New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
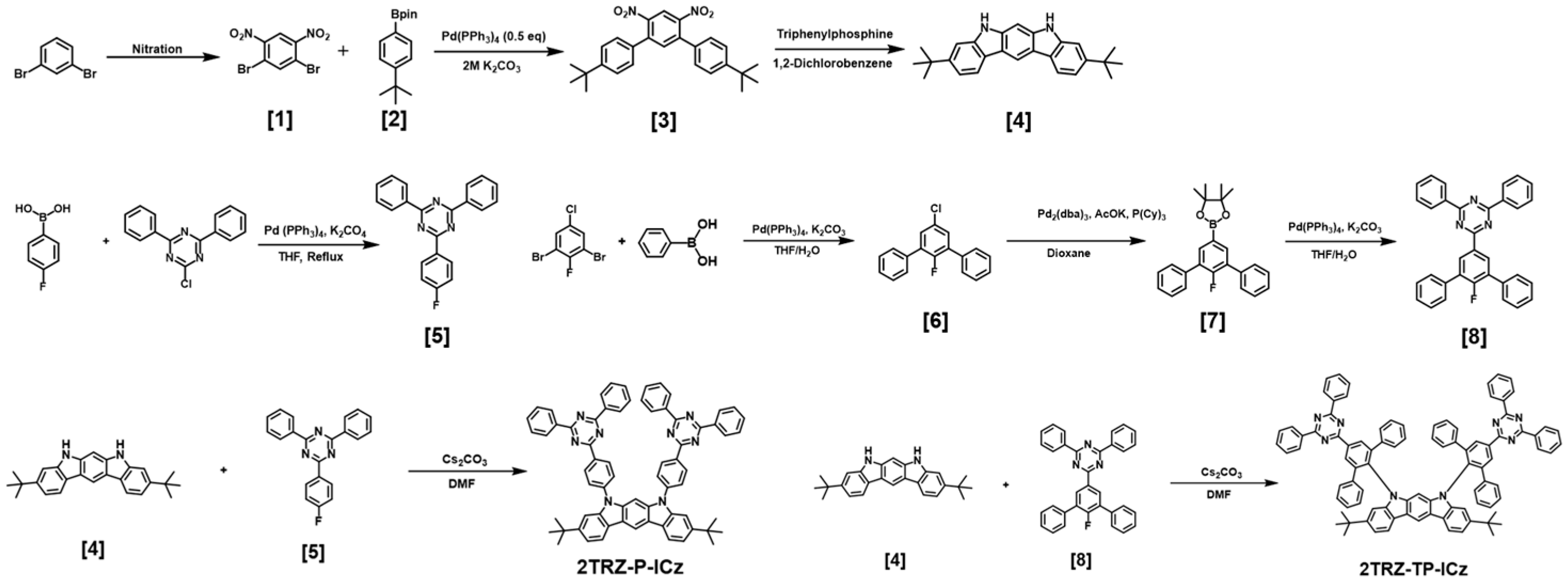
2.2. Synthesis
2.2.1. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (1)
2.2.2. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (2)
2.2.3. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (3)
2.2.4. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (4)
2.2.5. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (5)
2.2.6. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (6)
2.2.7. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (7)
2.2.8. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, (8)
2.2.9. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, 2TRZ-P-ICz
2.2.10. Synthesis of 1,5-Dibromo-2,4-dinitrobenzene, 2TRZ-TP-ICz
2.3. Lippert–Mataga Model
3. Results and Discussion
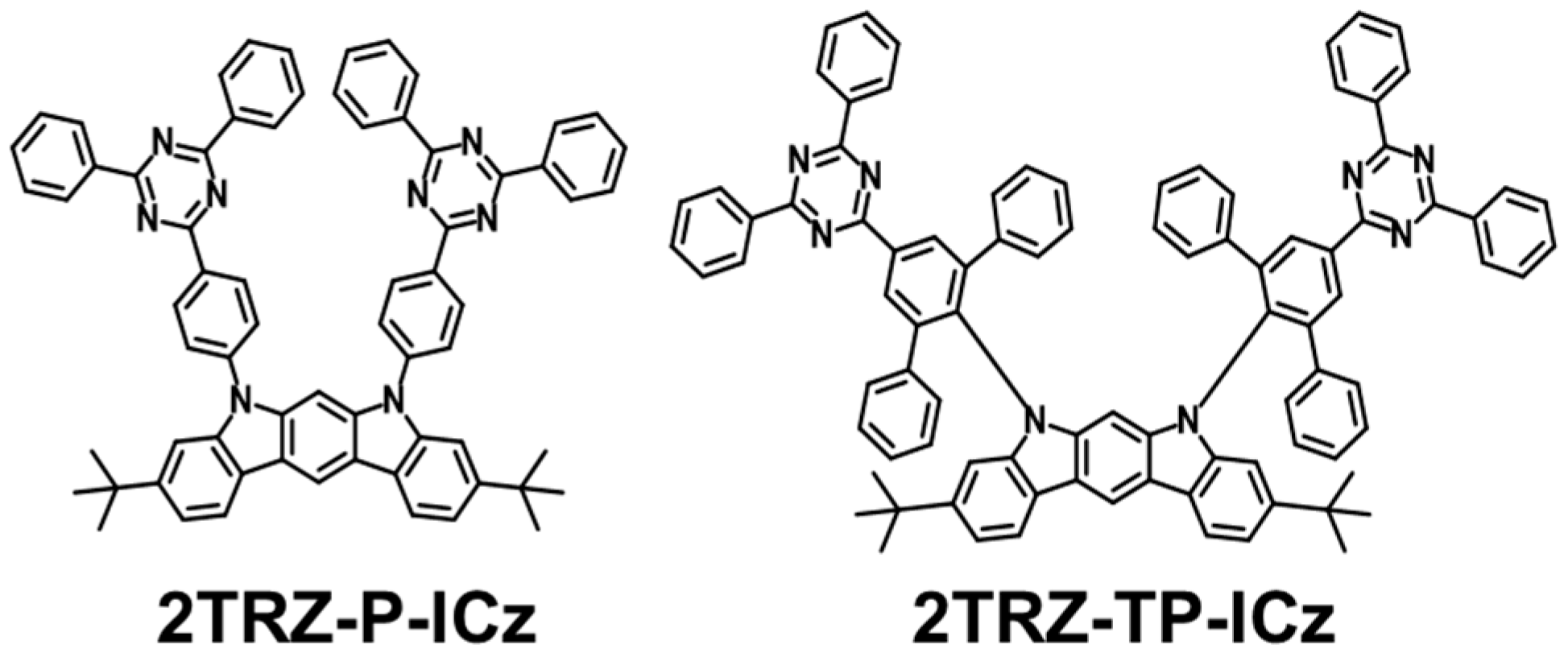
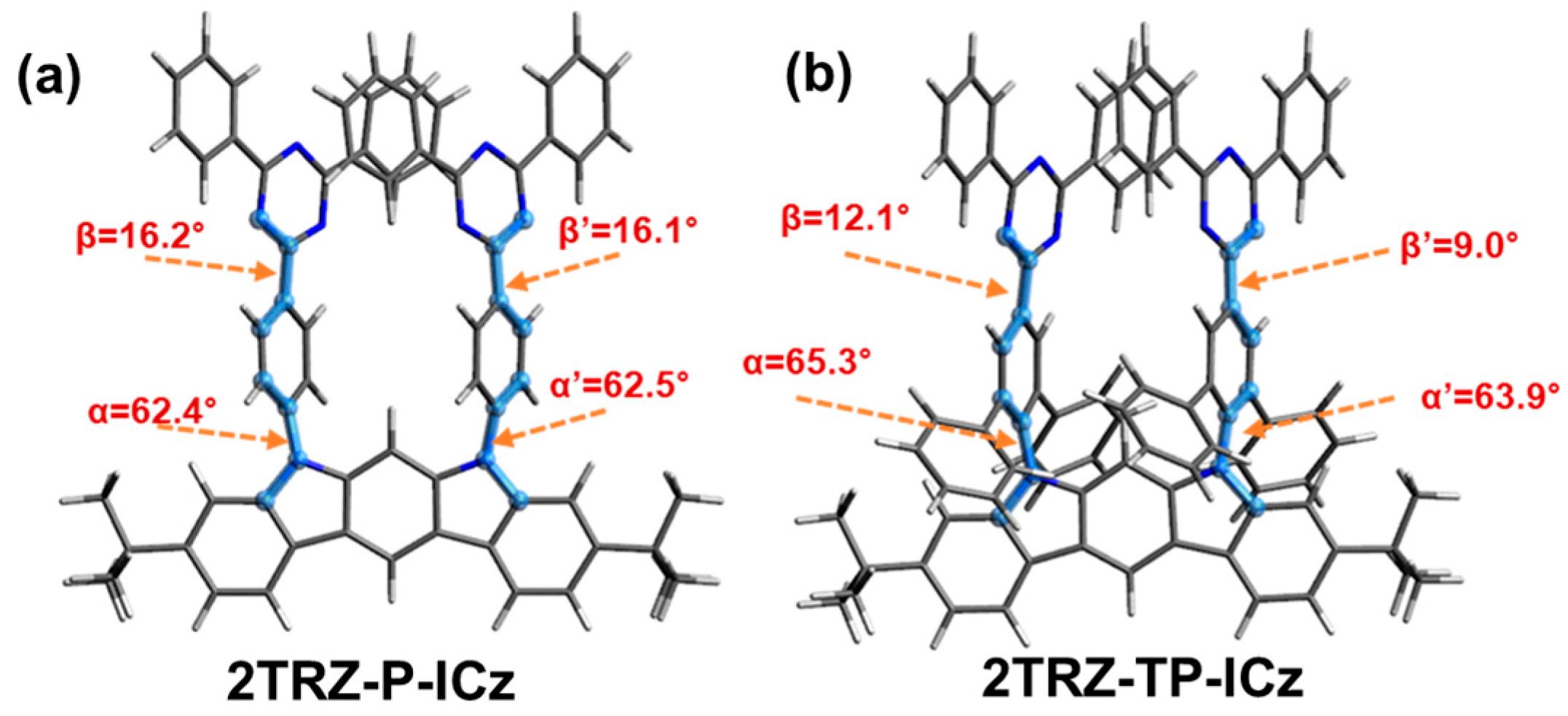
3.1. Molecular Design, Synthesis, and Characterization
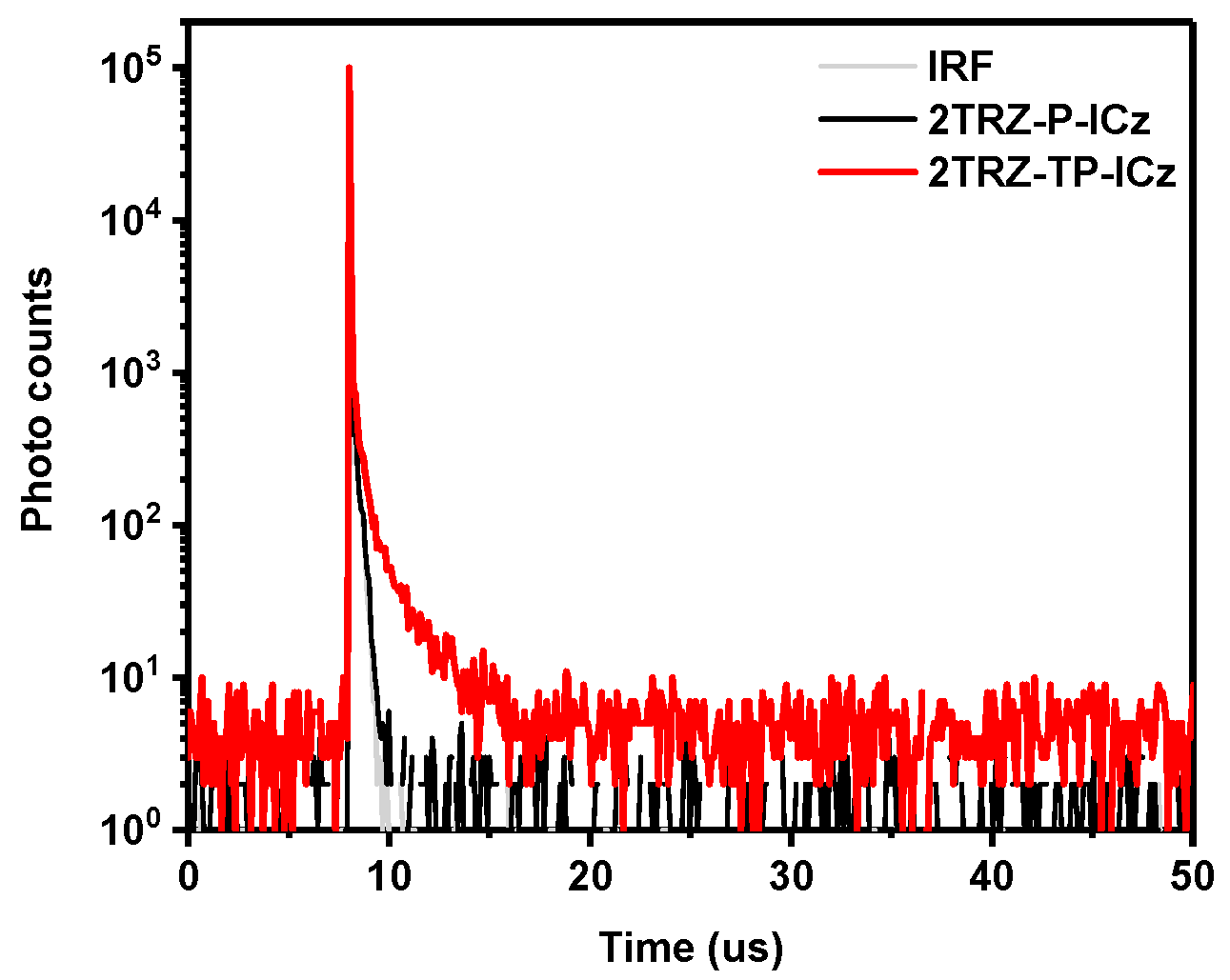
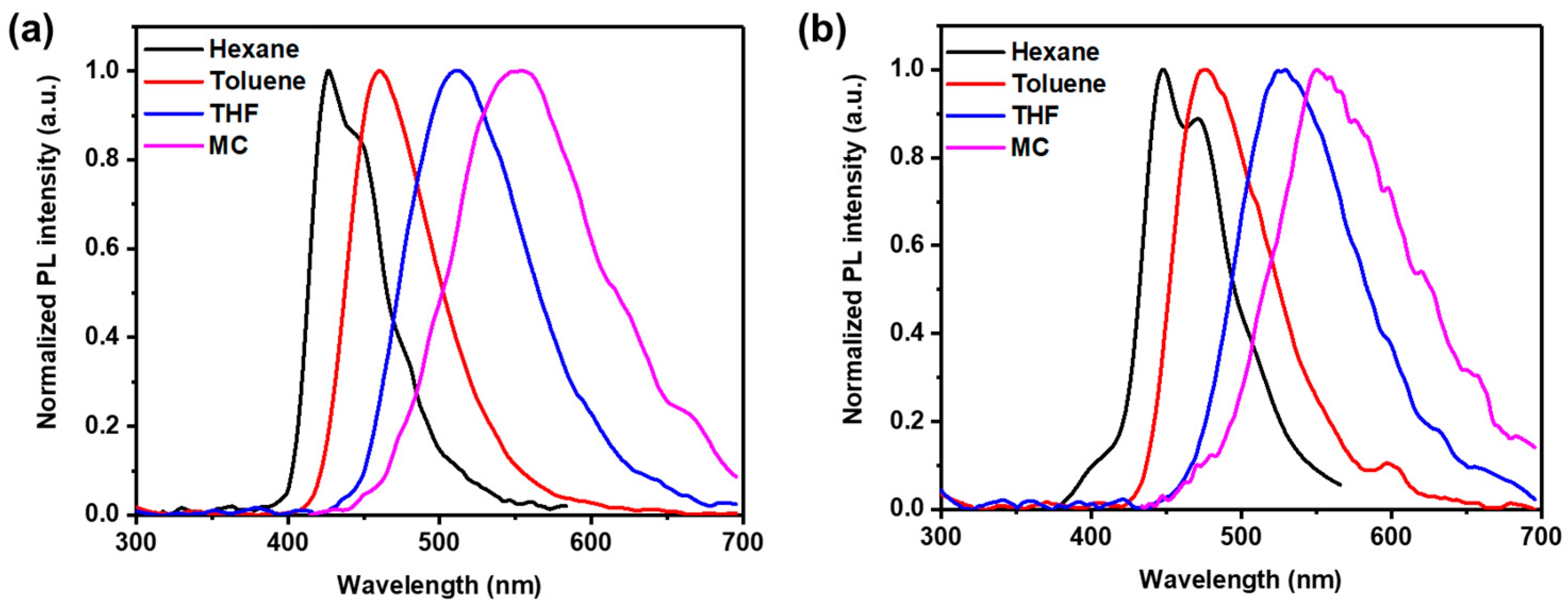
3.2. Photophysical Properties
3.3. Theoretical Calculation
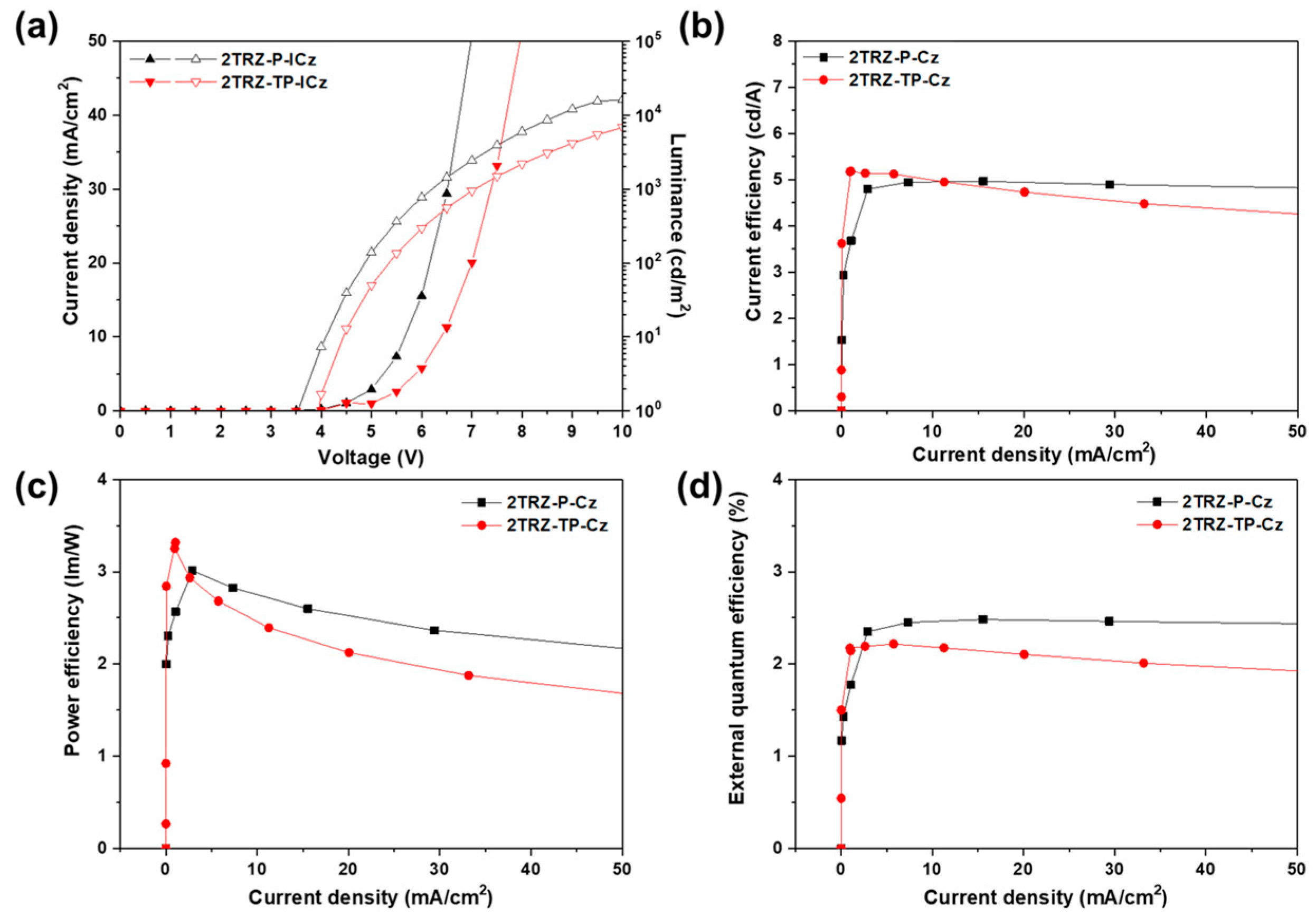
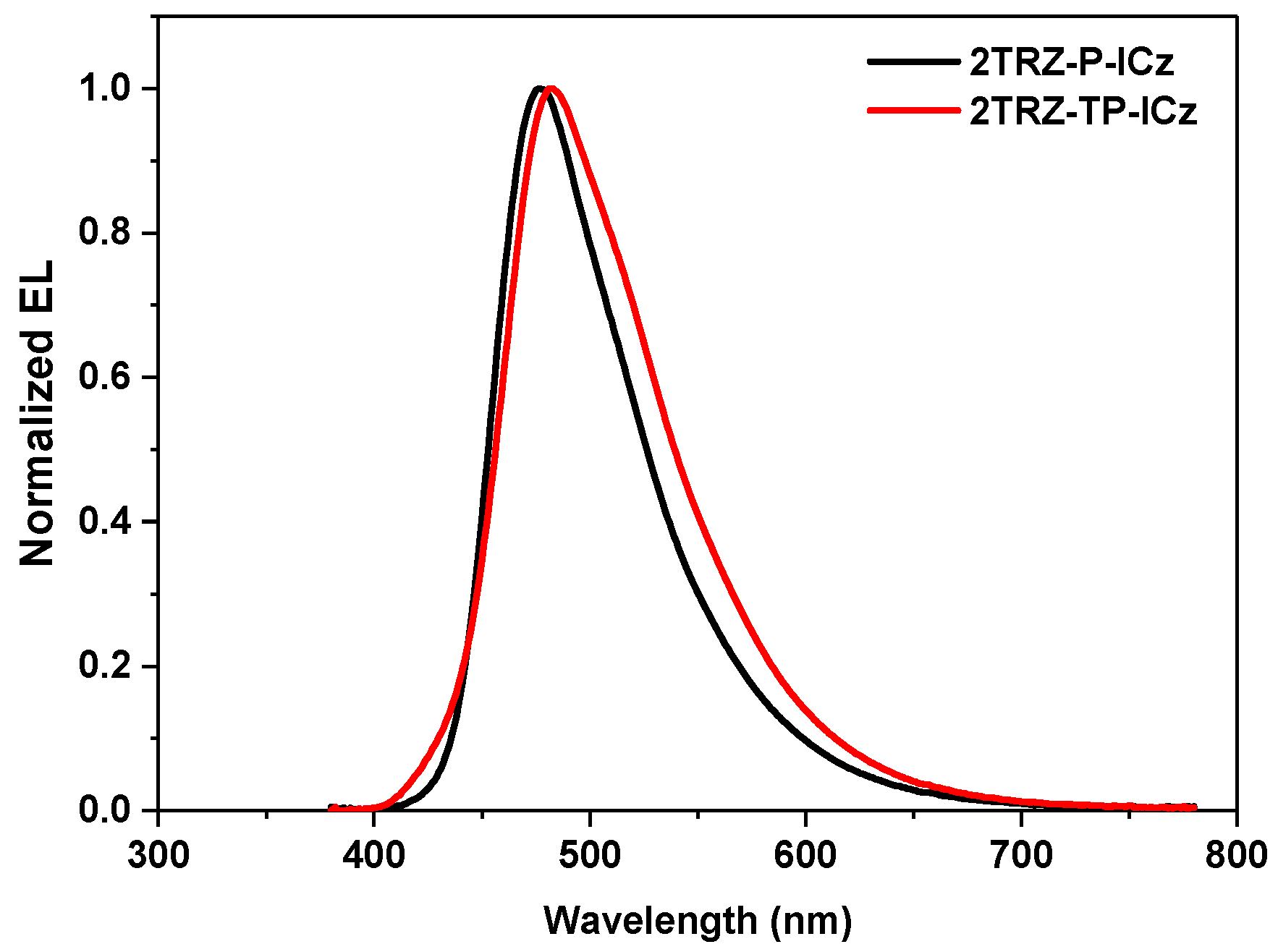
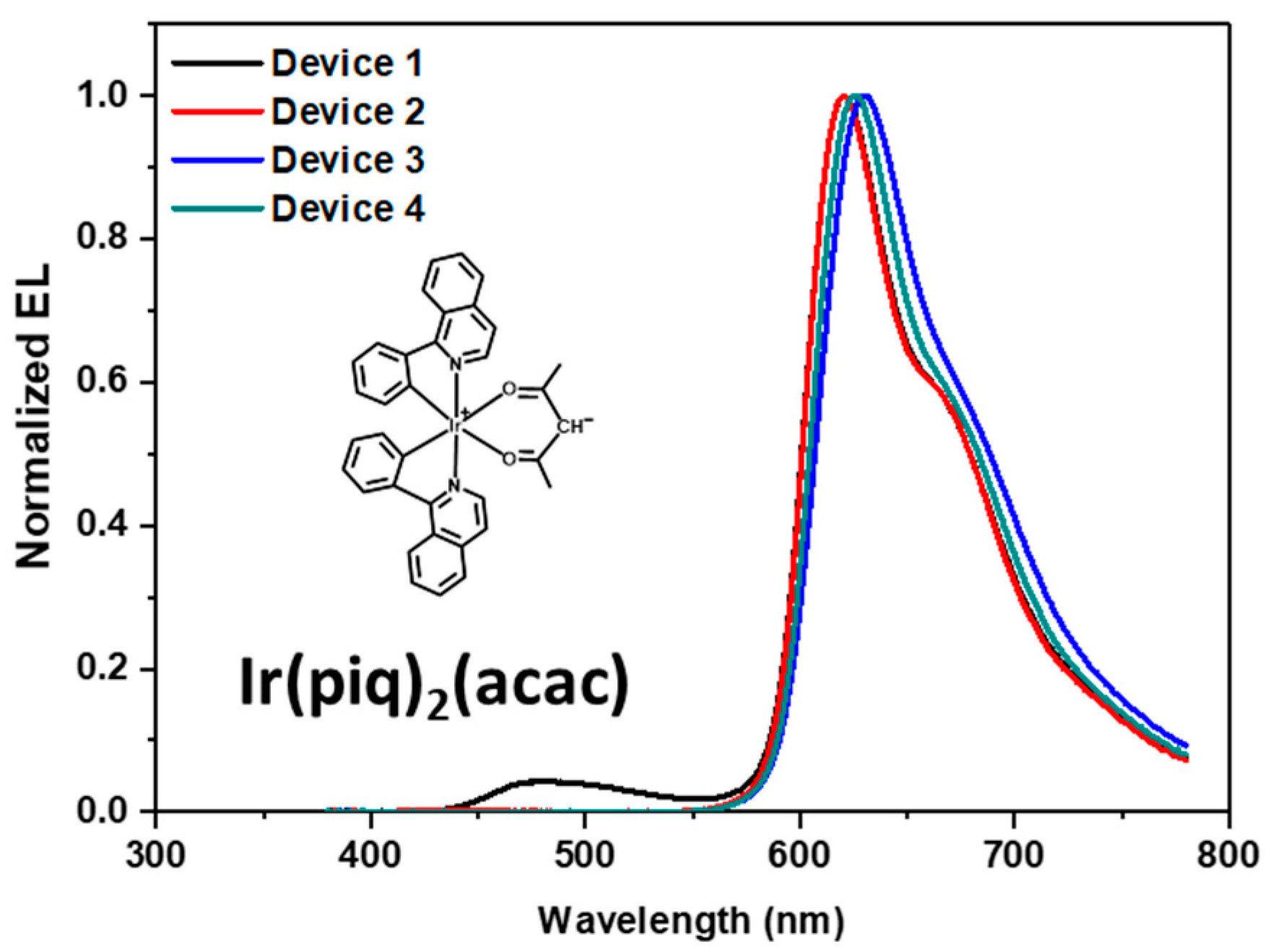
3.4. Electroluminescence (EL) Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.; Brown, A.; Marks, R.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Chen, F.-C.; Yang, Y.; Thompson, M.E.; Kido, J. High-performance polymer light-emitting diodes doped with a red phosphorescent iridium complex. Appl. Phys. Lett. 2002, 80, 2308–2310. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.; Lee, J.; Shin, H.; Park, Y.-I.; Park, J. Design of fluorescent blue light-emitting materials based on analyses of chemical structures and their effects. Mater. Sci. Eng. R Rep. 2016, 99, 1–22. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. In Electrophosphorescent Materials and Devices; Jenny Stanford Publishing: New Delhi, India, 2023; pp. 1–11. [Google Scholar]
- Kautny, P.; Wu, Z.; Eichelter, J.; Horkel, E.; Stöger, B.; Chen, J.; Ma, D.; Fröhlich, J.; Lumpi, D. Indolo[3,2,1-jk]carbazole based planarized CBP derivatives as host materials for PhOLEDs with low efficiency roll-off. Org. Electron. 2016, 34, 237–245. [Google Scholar] [CrossRef]
- Oner, S.; Bryce, M.R. A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applications. Mater. Chem. Front. 2023, 7, 4304–4338. [Google Scholar] [CrossRef]
- Shin, E.; Sung, B.; Joo, C.W.; Kim, D.; Kim, J.; Lee, J.; Kim, Y.H. Novel indolocarbazole-based bipolar host materials for fabricating green phosphorescent organic light-emitting diodes with high efficiency and low roll-off. Dye. Pigment. 2024, 226, 112147. [Google Scholar] [CrossRef]
- Wagner, D.; Hoffmann, S.T.; Heinemeyer, U.; Münster, I.; Köhler, A.; Strohriegl, P. Triazine based bipolar host materials for blue phosphorescent OLEDs. Chem. Mater. 2013, 25, 3758–3765. [Google Scholar] [CrossRef]
- Lee, D.R.; Han, S.H.; Lee, C.W.; Lee, J.Y. Bis (diphenyltriazine) as a new acceptor of efficient thermally activated delayed fluorescent emitters. Dye. Pigment. 2018, 151, 75–80. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, Y.; Du, B.; Li, Q.; Wang, S.; Wang, L. Benzimidazole-triazine based n-type hosts with twisted structure and high triplet energy level for efficient blue narrowband emitting OLEDs. Chem. Eng. J. 2023, 465, 142848. [Google Scholar] [CrossRef]
- Madushani, B.; Mamada, M.; Goushi, K.; Nguyen, T.B.; Nakanotani, H.; Kaji, H.; Adachi, C. Multiple donor-acceptor design for highly luminescent and stable thermally activated delayed fluorescence emitters. Sci. Rep. 2023, 13, 7644. [Google Scholar] [CrossRef]
- Cho, Y.J.; Jeon, S.K.; Chin, B.D.; Yu, E.; Lee, J.Y. The design of dual emitting cores for green thermally activated delayed fluorescent materials. Angew. Chem. Int. Ed. Engl. 2015, 54, 5201–5204. [Google Scholar] [CrossRef]
- Balijapalli, U.; Tanaka, M.; Auffray, M.; Chan, C.Y.; Lee, Y.T.; Tsuchiya, Y.; Nakanotani, H.; Adachi, C. Utilization of Multi-Heterodonors in Thermally Activated Delayed Fluorescence Molecules and Their High Performance Bluish-Green Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 9498–9506. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, C.; Zhang, Y.; Song, X.; Wei, P.; Cai, M.; Duan, L. Highly Efficient Full-Color Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes: Extremely Low Efficiency Roll-Off Utilizing a Host with Small Singlet-Triplet Splitting. ACS Appl. Mater. Interfaces 2017, 9, 4769–4777. [Google Scholar] [CrossRef]
- Park, J.; Han, S.; Jo, U.; Kim, S.C.; Lee, D.R.; Ahn, H.J.; Kim, J.Y.; Baek, J.-H.; Lee, J.Y. Boron-based thermally activated delayed fluorescence host materials as universal hosts for blue phosphorescent organic light-emitting diodes. Mater. Today 2024, 75, 27–36. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Mazumdar, A.K.; Nanda, G.P.; Yadav, N.; Deori, U.; Acharyya, U.; Sk, B.; Rajamalli, P. Thermally activated delayed fluorescence (TADF) emitters: Sensing and boosting spin-flipping by aggregation. Beilstein J. Org. Chem. 2022, 18, 1177–1187. [Google Scholar] [CrossRef]
- Sherefedin, U.; Belay, A.; Kebede, A.; Asemare, S.; Woldegiorges, K.; Kumela, A.G.; Gudishe, K. Determination of the ground and excited state dipole moments of ferulic and sinapic acids by solvatochromic effects and density function theory method. AIP Adv. 2023, 13, 105227. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Bai, H.; Zhang, Z.; Li, J. Novel bipolar hosts for highly efficient green phosphorescent organic light-emitting diodes with slow efficiency roll-off. Dye. Pigment. 2024, 223, 111920. [Google Scholar] [CrossRef]
- Kumar, K.; Thakur, D.; Karmakar, A.; Patra, S.; Kumar, A.; Banik, S.; Ghosh, S. Indolo[3,2-a]carbazoles as Engineered Materials for Optoelectronic Applications: Synthesis, Structural Insights, and Computational Screening. J. Org. Chem. 2024, 89, 7394–7407. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Park, D.; Kang, S.; Ryoo, C.H.; Jhun, B.H.; Jung, S.; Le, T.N.; Suh, M.C.; Lee, J.; Jun, M.E.; Chu, C.; et al. High-performance blue OLED using multiresonance thermally activated delayed fluorescence host materials containing silicon atoms. Nat. Commun. 2023, 14, 5589. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Chen, B.; Xie, Q.; Li, X.; Liu, H.; Wang, S. A Breakthrough in Solution-Processed Ultra-Deep-Blue HLCT OLEDs: A Record External Quantum Efficiency Exceeding 10% Based on Novel V-Shaped Emitters. Adv. Mater. 2023, 35, e2305310. [Google Scholar] [CrossRef]
- Park, S.; Kwon, H.; Lee, H.; Lee, K.; Kang, S.; Kim, K.J.; Kim, T.; Park, J. Novel blue multiresonance thermally activated delayed fluorescence host materials, including Ge-based bulky groups. J. Mater. Chem. C 2024, 12, 4384–4391. [Google Scholar] [CrossRef]
- Chaskar, A.; Chen, H.F.; Wong, K.T. Bipolar host materials: A chemical approach for highly efficient electrophosphorescent devices. Adv. Mater. 2011, 23, 3876–3895. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, Y.; Tagare, J.; Kwon, M.J.; Kim, T.; Hong, W.P. Strategic carbazole-cycloalkyl fused donors to induce TADF featured AIE for high efficiency deep blue emission. J. Mater. Chem. C 2024, 12, 11115–11126. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, J.; Liu, H.; Zhao, Z.; Tang, B.Z. A Multifunctional Bipolar Luminogen with Delayed Fluorescence for High-Performance Monochromatic and Color-Stable Warm-White OLEDs. Adv. Funct. Mater. 2020, 30, 2000019. [Google Scholar] [CrossRef]
- Miao, Z.; Ge, Z.; Gu, D.; Wang, L.; Zhang, T. Space charge limited current in organic materials with free and trapped charges. Phys. Lett. A 2024, 495, 129305. [Google Scholar] [CrossRef]
- Xie, Z.; Cao, C.; Zou, Y.; Cao, X.; Zhou, C.; He, J.; Lee, C.S.; Yang, C. Molecular Engineering Enables TADF Emitters Well Suitable for Non-Doped OLEDs with External Quantum Efficiency of Nearly 30%. Adv. Funct. Mater. 2022, 32, 2112881. [Google Scholar] [CrossRef]
- Godi, M.; Kwon, H.; Park, S.; Park, S.; Lee, H.; Lee, K.; Park, J. Enhancing OLED emitter efficiency through increased rigidity. RSC Adv. 2024, 14, 8135–8144. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Zheng, Y.; Zhang, Q.; Zhao, B.; Chen, L.; Huang, J. Novel bipolar host materials including carbazole and dioxy[2,3-b] pyrazine units for red phosphorescent organic light-emitting diodes. Dye. Pigment. 2023, 220, 111684. [Google Scholar] [CrossRef]
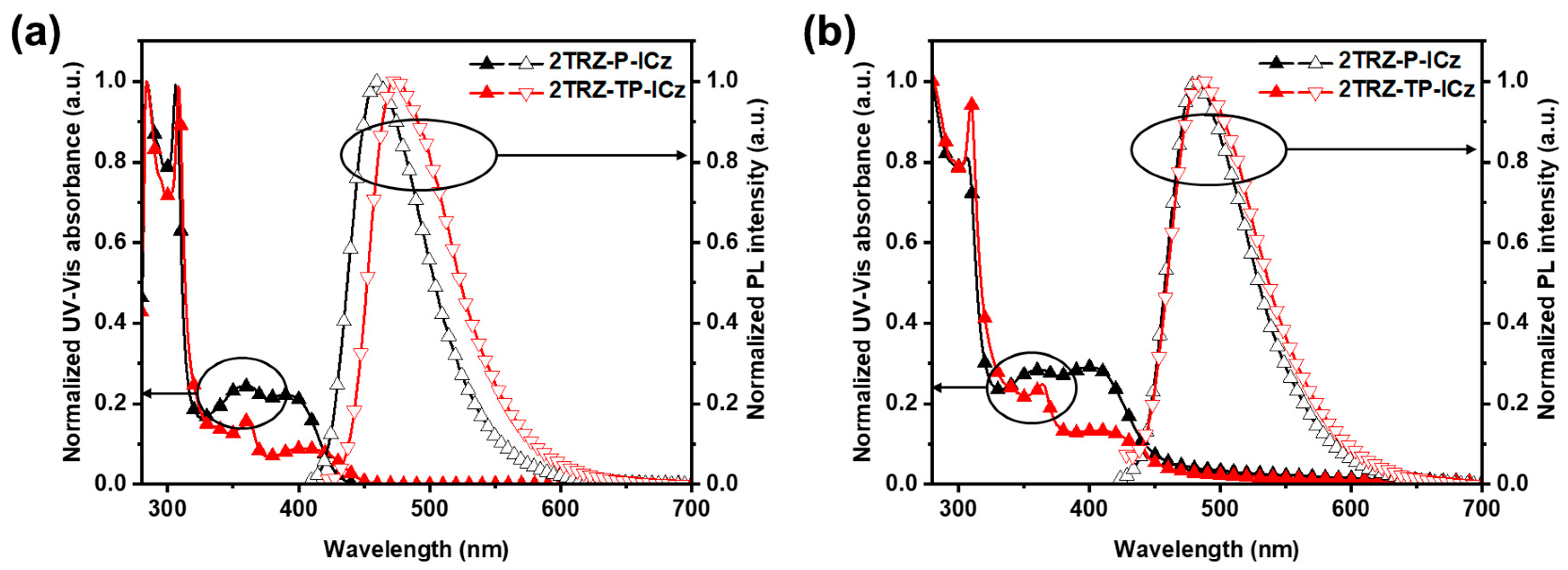


| Solution a | Film b | Φ c [%] | HO MO d [eV] | LU MO [eV] | Band Gap [eV] | S1 e [eV] | T1 f [eV] | ΔEST g [eV] | Td h [°C] | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λabs [nm] | λPL (FWHM) [nm] | λabs [nm] | λPL (FWHM) [nm] | |||||||||
| 2TRZ-P-ICz | 284, 306, 362, 390 | 460 (67) | 306, 363, 400 | 480 (71) | 57.1/33.8 | −5.50 | −2.70 | 2.80 | 2.93 | 2.72 | 0.21 | 504 |
| 2TRZ-TP-ICz | 284, 306, 362, 403 | 473 (71) | 309, 364, 404 | 488 (77) | 43.6/32 | −5.59 | −2.84 | 2.75 | 2.83 | 2.75 | 0.08 | 518 |
| Voltage a [V] | CEmax [cd/A] | PEmax [lm/W] | EQEmax [%] | CIE (x, y) a | ELmax a [nm] | |
|---|---|---|---|---|---|---|
| 2TRZ-P-ICz | 5.6 | 4.96 | 3.01 | 2.48 | (0.181, 0.304) | 477 (73) |
| 2TRZ-TP-ICz | 6.4 | 5.17 | 3.31 | 2.21 | (0.206, 0.363) | 482 (83) |
| @10mA/cm2 | Voltage [V] | CE [cd/A] | PE [lm/W] | EQE[%] | CIE (x, y) | ELmax (FWHM) [nm] | |
|---|---|---|---|---|---|---|---|
| Device 1 | 2TRZ-P-ICz: 4wt% Ir(piq)2(acac) | 5.10 | 5.77 | 3.57 | 6.27 | (0.635, 0.327) | 620 nm (78) |
| Device 2 | 2TRZ-P-ICz: 10wt% Ir(piq)2(acac) | 6.00 | 6.81 | 3.57 | 9.58 | (0.682, 0.316) | 630 nm (81) |
| Device 3 | 2TRZ-TP-ICz: 4wt% Ir(piq)2(acac) | 6.17 | 8.10 | 4.21 | 9.12 | (0.672, 0.324) | 620 nm (78) |
| Device 4 | 2TRZ-TP-ICz: 10wt% Ir(piq)2(acac) | 6.15 | 9.92 | 5.50 | 13.7 | (0.679, 0.319) | 626 nm (78) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kwon, H.; Park, S.; Oh, S.; Lee, K.; Lee, H.; Kang, S.; Park, D.; Park, J. New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs. Materials 2024, 17, 4347. https://doi.org/10.3390/ma17174347
Park S, Kwon H, Park S, Oh S, Lee K, Lee H, Kang S, Park D, Park J. New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs. Materials. 2024; 17(17):4347. https://doi.org/10.3390/ma17174347
Chicago/Turabian StylePark, Sunwoo, Hyukmin Kwon, Sangwook Park, Saeyoung Oh, Kiho Lee, Hayoon Lee, Seokwoo Kang, Dongmin Park, and Jongwook Park. 2024. "New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs" Materials 17, no. 17: 4347. https://doi.org/10.3390/ma17174347
APA StylePark, S., Kwon, H., Park, S., Oh, S., Lee, K., Lee, H., Kang, S., Park, D., & Park, J. (2024). New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs. Materials, 17(17), 4347. https://doi.org/10.3390/ma17174347







