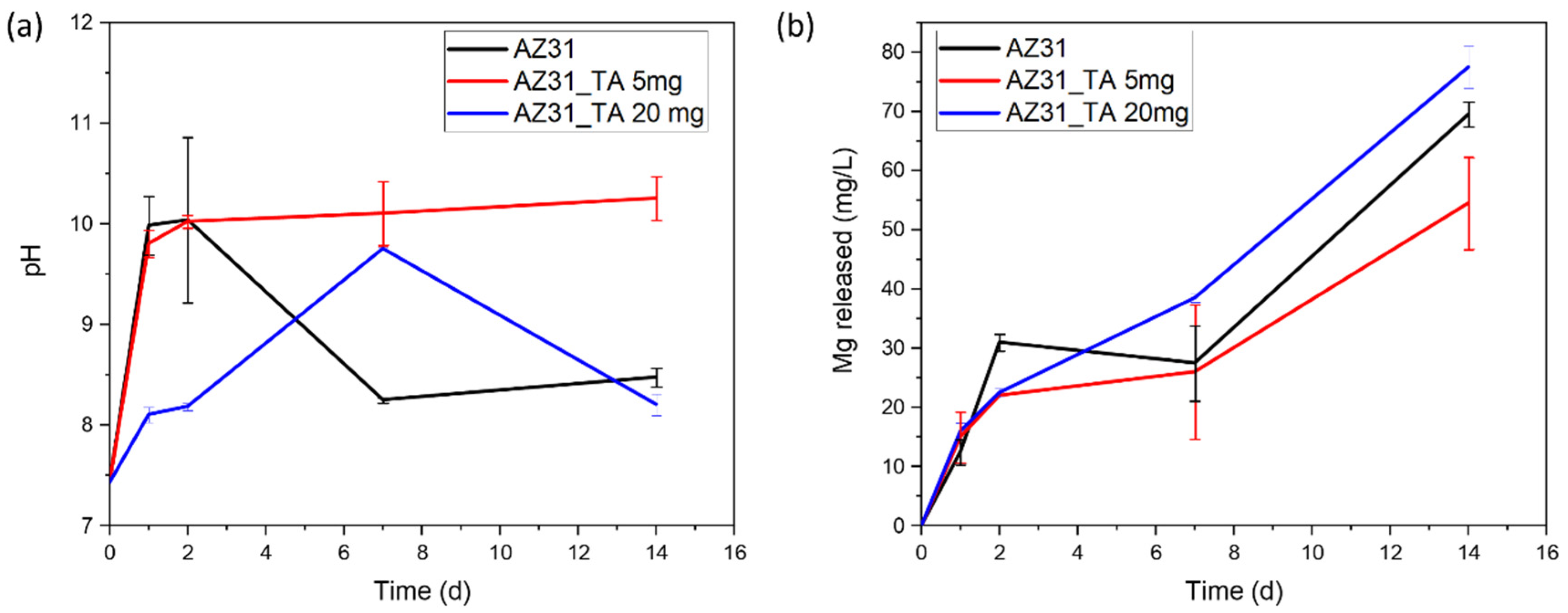
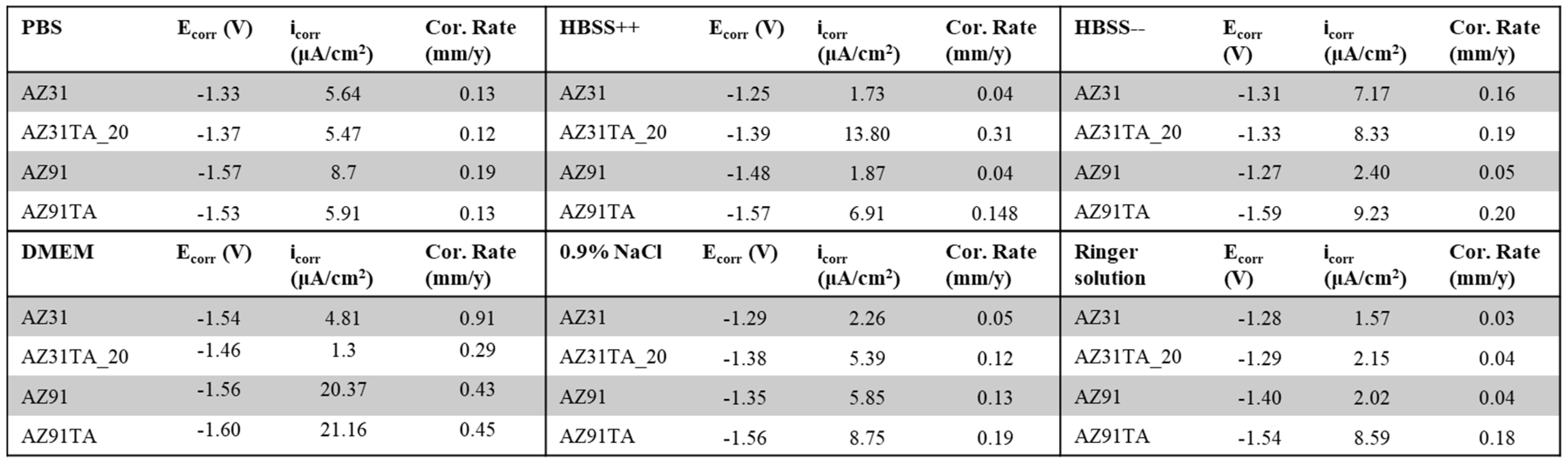
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: Material properties and animal models. Biomater. Transl. 2021, 2, 197–213. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Kim, J.; Pan, H. Effects of magnesium alloy corrosion on biological response—Perspectives of metal-cell interaction. Prog. Mater. Sci. 2023, 133, 101039. [Google Scholar] [CrossRef]
- de Oliveira, M.C.L.; da Silva, R.M.P.; Souto, R.M.; Antunes, R.A. Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review. J. Magnes. Alloys 2022, 10, 2997–3030. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Liu, X. Protection of magnesium alloys: From physical barrier coating to smart self-healing coating. J. Alloys Compd. 2021, 853, 157010. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol uses in biomaterials engineering. Biomaterials 2018, 167, 91–106. [Google Scholar] [CrossRef]
- Reitzer, F.; Allais, M.; Ball, V.; Meyer, F. Polyphenols at interfaces. Adv. Colloid Interface Sci. 2018, 257, 31–41. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, R.; Li, L.; Wang, Y.; Luo, R.; Yang, L.; Wang, Y. Green Tea Polyphenol Induced Mg2+-rich Multilayer Conversion Coating: Toward Enhanced Corrosion Resistance and Promoted in Situ Endothelialization of AZ31 for Potential Cardiovascular Applications. ACS Appl. Mater. Interfaces 2019, 11, 41165–41177. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Yang, Y.; Yang, S.; Yu, Z.; Yarlagadda, P.K.D.V.; Xiao, Y.; Li, Z. Mg-Phenolic Network Strategy for Enhancing Corrosion Resistance and Osteocompatibility of Degradable Magnesium Alloys. ACS Omega 2019, 4, 21931–21944. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Chen, Y.; Zhao, S.; Chen, M.; Li, X.; Maitz, M.F.; Wang, J.; Huang, N. Application of Phenol/Amine Copolymerized Film Modified Magnesium Alloys: Anticorrosion and Surface Biofunctionalization. ACS Appl. Mater. Interfaces 2015, 7, 24510–24522. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-P.; Lin, D.-J.; Yeh, M.-L. Phenolic modified ceramic coating on biodegradable Mg alloy: The improved corrosion resistance and osteoblast-like cell activity. Materials 2017, 10, 696. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, X.; Wang, J.; Huang, N.; Luo, R.; Zhang, B.; Wang, Y. Multistep Instead of One-Step: A Versatile and Multifunctional Coating Platform for Biocompatible Corrosion Protection. ACS Biomater. Sci. Eng. 2019, 5, 6541–6556. [Google Scholar] [CrossRef] [PubMed]
- Bertuola, M.; Miñán, A.; Grillo, C.; Cortizo, M.; de Mele, M.F.L. Corrosion protection of AZ31 alloy and constrained bacterial adhesion mediated by a polymeric coating obtained from a phytocompound. Colloids Surf. B Biointerfaces 2018, 172, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. Study of the formation and growth of tannic acid based conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2009, 204, 736–747. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, R.; Li, W.; Wang, J.; Maitz, M.F.; Wang, J.; Wan, G.; Chen, Y.; Sun, H.; Jiang, C.; et al. Epigallocatechin gallate (EGCG) induced chemical conversion coatings for corrosion protection of biomedical MgZnMn alloys. Corros. Sci. 2015, 94, 305–315. [Google Scholar] [CrossRef]
- Mena-Morcillo, E.; Veleva, L. Degradation of AZ31 and AZ91 magnesium alloys in different physiological media: Effect of surface layer stability on electrochemical behaviour. J. Magnes. Alloys 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Spriano, S.; Dmitruk, A.; Naplocha, K.; Ferraris, S. Tannic Acid Coatings to Control the Degradation of AZ91 Mg Alloy Porous Structures. Metals 2023, 13, 200. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS) Surface Texture: Areal. International Organization for Standardization: Geneva, Switzerland, 2021.
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Feliu, S.; Pardo, A.; Merino, M.; Coy, A.; Viejo, F.; Arrabal, R. Correlation between the surface chemistry and the atmospheric corrosion of AZ31, AZ80 and AZ91D magnesium alloys. Appl. Surf. Sci. 2009, 255, 4102–4108. [Google Scholar] [CrossRef]
- ASTM G31-21; Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- Saqib, M.; Kuzmin, O.S.; Kraskiewicz, H.; Wasyluk, L.; Cuniberti, G.; Ficai, A.A.; Pichugin, V.F.; Opitz, J.; Beshchasna, N. Evaluation of in Vitro Corrosion Behavior of Titanium Oxynitride Coated Stainless Steel Stents. IEEE Access 2021, 9, 59766–59782. [Google Scholar] [CrossRef]
- ASTM G102-89(2015)e1; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- Choi, K.; Shin, J.; Kang, H. The Effect of Solidification Rate on the Corrosion Resistance of Die-Cast AZ91 Magnesium Alloy. Materials 2022, 15, 1259. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hu, T.; Chu, P.K. Influence of Test Solutions on In Vitro Studies of Biomedical Magnesium Alloys. J. Electrochem. Soc. 2010, 157, C238. [Google Scholar] [CrossRef]
- Pokharel, D.B.; Liping, W.; Dong, J.; Wei, X.; Etim, I.-I.N.; Subedi, D.B.; Umoh, A.J.; Ke, W. Effect of D-fructose on the in-vitro corrosion behavior of AZ31 magnesium alloy in simulated body fluid. J. Mater. Sci. Technol. 2021, 66, 202–212. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, H.; Li, H.; Xu, Y.; Wang, C.; Yin, J.; Zhu, H. Enhanced Lithium Ion Storage Performance of Tannic Acid in LiTFSI Electrolyte. ACS Omega 2017, 2, 1273–1278. [Google Scholar] [CrossRef]
- El-Damhougy, T.K.; Ahmed, A.S.; Gaber, G.A.; Mazied, N.A.; Bassioni, G. Radiation synthesis for a highly sensitive colorimetric hydrogel sensor-based p(AAc/AMPS)-TA for metal ion detection. Results Mater. 2021, 9, 100169. [Google Scholar] [CrossRef]
- Rajar, K.; Soomro, R.A.; Ibupoto, Z.H.; Sirajuddin; Balouch, A. Tannic acid assisted copper oxide nanoglobules for sensitive electrochemical detection of bisphenol A. Int. J. Food Prop. 2017, 20, 1359–1367. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.; Guaita, M.; Spriano, S. Surface functionalization of Ti6Al4V with an extract of polyphenols from red grape pomace. Mater. Des. 2021, 206, 109776. [Google Scholar] [CrossRef]
- Reggio, C.; Barberi, J.; Ferraris, S.; Spriano, S. Functionalization of Ti6Al4V Alloy with Polyphenols: The Role of the Titanium Surface Features and the Addition of Calcium Ions on the Adsorption Mechanism. Metals 2023, 13, 1347. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.-P. XPS study of the surface chemistry on AZ31 and AZ91 magnesium alloys in dilute NaCl solution. Appl. Surf. Sci. 2010, 256, 5807–5812. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Zeng, S.; Zhang, G.; Liu, J.; Chen, C.; Suganuma, K. Effect of temperature on electrochemical corrosion of Zn-30Sn lead-free solder. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018; pp. 10–14. [Google Scholar] [CrossRef]
- Deng, F.; Huang, Y.; Azarmi, F. Corrosion Behavior Evaluation of Coated Steel Using Fiber Bragg Grating Sensors. Coatings 2019, 9, 55. [Google Scholar] [CrossRef]
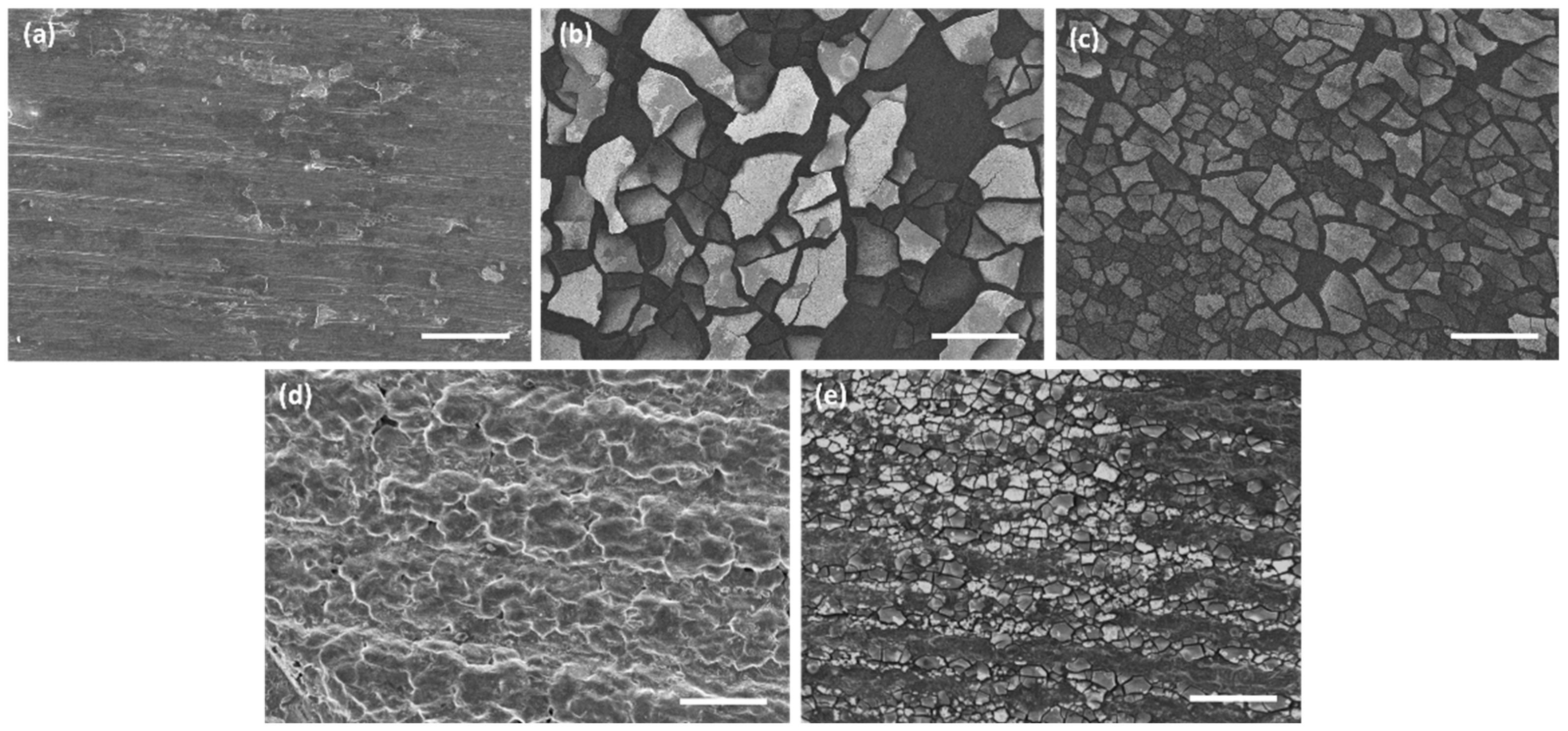

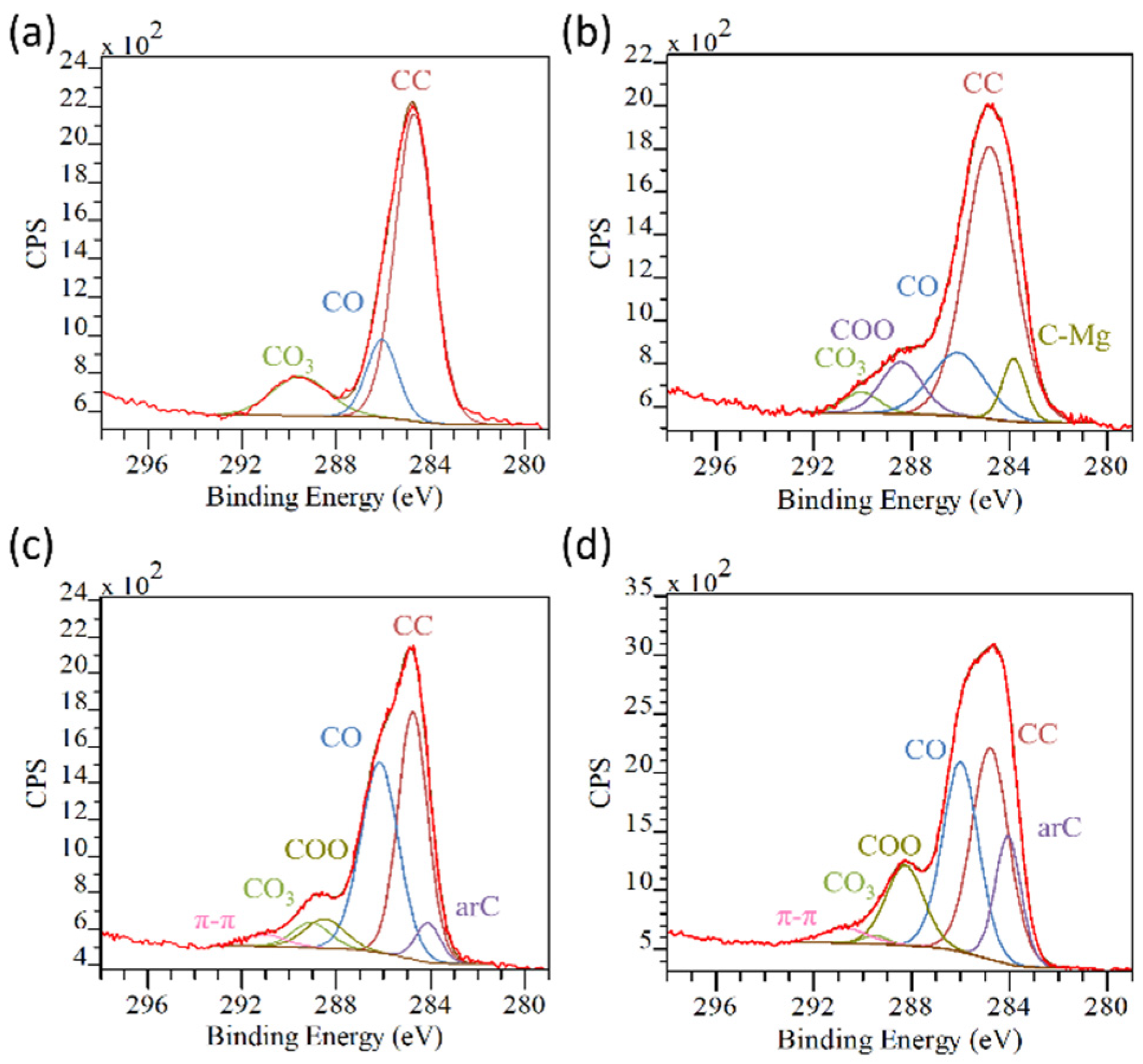
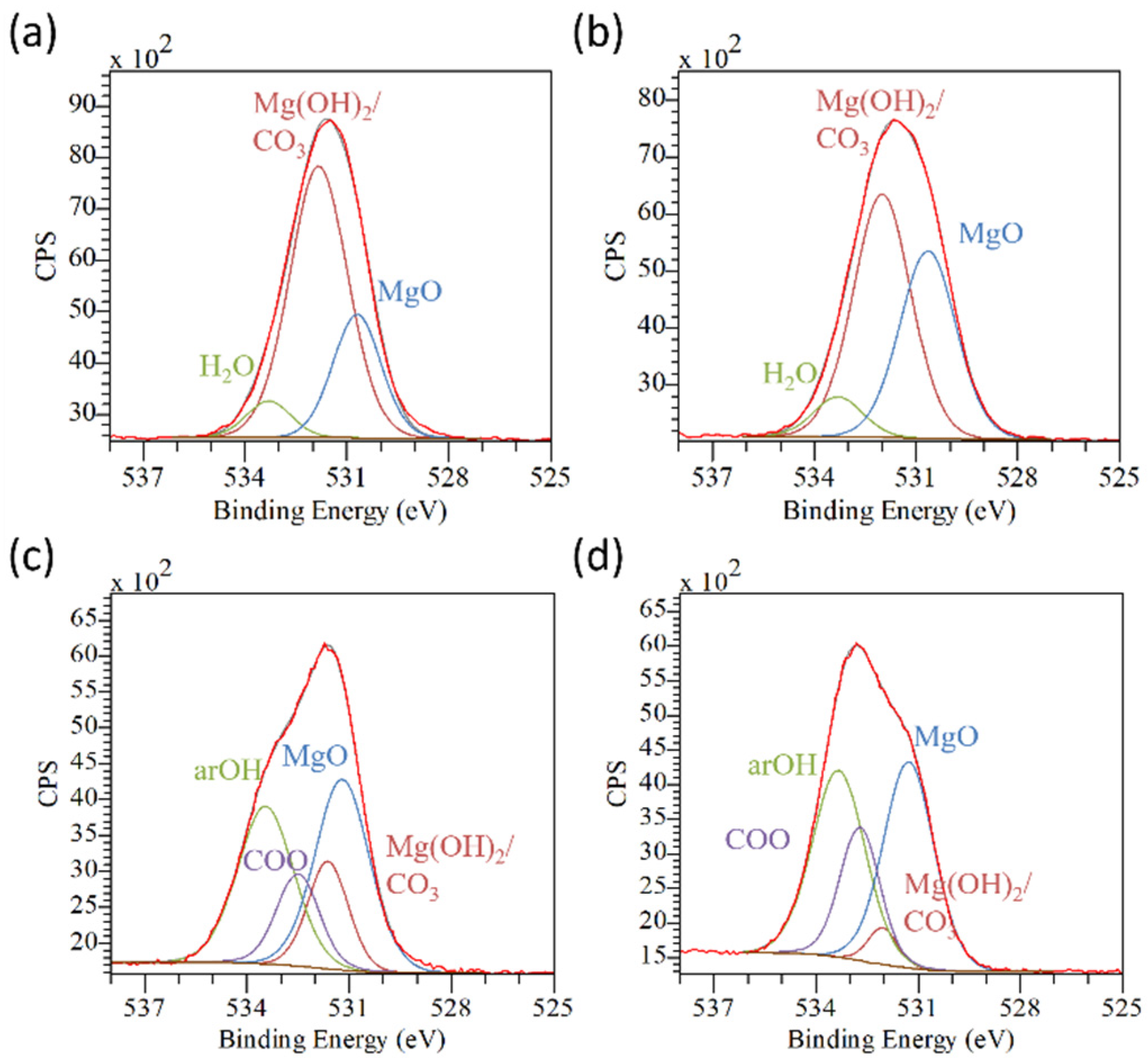
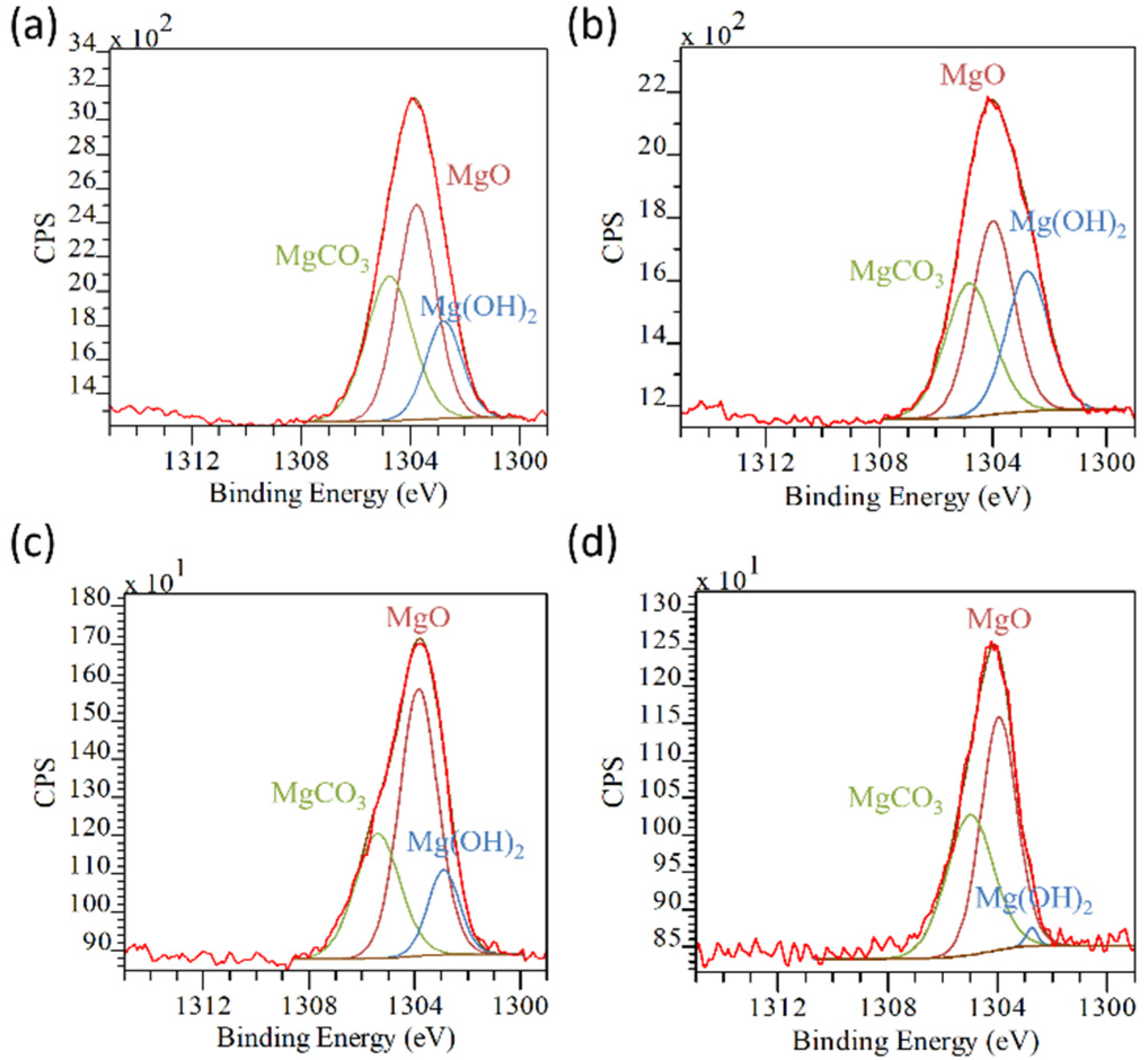
| AZ31 | AZ31TA_5 | AZ31TA_20 | AZ91 | AZ91TA | |
|---|---|---|---|---|---|
| Sa (µm) | 1.57 | 5.66 | 3.72 | 14.2 | 10.3 |
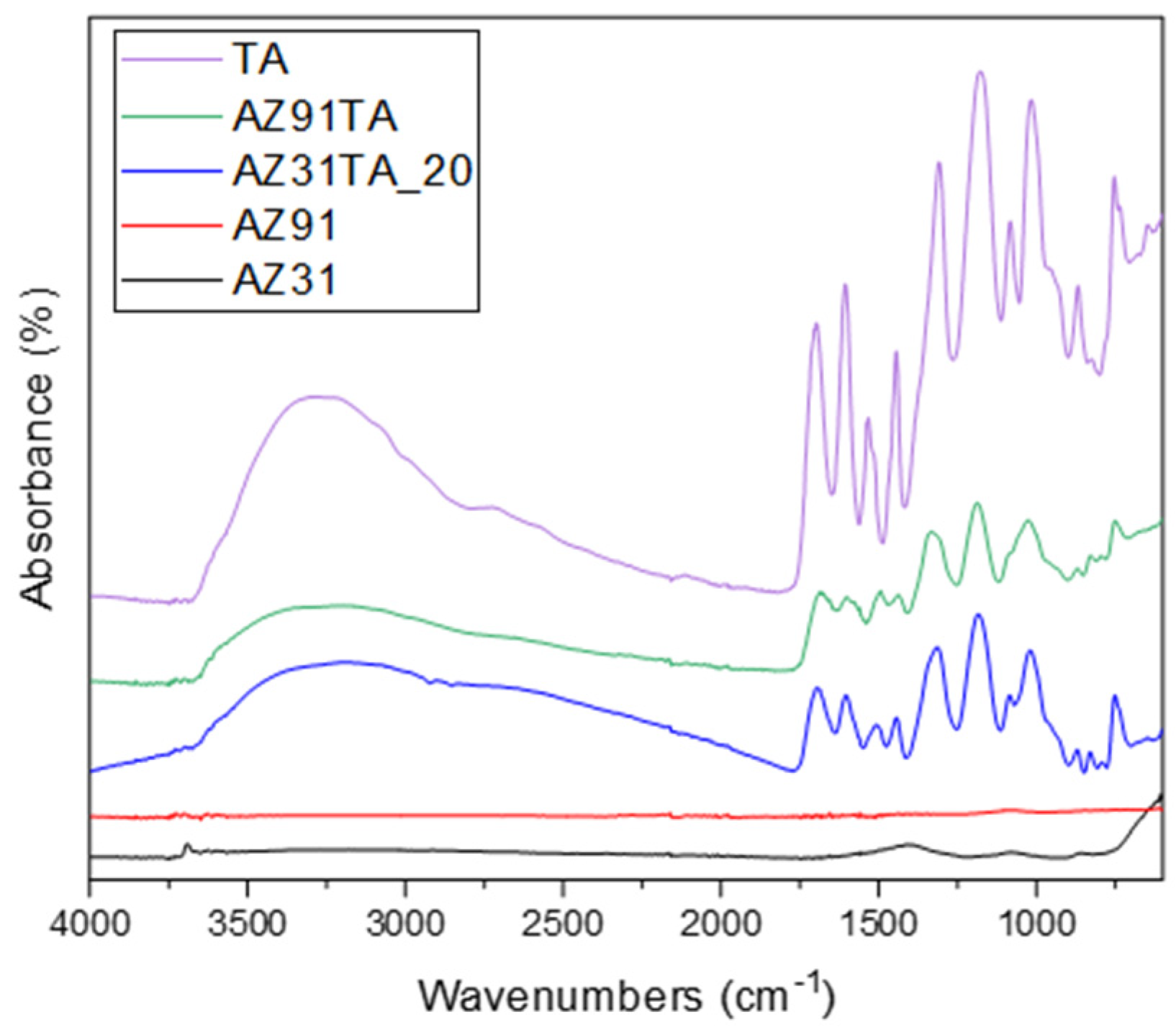
| Element (at%) | Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Mg | O | C | Al | Zn | Others | C/Mg | |
| AZ31 | 14.2 | 43.2 | 25.4 | 1.1 | - | 16.1 | 1.8 |
| AZ31TA_20 | 8.6 | 38.6 | 43.4 | 1.6 | 0.1 | 7.7 | 5.0 |
| AZ91 | 10.6 | 39.7 | 29.6 | 3.2 | - | 16.9 | 2.7 |
| AZ91TA | 2.9 | 33.7 | 61.4 | - | - | 2 | 21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberi, J.; Saqib, M.; Dmitruk, A.; Opitz, J.; Naplocha, K.; Beshchasna, N.; Spriano, S.; Ferraris, S. Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91. Materials 2024, 17, 343. https://doi.org/10.3390/ma17020343
Barberi J, Saqib M, Dmitruk A, Opitz J, Naplocha K, Beshchasna N, Spriano S, Ferraris S. Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91. Materials. 2024; 17(2):343. https://doi.org/10.3390/ma17020343
Chicago/Turabian StyleBarberi, Jacopo, Muhammad Saqib, Anna Dmitruk, Jörg Opitz, Krzysztof Naplocha, Natalia Beshchasna, Silvia Spriano, and Sara Ferraris. 2024. "Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91" Materials 17, no. 2: 343. https://doi.org/10.3390/ma17020343
APA StyleBarberi, J., Saqib, M., Dmitruk, A., Opitz, J., Naplocha, K., Beshchasna, N., Spriano, S., & Ferraris, S. (2024). Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91. Materials, 17(2), 343. https://doi.org/10.3390/ma17020343












