Use of a Glaciogene Marine Clay (Ilulissat, Greenland) in a Pilot Production of Red Bricks
Abstract
1. Introduction
2. Materials and Methods
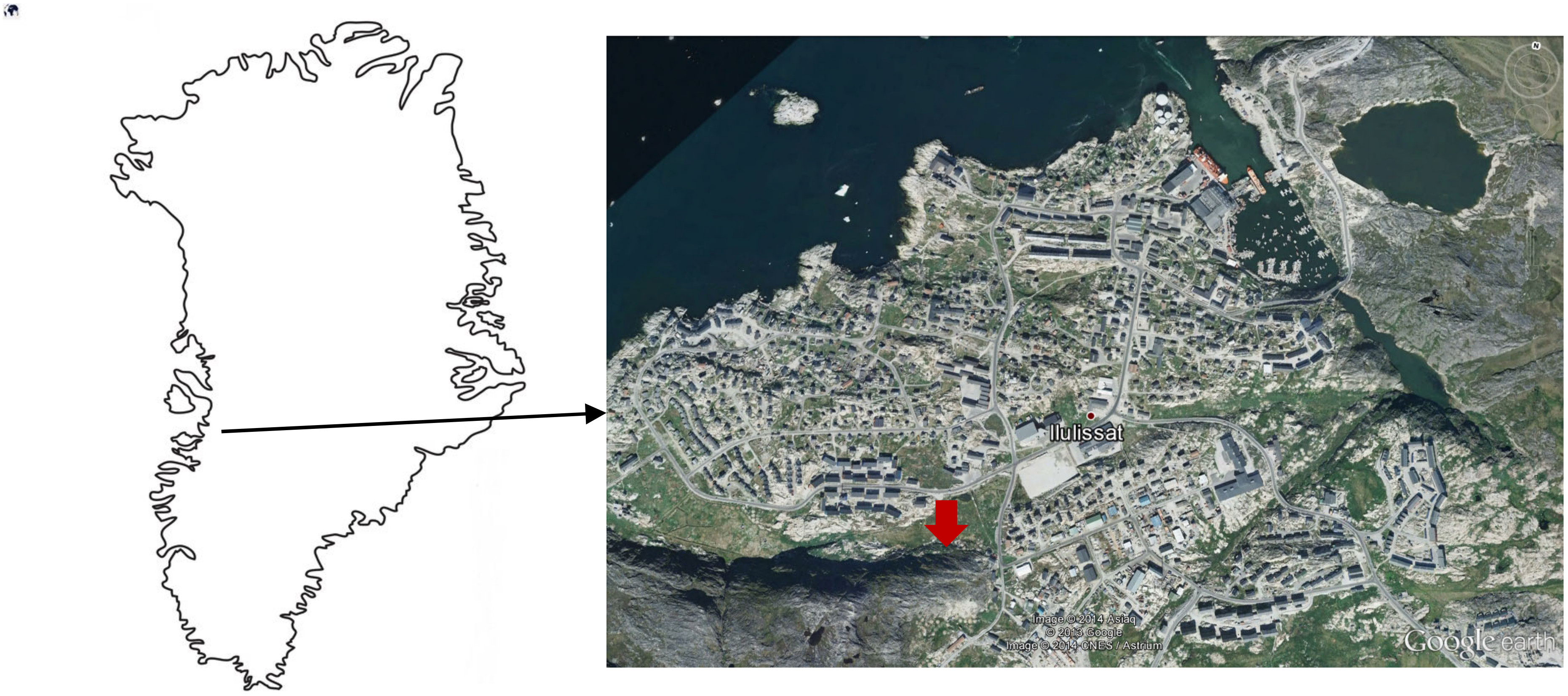
2.1. Materials and Sampling
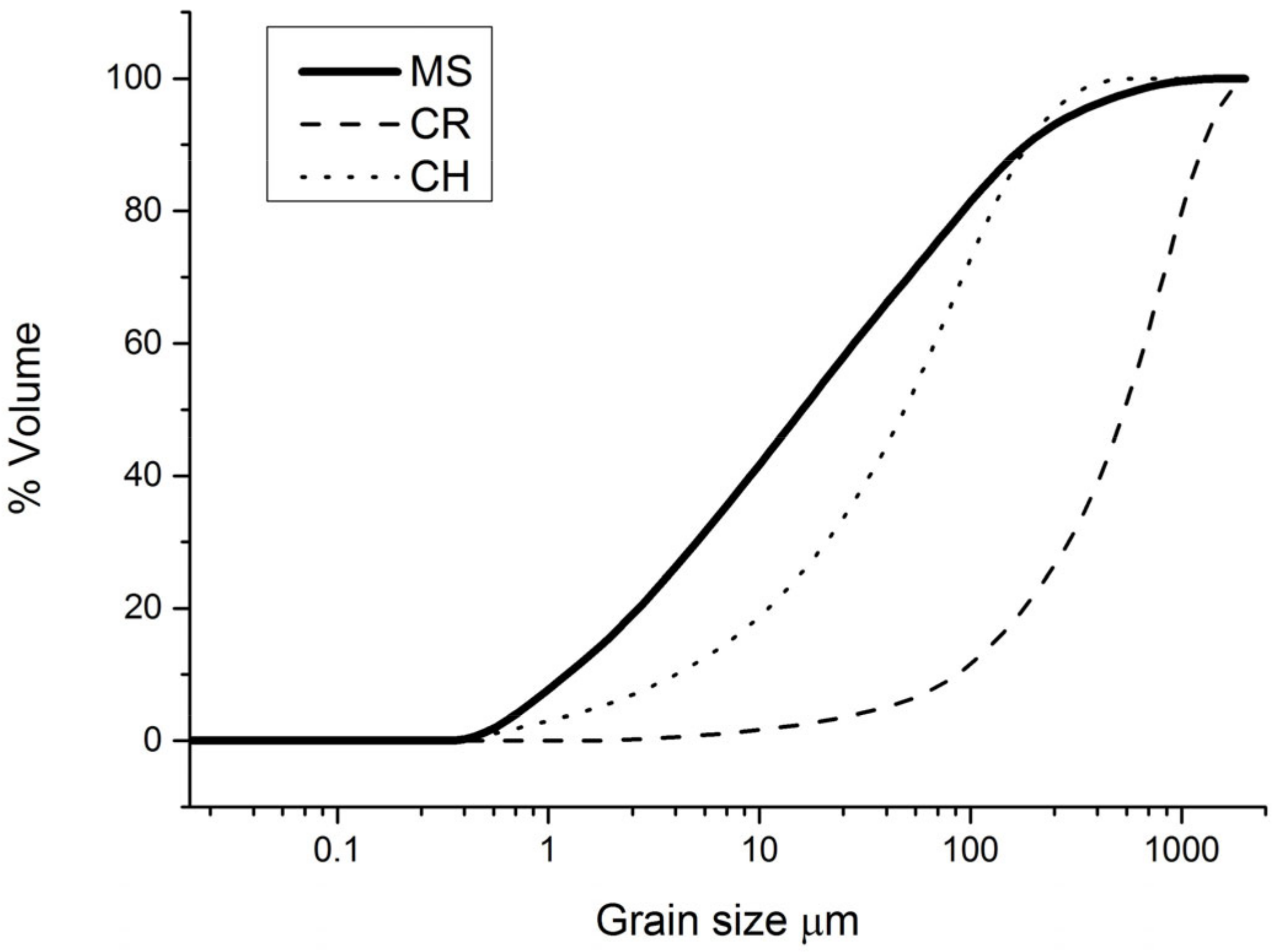
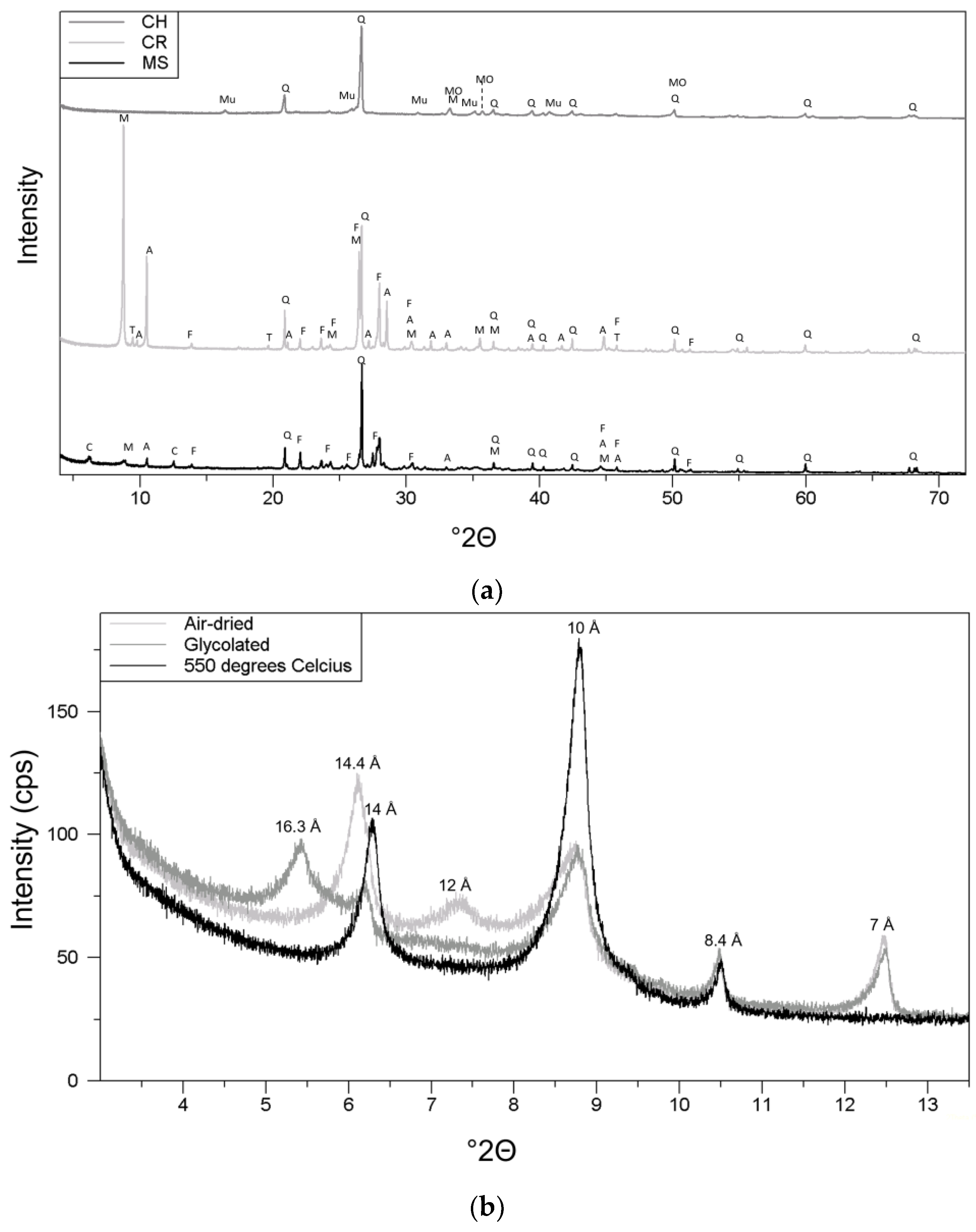
2.2. Characterization of Raw Material
2.3. Production of Fired Specimens
2.4. Characterization of Fired Specimens
3. Results
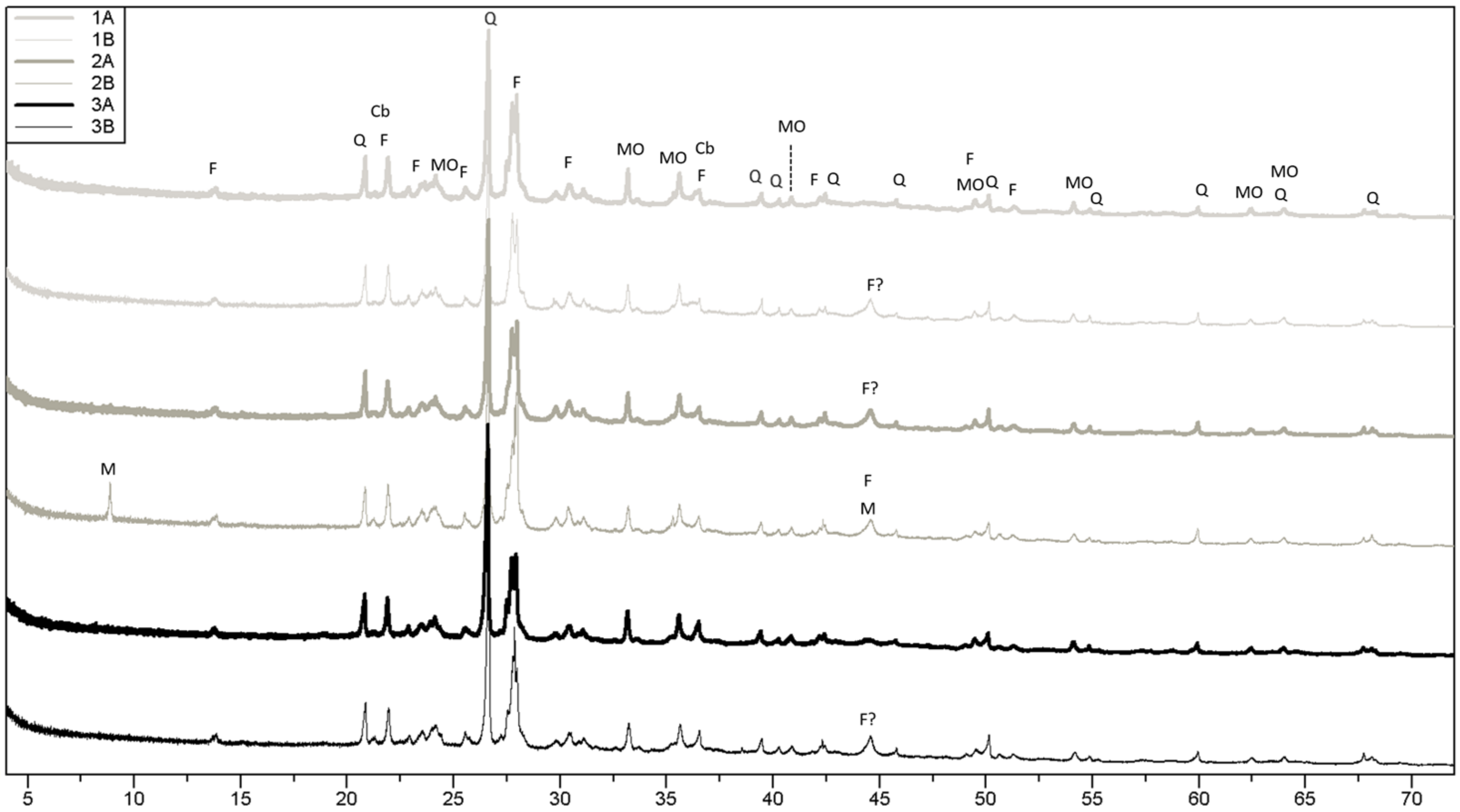
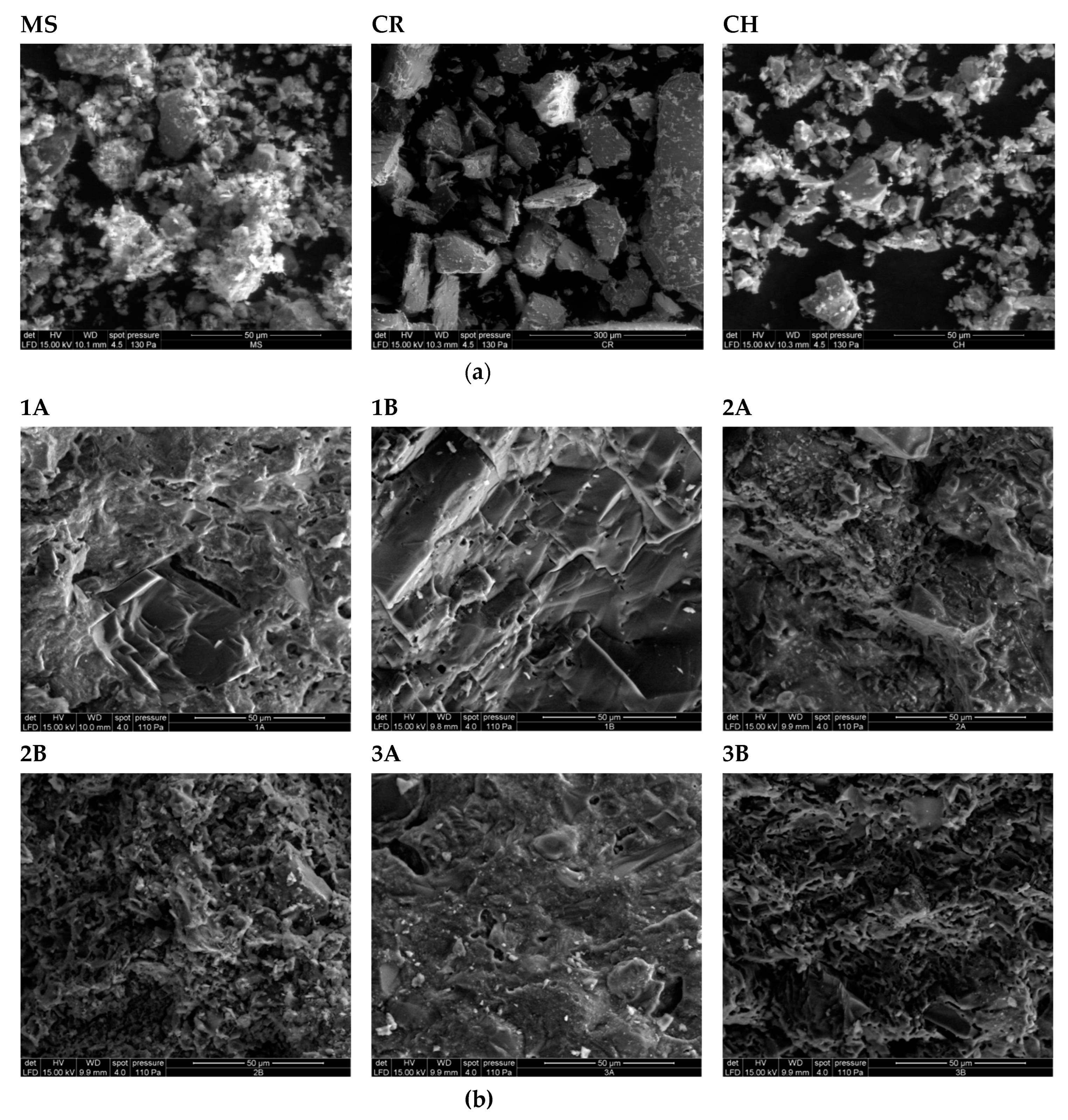
3.1. Raw Materials
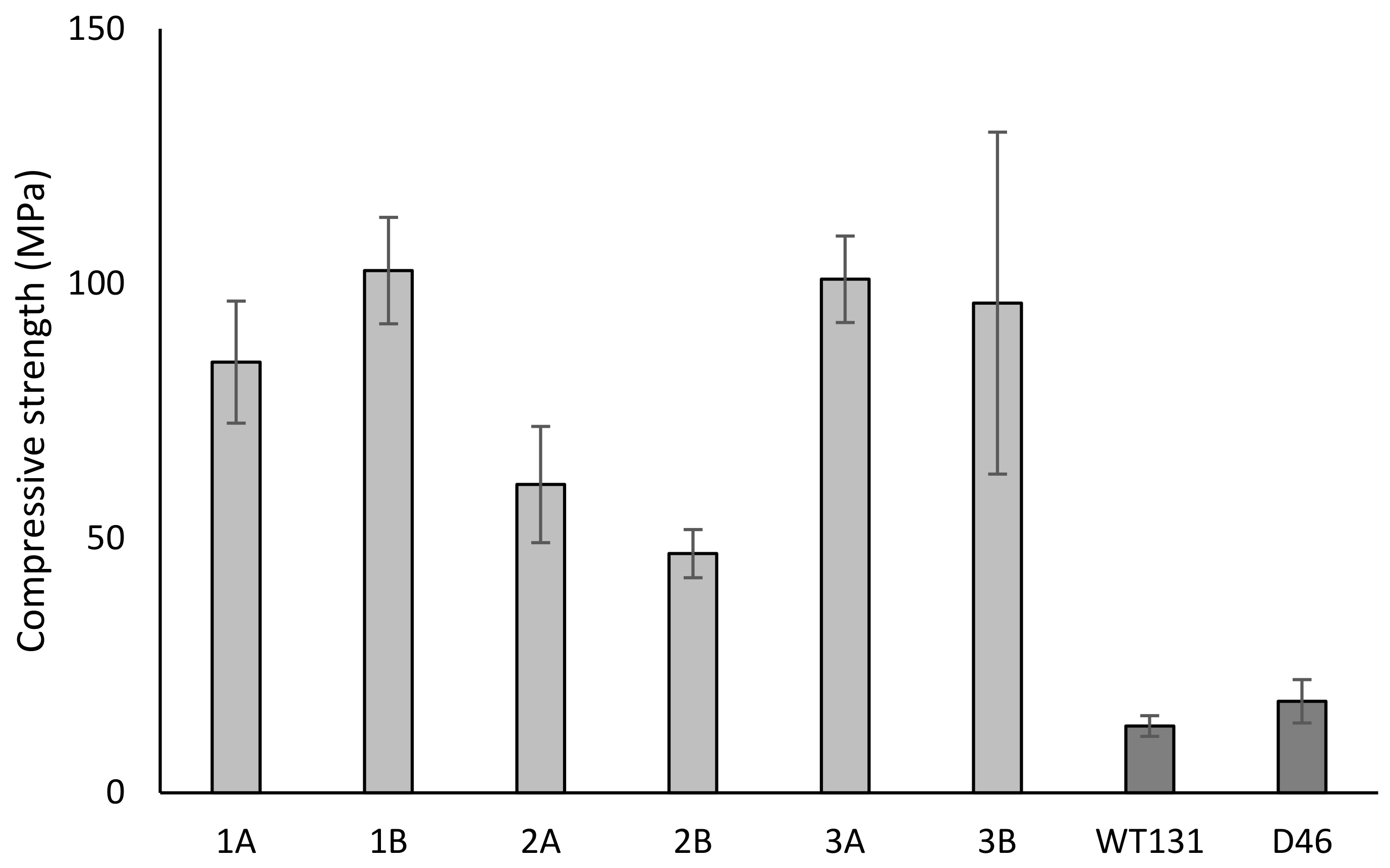
3.2. Fired Brick Specimens
4. Discussion
4.1. Raw Material Characterization
4.2. Influence of Production Settings
4.3. Shrinkage
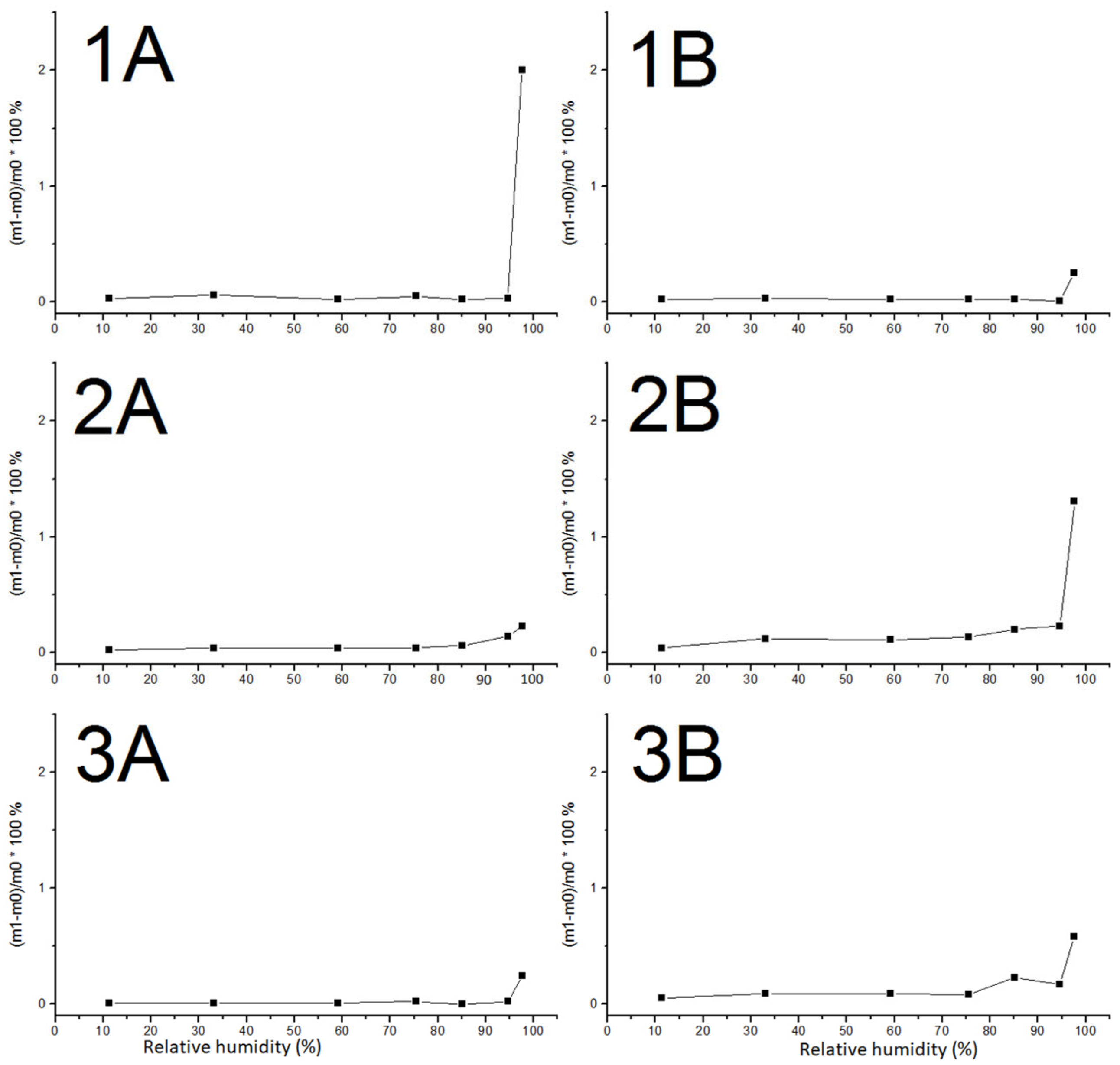
4.4. Initial Rate of Water Absorption and Hygroscopic Behavior
4.5. Degree of Sintering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foged, N. Ingeniørgeologiske Undersøgelser af Kvartære Marine Leraflejringer på Vestgrønland. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 1979. (In Danish). [Google Scholar]
- Gillott, J.E. Fabric, composition and properties of sensitive soils from Canada, Alaska and Norway. Eng. Geol. 1979, 14, 149–172. [Google Scholar] [CrossRef]
- Locat, J.; Lefebvre, G.; Ballivy, G. Mineralogy, chemistry, and physical properties interrelationships of some sensitive clays from Eastern Canada. Can. Geotech. J. 1984, 21, 530–540. [Google Scholar] [CrossRef]
- Ramesh, R.; d’Anglejan, B. Mineralogy, Chemistry and particle size interrelationships in some Post-Glacial Marine Deposits of the St. Lawrence Lowlands. J. Coast. Res. 1995, 11, 1167–1179. [Google Scholar]
- Roaldset, E. Mineralogy and geochemistry of Quaternary clays in the Numedal area, southern Norway. Nor. Geol. Tidsskr. 1972, 52, 335–369. [Google Scholar]
- Rosenqvist, I.T. Origin and mineralogy glacial and interglacial clays of southern Norway. Clays Clay Miner. 1975, 23, 153–159. [Google Scholar] [CrossRef]
- Bentley, S.P.; Smalley, I.J. Mineralogy of sensitive clays from Quebec. Can. Mineral. 1978, 16, 103–112. [Google Scholar]
- Pederstad, K.; Jørgensen, P. Weathering in a marine clay during postglacial time. Clay Miner. 1985, 20, 477–491. [Google Scholar] [CrossRef]
- Gunnarsen, K.C.; Jensen, L.S.; Rosing, M.T.; Dietzen, C. Greenlandic glacial rock flour improces crop yield in organic agricultural production. Nutr. Cycl. Agroecosystems 2023, 126, 51–66. [Google Scholar] [CrossRef]
- Belmonte, L.J.; Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Vestbø, A.P. Screening of heavy metal containing waste types for use as raw material in Arctic clay-based bricks. Environ. Sci. Pollut. Res. 2018, 25, 32831–32843. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, L.J.; Villumsen, A.; Ottosen, L.M.; Kirkelund, G.M. Use of clay from Kangerlussuaq in the Greenlandic construction industry. In Proceedings of the Rock Mechanics in the Nordic Countries, Kongsberg, Norway, 9–12 June 2010. [Google Scholar]
- Kalvig, P. Industrimineraler i Grønland—En Vurdering af Nogle Udnyttelsesmuligheder. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 1990. (In Danish). [Google Scholar]
- Hamer, K.; Karius, V. Brick production with dredged harbour sediments. An industrial-scale experiment. Waste Manag. 2002, 22, 521–530. [Google Scholar] [PubMed]
- Mezencevova, A.; Yeboah, N.N.; Burns, S.E.; Kahn, L.F.; Kurtis, K.E. Utilization of Savannah Harbor river sediments as the primary raw material in production of bricks. J. Environ. Manag. 2012, 113, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Andrés, A.; Alonso, R.; Viguri, J.; Rincón, J.M. Sintering behaviour of ceramic bodies from contaminated marine sediments. Ceram. Int. 2008, 34, 1917–1924. [Google Scholar] [CrossRef]
- Salim, W.S.W.; Sadikon, S.F.; Salleh, S.M.; Noor, N.A.M.; Arshad, M.F.; Wahid, N. Assessment of physical properties and chemical compositions of Kuala Perlis dredged marine sediment as a potential brick material. In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 509–512. [Google Scholar]
- Holmboe, T. Teglværksler i Danmark—Sammensætningen af Dansk Teglværksler, Hårdtbrændende Ler, Alternative Lertyper og Kortlægning Med Stang Slingram; Danmarks og Grønlands geologiske undersøgelse rapport 92; GEUS: Copenhagen, Denmark, 2001. (In Danish) [Google Scholar] [CrossRef]
- Overeem, I.; Hudson, B.; Syvitski, J.P.M.; Mikkelsen, A.B.; Hasholt, B.; Broeke, M.R.; Noël, B.P.Y.; Morlighem, M. Substantial export of suspended sediment to the global oceans from glacial erosion in Greenland. Nat. Geosci. 2017, 10, 859–863. [Google Scholar] [CrossRef]
- Stendahl, H.; Stensgaard, B.M.; Secher, K. Geological environments favourable for future mining. Geol. Ore 2009, 16, 1–12. [Google Scholar] [CrossRef]
- MacGregor, J.A.; Colgan, W.T.; Paxman, G.J.G.; Tinto, K.J.; Csathó, B.; Darbyshire, F.A.; Fahnestock, M.A.; Kokfelt, T.F.; MacKie, E.J.; Morlighem, M.; et al. Geologic provinces beneath the Greenland Ice Sheet constrained by geophysical data synthesis. Geophys. Res. Lett. 2024, 51, e2023GL107357. [Google Scholar] [CrossRef]
- Hollis, J.A.; Keiding, M.; Møller Stensgaard, B.; van Gool, J.A.; Garde, A.A. Evolution of Neoarchaean supracrustal belts at the northern margin of the North Atlantic Craton, West Greenland. GEUS Bull. 2006, 11, 9–32. [Google Scholar] [CrossRef]
- Bjarløv, S.P.; Vladykova, P. The potential need for energy saving in standard family detached and semi-detached wooden houses in arctic Greenland. Build. Environ. 2011, 46, 1525–1536. [Google Scholar] [CrossRef]
- Statbank Greenland. Available online: https://bank.stat.gl/pxweb/en/Greenland/Greenland__IE__IE10/IEXANV.px/ (accessed on 26 June 2024).
- Hornbostel, C. Construction Materials: Types, Uses and Applications, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1991. [Google Scholar]
- Kirkelund, G.M.; Skevi, L.; Ottosen, L.M. Electrodialytically treated MSWI fly ash use in clay bricks. Constr. Build. Mater. 2020, 254, 119286. [Google Scholar] [CrossRef]
- Brown, G.; Brindley, G.W. X-ray diffraction procedures for clay mineral identification. In Crystal Structures of Clay Minerals and Their X-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1980; pp. 305–359. [Google Scholar]
- DS/CEN ISO/TS 17892-12; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 12: Determination of Liquid and Plastic Limits. ISO: Geneva, Switzerland, 2018.
- DIN 18.122-1; Baugrund, Untersuchung von Bodenproben—Zustandsgrenzen (Konsistenzgrenzen)—Teil 1: Bestimmung der Fließ- und Ausrollgrenze. Deutsches Institut für Normung: Berlin, Germany, 1997.
- Brick Industry Association. Manufacturing of Brick; Technical notes on brick construction, 9; Brick Industry Association: Reston, VA, USA, 2006. [Google Scholar]
- DS/EN 772-11; Methods of Test for Masonry Units—Part 11: Determination of Water Absorption of Aggregate Concrete, Autoclaved Aerated Concrete, Manufactured Stone and Natural Stone Masonry Units Due to Capillary Action and the Initial Rate of Water Absorption of Clay Masonry Units. Dansk Standard: Nordhavn, Denmark, 2011.
- DS/EN 772-4; Methods of Test for Masonry Units—Part 4: Determination of Real and Bulk Density and of Total and Open Porosity for Natural Stone Masonry Units. Dansk Standard: Nordhavn, Denmark, 1998.
- ISO 12571; Hygrothermal Performance of Building Materials and Products—Determination of Hygroscopic Sorption Properties. ISO: Geneva, Switzerland, 2021.
- ASTM C216-23; Standard Specification for Facing Brick (Solid Masonry Units Made from Clay or Shale). ASTM: West Conshohocken, PA, USA, 2023.
- Vieira, C.M.F.; Monteiro, S.N. Characterization of granite waste for incorporation in red ceramic. Mater. Sci. Forum 2005, 498–499, 728–733. [Google Scholar] [CrossRef]
| Glaciogene Marine Sediments | ||||||
|---|---|---|---|---|---|---|
| Chemical Oxide (wt%) | Greenland (North Atlantic Craton) [9] | Greenland (Nagssugtoqidian Orogen) [10,11,12] | Norway [5,8] | Canada [2,3] | Marine Sediments Used for Clay Bricks [13,14,15,16] | Typical Clay for Danish Red Bricks [17] |
| SiO2 | 53.99 | 57.5–64.2 | 45.8–53.6 | 46.8–62.6 | 47.13–63 | 55.46–76.97 |
| TiO2 | 0.71 | 0.6–0.8 | 0.8–1.1 | 0.0–1.0 | 0.63–0.81 | |
| Al2O3 | 16.44 | 14.5–16.2 | 14.3–21.5 | 15.4–23.4 | 7.69–17.23 | 9.59–14.41 |
| Fe2O3T | 8.55 | 6.2–6.6 | 8.2–15.2 | 4.4–8.8 | 4.13–21.55 | 3.76–6.96 |
| MnO | 0.12 | 0.1 | 0.0–0.2 | 0.1 | 0.03–0.31 | |
| MgO | 4.94 | 2.4–3.8 | 3.9–5.1 | 2.1–4.9 | 0.05–3.6 | 0.87–1.71 |
| CaO | 3.72 | 2.4–4.4 | 1.0–4.1 | 2.9–5.4 | 0.68–7.49 | 0.68–2.48 |
| Na2O | 3.84 | 3.3–4.5 | 1.2–2.4 | 1.3–4.1 | 0.61–1.9 | 0.48–1.19 |
| K2O | 3.5 | 2.7–2.9 | 4.7–6.0 | 2.4–3.7 | 1.36–2.13 | 2.49–3.0 |
| P2O5 | 0.12 | 0.1–0.2 | 0.1–0.3 | 0.15–0.32 | 0.06–0.12 | |
| LOI | 3.72 | 2.4–5.1 | 3.5–10.3 | 1.6–11.9 | 10–19.35 | |
| Specimen Name | MS (wt%) | CR (wt%) | CH (wt%) | No. of Specimens | Coal Furnace | Electrical Furnace | Firing Temperature (°C) |
|---|---|---|---|---|---|---|---|
| 1A | 100 | 2 | X | 1045–1055 | |||
| 1B | 100 | 3 | X | 1070–1080 | |||
| 2A | 90 | 10 | 3 | X | 1045–1055 | ||
| 2B | 90 | 10 | 2 | X | 1030–1040 | ||
| 3A | 90 | 10 | 3 | X | 1045–1055 | ||
| 3B | 90 | 10 | 3 | X | 1030–1040 |
| MS | CR | CH | ||
|---|---|---|---|---|
| Major elements (XRF) | SiO2 (wt%) | 61.12 | 61.77 | 66.94 |
| TiO2 (wt%) | 0.59 | 0.39 | 1.28 | |
| Al2O3 (wt%) | 14.52 | 10.63 | 19.22 | |
| Fe2O3T (wt%) | 6.48 | 6.45 | 7.80 | |
| MnO (wt%) | 0.08 | 0.11 | 0.06 | |
| MgO (wt%) | 3.52 | 9.67 | 0.99 | |
| CaO (wt%) | 2.36 | 3.64 | 0.33 | |
| Na2O (wt%) | 3.31 | 3.11 | 0.43 | |
| K2O (wt%) | 2.81 | 2.51 | 2.59 | |
| P2O5 (wt%) | 0.12 | 0.04 | 0.06 | |
| LOI1000 °C (wt%) | 5.09 | 1.68 | 0.30 | |
| TC (wt%) | 1.41 ± 0.18 | N.M. | N.M. | |
| S (wt%) | 0.10 ± 0.08 | N.M. | N.M. | |
| Cl− (mg/L) | 3.29 ± 0.04 | N.M. | N.M. | |
| Atterberg limits | Water content (%) | 23.5 ± 1.7 | N.M. | N.M. |
| Liquid Limit (%) | 30.2 ± 0.2 | N.M. | N.M. | |
| Plastic Limit (%) | 20.5 | N.M. | N.M. | |
| Plasticity Index | 9.7 | N.M. | N.M. | |
| Activity | 0.23 | N.M. | N.M. |
| Total Shrinkage % | Bulk (Apparent) Density kg/m3 | Open Porosity % | IRA | k-Value | |
|---|---|---|---|---|---|
| 1A | 13.8 ± 0.5 | 2350 | 4.9 | 0.170 ± 0.060 | 0.018 ± 0.005 |
| 1B | 14.9 ± 0.4 | 2400 | 2.9 | 0.080 ± 0.050 | 0.008 ± 0.004 |
| 2A | 11.0 ± 0.5 | 2260 | 16.8 | 0.300 ± 0.010 | 0.035 ± 0.003 |
| 2B | 7.7 ± 0.8 | 1960 | 38.2 | 1.630 ± 0.060 | 0.183 ± 0.010 |
| 3A | 13.8 ± 0.2 | 2360 | 9.3 | 0.080 ± 0.020 | 0.009 ± 0.003 |
| 3B | 11.0 ± 0.4 | 2090 | 27.2 | 0.890 ± 0.180 | 0.094 ± 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte, L.J.; Ottosen, L.M.; Kirkelund, G.M. Use of a Glaciogene Marine Clay (Ilulissat, Greenland) in a Pilot Production of Red Bricks. Materials 2024, 17, 4365. https://doi.org/10.3390/ma17174365
Belmonte LJ, Ottosen LM, Kirkelund GM. Use of a Glaciogene Marine Clay (Ilulissat, Greenland) in a Pilot Production of Red Bricks. Materials. 2024; 17(17):4365. https://doi.org/10.3390/ma17174365
Chicago/Turabian StyleBelmonte, Louise J., Lisbeth M. Ottosen, and Gunvor M. Kirkelund. 2024. "Use of a Glaciogene Marine Clay (Ilulissat, Greenland) in a Pilot Production of Red Bricks" Materials 17, no. 17: 4365. https://doi.org/10.3390/ma17174365
APA StyleBelmonte, L. J., Ottosen, L. M., & Kirkelund, G. M. (2024). Use of a Glaciogene Marine Clay (Ilulissat, Greenland) in a Pilot Production of Red Bricks. Materials, 17(17), 4365. https://doi.org/10.3390/ma17174365








