Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production
Abstract
1. Introduction
2. Nickel
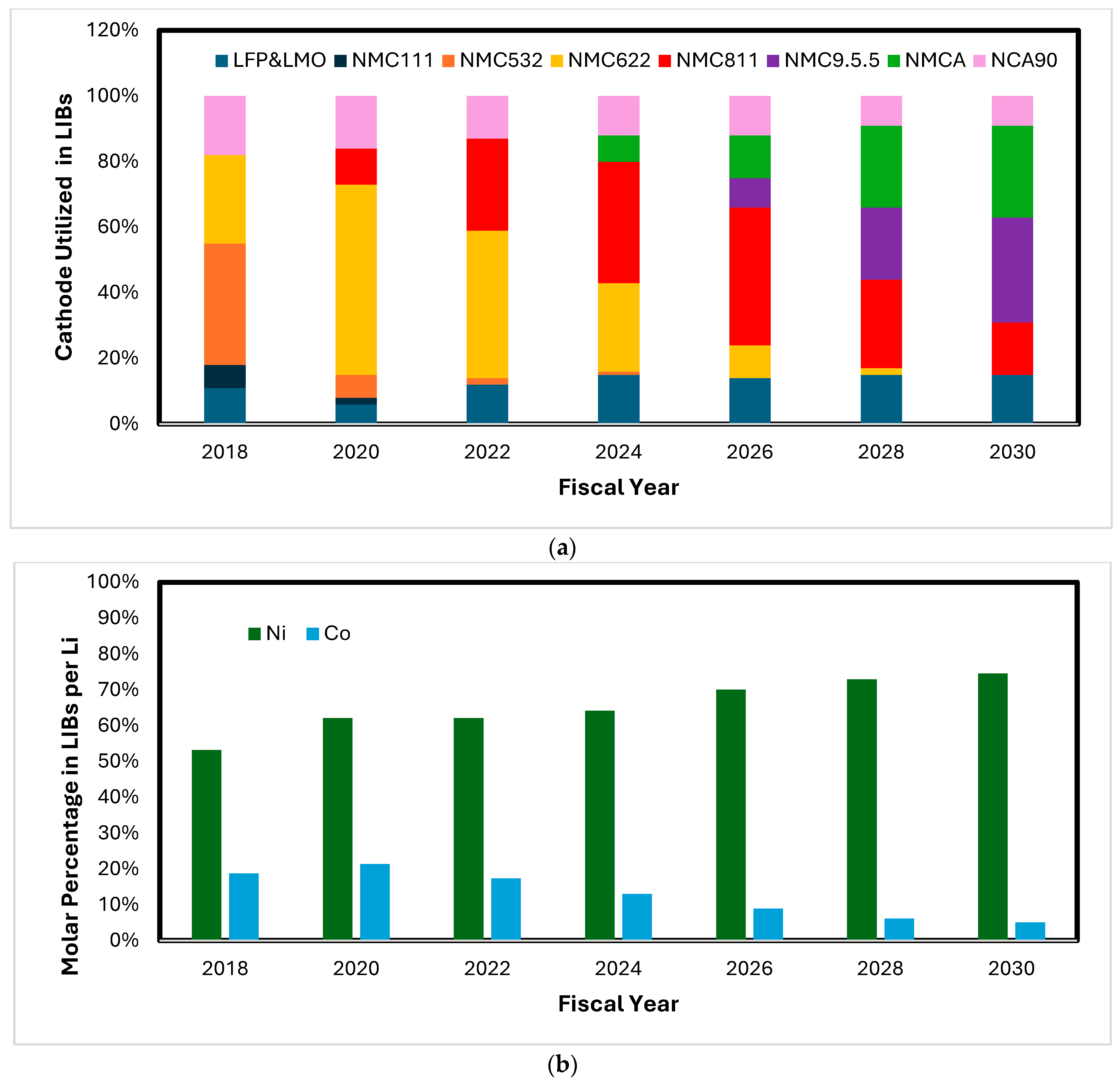
2.1. Demands and Land Reserves
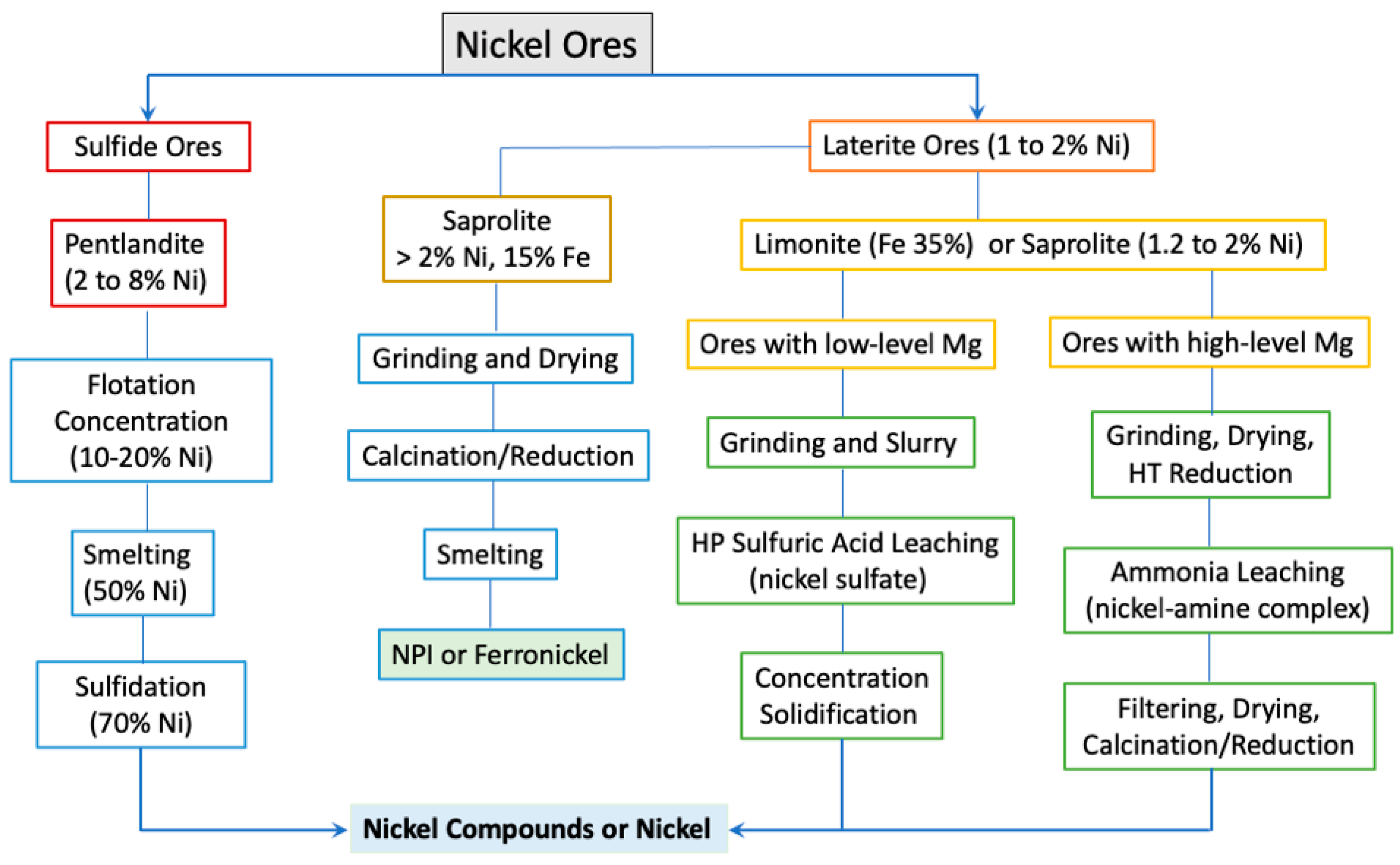
2.2. Nickel Production from Land Ores
2.2.1. Pre-Concentration
2.2.2. Processing of Sulfide Ores
2.2.3. Pyrometallurgical Processing of Saprolitic Laterite Ore
2.2.4. Hydrometallurgical Leaching of Limonite or Saprolite Ores
3. Cobalt
3.1. Demands and Land Reserves
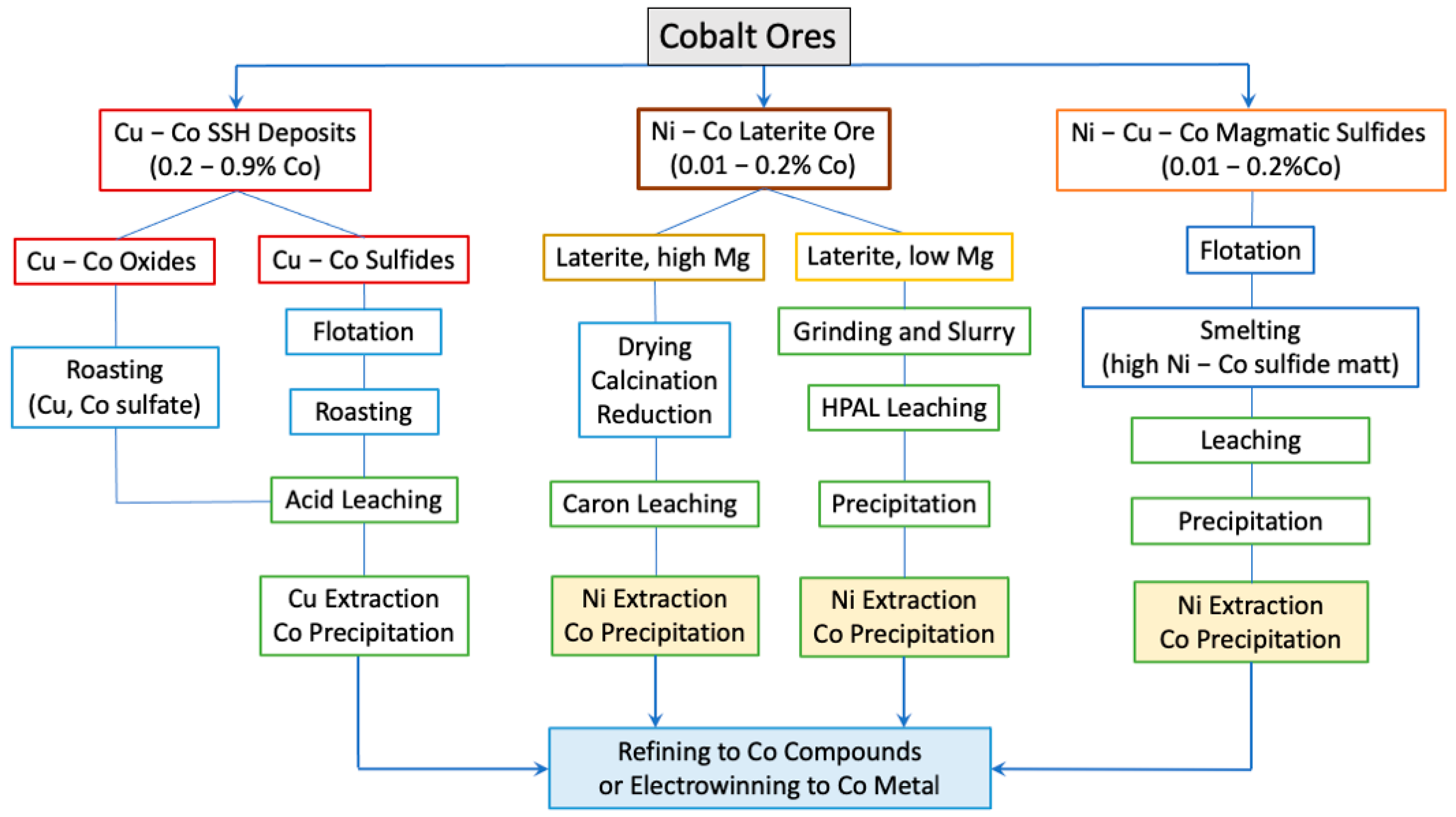
3.2. Cobalt Production from Land Ores
3.2.1. Processing of Cu−Co SSH Ores
3.2.2. Processing of Ni–Co Laterite and Ni–Co–Cu Magmatic Sulfide Ores
4. Lithium
4.1. Demands and Reserves
4.2. Production
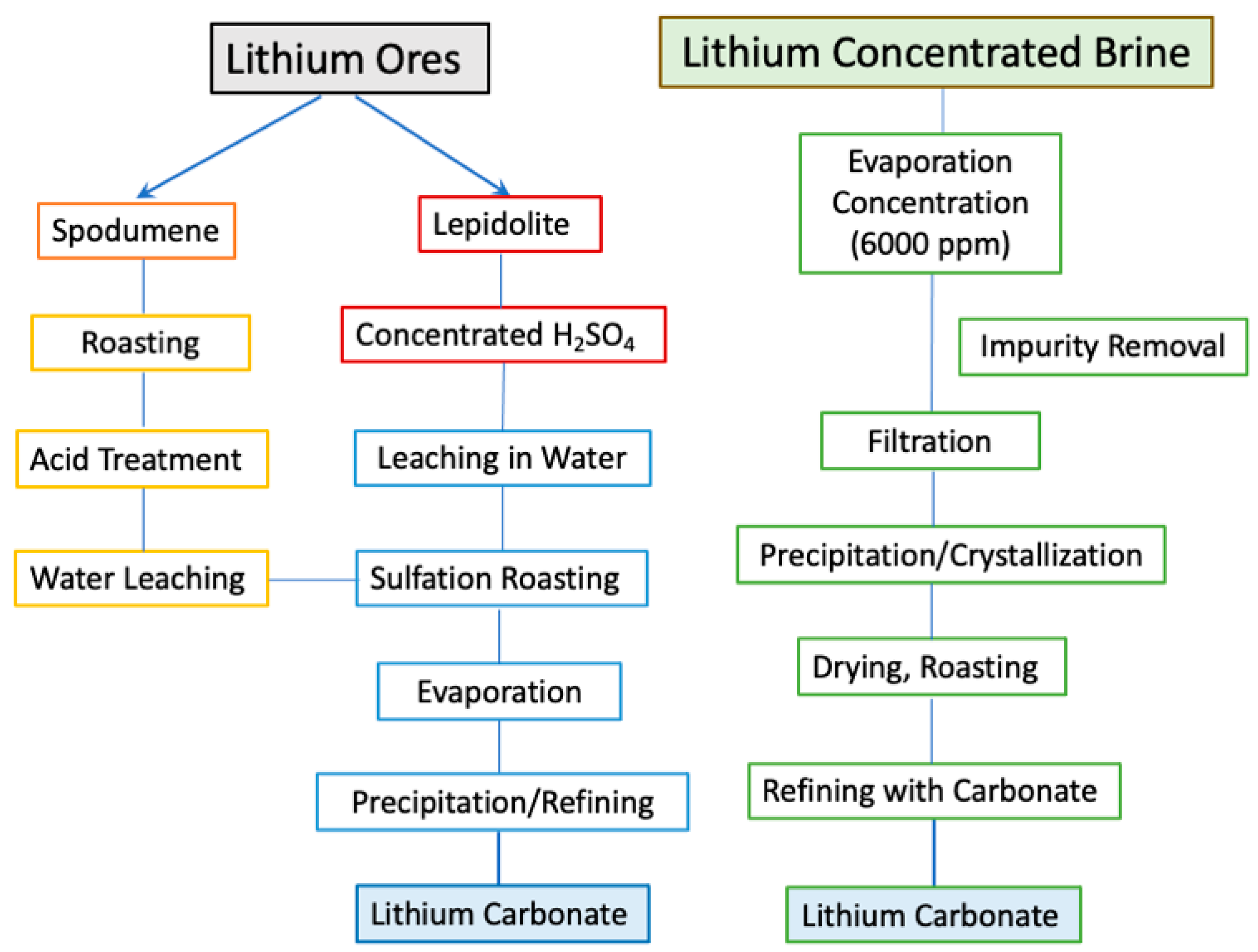
4.2.1. Production from Mineral Ores
Pre-Concentration
Processing Spodumene/Petalite Ores
Production from Lepidolite Ores
4.2.2. Lithium Extraction from Brines
5. Ocean Resources and Extraction
6. Secondary Resources and Extraction
6.1. Recycling Co and Ni from Metal Scraps and Wastes
6.2. Recycling Li, Co, and Ni from Spent LIB Batteries
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Global EV Outlook 2023: Catching up with Climate Ambitions; International Energy Agency: Paris, France, 2023.
- International Energy Agency. Global EV Outlook 2024: Moving towards Increased Affordability; International Energy Agency: Paris, France, 2024.
- Zhou, Y.; Gohlke, D.; Rush, L.; Kelly, J.; Dai, Q. Lithium-Ion Battery Supply Chain for e-Drive Vehicles in the United States: 2010–2020; ANL/ESD-21/3; Energy Systems Division, Argonne National Laboratory: Argonne, IL, USA, 2021.
- Cohen, A. Manufacturers Are Struggling to Supply Electric Vehicles with Batteries. Available online: https://www.forbes.com/sites/arielcohen/2020/03/25/manufacturers-are-struggling-to-supply-electric-vehicles-with-batteries/ (accessed on 1 January 2020).
- Grand View Research. Market Analysis Report: Energy Storage Systems Market Size, Share & Trends Analysis Report by Technology (Pumped Storage, Electrochemical Storage, Electromechanical Storage, Thermal Storage), by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- Mckinsey and Company. Market Analysis Report: Enabling Renewable Energy with Battery Energy Storage Systems; Mckinsey and Company: Chicago, IL, USA, 2023. [Google Scholar]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; Thomas, B., Ed.; McGraw-Hill Professional: New York, NY, USA, 2001. [Google Scholar]
- Ralls, A.M.; Leong, K.; Clayton, J.; Fuelling, P.; Mercer, C.; Navarro, V.; Menezes, P.L. The Role of Lithium-ion batteries in the growing trend of electric vehicles. Materials 2023, 16, 6063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Zhou, W.; Cleaver, C.; Dunant, C.; Allwood, J. Cost, range anxiety and future electricity supply: A review of how today’s technology trends may influence the future uptake of BEVs. Renew. Sustain. Energy Rev. 2023, 173, 113074. [Google Scholar] [CrossRef]
- Ragozzino, A. What’s Next for Electric-Vehicle Battery Technology? Wards Intelligence: Southfield, MI, USA, 2022. [Google Scholar]
- Biswal, B.K.; Balasubramanian, R. Recovery of valuable metals from spent lithium-ion batteries using microbial agents for bioleaching: A review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef]
- Saaid, F.I.; Kasim, M.F.; Winie, T.; Elong, K.A.; Azahidi, A.; Basri, N.D.; Yaakob, M.K.; Mastuli, M.S.; Shaffee, S.N.A.; Zolkiffly, M.Z.; et al. Ni-rich lithium nickel manganese cobalt oxide cathode materials: A review on the synthesis methods and their electrochemical performances. Heliyon 2024, 10, e23968. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future materials demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The recycling of spent lithium-ion batteries: A review of current processes and technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Jena, K.K.; AlFantazi, A.; Mayyas, A.T. Comprehensive Review on Concept and Recycling Evolution of Lithium-Ion Batteries (LIBs). Energy Fuels 2021, 35, 18257–18284. [Google Scholar] [CrossRef]
- Zhang, J.; Azimi, G. Recycling of lithium, cobalt, nickel, and manganese from end-of-life lithium-ion battery of an electric vehicle using supercritical carbon dioxide. Resour. Conserv. Recycl. 2022, 187, 106628. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Pan, C.; Shen, Y. Pyrometallurgical recycling of spent lithium-ion batteries from conventional roasting to synergistic pyrolysis with organic wastes. J. Energy Chem. 2023, 85, 547–561. [Google Scholar] [CrossRef]
- Ren, Z.; Li, H.; Yan, W.; Lv, W.; Zhang, G.; Lv, L.; Sun, L.; Gao, W. Comprehensive evaluation on production and recycling of lithium-ion batteries: A critical review. Renew. Sustain. Energy Rev. 2023, 185, 113585. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Zhang, L.; Liu, Q.; Wang, Y.; Lin, Y.; Xu, C.; Guo, J.; Chealic, P.; Xia, X. Progress, challenges, and prospects of spent lithium-ion batteries recycling: A review. J. Energy Chem. 2024, 89, 144–171. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, K.; Wang, X.; Wang, Q.; Wang, J. Examining green-sustainable approaches for recycling of lithium-ion batteries. DeCarbon 2024, 3, 100034. [Google Scholar] [CrossRef]
- Biswal, B.K.; Zhang, B.; Tran, P.; Zhang, J.; Balasubramanian, R. Recycling of spent lithium-ion batteries for a sustainable future: Recent advancements. Chem. Soc. Rev. 2024, 53, 5552–5592. [Google Scholar] [CrossRef]
- British Geological Survey. Nickel. 2008. Available online: https://core.ac.uk/download/pdf/58868.pdf (accessed on 4 August 2024).
- Schmidt, T.; Buchert, M.; Schebek, L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour. Conserv. Recycl. 2016, 112, 107–122. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D. Advanced review on extraction of Nickel from primary and secondary sources by pre-treatment, leaching, and separation: A comprehensive review. Metall. Rev. 2019, 40, 157–193. [Google Scholar]
- U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2024.
- Zhao, K.; Gao, F.; Yang, Q. Comprehensive review on metallurgical upgradation processes of nickel sulfide ores. J. Sustain. Metall. 2022, 8, 37–50. [Google Scholar] [CrossRef]
- Stankovic, S.; Topic, S.S.; Okic, M.; Markovic, B.; Friedrich, B. Review of the past, present, and future of the hydrometallurgical production of nickel and cobalt from lateritic ores. Metall. Mater. Eng. 2020, 26, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Munali Nickel Mine’s “Game-Changing” Dense Media Separator (DMS) Is Flying the Flag High for Zambia. 2019. Available online: https://miningforzambia.com/munali-nickel-mines-game-changing-dense-media-separator-dms-is-flying-the-flag-high-for-zambia/ (accessed on 4 August 2024).
- Quast, K.; Connor, J.N.; Skinner, W.; Robinson, D.J.; Addai-Mensh, J. Preconcentration strategies in the processing of nickel laterite ores Part 1: Literature review. Miner. Eng. 2015, 79, 261–268. [Google Scholar] [CrossRef]
- Rao, K.A.; Sreenivas, T.; Natarajan, R.; Rao, N.K. Preconcentration of nickel values from lateritic chromite ore overburden, Sukinda, Orissa, India. Miner. Process. Extract. Metall. Rev. 1995, 15, 37–45. [Google Scholar]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Application of Enhanced Gravity Separators for Fine Particle Processing: An Overview. J. Sustain. Metall. 2021, 7, 315–339. [Google Scholar] [CrossRef]
- Arrokhpay, S.; Filippov, L.; Fornasiero, D. Pre-concentration of nickel in laterite ores using physical separation methods. Min. Eng. 2019, 141, 105892. [Google Scholar] [CrossRef]
- Pillay, K.; Mainza, A.N.; Chetty, D.; Becker, M. Mineralogical Factors Affecting the Dense Medium Separation of Nickel Sulfide Ores. Minerals 2022, 12, 1311. [Google Scholar] [CrossRef]
- Iranmanesh, M.; Hulliger, J. Magnetic separation: Its application in mining, waste purification, medicine, biochemistry and chemistry. Chem. Soc. Rev. 2017, 46, 5925. [Google Scholar] [CrossRef]
- Xiao, J.; Ding, W.; Peng, Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of Nickel from Garnierite Laterite Ore Using Roasting and Magnetic Separation with Calcium Chloride and Iron Concentrate. Minerals 2020, 10, 352. [Google Scholar] [CrossRef]
- Srdjan Bulatovic, M. Handbook of Flotation Reagents Chemistry, Theory and Practice: Flotation of Sulfide Ores, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ian, D.; Wilson Colin, F. Poole Michael Cooke. In Encyclopedia of Separation Science, 1st ed.; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Eliana Mano, S.; Caner, L.; Petit, S.; Arthur Chaves, P.; André Mexias, S. Ni-smectitic ore behaviour during the Caron process. Hydrometallurgy 2019, 186, 200–209. [Google Scholar] [CrossRef]
- Pérez-Garibay, R.; Ramírez-Aguilera, N.; Bouchard, J.; Rubio, J. Froth flotation of sphalerite: Collector concentration, gas dispersion and particle size effects. Miner. Eng. 2014, 57, 72–78. [Google Scholar] [CrossRef]
- Masiya, T.T.; Nheta, W. Flotation of Nickel-Copper Sulphide Ore: Optimisation of Process Parameters Using Taguchi Method. In Proceedings of the International Conference on Mining, Material and Metallurgical Engineering, Prague, Czech Republic, 11–12 August 2014. Paper No. 113. [Google Scholar]
- Chernousenko, V.E.; Neradovsky, Y.N.; Kameneva, Y.S.; Vishnyakova, I.N.; Mitrofanova, G.V. Increasing Efficiency of Pechenga Rebellious, Copper–Nickel Sulphide Ore Flotation. J. Min. Sci. 2018, 54, 1035–1040. [Google Scholar] [CrossRef]
- Long, T.; Zhao, H.; Wang, Y.; Yang, W.; Deng, S.; Xiao, W.; Lan, X.; Wang, Q. Synergistic mechanism of acidified water glass and carboxymethyl cellulose in flotation of nickel sulfide ore. Miner. Eng. 2022, 181, 107547. [Google Scholar] [CrossRef]
- Faris, N.; Pownceby, M.I.; Bruckard, W.J.; Chen, M. The Direct Leaching of Nickel Sulfide Flotation Concentrates—A Historic and State-of-the-Art Review Part I: Piloted Processes and Commercial Operations. Miner. Process. Extr. Metall. Rev. 2023, 44, 407–435. [Google Scholar] [CrossRef]
- Anzoom, S.J.; Bournival, G.; Ata, S. Coarse particle flotation: A review. Miner. Eng. 2024, 206, 108499. [Google Scholar] [CrossRef]
- Mweene, L.; Gomex-Flores, A.; Jeong, H.E.; Ilyas, S.; Kim, H. Challenges and Future in Ni Laterite Ore Enrichment: A Critical Review. Miner. Process. Extr. Metall. Rev. 2023, 45, 539–563. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L. Challenges in processing nickel laterite ores by flotation. Int. J. Miner. Process. 2016, 151, 59–67. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Fornasiero, D.; Filippov, L. Upgrading nickel in laterite ores by flotation. Miner. Eng. 2018, 121, 100–106. [Google Scholar] [CrossRef]
- Quast, K.; Connor, J.N.; Skinner, W.; Robinson, D.J.; Addai-Mensh, J. Preconcentration strategies in the processing of nickel laterite ores part 3: Flotation testing. Miner. Eng. 2015, 79, 279–286. [Google Scholar] [CrossRef]
- Zappala, L.; McDonald, R.; Powceby, M.I. Nickel Laterite Beneficication and Potential for Upgrading Using High Temperature Methods: A Review. Miner. Process. Extr. Metall. Rev. 2023, 1–23. [Google Scholar] [CrossRef]
- Fang, X.; Peng, z.; Yin, T.; Rao, M.; Li, G. Microwave Treatment of Copper–Nickel Sulfide Ore for Promotion of Grinding and Flotation. Metals 2024, 14, 565. [Google Scholar] [CrossRef]
- Mu, W.; Cui, F.; Huang, Z.; Zhai, Y.; Xu, Q.; Luo, S. Synchronous extraction of nickel and copper from a mixed oxide-sulfide nickel ore in a low-temperature roasting system. J. Clean. Prod. 2018, 177, 371–377. [Google Scholar] [CrossRef]
- Cui, F.; Mu, W.; Wang, S.; Xin, H.; Xu, Q.; Zhai, Y.; Luo, S. Sodium sulfate activation mechanism on co-sulfating roasting to nickel copper sulfide concentrate in metal extractions, microtopography and kinetics. Min. Eng. 2018, 123, 104–116. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.W.; Chen, J. Recovery of copper, nickel and cobalt from acidic pressure leaching solutions of low-grade sulfide flotation concentrates. Min. Eng. 2007, 20, 722–728. [Google Scholar] [CrossRef]
- Marzoughi, O.; Pickles, C.A. Solid state reduction and magnetic separation of nickeliferous laterite ores: Review and analysis. J. Ind. Eng. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Keskinkilic, E. Nickel laterite smelting processes and some examples of recent possible modifications to the conventional route. Metals 2019, 9, 974. [Google Scholar] [CrossRef]
- Nurjaman, F.; Astuti, W.; Bahfie, F.; Suharno, B. Study of selective reduction in lateritic nickel ore: Saprolite versus limonite. Mater. Today Proc. 2021, 44, 1488–1494. [Google Scholar] [CrossRef]
- Norgate, T.; Jahanshahi, S. Low grade ores—Smelt, leach or concentrate? Miner. Eng. 2010, 23, 65–73. [Google Scholar] [CrossRef]
- Vahed, A.; Mackey, P.J.; Warner, E.M.A. A Review of Nickel Pyrometallurgy Over the Past 50 Years with Special Reference to the Former Inco Ltd and Falconbridge Ltd. In Proceedings of the 5th International Symposium on Nickel and Cobalt, Online, 15–18 March 2021; pp. 41–62. [Google Scholar]
- Ma, B.; Xing, P.; Yang, W.; Wang, C.; Chen, Y.; Wang, H. Solid-state metalized reduction of magnesium-rich low-nickel oxide ores using coal as the reductant based on thermodynamic analysis. Metall. Mater. Trans. B 2017, 48, 2037–2046. [Google Scholar] [CrossRef]
- Lv, X.; Lv, W.; Liu, M.; You, Z.; Lv, X.; Bai, C. Effect of sodium sulfate on preparation of ferronickel from nickel laterite by carbothermal reduction. ISIJ Int. 2018, 58, 799–807. [Google Scholar] [CrossRef]
- Hang, G.; Xue, Z.; Wang, J.; Wu, Y. Mechanism of calcium sulphate on the aggregation and growth of ferronickel particles in the self-reduction of saprolitic nickel laterite ore. Metals 2020, 10, 423. [Google Scholar] [CrossRef]
- Liu, S.; Yang, C.; Yang, S.; Yu, Z.; Wang, Z.; Yan, K.; Li, J.; Liu, X. A robust recovery of Ni from laterite ore promoted by sodium thiosulfate through hydrogen-thermal reduction. Front. Chem. 2021, 9, 704012. [Google Scholar] [CrossRef] [PubMed]
- Zulhan, Z.; Shalat, W. Evolution of ferronickel particles during the reduction of low-grade saprolitic laterite nickel ore by coal in the temperature range of 900–1250 °C with the addition of CaO-CaF2-H3BO3. Int. J. Miner. Metall. Mater. 2021, 28, 612–620. [Google Scholar] [CrossRef]
- Yang, W.; Ma, B.; Li, X.; Hu, D.; Wang, C.; Wang, H. Transferring behavior and reaction kinetics of saprolitic laterite during metalized reduction in the presence of calcium fluoride. Miner. Eng. 2022, 176, 107353. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Park, K.; Tian, Q.; Wu, Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation–roasting–leaching process. Hydrometallurgy 2009, 99, 144–150. [Google Scholar] [CrossRef]
- Basturkcu, H.; Acarkan, N. Saparation of Nickel and Iron from Laterite ore using digestion-roating-leaching-precipitation Process. Physicochem. Probl. Miner. Process. 2016, 52, 564–574. [Google Scholar]
- Ribeiro, P.P.M.; Santos, I.D.D.; Neumann, R.; Fernades, A.; Dutra, A.J.B. Roasting and Leaching Behavior of Nickel Laterite Ore. Metall. Mater. Trans. B 2021, 52, 1739. [Google Scholar] [CrossRef]
- Whttington, B.I.; Muir, D. Pressure acid leaching of nickel laterites: A Review, Mineral Processing and Extractive Metallurgy Review. Int. J. 2000, 21, 527–599. [Google Scholar]
- Akbar Rhamdhani, M.; Chen, J.; Hidayat, T.; Jak, E.; Hayes, P. Advances in research on nickel production through the Caron process. Proc. EMC 2009, 2009, 899–913. [Google Scholar]
- Gultom, T.; Sianipar, A. High pressure acid leaching: A newly introduced technology in Indonesia. Environ. Earth Sci. 2020, 413, 012015. [Google Scholar] [CrossRef]
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review: Part I. Sulphuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Luo, J.; Li, G.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Atmospheric leaching characteristics of nickel and iron in limonitic laterite with sulfuric acid in the presence of sodium sulfite. Miner. Eng. 2015, 78, 38–44. [Google Scholar] [CrossRef]
- Oxley, A.; Smith, M.E.; Caceres, O. Why heap leach nickel laterites? Miner. Eng. 2016, 88, 53–60. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Dimaki, D.; Zafiratos, J.; Tsakiridis, P.; Karidakis, T.; Frogoudakis, E.; Drougas, J. Heap leaching of greek low-grade nickel oxide ores by dilute sulphuric acid at a pilot-plant scale. Mater. Proc. 2021, 5, 65. [Google Scholar] [CrossRef]
- Robinson, D.J.; McDonald, R.; Zhang, W.; McCarthy, F. Developments in the hydrometallurgical processing of laterites. In Proceedings of the COM 2017-Nickel/Cobalt Hydrometallurgy Symposium, Vancouver, BC, Canada, 27–30 August 2017. [Google Scholar]
- Pandey, N.; Tripathy, S.K.; Patra, S.K.; Jha, G. Recent Progress in Hydrometallurgical Processing of Nickel Lateritic Ore. Trans. Indian Inst. Met. 2023, 76, 11–30. [Google Scholar] [CrossRef]
- Johnson, J.A.; Cashmore, B.C.; Hockridge, R.J. Optimization of nickel extraction from laterite ores by high pressure acid leaching with addition of sodium sulphate. Miner. Eng. 2005, 18, 1297–1303. [Google Scholar] [CrossRef]
- Loveday, B.K. The use of oxygen in high pressure acid leaching of nickel laterites. Miner. Eng. 2008, 21, 533–538. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, L.; Wang, H.; Cui, J.; Hao, J. Surfactant-assistant atmospheric acid leaching of laterite ore for the improvement of leaching efficiency of nickel and cobalt. J. Clean. Prod. 2019, 228, 1–7. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Hao, J.; Cui, J. Reinforcement of the two-stage leaching of laterite ores using surfactants. Front. Chem. Sci. Eng. 2021, 15, 562–570. [Google Scholar] [CrossRef]
- He, F.; Ma, B.; Qi, Z.; Wang, C.; Chen, Y.; Hu, X. Enhanced extraction of nickel from limonitic laterite via improved nitric acid pressure leaching process. Miner. Eng. 2023, 201, 108170. [Google Scholar] [CrossRef]
- Lakshmanan, V.I.; Sridhar, R.; DeLaat, R.; Chen, J.; Halim, M.A.; Roy, R. Extraction of nickel, cobalt and iron from laterite ores by mixed chloride leach process. In InNi–Co; Springer: Cham, Switzerland, 2013; pp. 97–106. [Google Scholar]
- Top, S.; Kusunoglu, S.; Ichlas, Z.T. Effects of leaching parameters on the dissolution of nickel, cobalt, manganese and iron from Caldag lateritic nickel ore in hydrochloric acid solution. Can. J. Metall. Mater. Sci. 2020, 59, 368–376. [Google Scholar] [CrossRef]
- Hosseini Nasab, M.; Noaparast, M.; Abdollahi, H. Dissolution of Nickel and Cobalt from Iron-Rich Laterite Ores Using Different Organic Acids. J. Min. Environ. (JME) 2020, 11, 779–797. [Google Scholar]
- Li, G.; Zhou, Q.; Zhu, Z.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Selective leaching of nickel and cobalt from limonitic laterite using phosphoric acid: An alternative for value-added processing of laterite. J. Clean. Prod. 2018, 189, 620–626. [Google Scholar] [CrossRef]
- Astuti, W.; Hirajima, T.; Sasaki, K.; Okibe, N. Comparison of effectiveness of citric acid and other acids in leaching of low-grade Indonesian saprolitic ores. Miner. Eng. 2015, 85, 1–16. [Google Scholar] [CrossRef]
- Astuti, W.; Nurjaman, F.; Mufakhir, F.R.; Sumardi, S.; Avista, D.; Wanta, K.C.; Petrus, H.T.B.M. A novel method: Nickel and cobalt extraction from citric acid leaching solution of nickel laterite ores using oxalate precipitation. Miner. Eng. 2023, 191, 107982. [Google Scholar] [CrossRef]
- Abdollahi, H.; Nasab, M.H.; Yadollahi, A. Bioleaching of Lateritic Nickel Ores. In Biotechnological Innovations in the Mineral-Metal Industry; Springer International Publishing: Cham, Switzerland, 2024; pp. 41–66. [Google Scholar]
- Le, L.; Tang, J.; Ryan, D.; Valix, M. Bioleaching nickel laterite ores using multi-metal tolerant Aspergillus foetidus organism. Miner. Eng. 2006, 19, 1259–1265. [Google Scholar] [CrossRef]
- Yang, Y.; Ferrier, J.; Csetenyi, L.; Gadd, G.M. Direct and indirect bioleaching of cobalt from low grade laterite and pyritic ores by Aspergillus niger. Geomicrobiol. J. 2019, 36, 940–949. [Google Scholar] [CrossRef]
- Nasab, M.H.; Noaparast, M.; Abdollahi, H.; Ali Amoozegar, M. Indirect bioleaching of Co and Ni from iron rich laterite ore, using metabolic carboxylic acids generated by P. putida, P. koreensis, P. bilaji and A. niger. Hydrometallurgy 2020, 193, 105309. [Google Scholar] [CrossRef]
- Carpen, H.L.; Giese, E.C. Enhancement of nickel laterite ore bioleaching by Burkholderia sp. using a factorial design. Appl. Water Sci. 2022, 12, 181. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries: Cobalt; U.S. Geological Survey: Reston, VA, USA, 2023.
- Fu, X.; Beatty, D.N.; Gaustad, G.G.; Ceder, G.; Roth, R.; Kirchain, R.E.; Bustamante, M.; Babbitt, C.; Olivetti, E.A. Perspectives on Cobalt Supply through 2030 in the Face of Changing Demand. Environ. Sci. Technol. 2020, 54, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Savinova, E.; Evans, C.; Lèbre, É.; Stringer, M.; Azadi, M.; Valenta, R.K. Will global cobalt supply meet demand? The geological, mineral processing, production and geographic risk profile of cobalt. Resour. Conserv. Recycl. 2023, 190, 106855. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, G.K.; Tormane, T. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Picazo-Rodriguez, N.; Toro, N.; Roman, M.; Soriano, D.; Madrid, F.; Jamett, J.; Galvez, E.; Cedillos, J. Cobalt metal: Overview of deposits, reserves, processings, and recycling. Preprints 2023, 2023, 061368. [Google Scholar]
- Huang, Y.; Chen, P.; Shu, X.; Fu, B.; Peng, W.; Liu, J.; Cao, Y.; Zhu, X. Extraction and recycling technologies of cobalt from primary and secondary resources. Int. J. Miner. Metall. Mater. 2024, 31, 628. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Miner. Eng. 2019, 138, 246–256. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Mineralogical Prediction of Flotation Performance for a Sediment-Hosted Copper–Cobalt Sulphide Ore. Minerals 2020, 10, 474. [Google Scholar] [CrossRef]
- Fisher, K.G.; Treadgold, L.G. Design Considerations for the Cobalt Recovery Circuit of the KOL (KOV) Copper/Cobalt Refinery, DRC. In Proceedings of the ALTA Nickel-Cobalt Conference, Perth, Australia, 25–30 May 2009; p. 16. [Google Scholar]
- Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G.; Crundwell, F.K.; Moats, M.S.; Ramachandran, V.; Robinson, T.G.; Davenport, W.G. Production of Cobalt from the Copper–Cobalt Ores of the Central African Copperbelt. In Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011; pp. 377–391. [Google Scholar]
- Ou, L.M.; Yin, B.Y. A Flotation Technique for a Sulfide-Oxidized Cu-Co Mixed Ore. Adv. Mater. Res. 2011, 402, 564–571. [Google Scholar] [CrossRef]
- Dehaine, Q.; Filippov, L.O.; Filippova, I.V.; Tijsseling, L.T.; Glass, H.J. Novel approach for processing complex carbonate-rich copper-cobalt mixed ores via reverse flotation. Miner. Eng. 2021, 161, 106710. [Google Scholar] [CrossRef]
- Swartz, B.; Donegan, S.; Amos, S.R. Processing considerations for cobalt recovery from Congolese copperbelt ores. In Hydrometallurgy Conference; The Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2009; pp. 385–400. [Google Scholar]
- Oraby, E.; Deng, Z.; Li, H.; Eksteen, J. Selective extraction of nickel and cobalt from disseminated sulfide flotation cleaner tailings using alkaline glycine-ammonia leaching solutions. Miner. Eng. 2023, 204, 108418. [Google Scholar] [CrossRef]
- Shengo, M.L.; Kime, M.-B.; Mambwe, M.P.; Nyembo, T.K. A review of the beneficiation of copper-cobalt-bearing minerals in the Democratic Republic of Congo. J. Sustain. Min. 2019, 18, 226–246. [Google Scholar] [CrossRef]
- Stuurman, S.; Ndlovu, S.; Sibanda, V. Comparing the extent of the dissolution of copper-cobalt ores from the DRC Region. Inst. Min. Metall. 2014, 114, 347–349. [Google Scholar]
- Morcali, M.H.; Khajavi, L.T.; Dreisinger, D.B. Extraction of nickel and cobalt from nickeliferous limonitic laterite ore using borax containing slags. Int. J. Miner. Process. 2017, 167, 27–34. [Google Scholar] [CrossRef]
- Dong, J.; Wei, Y.; Zhou, S.; Li, B.; Yang, Y.; Mclean, A. The Effect of Additives on Extraction of Ni, Fe and Cofrom Nickel Laterite Ores. JOM 2018, 70, 2365. [Google Scholar] [CrossRef]
- Peek, E.; Åkre, T.; Asselin, E. Technical and business considerations of cobalt hydrometallurgy. Sustain. Process. 2009, 61, 43–53. [Google Scholar] [CrossRef]
- Preston, J.S. Solvent extraction of cobalt and nickel by organophosphorus acids I. Comparison of phosphoric, phosphonic and phosphonic acid systems. Hydrometallurgy 1982, 9, 115133. [Google Scholar] [CrossRef]
- Sole, K.C. The Evolution of Cobalt–Nickel Separation and Purification Technologies: Fifty Years of Solvent Extraction and Ion Exchange. In Extraction 2018 Conference; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Flett, D.S. Cobalt-Nickel separation in hydrometallurgy: A review. Chem. Sustain. Dev. 2004, 12, 81–91. [Google Scholar]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 24262438. [Google Scholar] [CrossRef]
- Gupta, B.; Mudhar, N.; Singh, I. Separations and recovery of indium and gallium using bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272). Sep. Purif. Technol. 2007, 57, 294303. [Google Scholar] [CrossRef]
- Kurunoglu, S.; Kaya, M. Hydrometallurgical processing of Nickel laterites—A brief overview on the use of solvent extraction and Nickel/Cobalt Project for the separation and purification of nickle and cobalt. Mining 2019, 58, 131–144. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching, and separation: A comprehensive review. Hydrometallurgy 2014, 15, 192–208. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium resources and production: Critical assessment and global projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Kundu, T.; Rath, S.S.; Kanta Das, S.; Parhi, P.K.; Angadi, S.I. Recovery of lithium from spodumene-bearing pegmatites: A comprehensive review on geological reserves, beneficiation, and extraction. Powder Technol. 2023, 415, 118142. [Google Scholar] [CrossRef]
- Norman, J.; Gieseke, E.W. Beneficiation of Spodumene Rock by froth flotation. Trans. Am. Inst. Min. Metall. Eng. Min. Pract. 1940, 1, 347–355. [Google Scholar]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Yu, F.; Lu, D.; Wang, L.; Zhao, Y.; Zheng, H. Improving spodumene flotation using a mixed cationic and anionic collector. Physicochem. Prob. Miner. Process. 2018, 54, 567–577. [Google Scholar]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid-molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Huang, Y. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Filippov, L.O.; Filippova, I.V.; Crumiere, G.; Sousa, R.; Leite, M.M.; de Sousa, A.B.; Korbel, C.; Tripathy, S.K. Separation of lepidolite from hard-rock pegmatite ore via dry processing and flotation. Miner. Eng. 2022, 187, 107768. [Google Scholar] [CrossRef]
- Korbel, C.; Filippova, I.V.; Filippov, L.O. Froth flotation of lithium micas—A review. Miner. Eng. 2023, 192, 107986. [Google Scholar] [CrossRef]
- Liu, K. Research progress in flotation collectors for lepodolite monierals: An overview. Miner. Process. Extr. Metall. Rev. 2023, 1–15. [Google Scholar] [CrossRef]
- Vieceli, N.; Durão, F.O.; Guimarães, C.; Nogueira, C.A.; Pereira, M.F.C.; Margarido, F. Kinetic approach to the study of froth flotation applied to a lepidolite ore. Int. J. Miner. Metall. Mater. 2016, 23, 731. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Chen, C.; Hu, Y.; Yu, X.; He, G.; Fu, W. Froth flotation separation of lepidolite ore using a new Gemini surfactant as the flotation collector. Sep. Purif. Technol. 2022, 282, 119122. [Google Scholar] [CrossRef]
- Li, J.; Nie, G.; Li, J.; Zhu, Z.; Wang, Z. Flotation separation of quartz and dolomite from collophane using sodium N-dodecyl-β-amino propionate and its adsorption mechanism. Colloids Surf. A 2022, 641, 128586. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Li, W.; Song, Y.; Xu, W.; Li, K.; Zhang, Y. Mechanism of Modified Ether Amine Agents in Petalite and Quartz Flotation Systems under Weak Alkaline Conditions. Minerals 2023, 13, 825. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Sitando, O.; Crouse, P.L. Processing of a Zimbabwean petalite to obtain lithium carbonate. Int. J. Miner. Process. 2012, 102, 45–50. [Google Scholar] [CrossRef]
- Rioyo, J.; Tuset, S.; Grau, R. Lithium Extraction from spodumene by traditional sulfuric acid process: A review. Miner. Process. Extr. Metall. Rev. 2020, 43, 97–106. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market. Resources and Processe. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Karrecha, A.; Azadia, M.R.; Elchalakania, M.; Shahinb, M.A.; Seibic, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Han, S.; Sagzhanov, D.; Pan, J.; Hassas, B.V.; Rezaee, M.; Akbari, H.; Mensah-Biney, R. Direct Extraction of Lithium from α-Spodumene by Salt Roasting−Leaching Process. ACS Sustain. Chem. Eng. 2022, 10, 13495–13504. [Google Scholar] [CrossRef]
- Maliachova, K.; Doukas, N.; Tsakiri, D.; Samouhos, M.; Sakellariou, L.; Douni, I.; Taxiarchou, M.; Paspaliaris, I. Li Extraction from a-Spodumene Concentrate via Carbonizing Calcination. Mater. Proc. 2023, 15, 62. [Google Scholar] [CrossRef]
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium extraction from hard rock lithium ores (spodumene, lepidolite, zinnwaldite, petalite): Technology, resources, environment and cost. China Geol. 2023, 6, 137–153. [Google Scholar]
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Cheng, H. Recent advances in lithium extraction from lithium-bearing clay minerals. Hydrometallurgy 2023, 217, 106025. [Google Scholar] [CrossRef]
- Liu, J.-L.; Yin, Z.-L.; Li, X.-H.; Hu, Q.-Y.; Liu, W. Recovery of valuable metals from lepidolite by atmosphere leaching and kinetics on dissolution of lithium. Trans. Nonferrous Met. Soc. China 2019, 29, 641–649. [Google Scholar] [CrossRef]
- Liu, J.L.; Yin, Z.L.; Liu, W.; Li, X.H.; Hu, Q.Y. Treatment of aluminum and fluoride during hydrochloric acid leaching of lepidolite. Hydrometallurgy 2020, 191, 105222. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Guo, H.; Hu, Q.; Peng, W.; Wang, J. Extraction of valuable metals from lepidolite. Hydrometallurgy 2012, 117, 116–118. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; Ghahreman, A. Novel approaches for lithium extraction from salt-lake brines: A review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Dobson, P.F. Technology for the Recovery of Lithium from Geothermal Brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- Khalil, A.; Mohammed, S.; Hashaikeh, R.; Hilal, N. Lithium recovery from brine: Recent developments and challenges. Desalination 2022, 528, 115611. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, S.; Srinivasakannan, C.; Li, S.; Yin, S.; Zhang, L.; Jiang, X.; Zhou, G.; Zhang, N. Lithium extraction from salt lake brines with high magnesium/lithium ratio: A review. Environ. Chem. Lett. 2023, 21, 1611–1626. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.; Zhao, Z. Separating lithium and magnesium in brine by aluminum-based materials. Hydrometallurgy 2018, 176, 73–77. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Lithium recovery from salt lake brine by counter-current extraction using tributyl phosphate/FeCl3 in methyl isobutyl ketone. Hydrometallurgy 2017, 171, 27–32. [Google Scholar] [CrossRef]
- Song, J.; Huang, T.; Qiu, H.; Li, X.M.; He, T. Recovery of lithium from salt lake brine of high Mg/Li ratio using Na [FeCl4.2TBP] as extractant: Thermodynamics, kinetics and processes. Hydrometallurgy 2017, 173, 63–70. [Google Scholar] [CrossRef]
- Yang, F.; Chen, S.; Shi, C.; Xue, F.; Zhang, X.; Ju, S.; Xing, W. A Facile Synthesis of Hexagonal Spinel λ-MnO2 Ion-Sieves for Highly Selective Li+ Adsorption. Processes 2018, 6, 59. [Google Scholar] [CrossRef]
- Gao, A.; Sun, Z.; Li, S.; Hou, X.; Li, H.; Wu, Q.; Xi, X. The mechanism of manganese dissolution on Li1.6Mn1.6O4 ion sieves with HCl. Dalton Trans. 2018, 47, 3864–3871. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Soo, J.; Jo, K.; Kim, S.; Sung, Y.; Yoon, J. Lithium recovery from brine using a β-MnO2/activated carbon hybrid supercapacitor system. Chemosphere 2015, 125, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lawagon, C.P.; Nisola, G.M.; Cuevas, R.A.I.; Kim, H.; Lee, S.P.; Chung, W.J. Li1−xNi0.33Co1/3Mn1/3O2/Ag for electrochemical lithium recovery from brine. Chem. Eng. J. 2018, 348, 1000–1011. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Luo, Y.; Liu, X.; Zhao, Z. New insights into the application of lithium-ion battery materials: Selective extraction of lithium from brines via a rocking-chair lithium-ion battery system. Glob. Chall. 2018, 2, 1700079. [Google Scholar] [CrossRef]
- Atta Mends, E.L.; Chu, P. Lithium extraction from unconventional aqueous resources—A review on recent technological development for seawater and geothermal brines. J. Environ. Chem. Eng. 2023, 11, 110710. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Alan Hatton, T.; Lienhard, V. Lithium Recovery from Oil and Gas Produced Water: A Need for a Growing Energy Industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Z.; Qin, X.; Gao, X.; Wang, M.; Xian, X. Recent advances in lithium extraction from salt lake brine using coupled and tandem technologies. Desalination 2023, 547, 116225. [Google Scholar] [CrossRef]
- Miller, K.N.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 2018, 4, 418. [Google Scholar] [CrossRef]
- Hein, J.; Mizell, K.; Koschinsky, A.; Conrad, T. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium Metal Extraction from Seawater. Joule 2018, 2, 1648–1651. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ concentration aqueous solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- European Consortium Launches Blue Nodules Project. Press Release. 2020. Available online: https://www.mining-technology.com/contractors/data//pressreleases/pressblue-nodules-project/ (accessed on 4 August 2024).
- Brooks, C.S. Metal Recovery from Industrial Waste; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Cui, F.; Wang, G.; Yu, D.; Gan, X.; Tian, Q.; Guo, X. Towards “zero waste” extraction of nickel from scrap nickel-based superalloy using magnesium. J. Clean. Prod. 2020, 262, 121275. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.C.; Park, H.S.; Jun, M.J.; Kim, B.S. A multistep leaching of nickel-based superalloy scrap for selective dissolution of its constituent metals in hydrochloric acid solutions. Hydrometallurgy 2018, 176, 235–242. [Google Scholar] [CrossRef]
- Mamo, S.K.; Elie, M.; Baron, M.G.; Simons, A.M.; Gonzalez-Rodriguez, J. Leaching kinetics, separation, and recovery of rhenium and component metals from CMSX-4 superalloys using hydrometallurgical processes. Separ. Purif. Technol. 2019, 212, 150–160. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Yin, J.; Xia, W.; Yuan, X.; Xiang, X. Leaching kinetics of cobalt from the scraps of spent aerospace magnetic materials. Waste Manag. 2018, 76, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Pilone, D. Ni–MH spent batteries: A raw material to produce Ni–Co alloys. Waste Manag. 2002, 22, 871–874. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling─Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A comprehensive review and classification of unit operations with assessment of outputs quality in lithium-ion battery recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Niu, B.; Xu, Z.; Xiao, J.; Qin, Y. Recycling Hazardous and Valuable Electrolyte in Spent Lithium-Ion Batteries: Urgency, Progress, Challenge, and Viable Approach. Chem. Rev. 2023, 123, 8718–8735. [Google Scholar] [CrossRef]
- Mazurek, K.; Weidner, E.; Drużyński, S.; Ciesielczyk, F.; Kiełkowska, U.; Wróbel-Kaszanek, A.; Jesionowski, T. Lanthanum enriched TiO2-ZrO2 hybrid material with tailored physicochemical properties dedicated to separation of lithium and cobalt(II) raising from the hydrometallurgical stage of the recycling process of lithium-ion batteries. Hydrometallurgy 2020, 197, 105448. [Google Scholar] [CrossRef]
- Takano, M.; Asano, S.; Goto, M. Recovery of nickel, cobalt and rare-earth elements from spent nickel–metal-hydride battery: Laboratory tests and pilot trials. Hydrometallurgy 2022, 209, 105826. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Shang, Z.; Zhang, Y.; Xu, S. A review on comprehensive recycling of spent power lithium-ion battery in China. eTransportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Bruno, M.; Fiore, S. Material Flow Analysis of Lithium-Ion Battery Recycling in Europe: Environmental and Economic Implications. Batteries 2023, 9, 231. [Google Scholar] [CrossRef]
- Meng, K.; Xu, G.; Peng, X.; Youcef-Toumi, K.; Li, J. Intelligent disassembly of electric-vehicle batteries: A forward-looking overview. Resour. Conserv. Recycl. 2022, 182, 106207. [Google Scholar] [CrossRef]
- Zorn, M.; Ionescu, C.; Klohs, D.; Zähl, K.; Kisseler, N.; Daldrup, A.; Hams, S.; Zheng, Y.; Offermanns, C.; Flamme, S.; et al. An Approach for Automated Disassembly of Lithium-Ion Battery Packs and High-Quality Recycling Using ComputerVision, Labeling, and Material Characterization. Recycling 2022, 7, 48. [Google Scholar] [CrossRef]
- Ueda, T.; Koyanaka, S.; Oki, T. In-line sorting system with battery detection capabilities in e-waste using combination of X-ray transmission scanning and deep learning. Resources. Conserv. Recycl. 2024, 201, 107345. [Google Scholar] [CrossRef]
- Dunn, J.; Kendall, A.; Slattery, M. Electric vehicle lithium-ion battery recycled content standards for the US—Targets, costs, and environmental impacts. Resour. Conserv. Recycl. 2022, 185, 106488. [Google Scholar] [CrossRef]
- Gonzales-Calienes, G.; Kannangara, M.; Bensebaa, F. Economic and Environmental Viability of Lithium-Ion Battery Recycling—Case Study in Two Canadian Regions with Different Energy Mixes. Batteries 2023, 9, 375. [Google Scholar] [CrossRef]
- Kala, S.; Mishra, A. Battery recycling opportunity and challenges in India. Mater. Today Proc. 2021, 46, 1543–1556. [Google Scholar] [CrossRef]
- Gericke, M.; Nyanjowa, W.; Robertson, S. Technology Landscape Report and Business Case for the Recycling of Li-Ion Batteries in South Africa; Mintek Report; Mintek: Johannesburg, South Africa, 2021. [Google Scholar]
- Hernández, L.; Hilbert, I.; Castillero, L.G.; Manhart, A.; García, D.; Nkongdem, B.; Dumitrescu, R.; Sucre, C.G.; Herrera, C.F. Recycling and Reuse of Lithium Batteries in Latin America and the Caribbean Analytical Review of Global and Regional Practices; Viviana, Inter-American Development Bank, Technical Note No IDB-TN-2893; Infrastructure and Energy Department: Washington, DC, USA, 2024.
- Paulikas, D.; Katona, S.; Ilves, E.; Ali, S.H. Life cycle climate change impacts of producing battery metals from land ores versus deep-sea polymetallic nodules. J. Clean. Prod. 2020, 275, 123822. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Wei, W.; Samuelsson, P.B.; Tilliander, A.; Gyllenram, R.; Jonsson, P.G. Energy Consumption and Greenhouse Gas Emissions of Nickel Products. Energies 2020, 13, 5664. [Google Scholar] [CrossRef]
- Sovacool, B.K. When subterranean slavery supports sustainability transitions? power, patriarchy, and child labor in artisanal Congolese cobalt mining. Extr. Ind. Soc. 2021, 8, 271–293. [Google Scholar] [CrossRef]
- Chordia, M.; Wickerts, S.; Nordelöf, A.; Arvidsson, R. Life cycle environmental impacts of current and future battery-grade lithium supply from brine and spodumene. Resour. Conserv. Recycl. 2022, 187, 106634. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- Sharma, S.S.; Manthiram, A. Towards more environmentally and socially responsible batteries. Energy Environ. Sci. 2020, 13, 4087–4097. [Google Scholar] [CrossRef]


| Methods | Property and Facility | Example Results |
|---|---|---|
| Tradition [34] | Size, screening | 0.54% to 1.1% at 62% recovery [35] |
| Gravity [36] | Density, centrifugation or dense media | 1.1% Ni to 1.5% with 70% recovery using Falcon [37] 0.43% to 0.66% with 87% recovery using DMS [38] |
| Magnetic [39] | Magnetism, magnetic field w/o roasting oven | 0.89% to 1.08% with 57% recovery [34] laterite ore Ni 0.72%, Co 0.029%, Fe 8.65%; ferronickel 16.16% Ni, 73.67% Fe with 90% Ni recovery [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalungi, P.; Yao, Z.; Huang, H. Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production. Materials 2024, 17, 4389. https://doi.org/10.3390/ma17174389
Kalungi P, Yao Z, Huang H. Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production. Materials. 2024; 17(17):4389. https://doi.org/10.3390/ma17174389
Chicago/Turabian StyleKalungi, Paul, Zhuo Yao, and Hong Huang. 2024. "Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production" Materials 17, no. 17: 4389. https://doi.org/10.3390/ma17174389
APA StyleKalungi, P., Yao, Z., & Huang, H. (2024). Aspects of Nickel, Cobalt and Lithium, the Three Key Elements for Li-Ion Batteries: An Overview on Resources, Demands, and Production. Materials, 17(17), 4389. https://doi.org/10.3390/ma17174389








