Assessing the Potential of Rare Earth Elements in Bottom Ash from Coal Combustion in Poland
Abstract
:1. Introduction
2. Materials and Methods
3. Results
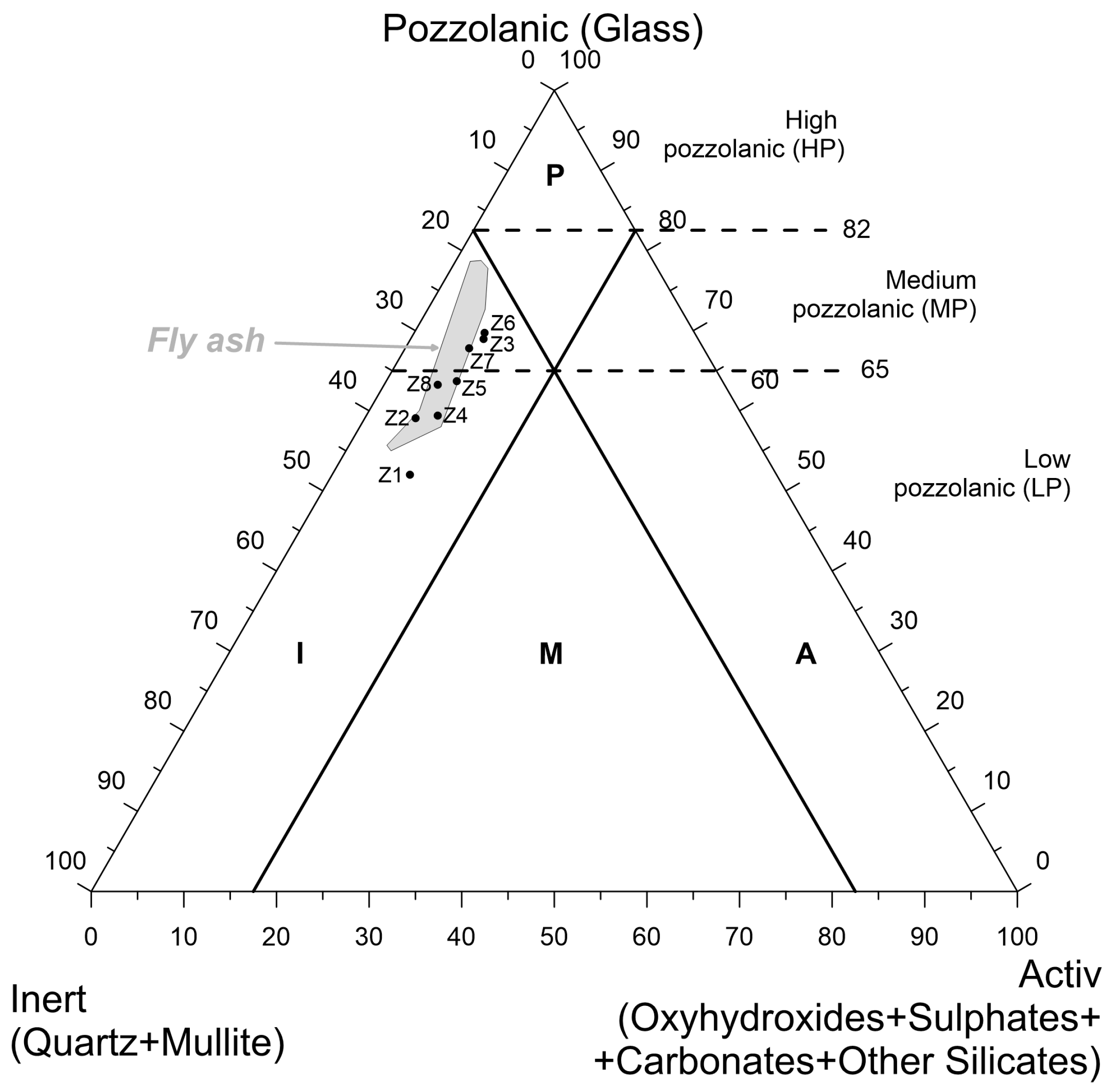
3.1. Phase Composition
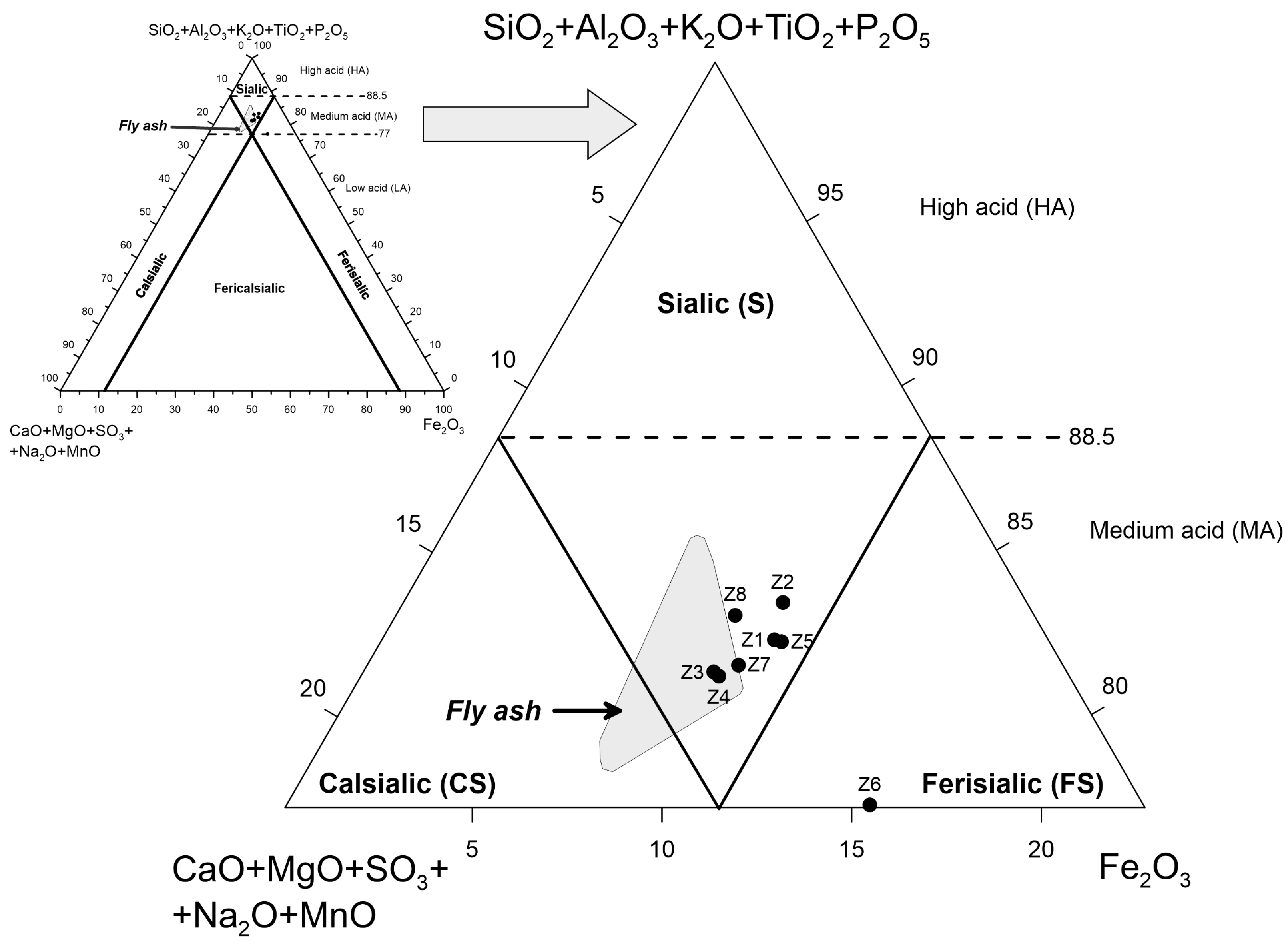
3.2. Chemical Composition
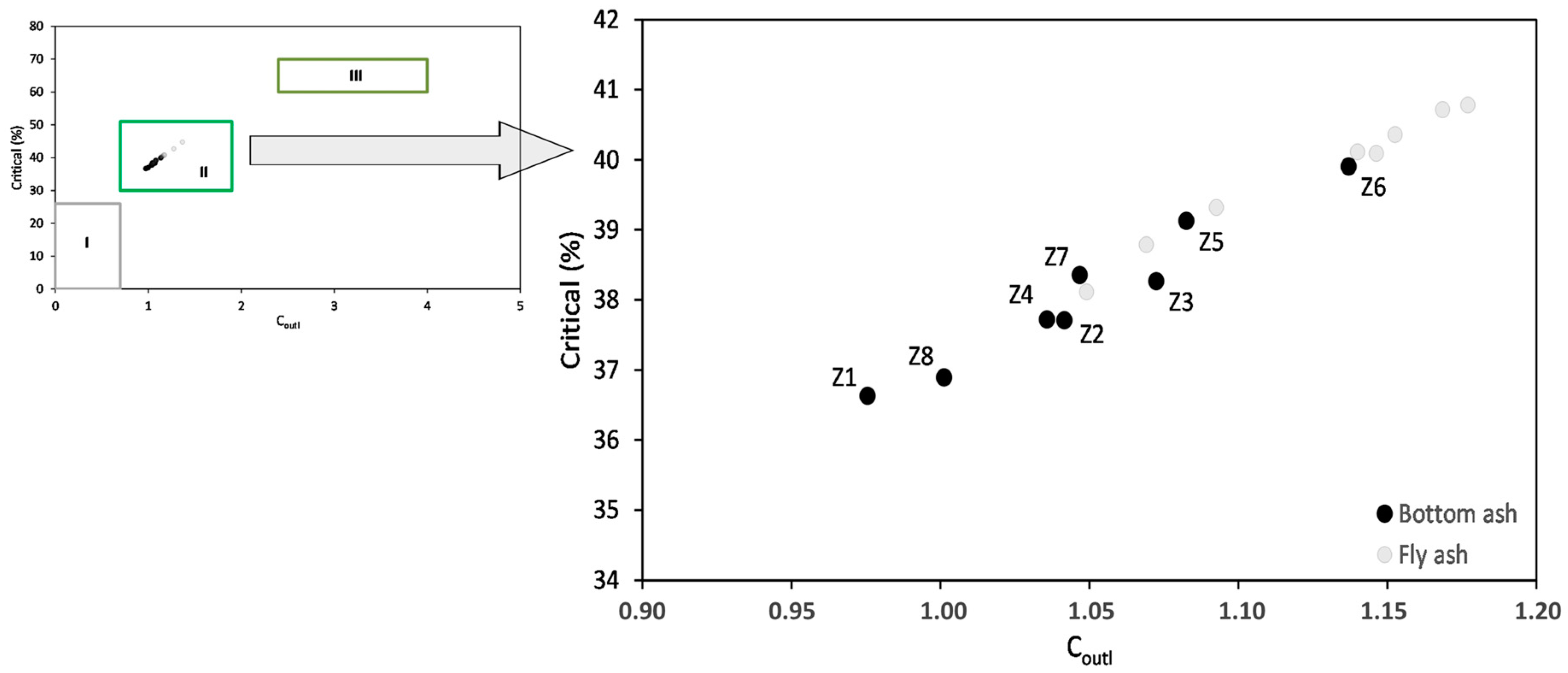
3.3. Rare Earth Elements
4. Discussion
4.1. Correlation between Phase Components and Chemical Components
4.2. The Relationship between REY and Phase Components
4.3. The Relationship between REY and Chemical Components
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Switzer, S.; Gerber, L.; Sindico, F. Access to Minerals: WTO Export Restrictions and Climate Change Considerations. Laws 2015, 4, 617–637. [Google Scholar] [CrossRef]
- Ascenzi, P.; Bettinelli, P.; Boffi, A.; Botta, P.; De Simeone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare earth elements (REE) in biology and medicine. Rend. Lincei Sci. Fis. Nat. 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Salfate, G.; Sánchez, J. Rare Earth Elements Uptake by Synthetic Polymeric and Cellulose-Based Materials: A Review. Polymers 2022, 14, 4786. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impast. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Sobri, N.A.; Yunus, M.Y.B.M.; Harun, N. A review of ion adsorption clay as a high potential source of rare earth minerals in Malaysia. Mater. Today Proc. 2023, 1–8. [Google Scholar] [CrossRef]
- El Azhari, H.; Cherif, E.K.; El Halimi, R.; Azzirgue, E.M.; Ou Larbi, Y.; Coren, F.; Salmoun, F. Predicting the Production and Depletion of Rare Earth Elements and Their Influence on Energy Sector Sustainability through the Utilization of Multilevel Linear Prediction Mixed-Effects Models with R Software. Sustainability 2024, 16, 1951. [Google Scholar] [CrossRef]
- Brown, R.M.; Struhs, E.; Mirkouei, A.; Raja, K.; Reed, D. Mixed rare earth metals production from surface soil in Idaho, USA: Techno-economic analysis and greenhouse gas emission assessment. Sci. Total Environ. 2024, 944, 173945. [Google Scholar] [CrossRef]
- Thomas, B.S.; Dimitriadis, P.; Kundu, C.; Vuppaladadiyam, S.S.V.; Raman, R.K.S.; Bhattacharya, S. Extraction and separation of rare earth elements from coal and coal fly ash: A review on fundamental understanding and on-going engineering advancements. J. Environ. Chem. Eng. 2024, 12, 112769. [Google Scholar] [CrossRef]
- Greinacher, E. History of rare earth applications, rare earth market today: Overview. In Industrial Applications of Rare Earth Elements; Gschneidner, K.A., Jr., Ed.; ACS Symposium Series 164; American Chemical Society: Washington, DC, USA, 1981; pp. 3–18. [Google Scholar]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2015. [Google Scholar]
- Sikander, A.; Savvilotidou, V.; Jia, X.; Nicomel, N. The presence of rare earth elements and critical metals in waste electric and electronic equipment: Challenges for recovery. Glob. NEST J. 2018, 20, 773–777. [Google Scholar]
- Löffler, G.R. Carl Auer von Welsbach (1858–1929)—A Famous Austrian Chemist Whose Services Have Been Forgotten for Modern Physics. Substantia 2019, 3, 91–107. [Google Scholar]
- Sager, M.; Wiche, O. Rare Earth Elements (REE): Origins, Dispersion, and Environmental Implications—A Comprehensive Review. Environments 2024, 11, 24. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Drobniak, A.; Mastalerz, M. Rare Earth Elements—A brief overview. Indiana J. Earth Sci. 2022, 4, 33628. [Google Scholar] [CrossRef]
- Willenbacher, M.; Wohlgemuth, V. Rebound Effects in the Use of Rare Earth Metals in ICT. Int. J. Environ. Sci. Nat. Res. 2022, 30, 556277. [Google Scholar]
- Patel, K.S.; Sharma, S.; Maity, J.P.; Martín-Ramos, P.; Fiket, Ž.; Bhattacharya, P.; Zhu, Y. Occurrence of uranium, thorium and rare earth elements in the environment: A review. Front. Environ. Sci. 2023, 10, 1058053. [Google Scholar] [CrossRef]
- Salehi, H.; Maroufi, S.; Mofarah, S.S.; Nekouei, R.K.; Sahajwalla, V. Recovery of Rare Earth Metals from Ni-MH Batteries: A Comprehensive Review. Renew. Sustain. Energy Rev. 2023, 178, 113248. [Google Scholar]
- Weng, Z.; Simon, M.J.; Gavin, M.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Soc. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Chen, C. Global Potential of Rare Earth Resources and Rare Earth Demand from Clean Technologies. Minerals 2017, 7, 203. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2024; Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2024; Volume 2024, pp. 144–145. [Google Scholar]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Hower, J.C.; Ruppert, L.F.; Eble, C.F. Lanthanide, yttrium, and zirconium anomalies in the Fire Clay coal bed, Eastern Kentucky. Int. J. Coal Geol. 1999, 39, 141–153. [Google Scholar] [CrossRef]
- Hower, J.C.; Dai, S.; Seredin, V.V.; Zhao, L.; Kostova, I.J.; Silva, L.F.; Mardon, S.M.; Gurdal, G. A note on the occurrence of yttrium and rare earth elements in coal combustion products. Coal Combust. Gasif. Prod. 2013, 5, 39–47. [Google Scholar]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Hood, M.M.; Eble, C.F.; Honaker, R.Q.; Zhang, W.; Qian, D. Notes on the potential for the concentration of rare earth elements and yttrium in coal combustion fly ash. Minerals 2015, 5, 356–366. [Google Scholar] [CrossRef]
- Hower, J.C.; Granite, E.J.; Mayfield, D.B.; Lewis, A.S.; Finkelman, R.B. Notes on Contributions to the Science of Rare Earth Element Enrichment in Coal and Coal Combustion Byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Henke, K.R.; Graham, U.M.; Hood, M.M.; Joshi, P.; Preda, D.V. Ponded and landfilled fly ash as a source of rare earth elements from a Kentucky power plant. Coal Comb. Gasific. Prod. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Mardon, S.M.; Hower, J.C. Impact of coal properties on coal combustion by-product quality: Examples from a Kentucky power plant. Int. J. Coal Geol. 2004, 59, 153–169. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef]
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A Review of the Occurrence and Promising Recovery Methods of Rare Earth Elements from Coal and Coal By-Products. Int. J. Coal Prep. Util. 2015, 35, 295–330. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Dai, S.; Yan, X.; Ward, C.R.; Hower, J.C.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R.B. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Dwyer, G.S.; Hsu-Kim, H. Trends in the Rare Earth Element Content of U.S.-Based Coal Combustion Fly Ashes. Environ. Sci. Technol. 2016, 50, 5919–5926. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Lewandowska, M.; Nowak, J.; Białecka, B.; Całusz-Moszko, J.; Klupa, A. Ashes from bituminous coal burning in fluidized bed boilers as a potential source of rare earth elements. Gospod. Surowcami Miner.-Miner. Resour. Manag. 2018, 34, 21–36. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Lewandowska, M. The high temperature ashes (HTA) from bituminous coal combustion as a potential resource of rare earth elements. Gospod. Surowcami Miner.-Miner. Resour. Manag. 2018, 34, 135–150. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Białecka, B.; Nowak, J.; Klupa, A. Assessment of the potential of polish fly ashes as a source of rare earth elements. Ore Geol. Rev. 2020, 124, 103638. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Kokowska-Pawłowska, M.; Nowak, J. Distribution of Rare-Earth Elements in Ashes Produced in the Coal Combustion Process from Power Boilers. Energies 2023, 16, 2696. [Google Scholar] [CrossRef]
- Hussain, R.; Luo, K. Geochemical Evaluation of Enrichment of Rare-Earth and Critical Elements in Coal Wastes from Jurassic and Permo-Carboniferous Coals in Ordos Basin, China. Nat. Resour. Res. 2020, 29, 1731–1754. [Google Scholar] [CrossRef]
- Pyrgaki, K.; Gemeni, V.; Karkalis, C.; Koukouzas, N.; Koutsovitis, P.; Petrounias, P. Geochemical Occurrence of Rare Earth Elements in Mining Waste and Mine Water: A Review. Minerals 2021, 11, 860. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A review of rare elements and yttrium in coal ash: Content, modes of occurrences, combustion behavior, and extraction methods. Prog. Energy Combust. Sci. 2022, 88, 100954. [Google Scholar] [CrossRef]
- Strzałkowska, E. Rare earth elements and other critical elements in the magnetic fraction of fly ash from several Polish power plants. Int. J. Coal Geol. 2022, 258, 104015. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, X.; Cheng, F. Distribution Characteristics of Valuable Elements, Al, Li, and Ga, and Rare Earth Elements in Feed Coal, Fly Ash, and Bottom Ash from a 300 MW Circulating Fluidized Bed Boiler. ACS Omega 2019, 4, 6854–6863. [Google Scholar] [CrossRef]
- Tuan, L.Q.; Thenepalli, T.; Chilakala, R.; Vu, H.H.T.; Ahn, J.W.; Kim, J. Leaching Characteristics of Low Concentration Rare Earth Elements in Korean (Samcheok) CFBC Bottom Ash Samples. Sustainability 2019, 11, 2562. [Google Scholar] [CrossRef]
- Firman, H. A Study on the Potential Rare Earth Elements in Coal Combustion Product from Banjarsari Power Plant, South Sumatera. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1125, 012003. [Google Scholar] [CrossRef]
- Besari, D.A.A.; Anggara, F.; Rosita, W.; Petrus, T.B.M. Characterization and mode of occurrence of rare earth elements and yttrium in fly and bottom ash from coal-fired power plants in Java, Indonesia. Int. J. Coal Sci. Technol. 2022, 9, 20. [Google Scholar] [CrossRef]
- Bishop, B.A.; Shivacumar, K.R.; Alessi, D.S.; Robbins, L. Insights into the rare earth element potential of coal combustion by-products from western Canada. Environ. Sci. Adv. 2023, 2, 529–542. [Google Scholar] [CrossRef]
- Huang, S.; Ning, S.; Zhang, D.; Cai, Y.; Yan, X.; Liu, K.; Xu, X. Rare Earth Element Characteristics in Coal Ash from the Jungar Energy Gangue Power Plant, Inner Mongolia, China. Minerals 2023, 3, 1212. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Gkika, D.A.; Chalaris, M.; Kyzas, G.Z. Review of Methods for Obtaining Rare Earth Elements from Recycling and Their Impact on the Environment and Human Health. Processes 2024, 12, 1235. [Google Scholar] [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.I.; Al-Otaibi, J.S.; Cheira, M.F. Cetylpyridinium Bromide/Polyvinyl Chloride for Substantially Efficient Capture of Rare Earth Elements from Chloride Solution. Polymers 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Seredin, V.V. A new method for primary evaluation of the outlook for rare earth element ores. Geol. Ore Depos. 2010, 52, 428–433. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Suarez-Ruiz, I.; Valentim, B.; Borrego, A.G.; Bouzinos, A.; Flores, D.; Kalaitzidis, S.; Malinconico, M.L.; Marques, M.; Misz-Kennan, M.; Predeanu, G.; et al. Development of a petrographic classification of fly-ash components from coal combustion and co-combustion. (An ICCP Classification System, Fly-Ash Working Group—Commission III.). Int. J. Coal Geol. 2017, 183, 188–203. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World average for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Jarosiński, A. Możliwości pozyskiwania metali ziem rzadkich w Polsce. Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią Pol. Akad. Nauk 2016, 92, 75–88. [Google Scholar]
- Taylor, S.R.; McLennan, S.H. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, UK, 1985; pp. 1–312. [Google Scholar]
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMPRG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Götze, J.; Plötze, M.; Graupner, T.; Hallbauer, D.K.; Bray, C. Trace element incorporation into quartz: A combined study by ICP-MS, electron spin resonance, cathodoluminescence, capillary ion analysis and gas chromatography. Geochim. Cosmochim. Acta 2004, 68, 3741–3759. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]









| Power Plant | Sample |
|---|---|
| Łagisza | Z1 |
| Z2 | |
| Siersza | Z3 |
| Z4 | |
| Jaworzno III | Z5 |
| Z6 | |
| Łaziska | Z7 |
| Z8 |
| Power Plant | Sample | Q | Mu | An | Cc | He | Mgt | Mgh | SpFe | Am | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Łagisza | Z1 | 11.9 | 27.7 | 2.5 | 0.5 | 1.5 | 0.8 | 1.0 | 2.1 | 52.0 | 100.0 |
| Z2 | 11.5 | 23.9 | 2.6 | 0.4 | 0.4 | 0.6 | 1.5 | 59.1 | 100.0 | ||
| Siersza | Z3 | 11.2 | 11.9 | 4.8 | 0.5 | 0.6 | 0.5 | 1.5 | 69.0 | 100.0 | |
| Z4 | 15.7 | 17.2 | 3.7 | 0.7 | 1.1 | 0.7 | 0.3 | 1.2 | 59.4 | 100.0 | |
| Jaworzno III | Z5 | 8.8 | 19.9 | 4.2 | 1.0 | 0.3 | 2.1 | 63.7 | 100.0 | ||
| Z6 | 8.4 | 14.3 | 3.6 | 0.4 | 1.6 | 0.3 | 0.8 | 0.9 | 69.7 | 100.0 | |
| Łaziska | Z7 | 5.7 | 19.6 | 3.1 | 0.6 | 0.3 | 0.4 | 2.5 | 67.8 | 100.0 | |
| Z8 | 4.3 | 26.6 | 2.2 | 0.7 | 0.3 | 0.1 | 0.5 | 2.0 | 63.3 | 100.0 | |
| Min. | 4.3 | 11.9 | 2.2 | 0.0 | 0.3 | 0.3 | 0.9 | 52.0 | |||
| Max. | 15.7 | 27.7 | 4.8 | 0.7 | 1.6 | 0.8 | 1.0 | 2.5 | 69.7 | ||
| S | 9.7 | 20.1 | 3.3 | 0.5 | 0.9 | 0.2 | 0.6 | 1.7 | 62.6 | ||
| SD | 3.4 | 5.3 | 0.8 | 0.2 | 0.5 | 0.3 | 0.2 | 0.5 | 5.8 | ||
| V | 35.4 | 26.3 | 25.2 | 44.4 | 57.9 | 131.4 | 41.6 | 29.1 | 9.2 | ||
| Power Plant | Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | Mn3O4 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | LOI | DAI | SiO2/ Al2O3 | (MgO + CaO)/ (K2O + Na2O) | CaO/ MgO | K2O/ Na2O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Łagisza | Z1 | 46.37 | 0.91 | 23.04 | 9.19 | 0.11 | 2.23 | 2.86 | 1.05 | 2.08 | 0.22 | 0.21 | 11.73 | 4.96 | 2.01 | 1.63 | 1.28 | 1.98 |
| Z2 | 45.31 | 0.82 | 21.75 | 8.47 | 0.09 | 1.89 | 2.27 | 0.99 | 2.08 | 0.21 | 0.25 | 15.87 | 5.38 | 2.08 | 1.36 | 1.20 | 2.10 | |
| Siersza | Z3 | 48.80 | 0.81 | 20.34 | 8.26 | 0.10 | 2.57 | 3.72 | 1.82 | 2.13 | 0.10 | 0.14 | 11.21 | 4.96 | 2.40 | 1.59 | 1.45 | 1.17 |
| Z4 | 46.34 | 0.73 | 18.51 | 7.93 | 0.10 | 2.50 | 3.58 | 1.44 | 2.06 | 0.09 | 0.17 | 16.55 | 4.81 | 2.50 | 1.74 | 1.43 | 1.43 | |
| Jaworzno | Z5 | 50.23 | 1.01 | 24.21 | 10.06 | 0.09 | 2.08 | 2.97 | 1.45 | 2.19 | 0.17 | 0.19 | 5.35 | 5.08 | 2.07 | 1.39 | 1.43 | 1.51 |
| Z6 | 49.93 | 0.93 | 23.33 | 15.32 | 0.09 | 2.19 | 3.74 | 0.80 | 2.24 | 0.21 | 0.43 | 0.79 | 3.51 | 2.14 | 1.95 | 1.71 | 2.80 | |
| Łaziska | Z7 | 48.76 | 0.95 | 25.94 | 9.54 | 0.12 | 2.87 | 3.84 | 1.18 | 2.96 | 0.29 | 0.36 | 3.19 | 4.69 | 1.88 | 1.62 | 1.34 | 2.51 |
| Z8 | 46.81 | 0.96 | 25.48 | 8.32 | 0.12 | 2.89 | 3.35 | 0.73 | 3.08 | 0.33 | 0.27 | 7.66 | 5.04 | 1.84 | 1.64 | 1.16 | 4.22 | |
| Min. | 45.31 | 0.73 | 18.51 | 7.93 | 0.09 | 1.89 | 2.27 | 0.73 | 2.06 | 0.09 | 0.14 | 0.79 | 3.51 | 1.84 | 1.36 | 1.16 | 1.17 | |
| Max | 50.21 | 1.01 | 25.94 | 15.32 | 0.12 | 2.89 | 3.84 | 1.82 | 3.08 | 0.33 | 0.43 | 16.55 | 5.38 | 2.50 | 1.95 | 1.71 | 4.22 | |
| S | 47.81 | 0.89 | 22.82 | 9.64 | 0.10 | 2.40 | 3.29 | 1.18 | 2.35 | 0.20 | 0.25 | 9.05 | 4.80 | 2.12 | 1.61 | 1.37 | 2.22 | |
| SD | 1.71 | 0.09 | 2.37 | 2.25 | 0.01 | 0.34 | 0.51 | 0.35 | 0.39 | 0.08 | 0.09 | 5.39 | 0.56 | 0.23 | 0.19 | 0.17 | 0.98 | |
| V | 3.59 | 9.84 | 10.39 | 23.37 | 11.51 | 14.23 | 15.62 | 29.16 | 16.59 | 38.35 | 36.51 | 59.63 | 11.66 | 10.98 | 11.67 | 12.57 | 44.22 |
| PP | S | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Type REE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ppm] | |||||||||||||||||
| Łaziska | Z1 | 31.80 | 34.10 | 77.04 | 9.40 | 36.50 | 6.90 | 1.70 | 6.60 | 1.10 | 6.30 | 1.20 | 3.50 | 0.60 | 3.60 | 0.50 | H |
| Z2 | 34.00 | 33.40 | 75.90 | 9.30 | 37.30 | 8.70 | 1.80 | 7.60 | 1.10 | 7.20 | 1.30 | 3.90 | 0.60 | 3.60 | 0.50 | H | |
| Siersza | Z3 | 37.30 | 33.80 | 73.35 | 8.80 | 34.00 | 7.80 | 1.80 | 7.60 | 1.10 | 7.30 | 1.40 | 3.70 | 0.50 | 3.60 | 0.60 | H |
| Z4 | 33.80 | 30.30 | 67.31 | 8.00 | 30.20 | 6.80 | 1.50 | 6.50 | 1.10 | 5.60 | 1.20 | 3.10 | 0.50 | 3.20 | 0.50 | H | |
| Jaworzno | Z5 | 36.60 | 31.10 | 73.72 | 8.90 | 35.30 | 7.30 | 1.60 | 7.30 | 1.20 | 7.30 | 1.50 | 4.40 | 0.50 | 3.60 | 0.50 | H |
| Z6 | 41.00 | 32.50 | 73.95 | 8.80 | 35.60 | 8.10 | 1.90 | 8.10 | 1.30 | 7.60 | 1.50 | 4.40 | 0.60 | 4.00 | 0.70 | H | |
| Łaziska | Z7 | 45.90 | 41.20 | 96.31 | 11.20 | 46.50 | 9.50 | 2.10 | 9.60 | 1.50 | 8.90 | 1.80 | 4.80 | 0.80 | 5.10 | 0.80 | H |
| Z8 | 39.30 | 41.90 | 92.89 | 11.30 | 45.60 | 9.40 | 1.90 | 8.70 | 1.30 | 7.90 | 1.50 | 4.20 | 0.70 | 4.40 | 0.60 | H | |
| Min. | 31.80 | 30.30 | 67.31 | 8.00 | 30.20 | 6.80 | 1.50 | 6.50 | 1.10 | 5.60 | 1.20 | 3.10 | 0.50 | 3.20 | 0.50 | ||
| Max | 45.90 | 41.90 | 96.31 | 11.30 | 46.50 | 9.50 | 2.10 | 9.60 | 1.50 | 8.90 | 1.80 | 4.80 | 0.80 | 5.10 | 0.80 | ||
| S | 37.46 | 34.79 | 78.81 | 9.46 | 37.63 | 8.06 | 1.79 | 7.75 | 1.21 | 7.26 | 1.43 | 4.00 | 0.60 | 3.89 | 0.59 | ||
| SD | 4.56 | 4.38 | 10.20 | 1.18 | 5.63 | 1.06 | 0.19 | 1.04 | 0.15 | 0.99 | 0.20 | 0.56 | 0.11 | 0.60 | 0.11 | ||
| V | 12.16 | 12.58 | 12.94 | 12.48 | 14.95 | 13.14 | 10.55 | 13.43 | 12.02 | 13.67 | 13.91 | 13.89 | 17.82 | 15.52 | 19.17 | ||
| PP | S | REY | LREY | MREY | HREY | LREY | MREY | HREY | C | U | E | C | U | E | REO | Coutl | |
| [ppm] | [%] | [ppm] | [%] | [ppm] | |||||||||||||
| Łaziska | Z1 | 220.84 | 163.94 | 47.50 | 9.40 | 74 | 22 | 4 | 80.90 | 57.00 | 82.94 | 37 | 26 | 38 | 261.00 | 0.98 | |
| Z2 | 226.20 | 164.60 | 51.70 | 9.90 | 73 | 23 | 4 | 85.30 | 59.00 | 81.90 | 38 | 26 | 36 | 267.42 | 1.04 | ||
| Siersza | Z3 | 222.65 | 157.75 | 55.10 | 9.80 | 71 | 25 | 4 | 85.20 | 58.00 | 79.45 | 38 | 26 | 36 | 263.61 | 1.07 | |
| Z4 | 199.61 | 142.61 | 48.50 | 8.50 | 71 | 24 | 4 | 75.30 | 51.60 | 72.71 | 38 | 26 | 36 | 236.41 | 1.04 | ||
| Jaworzno | Z5 | 220.82 | 156.32 | 54.00 | 10.50 | 71 | 24 | 5 | 86.40 | 54.60 | 79.82 | 39 | 25 | 36 | 261.38 | 1.08 | |
| Z6 | 230.05 | 158.95 | 59.90 | 11.20 | 69 | 26 | 5 | 91.80 | 57.50 | 80.75 | 40 | 25 | 35 | 272.57 | 1.14 | ||
| Łaziska | Z7 | 286.01 | 204.71 | 68.00 | 13.30 | 72 | 24 | 5 | 109.70 | 71.50 | 104.81 | 38 | 25 | 37 | 338.41 | 1.05 | |
| Z8 | 271.59 | 201.09 | 59.10 | 11.40 | 74 | 22 | 4 | 100.20 | 71.30 | 100.09 | 37 | 26 | 37 | 320.98 | 1.00 | ||
| Min. | 199.61 | 142.61 | 47.50 | 8.50 | 69.09 | 21.51 | 4.20 | 75.30 | 51.60 | 72.71 | 36.63 | 24.73 | 35.10 | 236.41 | 0.98 | ||
| Max | 286.01 | 204.71 | 68.00 | 13.30 | 74.23 | 26.04 | 4.87 | 109.70 | 71.50 | 104.81 | 39.90 | 26.25 | 37.56 | 338.41 | 1.14 | ||
| S | 234.72 | 168.75 | 55.48 | 10.50 | 71.85 | 23.68 | 4.47 | 89.35 | 60.06 | 85.31 | 38.08 | 25.60 | 36.33 | 277.72 | 1.05 | ||
| SD | 28.91 | 22.15 | 6.74 | 1.47 | 1.74 | 1.55 | 0.25 | 11.01 | 7.37 | 11.08 | 1.09 | 0.59 | 0.74 | 34.10 | 0.05 | ||
| V | 12.31 | 13.13 | 12.16 | 14.03 | 2.43 | 6.54 | 5.68 | 12.33 | 12.27 | 12.99 | 2.86 | 2.32 | 2.05 | 12.28 | 4.74 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, Z.; Komorek, J.; Białecka, B.; Nowak, J. Assessing the Potential of Rare Earth Elements in Bottom Ash from Coal Combustion in Poland. Materials 2024, 17, 4323. https://doi.org/10.3390/ma17174323
Adamczyk Z, Komorek J, Białecka B, Nowak J. Assessing the Potential of Rare Earth Elements in Bottom Ash from Coal Combustion in Poland. Materials. 2024; 17(17):4323. https://doi.org/10.3390/ma17174323
Chicago/Turabian StyleAdamczyk, Zdzisław, Joanna Komorek, Barbara Białecka, and Jacek Nowak. 2024. "Assessing the Potential of Rare Earth Elements in Bottom Ash from Coal Combustion in Poland" Materials 17, no. 17: 4323. https://doi.org/10.3390/ma17174323
APA StyleAdamczyk, Z., Komorek, J., Białecka, B., & Nowak, J. (2024). Assessing the Potential of Rare Earth Elements in Bottom Ash from Coal Combustion in Poland. Materials, 17(17), 4323. https://doi.org/10.3390/ma17174323






