Unraveling Asymmetric Electrochemical Kinetics in Low-Mass-Loading LiNi1/3Mn1/3Co1/3O2 (NMC111) Li-Metal All-Solid-State Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of a Low-Mass-Loading NMC111 ASSB
2.2. Fabrication of LATP Solid Electrolyte Pellet
2.3. Electrochemical and Structural Characterizations
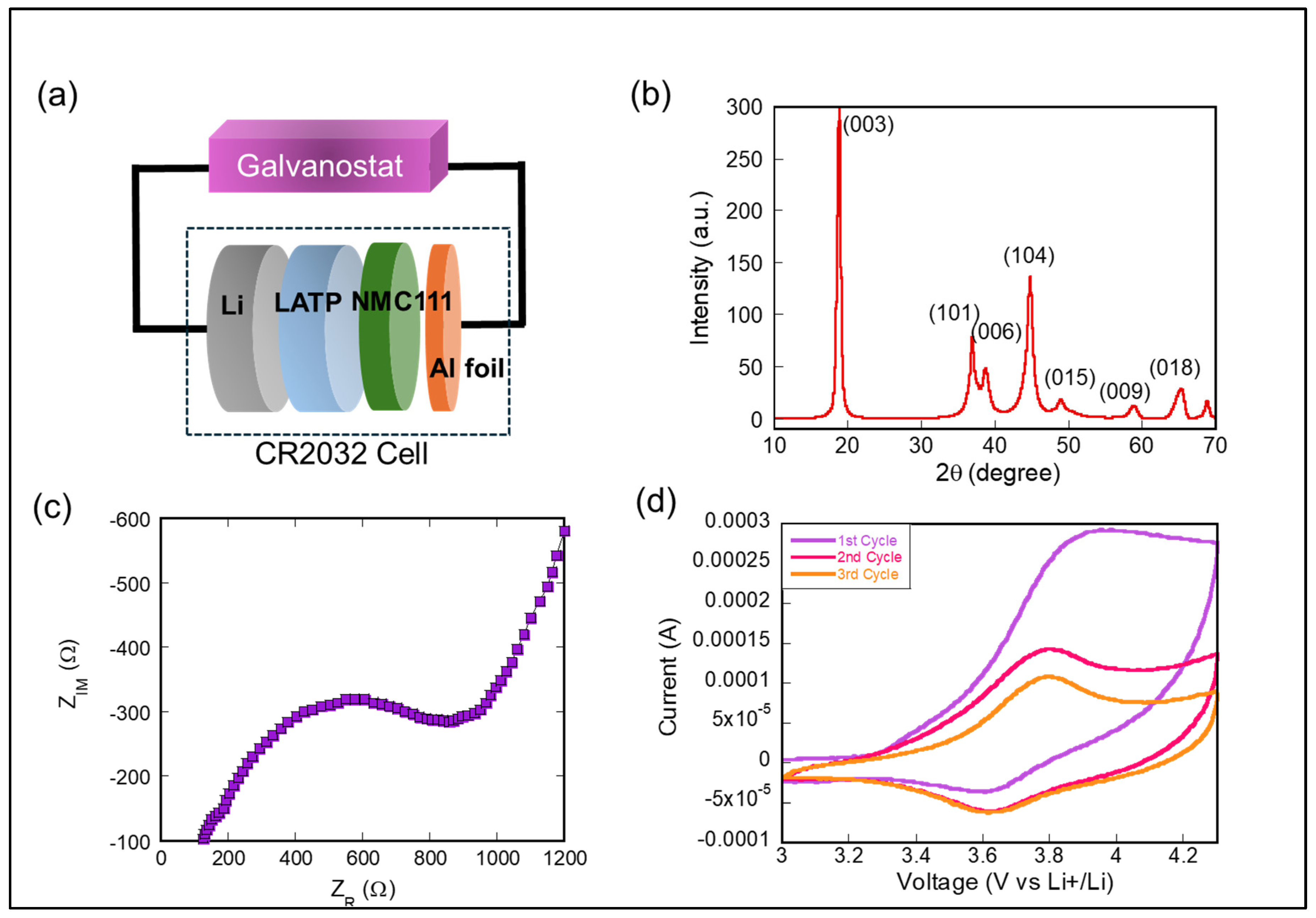
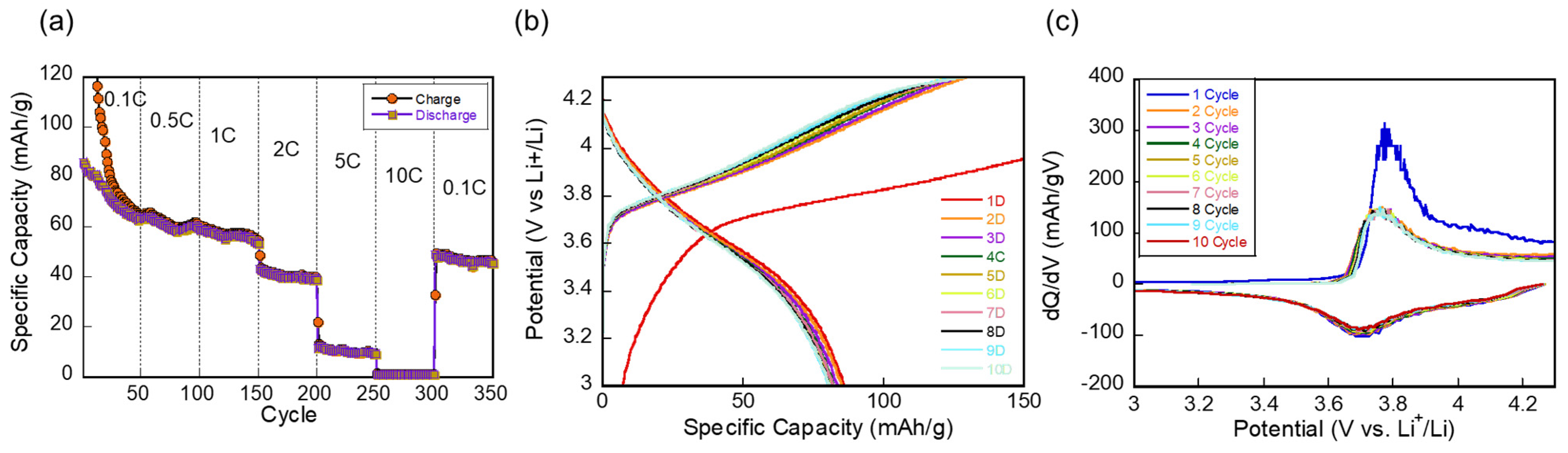
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Chem. Soc. Rev. 2020, 49, 5407–5445. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.P.; McShane, E.J.; Colclasure, A.M.; Balsara, N.; Brown, D.E.; Cao, C.; Chen, B.R.; Chinnam, P.R.; Cui, Y.; Dufek, E.J. A review of existing and emerging methods for lithium detection and characterization in Li-ion and Li-metal batteries. Adv. Energy Mater. 2021, 11, 2100372. [Google Scholar] [CrossRef]
- Duan, H.; Yin, Y.-X.; Shi, Y.; Wang, P.-F.; Zhang, X.-D.; Yang, C.-P.; Shi, J.-L.; Wen, R.; Guo, Y.-G.; Wan, L.-J. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. J. Am. Chem. Soc. 2018, 140, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierarchical structuring of NMC111-cathode materials in lithium-ion batteries: An in-depth study on the influence of primary and secondary particle sizes on electrochemical performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Pang, Y.; Pan, J.; Yang, J.; Zheng, S.; Wang, C. Electrolyte/electrode interfaces in all-solid-state lithium batteries: A review. Electrochem. Energy Rev. 2021, 4, 169–193. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; Eck, E.R.v.; Wang, H.; Basak, S.; Li, Z.; Wagemaker, M. Accessing the bottleneck in all-solid state batteries, lithium-ion transport over the solid-electrolyte-electrode interface. Nat. Commun. 2017, 8, 1086. [Google Scholar] [CrossRef]
- Zahiri, B.; Patra, A.; Kiggins, C.; Yong, A.X.B.; Ertekin, E.; Cook, J.B.; Braun, P.V. Revealing the role of the cathode–electrolyte interface on solid-state batteries. Nat. Mater. 2021, 20, 1392–1400. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-solid-state garnet type sulfurized polyacrylonitrile/lithium-metal battery enabled by an inorganic lithium conductive salt and a bilayer electrolyte architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Z.; Du, P.; Su, C. Carbon nanomaterials used as conductive additives in lithium ion batteries. Recent Pat. Nanotechnol. 2010, 4, 100–110. [Google Scholar] [CrossRef]
- Ke, L.; Lv, W.; Su, F.-Y.; He, Y.-B.; You, C.-H.; Li, B.; Li, Z.; Yang, Q.-H.; Kang, F. Electrode thickness control: Precondition for quite different functions of graphene conductive additives in LiFePO4 electrode. Carbon 2015, 92, 311–317. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-F.; Song, K.-Y.; Joo, S.-K. Ultra-thick Li-ion battery electrodes using different cell size of metal foam current collectors. Rsc Adv. 2015, 5, 16702–16706. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2019, 4, 45–60. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, Y. 3D nanostructures for the next generation of high-performance nanodevices for electrochemical energy conversion and storage. Adv. Energy Mater. 2020, 10, 2001460. [Google Scholar] [CrossRef]
- Ni, W.; Xue, Y.; Zang, X.; Li, C.; Wang, H.; Yang, Z.; Yan, Y.-M. Fluorine doped cagelike carbon electrocatalyst: An insight into the structure-enhanced CO selectivity for CO2 reduction at high overpotential. ACS Nano 2020, 14, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.-N. Inducing and Understanding Pseudocapacitive Behavior in an Electrophoretically Deposited Lithium Iron Phosphate Li-Metal Battery as an Electrochemical Test Platform. J. Phys. Chem. Lett. 2024, 15, 7095–7102. [Google Scholar] [CrossRef]
- Hong, J.; Park, B. Additive-free Electrophoretic-deposited Ti3AlC2 MAX phase Li-ion battery anode. Mater. Lett. 2023, 330, 133227. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Broughton, J.; Brett, M. Investigation of thin sputtered Mn films for electrochemical capacitors. Electrochim. Acta 2004, 49, 4439–4446. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Ullah, N.; Hai, A.; Muhammad, A.; Younas, M.; Rezakazemi, M. Synergistic properties of molybdenum disulfide (MoS2) with electro-active materials for high-performance supercapacitors. Int. J. Hydrogen Energy 2019, 44, 17470–17492. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Qiu, J. Toward commercial-level mass-loading electrodes for supercapacitors: Opportunities, challenges and perspectives. Energy Environ. Sci. 2021, 14, 576–601. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, R.; Fu, J.; Liu, X.; Yang, H.; Wang, D.; Xu, X.; Cao, J.; Wen, G.; Wang, D. Mass Loading-Independent Lithium Storage of Transitional Metal Compounds Achieved by Multi-Dimensional Synergistic Nanoarchitecture. Small 2023, 19, 2303019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, T.-T.; Popovic, J.; Lim, K.; Yin, Y.-X.; Maier, J.; Guo, Y.-G. Towards better Li metal anodes: Challenges and strategies. Mater. Today 2020, 33, 56–74. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Lee, S.J.; Seo, S.; Ryu, K.; Kim, C.H.; Choi, J.W. Lithium-metal batteries: From fundamental research to industrialization. Adv. Mater. 2023, 35, 2206625. [Google Scholar] [CrossRef]
- Wang, J.; Ge, B.; Li, H.; Yang, M.; Wang, J.; Liu, D.; Fernandez, C.; Chen, X.; Peng, Q. Challenges and progresses of lithium-metal batteries. Chem. Eng. J. 2021, 420, 129739. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Bo, S.-H.; Kim, J.C.; Miara, L.J.; Ceder, G. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 2020, 5, 105–126. [Google Scholar] [CrossRef]
- Lim, H.-D.; Park, J.-H.; Shin, H.-J.; Jeong, J.; Kim, J.T.; Nam, K.-W.; Jung, H.-G.; Chung, K.Y. A review of challenges and issues concerning interfaces for all-solid-state batteries. Energy Storage Mater. 2020, 25, 224–250. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Miao, X.; Guan, S.; Ma, C.; Li, L.; Nan, C.W. Role of Interfaces in Solid-State Batteries. Adv. Mater. 2023, 35, 2206402. [Google Scholar] [CrossRef]
- Miao, X.; Wang, H.; Sun, R.; Wang, C.; Zhang, Z.; Li, Z.; Yin, L. Interface engineering of inorganic solid-state electrolytes for high-performance lithium metal batteries. Energy Environ. Sci. 2020, 13, 3780–3822. [Google Scholar] [CrossRef]
- Nie, K.; Hong, Y.; Qiu, J.; Li, Q.; Yu, X.; Li, H.; Chen, L. Interfaces between cathode and electrolyte in solid state lithium batteries: Challenges and perspectives. Front. Chem. 2018, 6, 616. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Yamamoto, K.; Hirayama, T.; Ouchi, S.; Igaki, E.; Saitoh, K. Direct observation of a Li-ionic space-charge layer formed at an electrode/solid-electrolyte interface. Angew. Chem. 2019, 131, 5346–5350. [Google Scholar] [CrossRef]
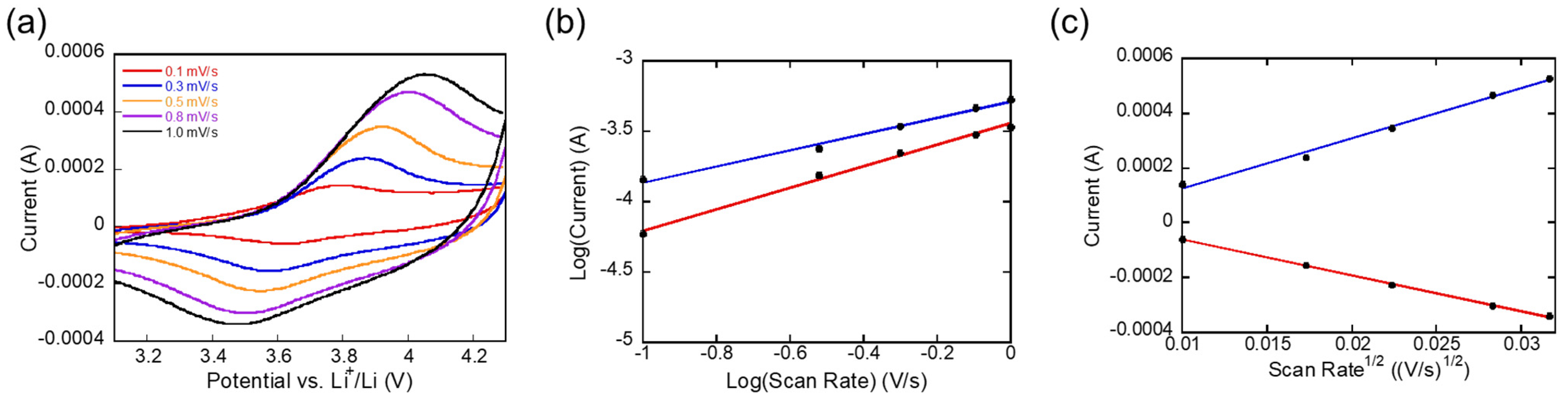

| b-Value | Diffusion Coefficient (cm2⋅s−1) | Fraction of Capacitive to Diffusive Charge Storage (1.0 mV/s) | Average Specific Capacity (mAh⋅g−1) (0.5 C) | Average Specific Capacity (mAh⋅g−1) (2 C) | Average Specific Capacity (mAh⋅g−1) (5 C) | ||
|---|---|---|---|---|---|---|---|
| With Liquid Electrolyte | Anodic | 0.70 | 1.64 × 10−8 | 1.78 | 61 | 40 | 11 |
| Cathodic | 0.77 | ||||||
| With Solid Electrolyte | Anodic | 0.76 | 1.50 × 10−9 | 1.38 | 90 | 54 | 28 |
| Cathodic | 0.58 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.-N. Unraveling Asymmetric Electrochemical Kinetics in Low-Mass-Loading LiNi1/3Mn1/3Co1/3O2 (NMC111) Li-Metal All-Solid-State Batteries. Materials 2024, 17, 5014. https://doi.org/10.3390/ma17205014
Park B-N. Unraveling Asymmetric Electrochemical Kinetics in Low-Mass-Loading LiNi1/3Mn1/3Co1/3O2 (NMC111) Li-Metal All-Solid-State Batteries. Materials. 2024; 17(20):5014. https://doi.org/10.3390/ma17205014
Chicago/Turabian StylePark, Byoung-Nam. 2024. "Unraveling Asymmetric Electrochemical Kinetics in Low-Mass-Loading LiNi1/3Mn1/3Co1/3O2 (NMC111) Li-Metal All-Solid-State Batteries" Materials 17, no. 20: 5014. https://doi.org/10.3390/ma17205014
APA StylePark, B.-N. (2024). Unraveling Asymmetric Electrochemical Kinetics in Low-Mass-Loading LiNi1/3Mn1/3Co1/3O2 (NMC111) Li-Metal All-Solid-State Batteries. Materials, 17(20), 5014. https://doi.org/10.3390/ma17205014






