Preparation and Performance Study of Ultraviolet-Responsive Self-Healing Epoxy Asphalt
Abstract
:1. Introduction
2. Experimental Part
2.1. Experimental Materials and Equipment
2.2. Preparation of Epoxy Asphalt and Self-Healing Epoxy Asphalt
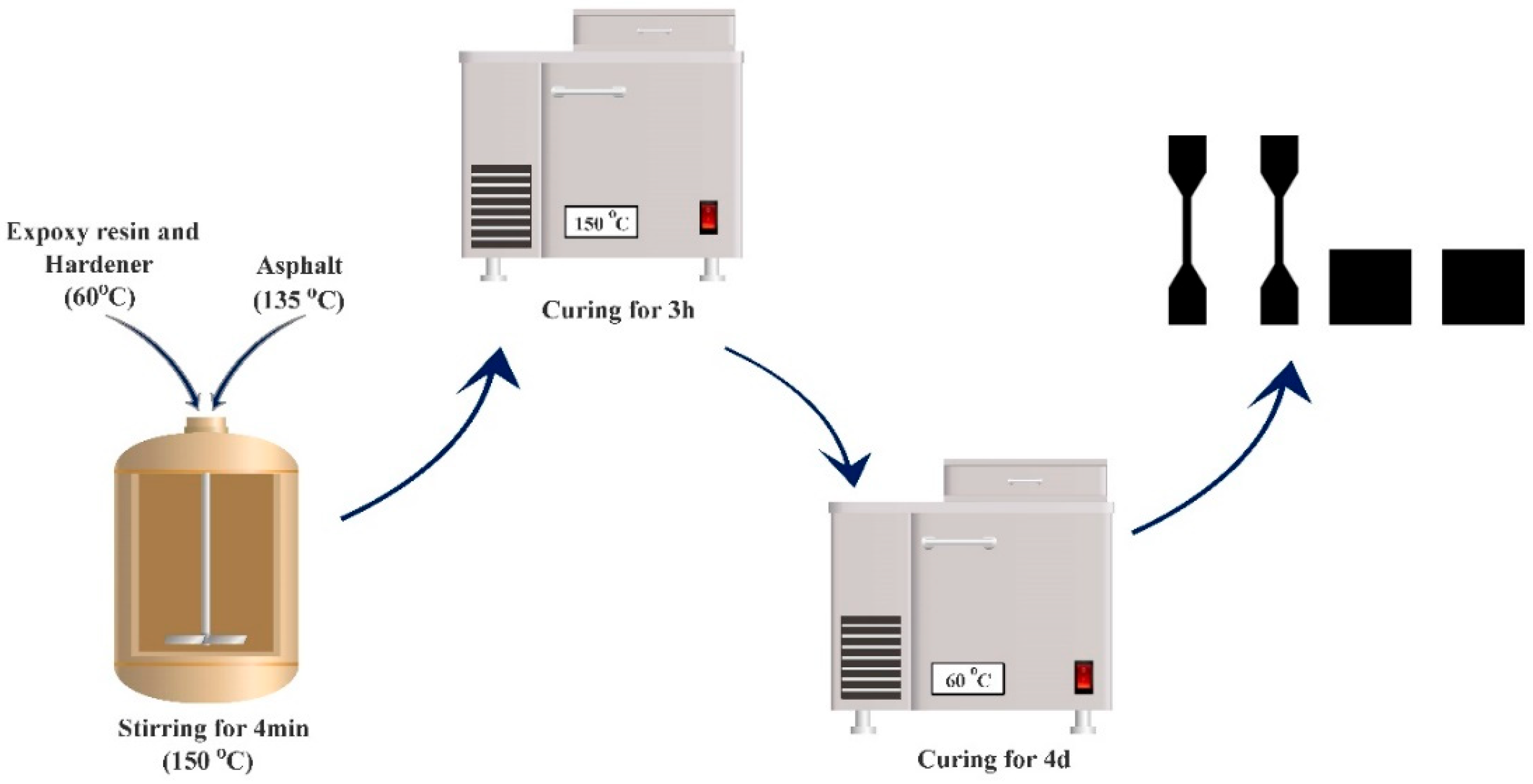
2.2.1. Preparation of Epoxy Asphalt
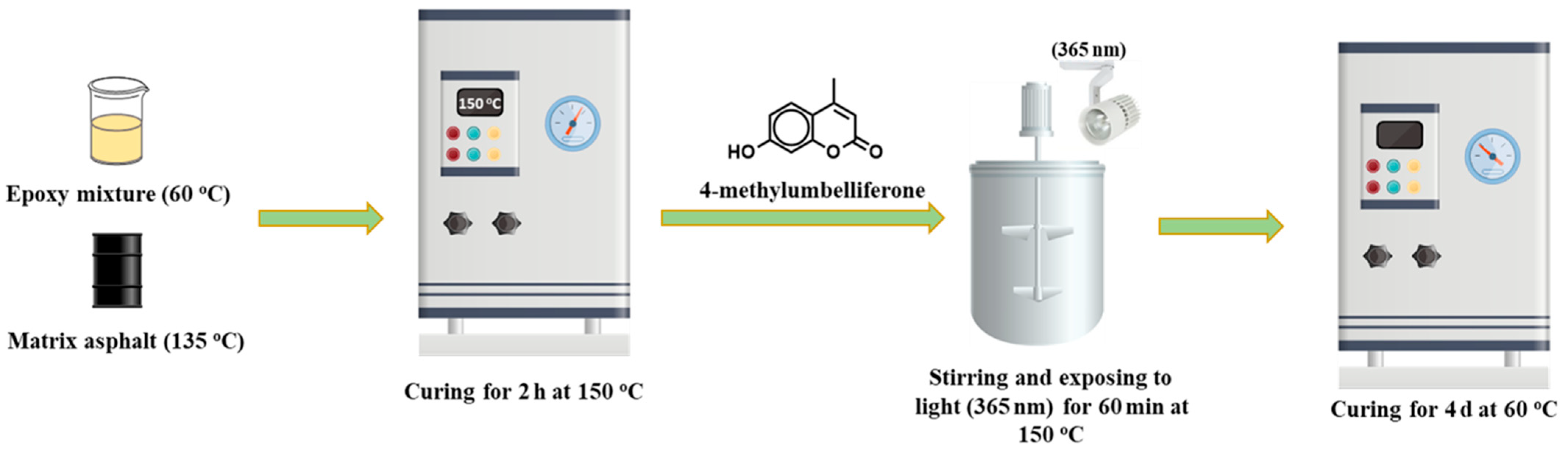
2.2.2. Preparation of Self-Healing Epoxy Asphalt
3. Experimental Results and Discussion
3.1. Microscopic Analysis
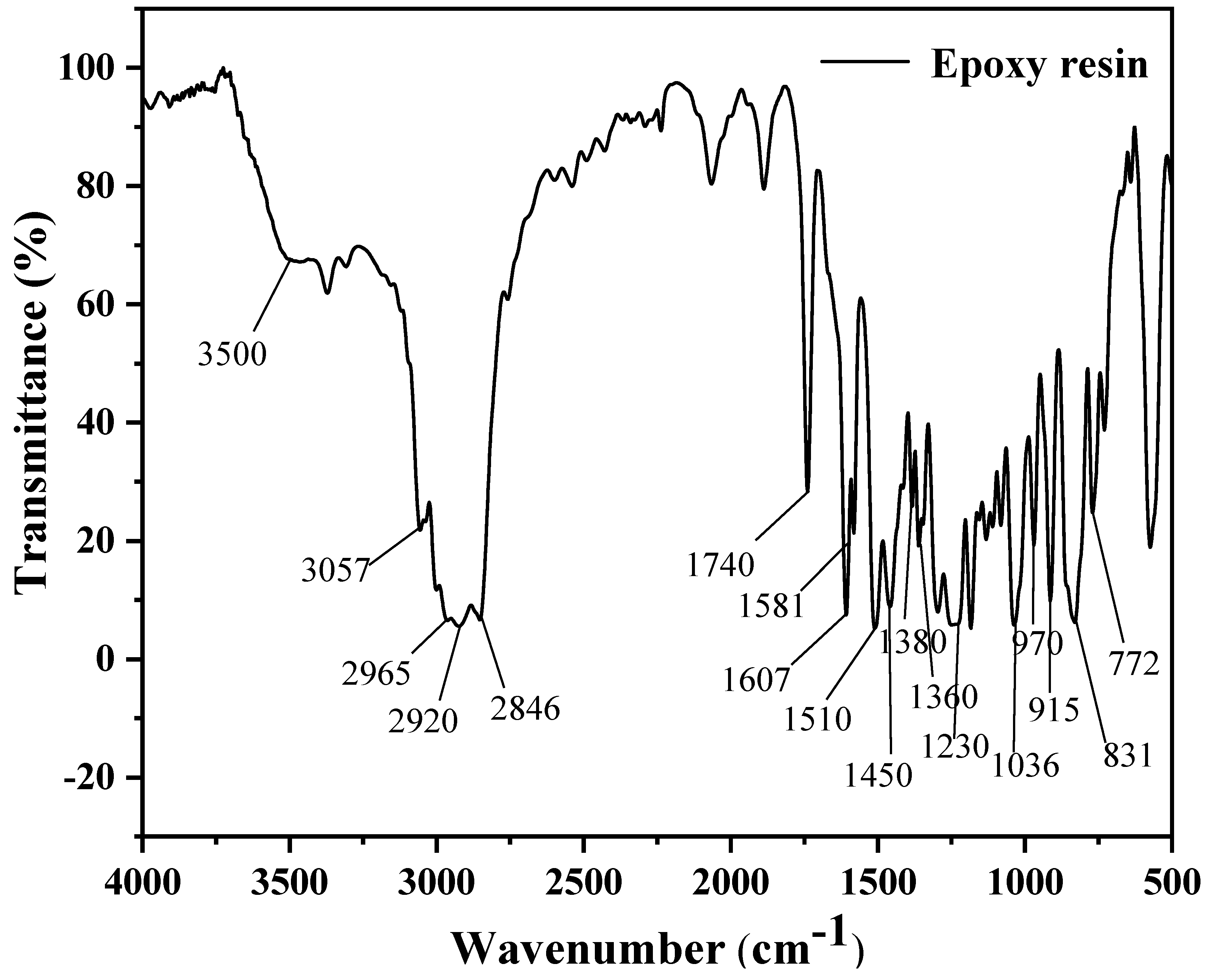
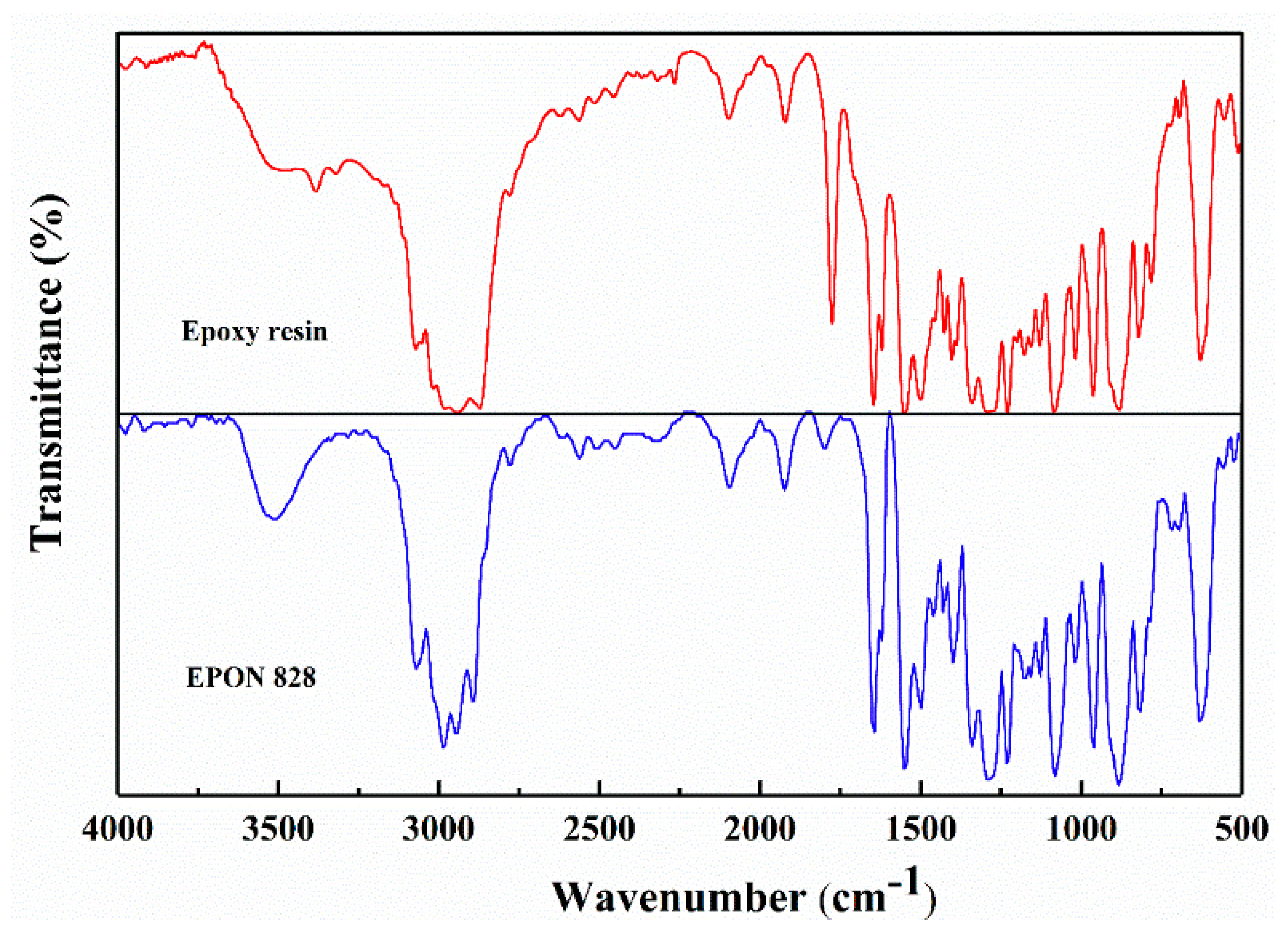
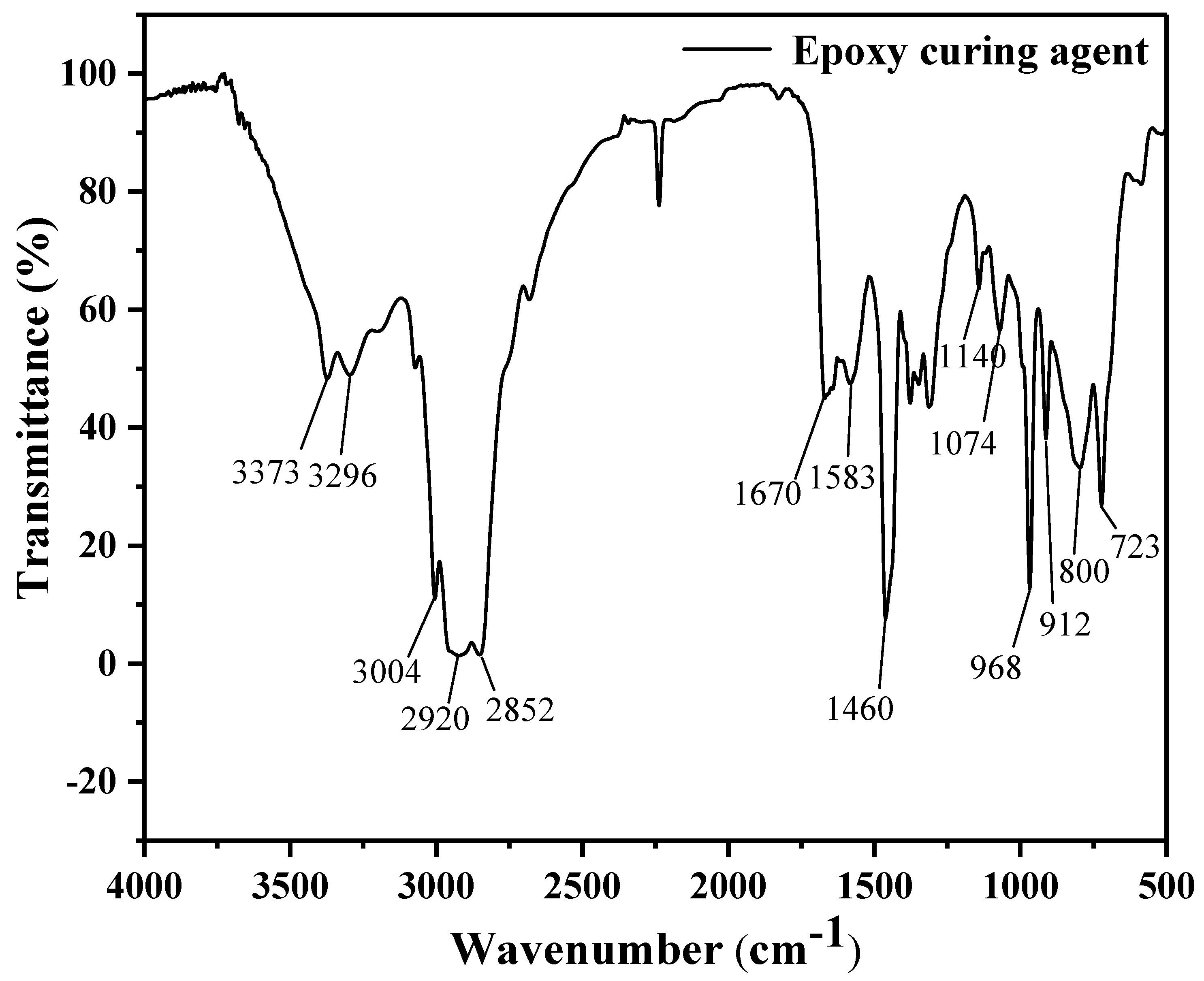
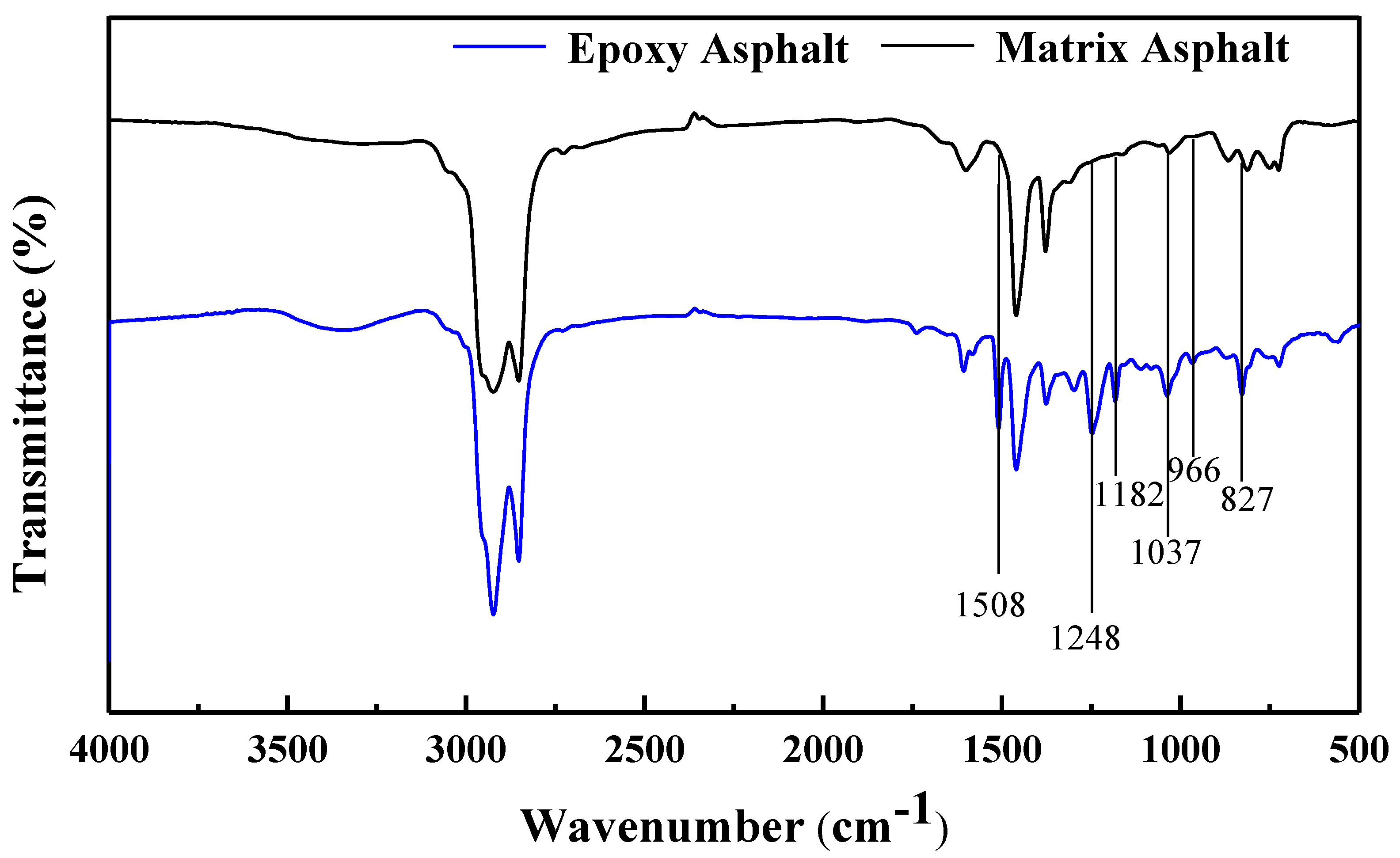
3.1.1. FTIR Spectroscopy
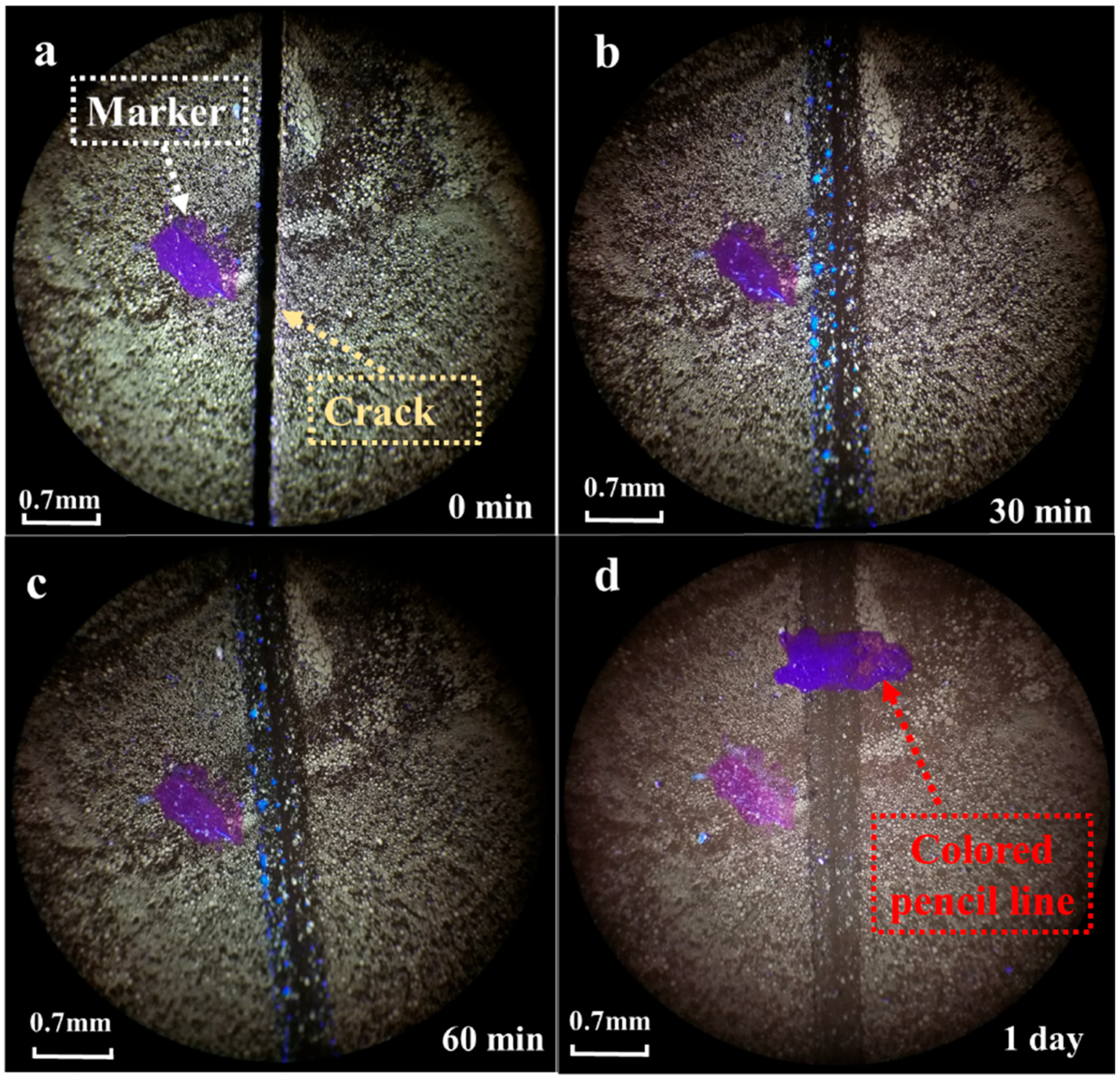
3.1.2. Fluorescence Microanalysis
3.2. Macroscopic Performance Analysis
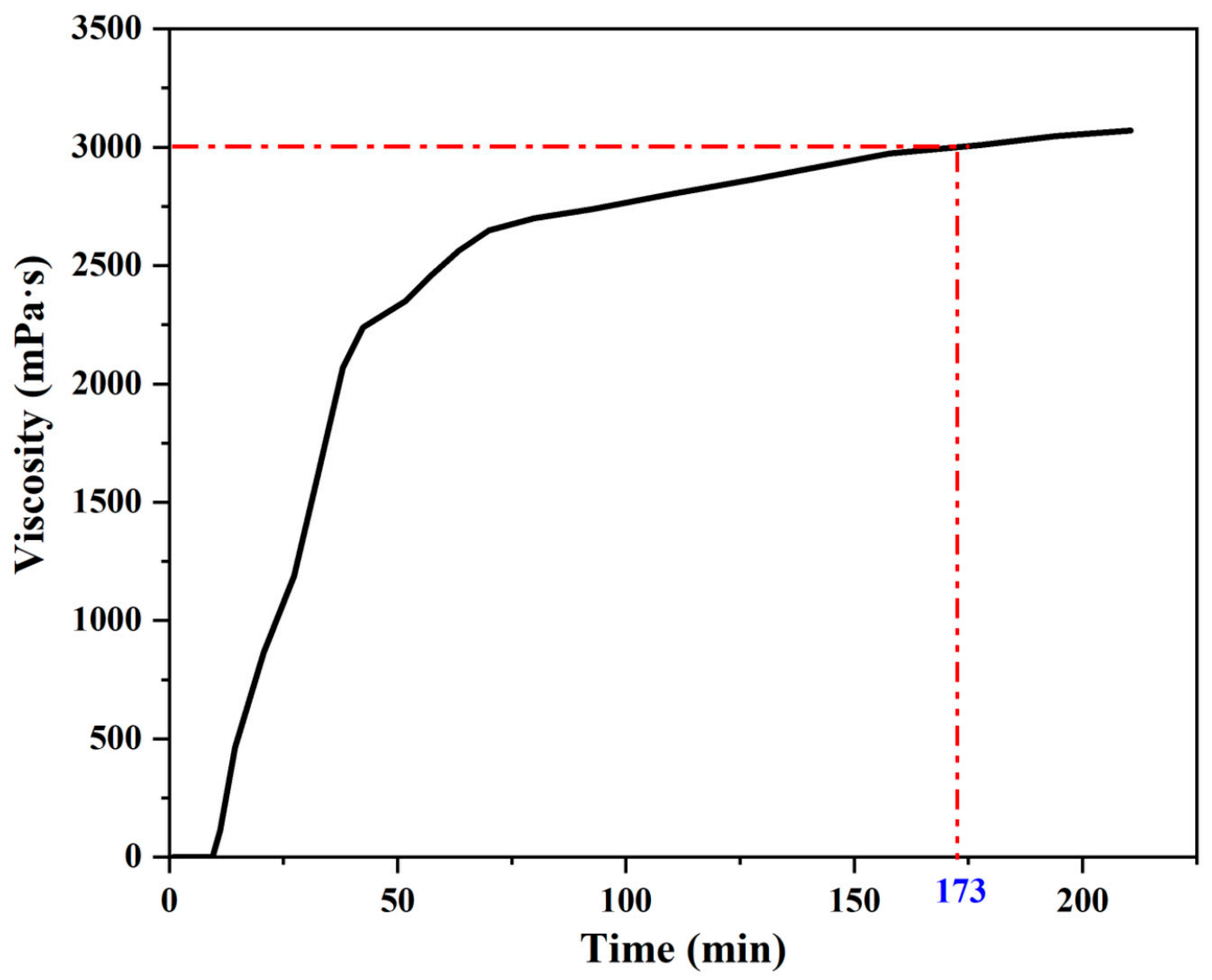
3.2.1. Viscosity Characteristics
3.2.2. Tensile Test Analysis
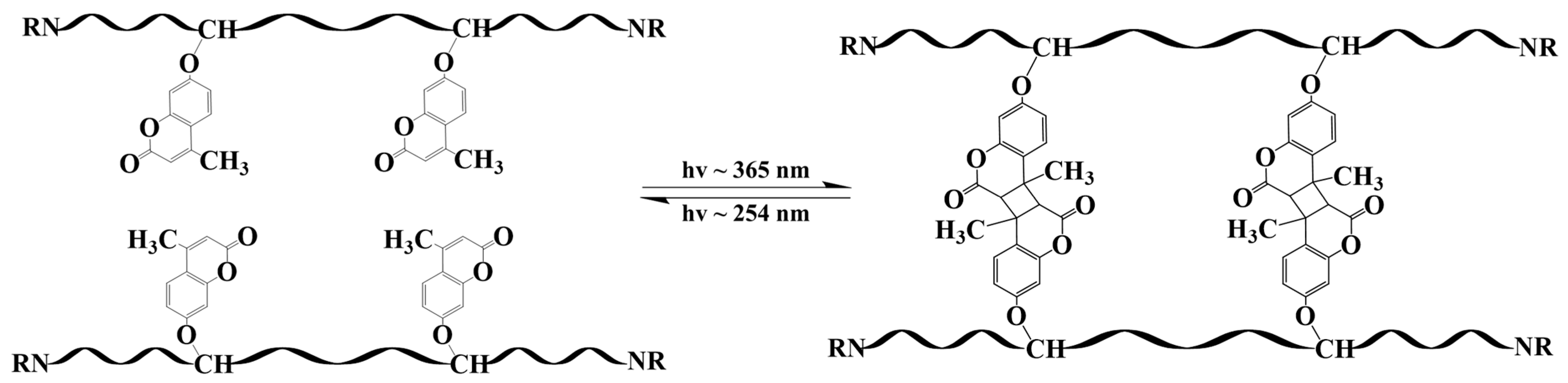
4. Analysis of the Self-Healing Mechanism
5. Conclusions
- In this study, an epoxy asphalt material containing coumarin groups was prepared, which could achieve self-healing of microcracks under UV irradiation at 50 °C.
- The fluorescence microscopy observations showed that during the healing process, the cracks gradually filled in and eventually disappeared, indicating that the self-healing material exhibited a significant healing effect at the crack locations.
- The mechanical performance test results indicated that the self-healing epoxy asphalt experienced minimal degradation in mechanical properties after healing, with the post-healing fracture toughness maintaining approximately 69% of that of the initial state, demonstrating superior performance compared to conventional epoxy asphalt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaute-Alonso, A.; Garcia-Sanchez, D.; Ramos-Gutierrez, Ó.R.; Ntertimanis, V. Enhancing stress measurements accuracy control in the construction of long-span bridges. Sci. Rep. 2024, 14, 10961. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.A.; Ansari, M.I.; Islam, N.; Umair, M. Effect of lead rubber bearing on seismic performance of steel box girder bridge. Mater. Today Proc. 2022, 64, 468–480. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z.; Liu, J.; Chen, S.; Wang, J.; Wang, X. Advances in the Application and Research of Steel Bridge Deck Pavement, Structures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1156–1174. [Google Scholar]
- Ren, J.; Chen, Y.; Sun, Z.; Zhang, Y. A vehicle-bridge interaction vibration model considering bridge deck pavement. J. Low Freq. Noise Vib. Act. Control. 2023, 42, 146–172. [Google Scholar] [CrossRef]
- Chen, Y.; Hossiney, N.; Yang, X.; Wang, H.; You, Z. Application of Epoxy-Asphalt Composite in Asphalt Paving Industry: A Review with Emphasis on Physicochemical Properties and Pavement Performances. Adv. Mater. Sci. Eng. 2021, 2021, 3454029. [Google Scholar] [CrossRef]
- Yeon, J. Deformability of bisphenol A-type epoxy resin-based polymer concrete with different hardeners and fillers. Appl. Sci. 2020, 10, 1336. [Google Scholar] [CrossRef]
- Lu, Q.; Bors, J. Alternate uses of epoxy asphalt on bridge decks and roadways. Constr. Build. Mater. 2015, 78, 18–25. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, H.; Pan, Y. Cracking mechanism and repair techniques of epoxy asphalt on steel bridge deck pavement. Transp. Res. Rec. 2016, 2550, 123–130. [Google Scholar] [CrossRef]
- Mohan, P. A critical review: The modification, properties, and applications of epoxy resins. Polym.-Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Montenegro, P.; Carvalho, H.; Ribeiro, D.; Calçada, R.; Tokunaga, M.; Tanabe, M.; Zhai, W. Assessment of train running safety on bridges: A literature review. Eng. Struct. 2021, 241, 112425. [Google Scholar] [CrossRef]
- Hopper, T.; Manafpour, A.; Warn, G.; Rajabipour, F.; Morian, D.; Jahangirnejad, S. Bridge Deck Cracking: Effects on In-Service Performance, Prevention, and Remediation; Department of Transportation, Bureau of Planning and Research: Lancaster, PA, USA, 2015. [Google Scholar]
- Lv, Q.; Huang, W.; Xiao, F. Laboratory evaluation of self-healing properties of various modified asphalt. Constr. Build. Mater. 2017, 136, 192–201. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, M.; Li, P.; Zhang, J.; Qiao, R.; Cheng, C.; Xu, H. Preparation and characterization of self-healing microcapsules of asphalt. Constr. Build. Mater. 2020, 263, 120174. [Google Scholar] [CrossRef]
- Kim, Y.R.; Little, D.N.; Benson, F.C. Chemical and mechanical evaluation on healing mechanism of asphalt concrete (with discussion). J. Assoc. Asph. Paving Technol. 1990, 59, 240–275. [Google Scholar]
- Bhasin, A.; Motamed, A. Analytical models to characterise crack growth in asphaltic materials and healing in asphalt binders. Int. J. Pavement Eng. 2011, 12, 371–383. [Google Scholar] [CrossRef]
- Sun, D.; Sun, G.; Zhu, X.; Guarin, A.; Li, B.; Dai, Z.; Ling, J. A comprehensive review on self-healing of asphalt materials: Mechanism, model, characterization and enhancement. Adv. Colloid Interface Sci. 2018, 256, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Schlangen, E.; Sangadji, S. Addressing infrastructure durability and sustainability by self healing mechanisms-Recent advances in self healing concrete and asphalt. Procedia Eng. 2013, 54, 39–57. [Google Scholar] [CrossRef]
- Su, J.-F.; Han, S.; Wang, Y.-Y.; Schlangen, E.; Han, N.-X.; Liu, B.; Zhang, X.-L.; Yang, P.; Li, W. Experimental observation of the self-healing microcapsules containing rejuvenator states in asphalt binder. Constr. Build. Mater. 2017, 147, 533–542. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Yaseen, M.; Huang, K. A review on the self-healing ability of epoxy polymers. J. Appl. Polym. Sci. 2021, 138, 50260. [Google Scholar] [CrossRef]
- Peñas-Caballero, M.; Santana, M.H.; Verdejo, R.; Lopez-Manchado, M.A. Measuring self-healing in epoxy matrices: The need for standard conditions. React. Funct. Polym. 2021, 161, 104847. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Hosur, M.V.; Nuruddin, M.; Tcherbi-Narteh, A.; Kumar, A.; Boddu, V.; Jeelani, S. Self-healing epoxy composites: Preparation, characterization and healing performance. J. Mater. Res. Technol. 2015, 4, 33–43. [Google Scholar] [CrossRef]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef]
- Patil, R.S.; Thomas, J.; Patil, M.; John, J. To shed light on the UV curable coating technology: Current state of the art and perspectives. J. Compos. Sci. 2023, 7, 513. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+ 2] photocycloaddition reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef] [PubMed]
- Stukalin, E.B.; Cai, L.-H.; Kumar, N.A.; Leibler, L.; Rubinstein, M. Self-healing of unentangled polymer networks with reversible bonds. Macromolecules 2013, 46, 7525–7541. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Bode, S.; Hager, M.D.; Schubert, U.S. Self-healing polymers based on reversible covalent bonds. In Self-Healing Materials; Springer: Cham, Switzerland, 2016; pp. 1–58. [Google Scholar]
- Banerjee, S.; Tripathy, R.; Cozzens, D.; Nagy, T.; Keki, S.; Zsuga, M.; Faust, R. Photoinduced smart, self-healing polymer sealant for photovoltaics. ACS Appl. Mater. Interfaces 2015, 7, 2064–2072. [Google Scholar] [CrossRef]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels–Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2013, 4, 724–730. [Google Scholar] [CrossRef]
- Pipintakos, G.; Sreeram, A.; Mirwald, J.; Bhasin, A. Engineering bitumen for future asphalt pavements: A review of chemistry, structure and rheology. Mater. Des. 2024, 244, 113157. [Google Scholar] [CrossRef]
- ASTM D638-2010; Standard Test Methods for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA.







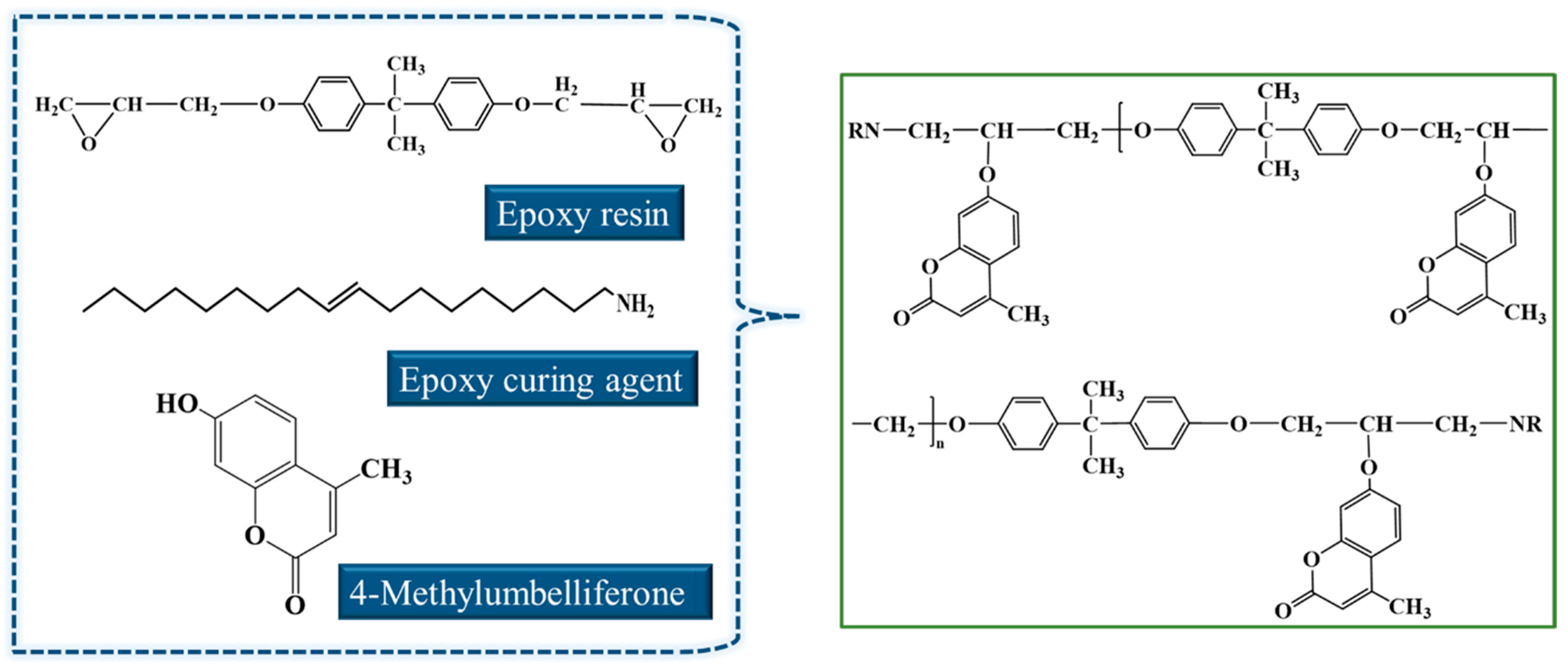
| Material | Specifications | Manufacturer |
|---|---|---|
| 4-Methylumbelliferone | 98% (Analytical purity) | Shanghai Aladdin Biochemical Technology Co., Ltd. Shanghai China |
| Glycerol | 99% (Analytical purity) | Shanghai Aladdin Biochemical Technology Co., Ltd. Shanghai China |
| Talc | 800 mesh | Sinopharm Chemical Reagent Co., Ltd. Beijing China |
| Potassium bromide | 99.95% (Analytical purity) | Sinopharm Chemical Reagent Co., Ltd. Beijing China |
| Device Name | Device Model | Manufacturer |
|---|---|---|
| Collector thermostatically heated magnetic stirrer | DF-102S | Gongyi City to China Instrument Co., Ltd. Gongyi China |
| Constant-temperature blast-drying oven | LABOROTA 4000 | Shanghai Danding International Trade Co., Ltd. Shanghai China |
| Ultraviolet analyzer | ZNHW | Shanghai Lingke Development Co., Ltd. Shanghai China |
| Type | Technical Indicators | Technical Requirement | Result |
|---|---|---|---|
| Epoxy resin | Viscosity (25 °C/mPa·s) | 10–12.5 | 11.2 |
| Density (25)/g·cm−3 | 1.0–1.2 | 1.1 | |
| Appearance | Transparent liquid | - |
| Type | Technical Indicators | Technical Requirement | Result |
|---|---|---|---|
| Epoxy curing agent | Viscosity (25 °C/mPa·s) | 8–10 | 9 |
| Density (25)/g·cm−3 | 0.75–1.0 | 0.85 | |
| Appearance | Light yellow liquid | - |
| Technical Indicators | Prescribed Value | Result |
|---|---|---|
| Penetration (25 °C, 0.1 mm) | 60–80 | 70.5 |
| Ductility (5 cm/min) 10 °C (cm) | ≥25 | 90.3 |
| Softening point (°C) | ≥45 | 55 |
| Category | Ordinary Epoxy Asphalt | Ordinary Epoxy Asphalt with Healing | Self-Healing Epoxy Asphalt | Self-Healing Epoxy Asphalt with Healing |
|---|---|---|---|---|
| Fracture Stress (MPa) | 2.44 ± 0.13 | 1.34 ± 0.09 | 5.87 ± 0.21 | 4.92 ± 0.30 |
| Fracture Strain | 2.09 ± 0.20 | 1.42 ± 0.11 | 1.64 ± 0.18 | 1.01 ± 0.27 |
| Fracture Toughness (J/m3) | 2.19 ± 0.14 | 1.17 ± 0.15 | 5.32 ± 0.25 | 3.67 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, M.; Xu, S.; Zhang, F. Preparation and Performance Study of Ultraviolet-Responsive Self-Healing Epoxy Asphalt. Materials 2024, 17, 4403. https://doi.org/10.3390/ma17174403
Wang J, Wang M, Xu S, Zhang F. Preparation and Performance Study of Ultraviolet-Responsive Self-Healing Epoxy Asphalt. Materials. 2024; 17(17):4403. https://doi.org/10.3390/ma17174403
Chicago/Turabian StyleWang, Jian, Maoan Wang, Shuwen Xu, and Fenglei Zhang. 2024. "Preparation and Performance Study of Ultraviolet-Responsive Self-Healing Epoxy Asphalt" Materials 17, no. 17: 4403. https://doi.org/10.3390/ma17174403
APA StyleWang, J., Wang, M., Xu, S., & Zhang, F. (2024). Preparation and Performance Study of Ultraviolet-Responsive Self-Healing Epoxy Asphalt. Materials, 17(17), 4403. https://doi.org/10.3390/ma17174403





