Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model
Abstract
:1. Introduction
2. Materials and Methods
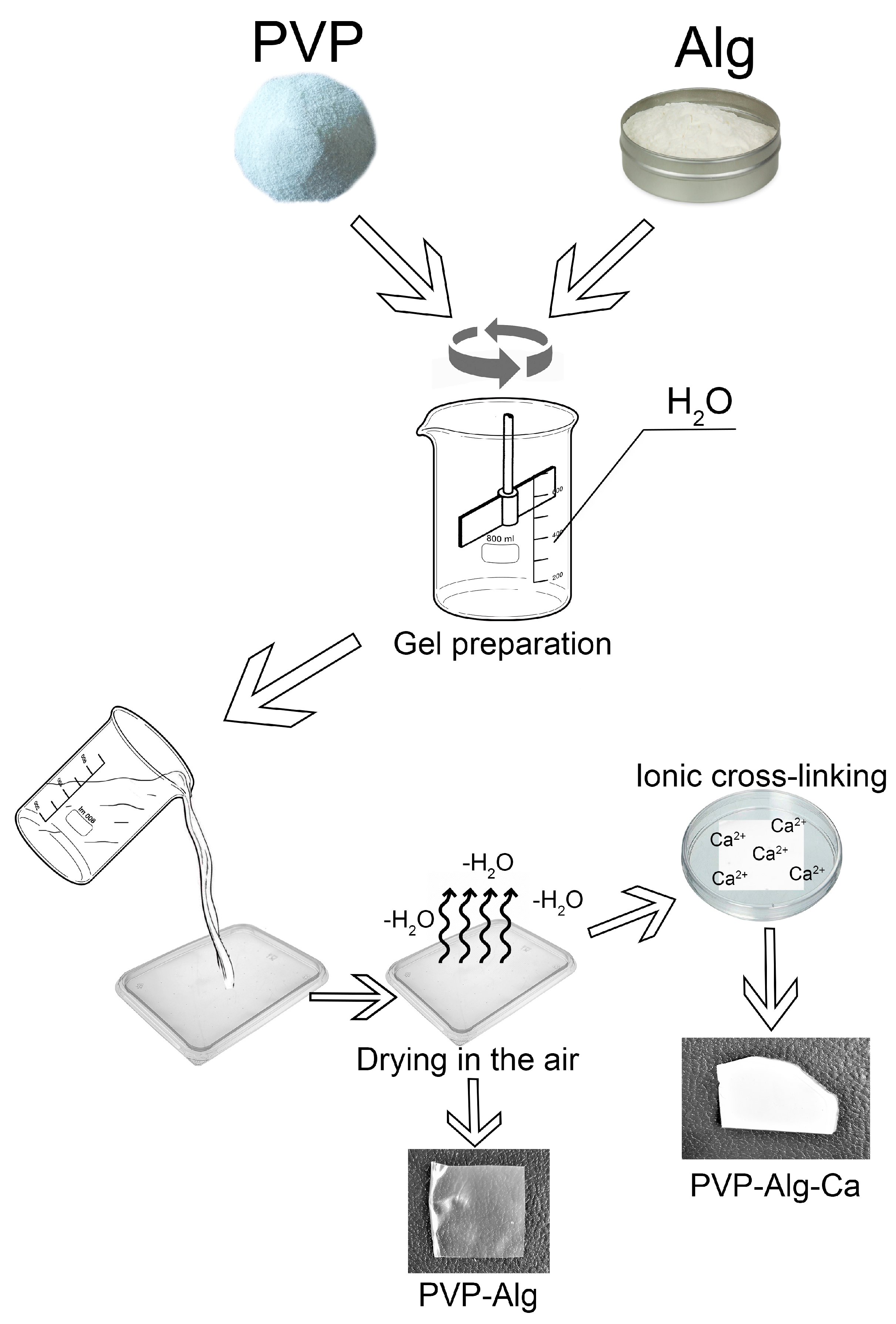
2.1. PVP-Alg Film and PVP-Alg-Ca Film Preparation
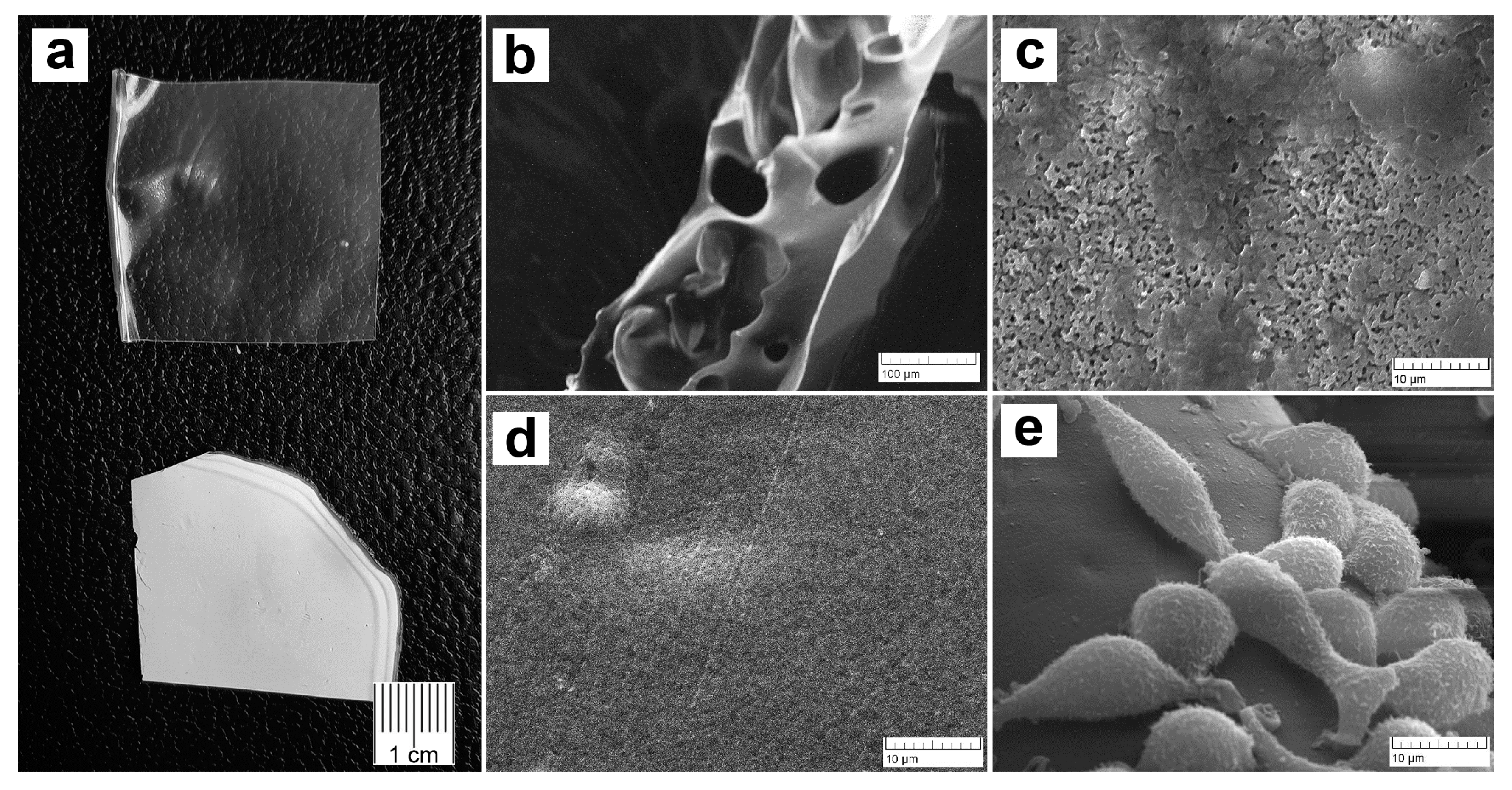
2.2. Scanning Electron Microscopy (SEM) Investigation
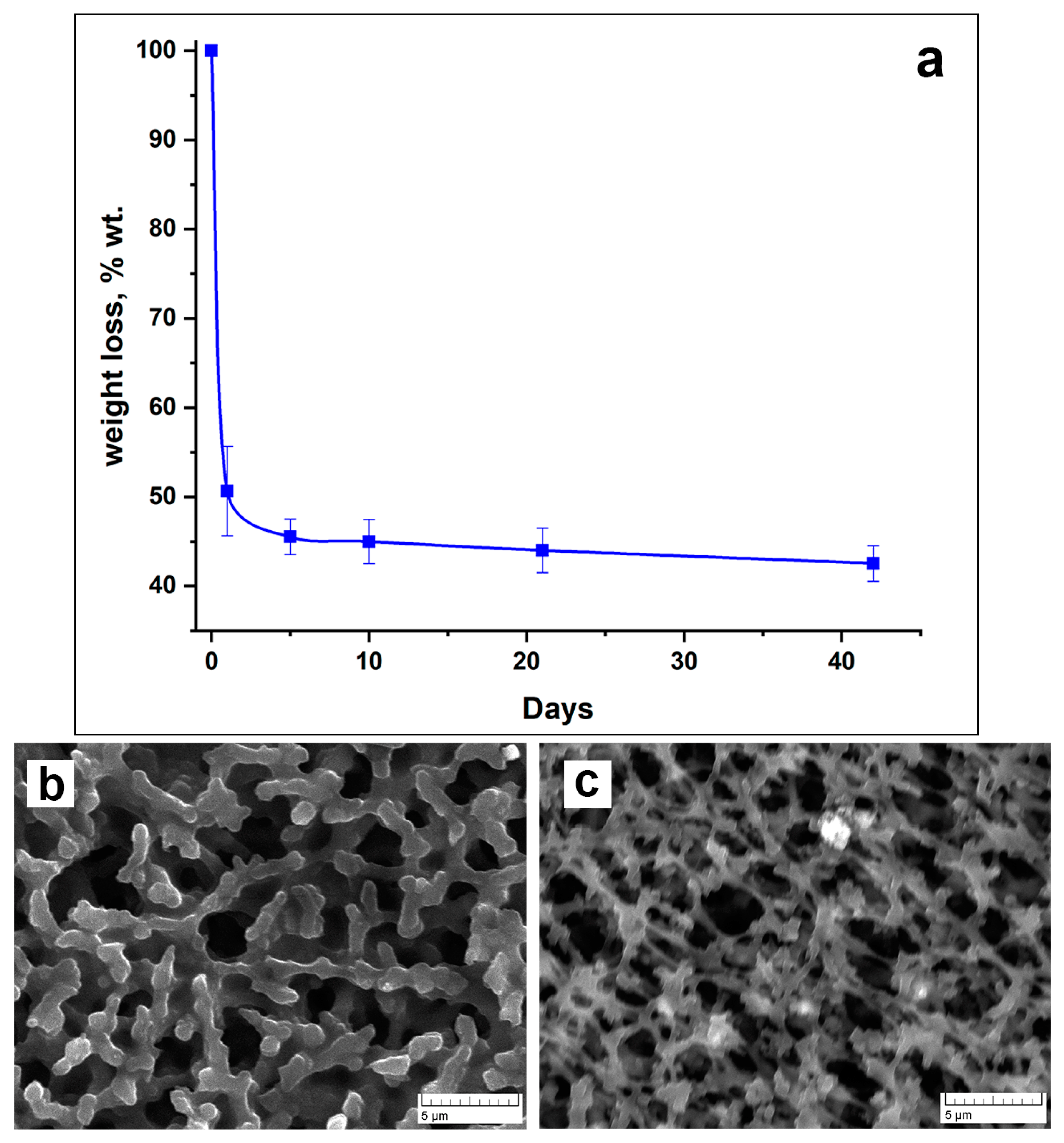
2.3. Investigation of PVP-Alg-Ca Film Solubility
2.4. Investigation of Fibroblast L929 Attachment
2.5. Anti-Adhesive Activity of PVP-Alg and PVP-Alg-Ca Films in Murine Model
2.6. Analysis of Gene Expression
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. Microstructure, Cells Attachment, and Solubility of PVP-Alg and PVP-Alg-Ca Films
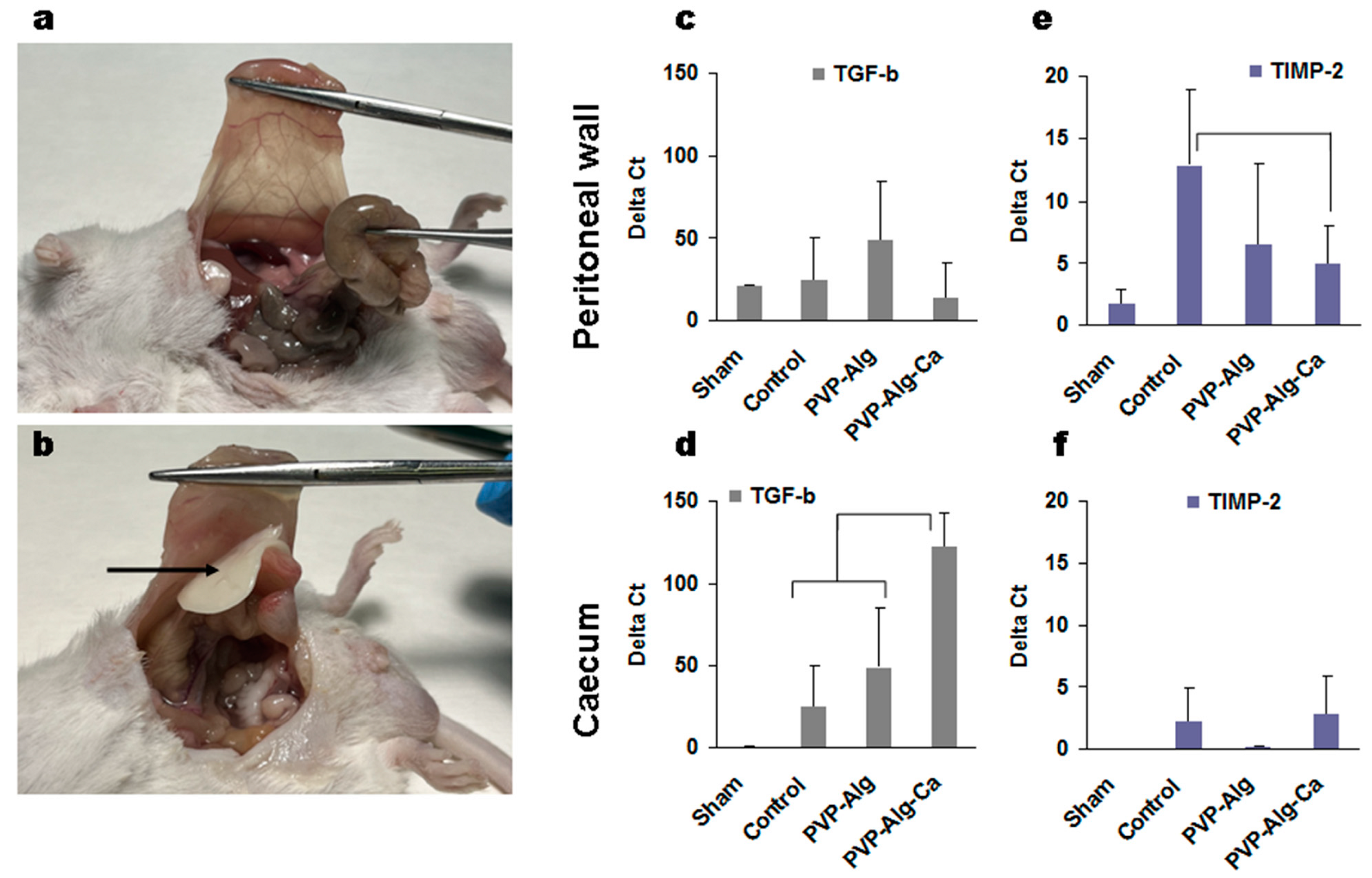
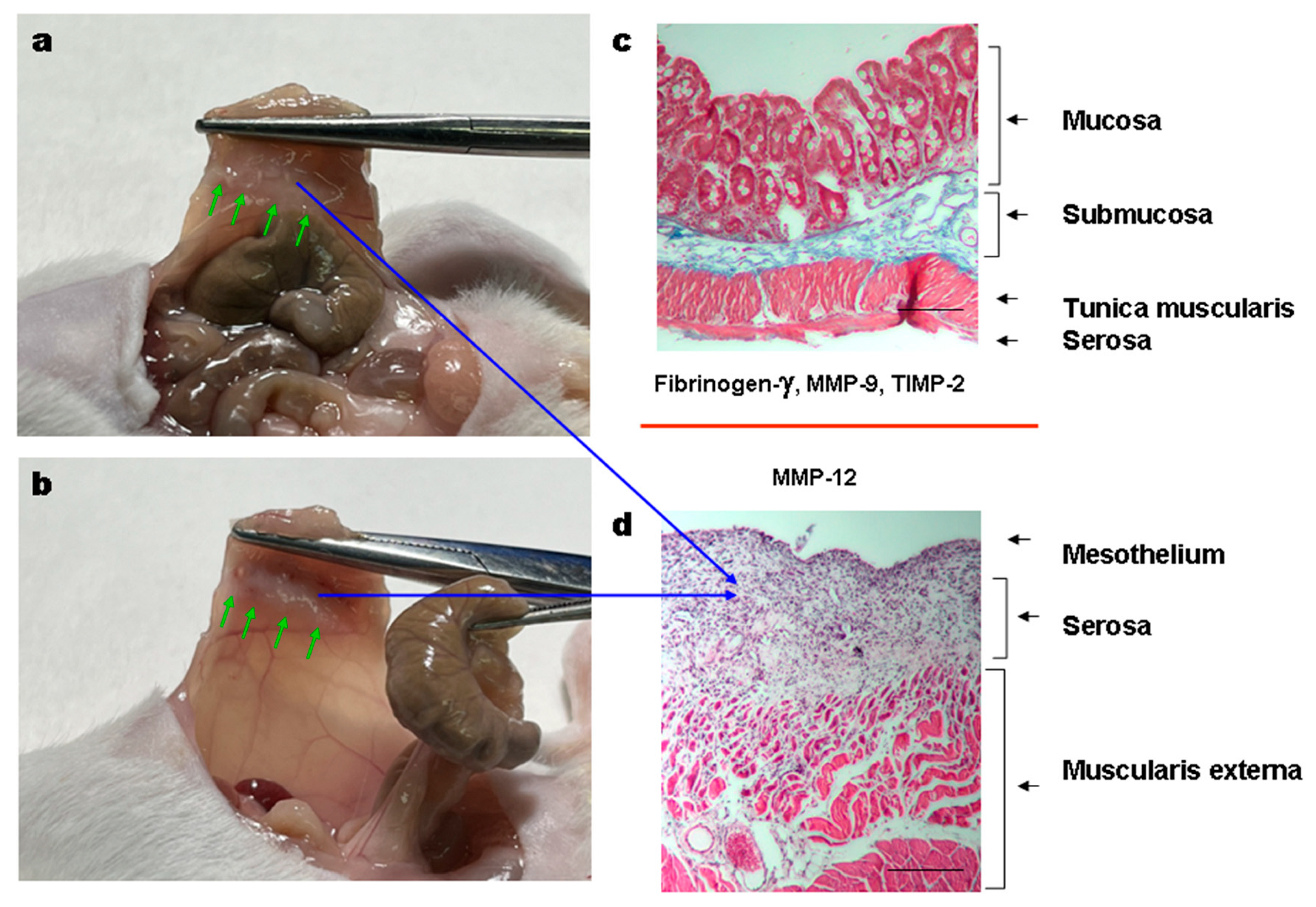
3.2. Prevention of Adhesion Formation by PVP-Alg and PVP-Alg-Ca Films
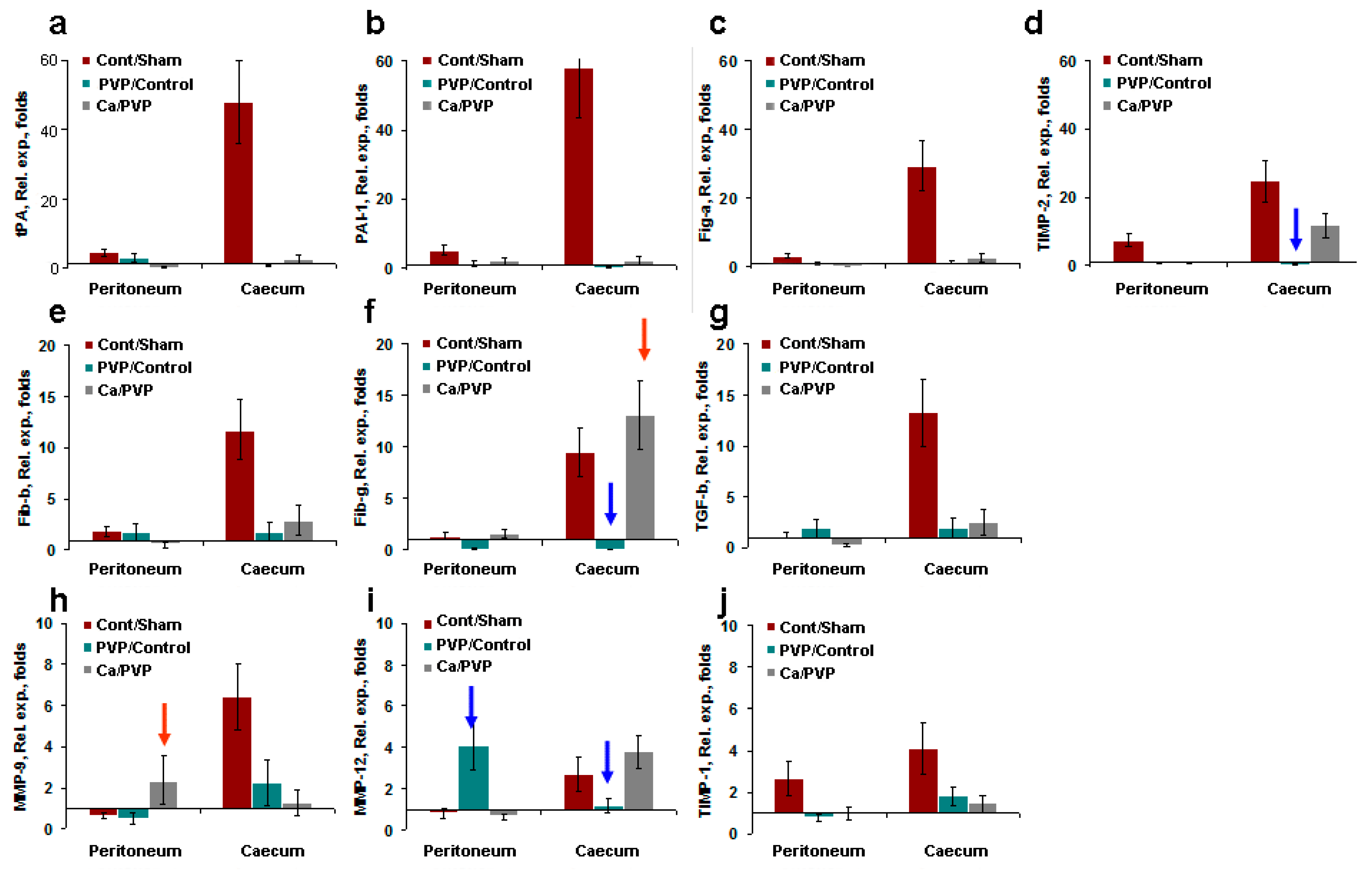
3.3. Gene Expression Induced by Model Surgery
3.3.1. Transforming Growth Factor β
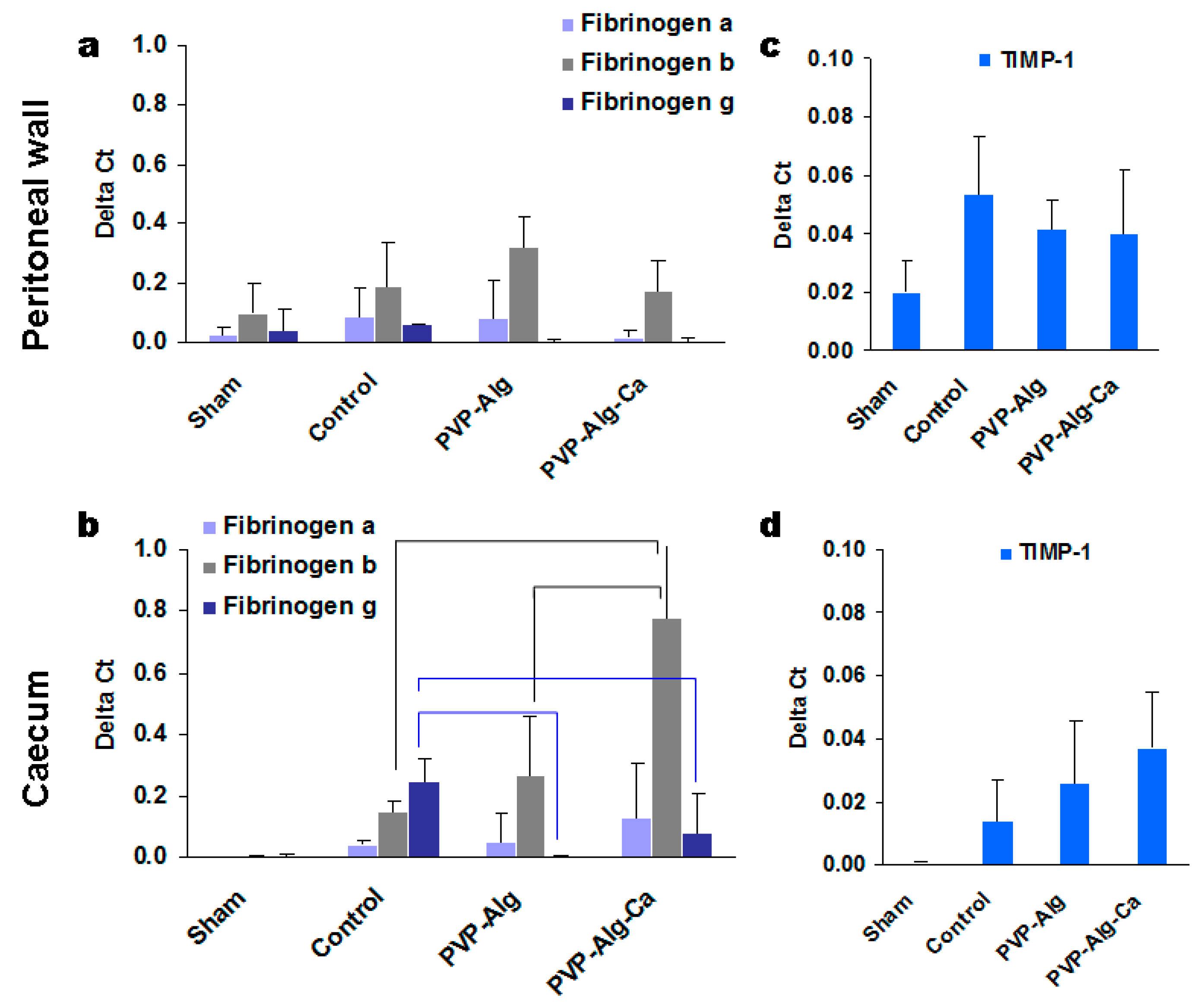
3.3.2. Fibrinogens α, β, and γ
3.3.3. Fibrinolytic Systems tPA and PAI-1
3.3.4. Matrix Metalloproteinases
3.3.5. Histology of the Adhesions
3.3.6. Comparison of Gene Expression in Different Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limperg, T.; Chaves, K.; Jesse, N.; Zhao, Z.; Yunker, A. Ultrasound Visceral Slide Assessment to Evaluate for Intra-Abdominal Adhesions in Patients Undergoing Abdominal Surgery—A Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2021, 28, 1993–2003.e10. [Google Scholar] [CrossRef] [PubMed]
- Kheilnezhad, B.; Hadjizadeh, A. Biomaterials Science from a Biomaterial Perspective. Biomater. Sci. 2021, 9, 2850–2873. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Baek, S.; Kang, H. Biomaterials to Prevent Post-Operative Adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of Biomaterial-Based Postoperative Adhesion Barriers. Macromol. Biosci. 2021, 21, e2000395. [Google Scholar] [CrossRef] [PubMed]
- Brochhausen, C.; Schmitt, V.H.; Hollemann, D.; Tapprich, C.; Krämer, B.; Wallwiener, C.; Hierlemann, H.; Zehbe, R.; Planck, H.; Kirkpatrick, C.J. Current Strategies and Future Perspectives for Intraperitoneal Adhesion Prevention. J. Gastrointestig. Surg. 2012, 16, 1256–1274. [Google Scholar] [CrossRef]
- Dhall, S.; Coksaygan, T.; Ho, T.; Moorman, M.; Lerch, A.; Kuang, J.; Sathyamoorthy, M.; Danilkovitch, A. Bioactive Materials Viable Cryopreserved Umbilical Tissue (VCUT) Reduces Post-Operative Adhesions in a Rabbit Abdominal Adhesion Model. Bioact. Mater. 2018, 4, 97–106. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: Polymer Chemistry Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion Mechanism and Application Progress of Hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di, D.; Martina, F.; Francesca, R. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y. Biomaterials Biodegradable and Injectable in Situ Cross-Linking Chitosan-Hyaluronic Acid Based Hydrogels for Postoperative Adhesion Prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Park, S.; Baek, I.; Song, Y.; Kim, S.; Choi, D.; Kim, J.; Lee, Y. Materials Science & Engineering C Development of Poly (D,L-Lactic-Co-Glycolic Acid) Films Coated with Biomembrane-Mimicking Polymers for Anti-Adhesion Activity. J. Gastrointest. Surg. 2021, 120, 111780. [Google Scholar] [CrossRef]
- Yong, K.; Mooney, D.J. Progress in Polymer Science Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Zebiri, H.; Van Den Berghe, H.; Sayegh, S.; Chammas, P.E.; Pompée, C.; Chammas, M.; Garric, X. Synthesis of PLA–poly (ether urethane)–PLA copolymers and design of biodegradable anti-adhesive membranes for orthopaedic applications. J. Mat. Chem. B 2021, 9, 832–845. [Google Scholar] [CrossRef]
- Lo, H.Y.; Kuo, H.T.; Huang, Y.Y. Application of polycaprolactone as an anti-adhesion biomaterial film. Artif. Org. 2010, 34, 648–653. [Google Scholar] [CrossRef]
- Osadchenko, S.V.; Sten’kina, M.V.; Mezhuev, Y.O.; Shtil’man, M.I. A New Biocompatible Release Material Based on Branched Polyvinyl Alcohol. Polym. Sci. D 2022, 15, 436–440. [Google Scholar] [CrossRef]
- Anisha, A.D.; Shegokar, R. Expert Opinion on Drug Delivery Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-Based Thermosensitive and Biodegradable Hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Materials Science & Engineering C Surface Potential and Charges Impact on Cell Responses on Biomaterials Interfaces for Medical Applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawada, A.M.; Lang, T.; Ottillinger, B.; Kircelli, F.; Stauss-grabo, M.; Kennedy, J.P. Impact of Hydrophilic Modification of Synthetic Dialysis Membranes on Hemocompatibility and Performance. Membranes 2022, 12, 932. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W. PVP: A Critical Review of the Kinetics and Toxicology of Polyvinylpyrrolidone (Povidone); CRC Press: Boca Raton, FL, USA, 1990; ISBN 0873712889. [Google Scholar]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Review Article Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Trofimchuk, E.S.; Forysenkova, A.A.; Ahmed, A.I.; Gnezdilov, O.I.; Davydova, G.A.; Kozlova, S.G.; Antoniac, A.; Rau, J.V. Composite Polyvinylpyrrolidone—Sodium Alginate—Hydroxyapatite Hydrogel Films for Bone Repair and Wound Dressings Applications. Polymers 2021, 13, 3989. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Forysenkova, A.A.; Gafurov, M.R.; Ahmed, A.I.; Davidova, G.A.; Antonova, O.S.; Barinov, S.M.; Federation, R.; Biophysics, E.; Federation, R. Porous Matrixes Based on Polyvinylpyrrolidone Containing Calcium Phosphates for Medical Application. Russ. Chem. Bull. 2022, 71, 543–548. [Google Scholar] [CrossRef]
- Forysenkova, A.A.; Ivanova, V.A.; Fadeeva, I.V.; Mamin, G.V.; Rau, J.V. NMR and EPR Spectroscopies Investigation of Alginate Cross-Linking by Divalent Ions. Materials 2023, 16, 2832. [Google Scholar] [CrossRef]
- Konovalova, M.V.; Tsaregorodtseva, D.S.; Venzhik, A.N.; Poltavtseva, R.A.; Svirshchevskya, E.V. Antiadhesion Effect of Materials Based on Carboxymethylchitosan and Carboxymethylcellulose. Appl. Biochem. Microbiol. 2022, 58, 155–160. [Google Scholar] [CrossRef]
- Scott-Coombes, D.M.; Vipond, M.N.; Thompson, J.N. General Surgeons’ Attitudes to the Treatment and Prevention of Abdominal Adhesions. Ann. R. Coll. Surg. Engl. 1993, 75, 123. [Google Scholar]
- Esser, E.; Tessmar, J.K. V Preparation of Well-Defined Calcium Cross-Linked Alginate Films for the Prevention of Surgical Adhesions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 826–839. [Google Scholar] [CrossRef]
- Łojszczyk, I.; Kuźmińska, A.; Butruk-raszeja, B.A.; Ciach, T. Materials Science & Engineering C Fenton-Type Reaction Grafting of Polyvinylpyrrolidone onto Polypropylene Membrane for Improving Hemo- and Biocompatibility. Mater. Sci. Eng. C 2020, 113, 110960. [Google Scholar] [CrossRef]
- Doria-Serrano, M.C.; Riva-Palacio, G.; Ruiz-Treviño, F.A.; Hernández-Esparza, M. Poly (N-Vinyl Pyrrolidone)-Calcium Alginate (PVP-Ca-Alg) Composite Hydrogels: Physical Properties and Activated Sludge Immobilization for Wastewater Treatment. Ind. Eng. Chem. Res. 2002, 41, 3163–3168. [Google Scholar] [CrossRef]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Ann. Rev. Microb. 2000, 54, 289–340. [Google Scholar] [CrossRef]
- Cheng, D.; Jiang, C.; Xu, J.; Liu, Z.; Mao, X. Characteristics and applications of alginate lyases: A review. Int. J. Biol. Macromol. 2020, 164, 1304–1320. [Google Scholar] [CrossRef]
- Seltana, A.; Cloutier, G.; Nicolas, V.R.; Khalfaoui, T.; Teller, I.C.; Perreault, N.; Beaulieu, J.F. Fibrin(Ogen) Is Constitutively Expressed by Differentiated Intestinal Epithelial Cells and Mediates Wound Healing. Front. Immunol. 2022, 13, 916187. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Sun, S.; Xu, Y.; Fang, Z.; Cong, L. Expression and Potential Role of MMP-9 in Intrauterine Adhesion. Mediators Inflamm. 2021, 2021, 6676510. [Google Scholar] [CrossRef]
- Yi, C.; Liu, J.; Deng, W.; Luo, C.; Qi, J.; Chen, M.; Xu, H. Macrophage Elastase (MMP12) Critically Contributes to the Development of Subretinal Fibrosis. J. Neuroinflamm. 2022, 19, 78. [Google Scholar] [CrossRef]
- Whawell, S.A.; Vipond, M.N.; Scott-Coombes, D.M.; Thompson, J.N. Plasminogen Activator Inhibitor 2 Reduces Peritoneal Fibrinolytic Activity in Inflammation. Br. J. Surg. 1993, 80, 107–109. [Google Scholar] [CrossRef]
- Hong, G.-S.; Schwandt, T.; Stein, K.; Schneiker, B.; Kummer, M.P.; Heneka, M.T.; Kitamura, K.; Kalff, J.C.; Wehner, S. Effects of Macrophage-Dependent Peroxisome Proliferator-Activated Receptor γ Signalling on Adhesion Formation after Abdominal Surgery in an Experimental Model. Br. J. Surg. 2015, 102, 1506–1516. [Google Scholar] [CrossRef]
- Arung, W.; Meurisse, M.; Detry, O. Pathophysiology and Prevention of Postoperative Peritoneal Adhesions. World J. Gastroenterol. 2011, 17, 4545–4553. [Google Scholar] [CrossRef]
- Fometescu, S.G.; Costache, M.; Coveney, A.; Oprescu, S.M.; Serban, D.; Savlovschi, C. Peritoneal Fibrinolytic Activity and Adhesiogenesis. Chirurgia 2013, 108, 331–340. [Google Scholar]
- Moris, D.; Chakedis, J.; Rahnemai-azar, A.A.; Wilson, A.; Hennessy, M.M.; Athanasiou, A.; Beal, E.W.; Argyrou, C.; Felekouras, E.; Pawlik, T.M. Postoperative Abdominal Adhesions: Clinical Significance and Advances in Prevention and Management. J. Gastrointest. Surg. 2017, 21, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Augustin, M.; Dietlein, M.; Faust, U.; Keuthage, W.; Lobmann, R.; Münter, K.C.; Strohal, R.; Stücker, M.; Traber, J.; et al. Efficacy of MMP-Inhibiting Wound Dressings in the Treatment of Hard-to-Heal Wounds: A Systematic Review. J. Wound Care 2020, 29, 102–118. [Google Scholar] [CrossRef]
- Falk, P.; Ma, C.; Chegini, N.; Holmdahl, L. Differential Regulation of Mesothelial Cell Fibrinolysis by Transforming Growth Factor Beta 1. Scand. J. Clin. Lab. Investig. 2000, 60, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Atta, H.M. Prevention of Peritoneal Adhesions: A Promising Role for Gene Therapy. World J. Gastroenterol. 2011, 17, 5049. [Google Scholar] [CrossRef]
- Van Rooijen, N.; Sanders, A. Elimination, Blocking, and Activation of Macrophages: Three of a Kind? J. Leukoc. Biol. 1997, 62, 702–709. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, Y.; Deng, Y.; Sun, Y.; Yang, H.; Wei, S. Peptide-Incorporated 3D Porous Alginate Scaffolds with Enhanced Osteogenesis for Bone Tissue Engineering. Coll. Surf. B Biointerf. 2016, 143, 243–251. [Google Scholar] [CrossRef]
- Kameda, T.; Mano, H.; Yamada, Y.; Takai, H.; Amizuka, N.; Kobori, M.; Izumi, N.; Kawashima, H.; Ozawa, H.; Ikeda, K.; et al. Calcium-Sensing Receptor in Mature Osteoclasts, Which Are Bone Resorbing Cells. Biochem. Biophys. Res. Commun. 1998, 245, 419–422. [Google Scholar] [CrossRef]
- Weber, D.E.; Semaan, M.T.; Wasman, J.K.; Beane, R.; Bonassar, L.J.; Megerian, C.A. Tissue-Engineered Calcium Alginate Patches in the Repair of Chronic Chinchilla Tympanic Membrane Perforations. Laryngoscope 2006, 116, 700–704. [Google Scholar] [CrossRef]
- Johns, D.B.; Rodgers, K.E.; Donahue, W.D.; Kiorpes, T.C.; DiZerega, G.S. Reduction of adhesion formation by postoperative administration of ionically crosslinked hyaluronic acid. Fertil. Steril. 1997, 68, 37–42. [Google Scholar] [CrossRef]
- Yeo, Y.; Highley, C.B.; Bellas, E.; Ito, T.; Marini, R.; Langer, R.; Kohane, D.S. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials 2006, 27, 4698–4705. [Google Scholar] [CrossRef] [PubMed]


| Points | Description |
|---|---|
| 0 | No adhesion |
| 1 | Thin-film adhesion |
| 2 | Dense adhesion with small point attachment |
| 3 | More than one thin adhesion |
| 4 | Tight adhesion with flat attachment |
| 5 | A dense blood-supplied adhesion or more than one adhesion with a flat attachment |
| No | Gene | Direct Primer | Reverse Primer |
|---|---|---|---|
| 1 | Actin-β | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| 2 | tPA | TGCTGTGTGTACTGCTGCTT | TCTGCGTTGGCTCATCTCTG |
| 3 | PAI-1 | AGTGTTTCAGCAGGTGGTCC | GACAAAGATGGCATCCGCAG |
| 4 | Fibrinogen α | GCCATCCCTAAACGCAGACA | AATCCTGGTTGGCTTCGTCA |
| 5 | Fibrinogen β | GAAAGTAGAACGGAGACCCCC | AGCGGAGCACACGAAGATT |
| 6 | Fibrinogen γ | CGGCTGGTGGATGAACAAATG | TGAAAATGAAGTGAGGTCCTGAAAG |
| 7 | MMP-9 | GGGTCTAGGCCCAGAGGTAA | AGACACGCCCCTTGCTGA |
| 8 | MMP-12 | TGCACTCTGCTGAAAGGAGTC | TGAGTTGTCCAGTTGCCCAG |
| 9 | TIMP-1 | GGACCTGGTCATAAGGGCTA | GGCATATCCACAGAGGCTTT |
| 10 | TIMP-2 | TTCCGGGAATGACATCTATGG | GGGCCGTGTAGATAAACTCGAT |
| 11 | TGF-β1 | ACTGGAGTTGTACGGCAGTG | GGGGCTGATCCCGTTGATTT |
| Group | Number of Animals | Adhesion Score | The Median Score | Standard Deviation | p-Value vs. Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||
| Animal distribution | ||||||||||
| Control | 5 | 1 | 0 | 0 | 0 | 4 | 0 | 4 | 1.79 | |
| PVP-Alg gel | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.019 |
| PVP-Alg film | 8 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0.35 | 0.011 |
| PVP-Alg-Ca film | 5 | 0 | 0 | 0 | 0 | 0 | 5 | 5 | 0 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forysenkova, A.A.; Konovalova, M.V.; Fadeeva, I.V.; Antonova, O.S.; Kotsareva, O.D.; Slonskaya, T.K.; Rau, J.V.; Svirshchevskaya, E.V. Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model. Materials 2023, 16, 5532. https://doi.org/10.3390/ma16165532
Forysenkova AA, Konovalova MV, Fadeeva IV, Antonova OS, Kotsareva OD, Slonskaya TK, Rau JV, Svirshchevskaya EV. Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model. Materials. 2023; 16(16):5532. https://doi.org/10.3390/ma16165532
Chicago/Turabian StyleForysenkova, Anna A., Mariya V. Konovalova, Inna V. Fadeeva, Olga S. Antonova, Olga D. Kotsareva, Tatiana K. Slonskaya, Julietta V. Rau, and Elena V. Svirshchevskaya. 2023. "Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model" Materials 16, no. 16: 5532. https://doi.org/10.3390/ma16165532
APA StyleForysenkova, A. A., Konovalova, M. V., Fadeeva, I. V., Antonova, O. S., Kotsareva, O. D., Slonskaya, T. K., Rau, J. V., & Svirshchevskaya, E. V. (2023). Polyvinylpyrrolidone–Alginate Film Barriers for Abdominal Surgery: Anti-Adhesion Effect in Murine Model. Materials, 16(16), 5532. https://doi.org/10.3390/ma16165532









