A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Neutron Diffraction
2.3. Thermodynamic Modelling in ThermoCalcTM
3. Results
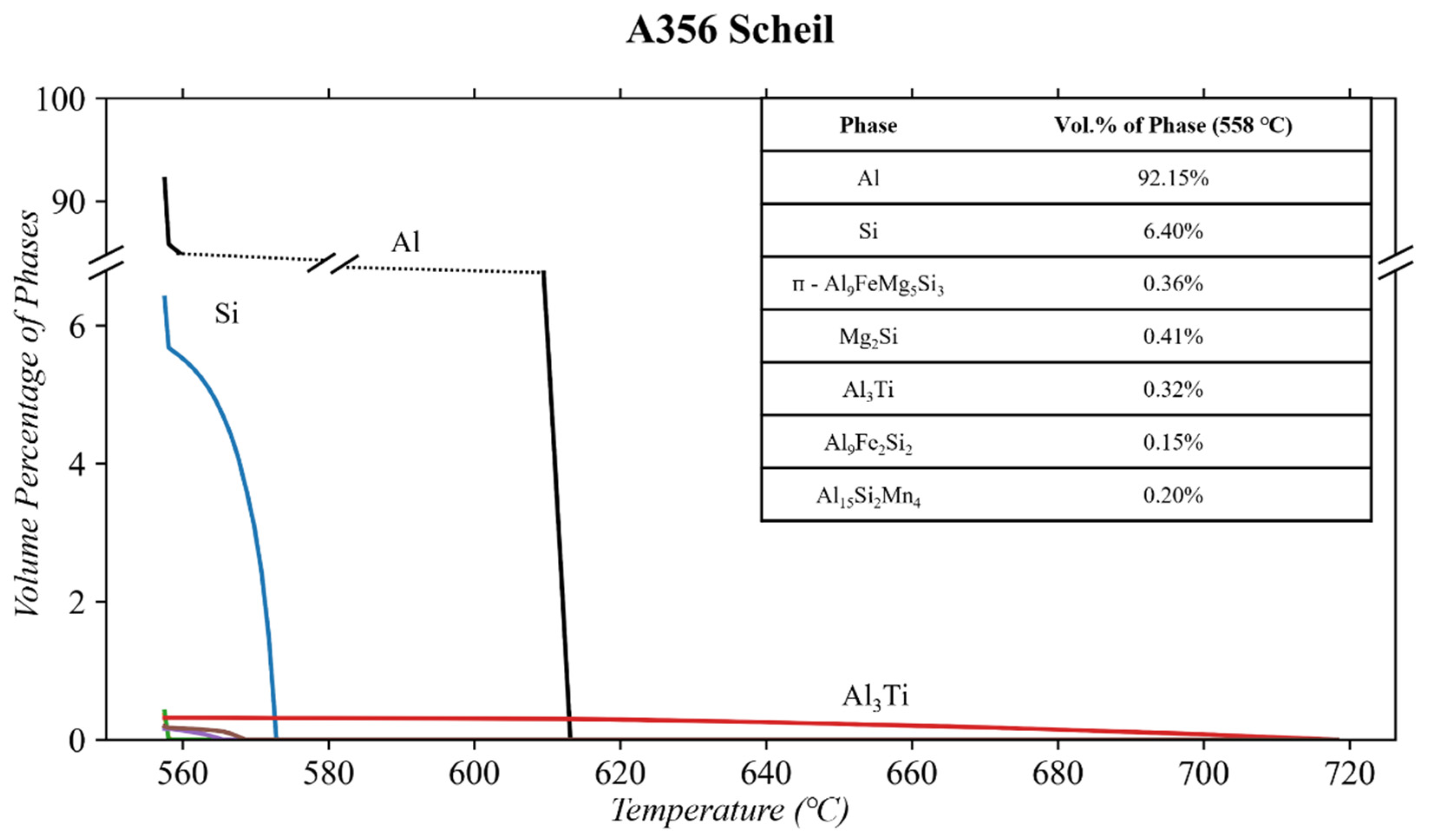
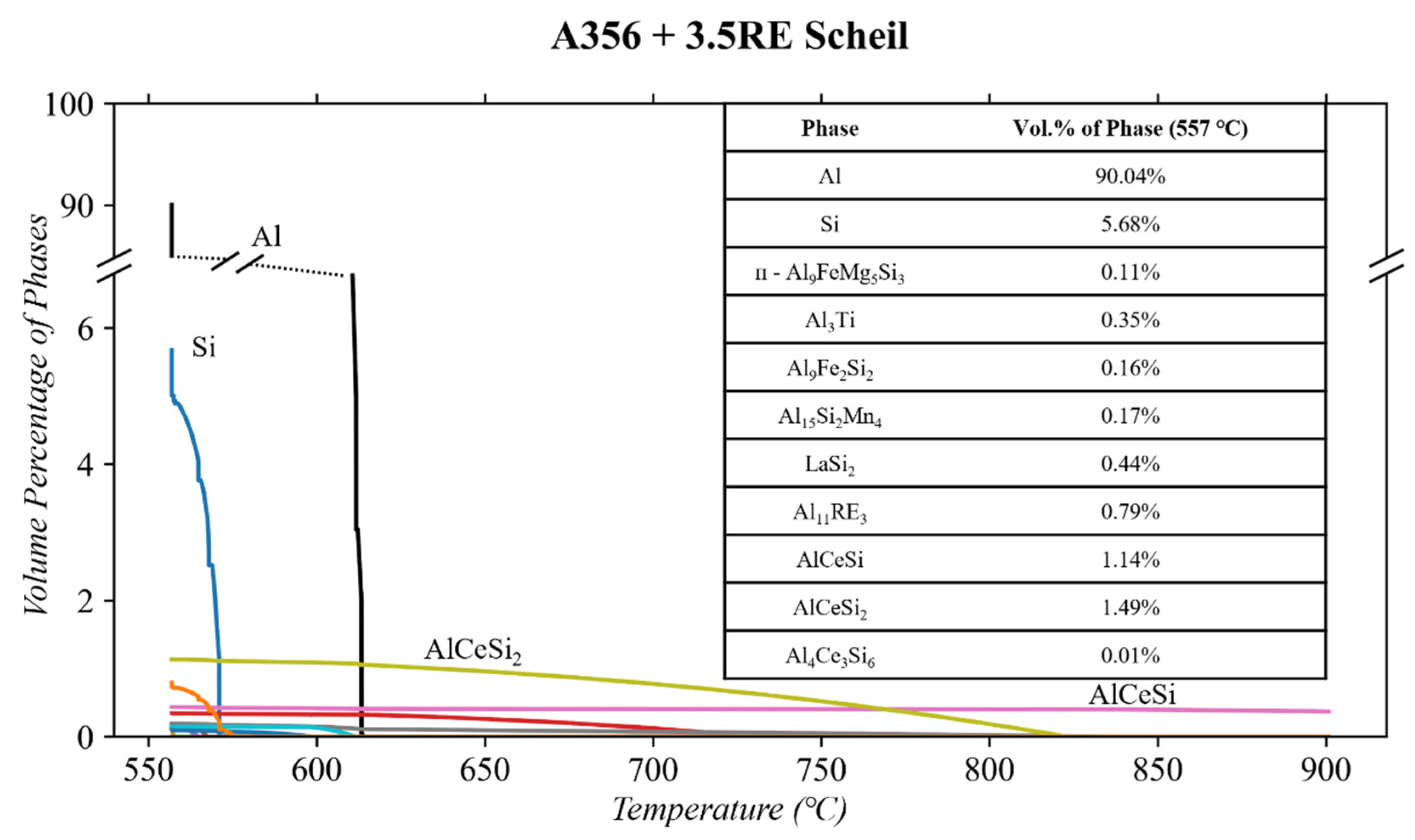
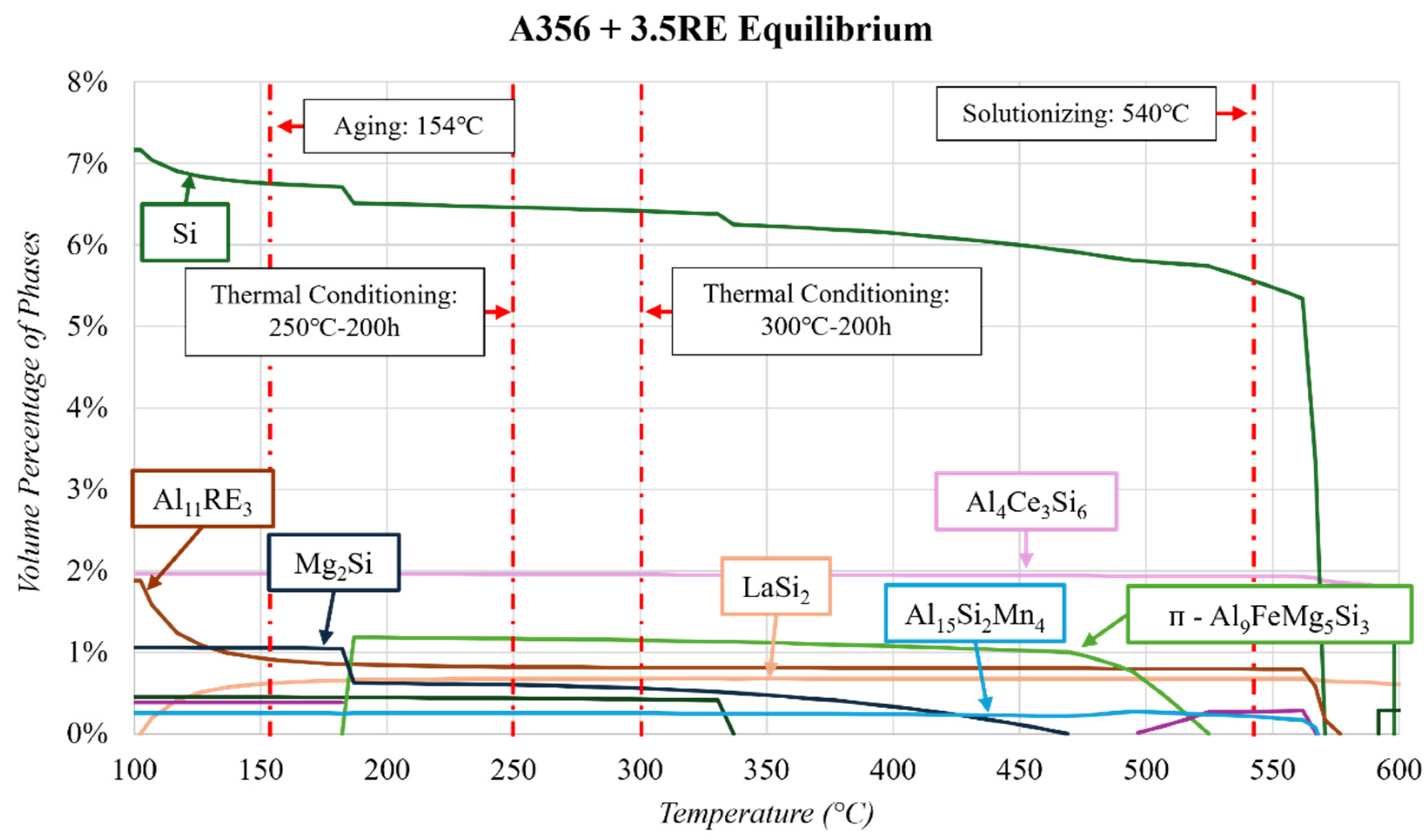
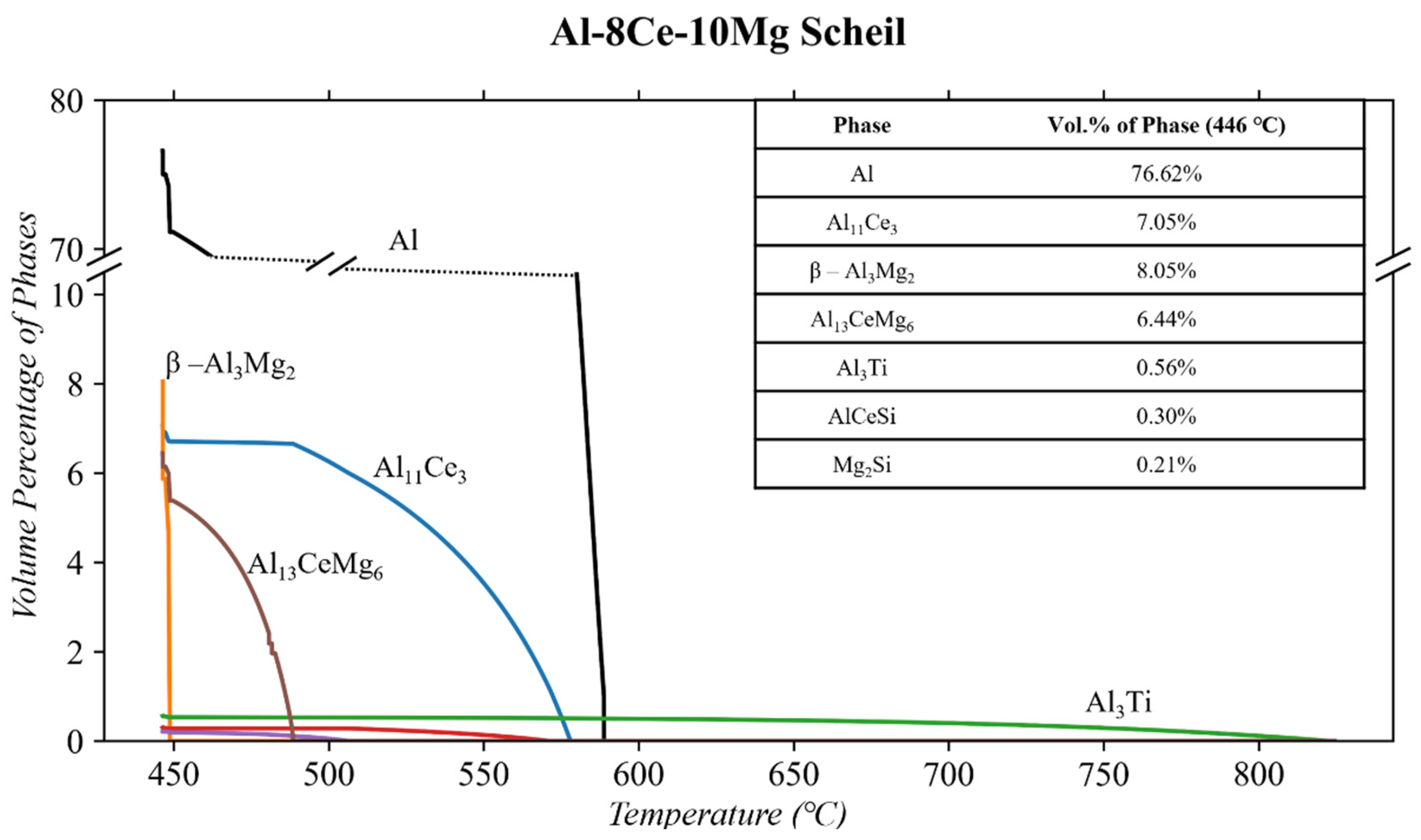
3.1. Thermodynamic Simulations
3.2. Quantitative Phase Analysis in MAUD
4. Discussion
4.1. T6 A356
4.2. As-Cast and T6 A356 + 3.5RE
4.3. As-Cast Al-8Ce-10Mg
5. Conclusions
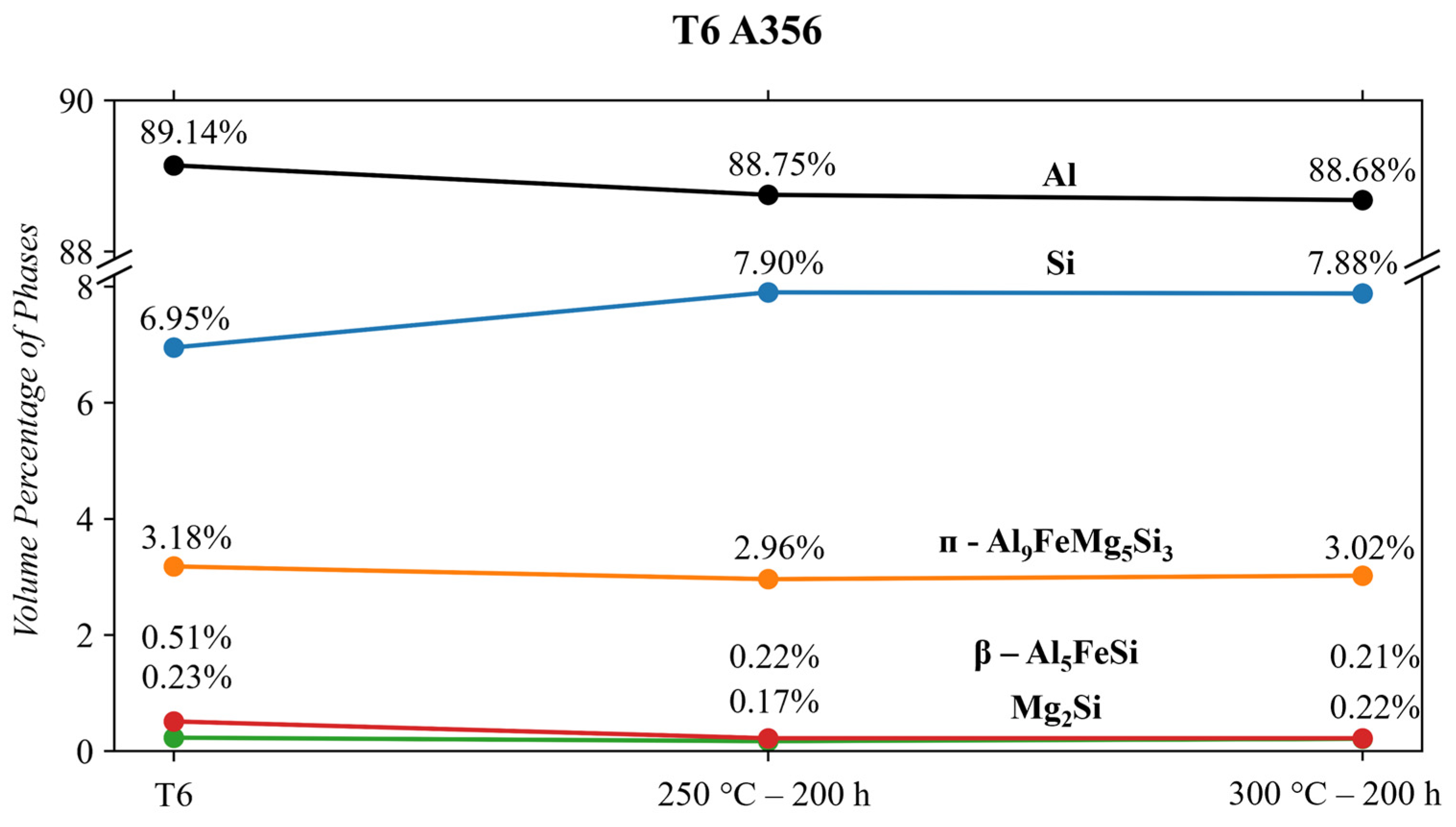
- The T6 A356 alloy contained the following phases, confirmed via Rietveld analysis: cubic Al, Si, Mg2Si, ᴨ-Al9FeSi3Mg5, β-Al5FeSi. Conditioning at 250 and 300 °C decreases the β-Al5FeSi phase from 0.51 to 0.22 vol.%. The Si phase grows significantly by ~13% when subjected to either condition temperature. The volume of other phases remained relatively stable when subjected to thermal conditioning. The growth of the Si phase significantly weakens this alloy and makes it appealing for this temperature region.
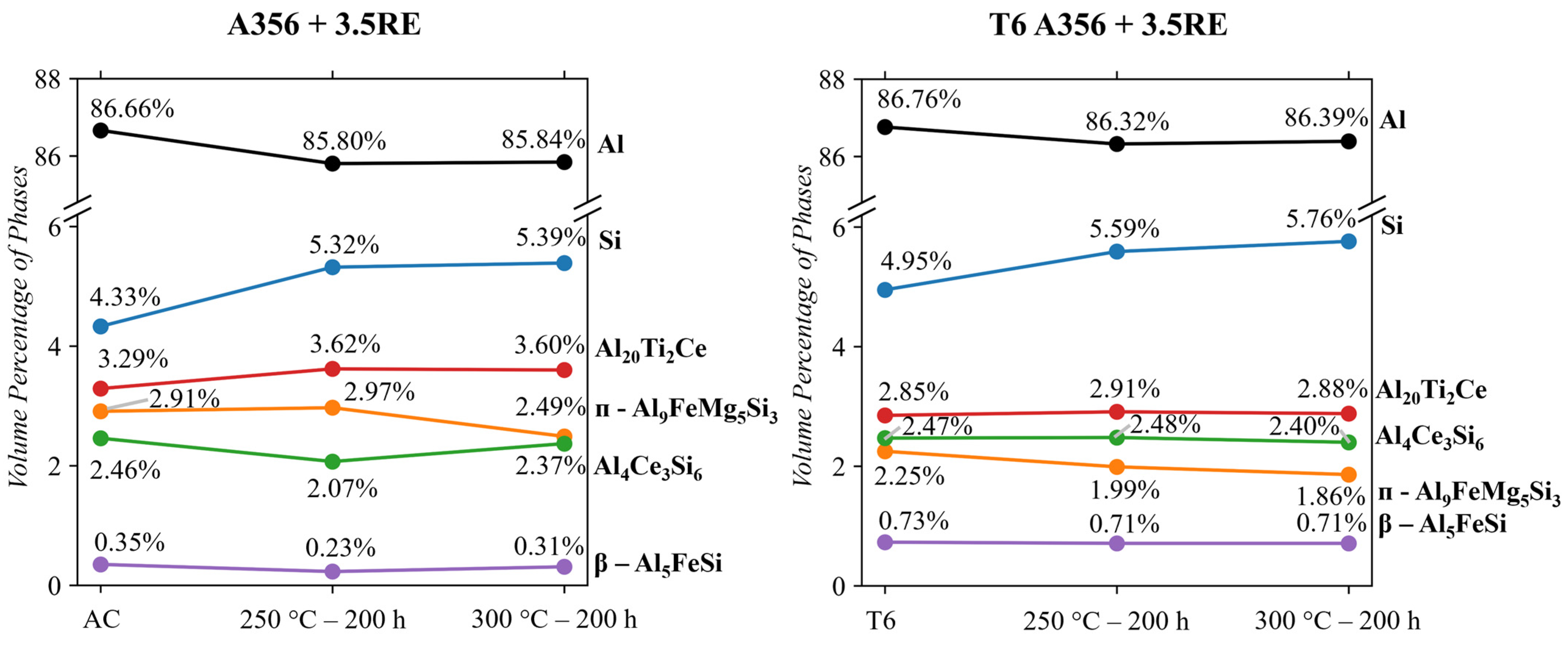
- The A356 + 3.5RE alloy responded to 250 °C conditioning with an increase in the Si, ᴨ-Al9FeSi3Mg5, and Al20Ti2Ce phases, causing a reduction in Al4Ce3Si6. When subjected to 300 °C thermal conditioning, the Al4Ce3Si6 and β-Al5FeSi stabilize, the Si and Al20Ti2Ce phases increase similarly to the 250 °C condition, and the ᴨ-Al9FeSi3Mg5 partially dissolves. The benefits of increasing the Al4Ce3Si6 and decreasing the ᴨ-Al9FeSi3Mg5 are beneficial for mechanical properties. However, the growth effects of Si and Al20Ti2Ce are typically detrimental to mechanical properties.
- When subjecting the A356 + 3.5RE alloy to the same T6 heat treatment as the A356 alloy, the Al4Ce3Si6 phase stabilizes, the Al20Ti2Ce decreases by ~13% from 3.3 to 2.9 vol.%, and the volume of β-Al5FeSi doubles from 0.35 to 0.7 vol.%. These phases become thermally stable and have negligible responses to thermal conditioning at 250 and 300 °C. The ᴨ-Al9FeSi3Mg5 phase decreases by 23% from 2.91 to 2.25 vol.%. This phase is thermally unstable due to the lack of Mg mobility and decreases by ~12% and 18% in response to thermal conditions at 250 and 300 °C, respectively. The volume of the Si phase within the alloy increases similarly to the T6 A356 and the as-cast A356 + 3.5RE alloys. All these refinements in the phases explain why the T6 A356 + 3.5RE alloy significantly outperforms the T6 A356 alloy in the desirable temperature region in other studies.
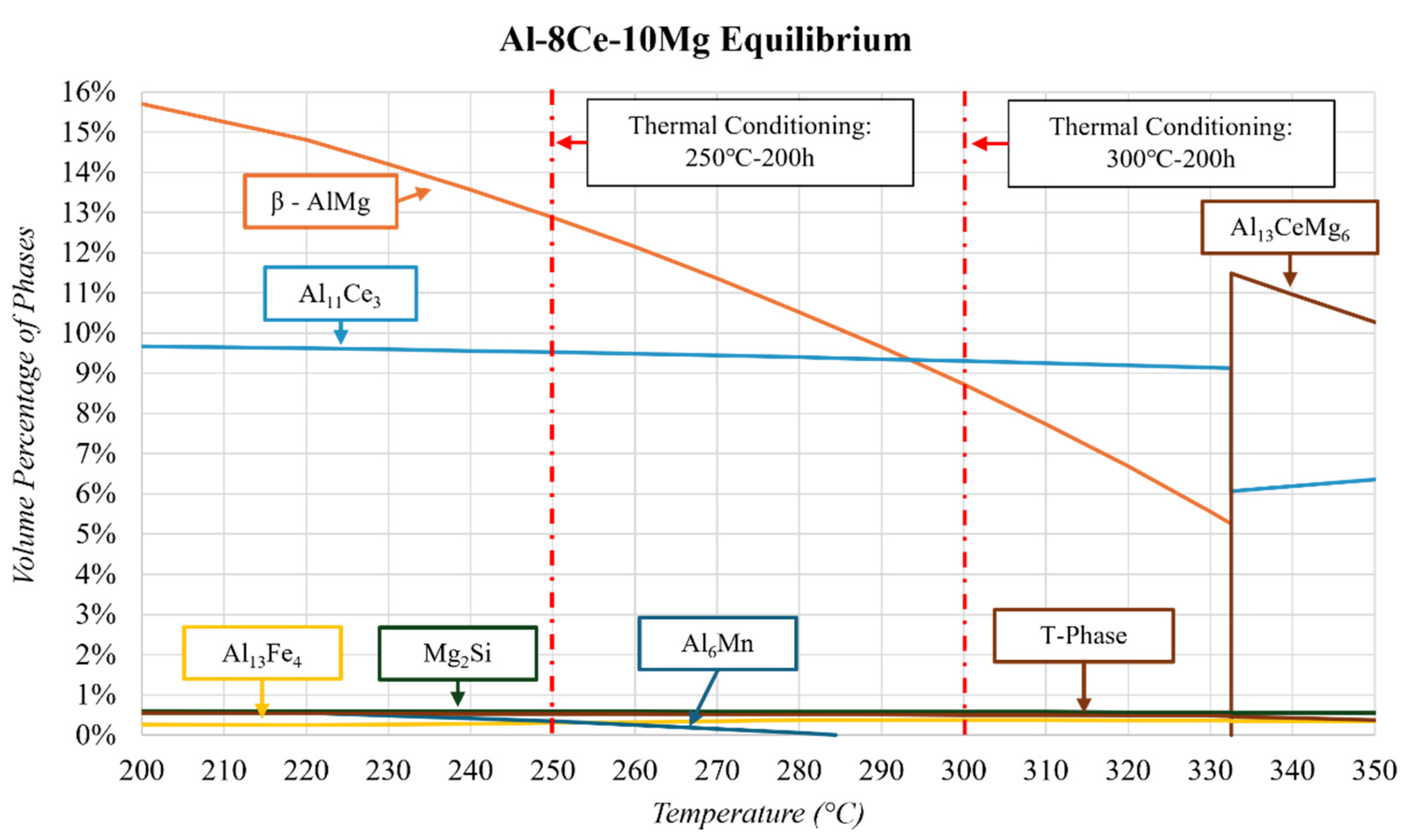
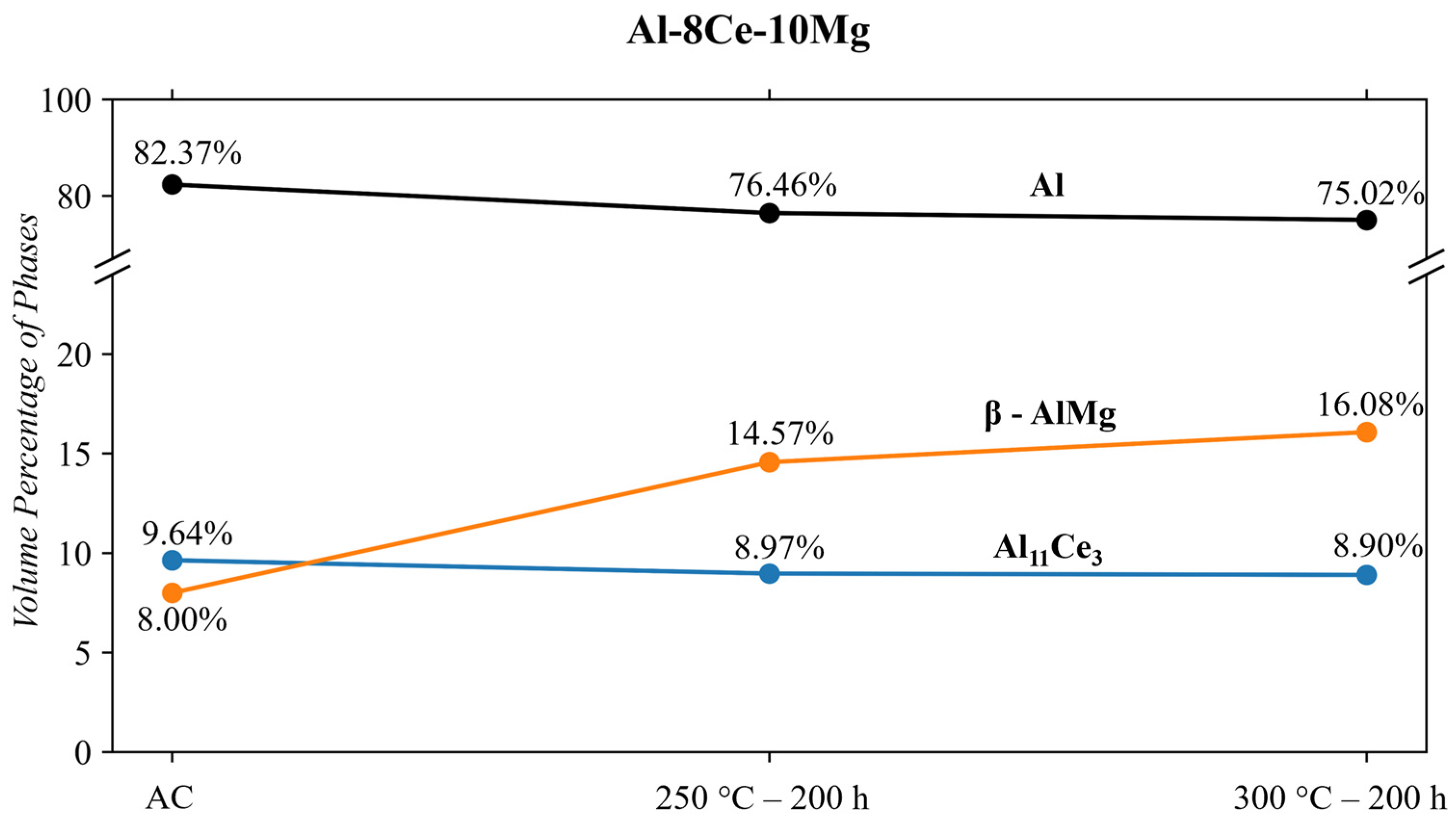
- The phases of the Al-8Ce-10Mg alloy in the as-cast state consist of Al (with Mg in solid solution), β-Al3Mg2, and Al11Ce3. The Al11Ce3 phase changes negligibly in response to thermal conditioning at 250 and 300 °C. The β-AlMg phase grows by 82% at 250 °C, and its volume doubles at 300 °C. This growth results from the alloy having little internal resistance to the migration of Mg from the solid solution with the matrix, evidenced by the decrease in the Al phase in response to the thermal conditions. The benefits from the stable Al11Ce3 are negatively affected by the increase in β-Al3Mg2 at 250 and 300 °C, making utilizing the alloy at this temperature region unfavourable. The full benefits of the stable Al11Ce3 phase will only be obtained at the temperature point where it is more favourable for the β-Al3Mg2 to dissolve back into the matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stroh, J.; Sediako, D.; Weiss, D. The Effects of Iron-Bearing Intermetallics on the Fitness-for-Service Performance of a Rare-Earth-Modified A356 Alloy for Next Generation Automotive Powertrains. Metals 2021, 11, 788. [Google Scholar] [CrossRef]
- Di Giovanni, M.T.; Cerri, E.; Casari, D.; Merlin, M.; Arnberg, L.; Garagnani, G.L. The Influence of Ni and V Trace Elements on High-Temperature Tensile Properties and Aging of A356 Aluminum Foundry Alloy. Metall. Mater. Trans. A 2016, 47, 2049–2057. [Google Scholar] [CrossRef]
- Stroh, J.; Sediako, D. Elevated Temperature Tensile and Creep Performance of Conditioned T7 319 Aluminum Powertrain Alloy for Next Generation Diesel Engine Blocks. Int. J. Met. 2023, 17, 2702–2715. [Google Scholar] [CrossRef]
- Elsayed, A.; Kotiadis, S. Utilizing Low Solubility, Light Metal Eutectic Systems for Castable, High Electrical/Thermal Conductivity Alloys. Trans. Indian Inst. Met. 2024. [Google Scholar] [CrossRef]
- Sims, Z.C.; Rios, O.R.; Weiss, D.; Turchi, P.E.A.; Perron, A.; Lee, J.R.I.; Li, T.T.; Hammons, J.A.; Bagge-Hansen, M.; Willey, T.M.; et al. High Performance Aluminum-Cerium Alloys for High-Temperature Applications. Mater. Horiz. 2017, 4, 1070–1078. [Google Scholar] [CrossRef]
- Nguyen, R.T.; Imholte, D.D.; Rios, O.R.; Weiss, D.; Sims, Z.; Stromme, E.; McCall, S.K. Anticipating Impacts of Introducing Aluminum-Cerium Alloys into the United States Automotive Market. Resour. Conserv. Recycl. 2019, 144, 340–349. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in Aluminum Alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced Lightweight Materials for Automobiles: A Review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- CiNiViZ, M.; Köse, H. Hydrogen Use in Internal Combustion Engine: A Review. Acad. Pap. 2012, 1, 1–15. [Google Scholar]
- Yip, H.L.; Srna, A.; Yuen, A.C.Y.; Kook, S.; Taylor, R.A.; Yeoh, G.H.; Medwell, P.R.; Chan, Q.N. A Review of Hydrogen Direct Injection for Internal Combustion Engines: Towards Carbon-Free Combustion. Appl. Sci. 2019, 9, 4842. [Google Scholar] [CrossRef]
- Qin, J.; Nagaumi, H.; Yu, C.; Liu, F.; Li, Y.; Wang, L. Coarsening Behavior of Mg2Si Precipitates during Post Homogenization Cooling Process in Al-Mg-Si Alloy. J. Alloys Compd. 2022, 902, 162851. [Google Scholar] [CrossRef]
- Choi, H.; Li, X. Refinement of Primary Si and Modification of Eutectic Si for Enhanced Ductility of Hypereutectic Al–20Si–4.5Cu Alloy with Addition of Al2O3 Nanoparticles. J. Mater. Sci. 2012, 47, 3096–3102. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Y.; Zheng, X.; Zhang, Y.; Chen, L.; Wang, J. Research Progress on Multi-Component Alloying and Heat Treatment of High Strength and Toughness Al–Si–Cu–Mg Cast Aluminum Alloys. Materials 2023, 16, 1065. [Google Scholar] [CrossRef]
- Peng, J.; Tang, X.; He, J.; Xu, D. Effect of Heat Treatment on Microstructure and Tensile Properties of A356 Alloys. Trans. Nonferr. Met. Soc. China 2011, 21, 1950–1956. [Google Scholar] [CrossRef]
- Mahidhara, R.K. Elevated-Temperature Coarsening Behavior in Aluminum Alloys. J. Mater. Eng. Perform. 1997, 6, 102–105. [Google Scholar] [CrossRef]
- Lombardi, A.; Ravindran, C.; Sediako, D.; MacKay, R. Determining the Mechanism of In-Service Cylinder Distortion in Aluminum Engine Blocks with Cast-In Gray Iron Liners. Metall. Mater. Trans. A 2014, 45, 6291–6303. [Google Scholar] [CrossRef]
- Stroh, J.; Sediako, D.; Weiss, D. The Effect of Rare Earth Mischmetal on the High Temperature Tensile Properties of an A356 Aluminum Allo. In Proceedings of the Light Metals 2021; The Minerals, Metals & Material Series; Springer International Publishing: Cham, Switzerland, 2021; pp. 184–191. [Google Scholar]
- Stroh, J. Development of Precipitation-Strengthened Aluminum Alloys and Manufacturing Processes for Next Generation Automotive Powertrains. Ph.D. Thesis, University of British Columbia, Kelowna, BC, Canada, 2021. [Google Scholar]
- Belov, N.A.; Naumova, E.A.; Eskin, D.G. Casting Alloys of the Al–Ce–Ni System: Microstructural Approach to Alloy Design. Mater. Sci. Eng. A 1999, 271, 134–142. [Google Scholar] [CrossRef]
- Stroh, J.; Sediako, D.; Weiss, D.; Peterson, V.K. In Situ Neutron Diffraction Solidification Analyses of Rare Earth Reinforced Hypoeutectic and Hypereutectic Aluminum-Silicon Alloys. In Proceedings of the Light Metals 2020; The Minerals, Metals & Material Series; Springer International Publishing: Cham, Switzerland, 2020; pp. 174–178. [Google Scholar]
- Elgallad, E.M.; Ibrahim, M.F.; Doty, H.W.; Samuel, F.H. Microstructural Characterisation of Al–Si Cast Alloys Containing Rare Earth Additions. Philos. Mag. 2018, 98, 1337–1359. [Google Scholar] [CrossRef]
- Sims, Z.C.; Kesler, M.S.; Henderson, H.B.; Castillo, E.; Fishman, T.; Weiss, D.; Singleton, P.; Eggert, R.; McCall, S.K.; Rios, O. How Cerium and Lanthanum as Coproducts Promote Stable Rare Earth Production and New Alloys. J. Sustain. Metall. 2022, 8, 1225–1234. [Google Scholar] [CrossRef]
- Stromme, E.T.; Henderson, H.B.; Sims, Z.C.; Kesler, M.S.; Weiss, D.; Ott, R.T.; Meng, F.; Kassoumeh, S.; Evangelista, J.; Begley, G.; et al. Ageless Aluminum-Cerium-Based Alloys in High-Volume Die Casting for Improved Energy Efficiency. JOM 2018, 70, 866–871. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef] [PubMed]
- Aniolek, M.; Smith, T.; Czerwinski, F. Combining Differential Scanning Calorimetry and Cooling-Heating Curve Thermal Analysis to Study the Melting and Solidification Behavior of Al-ce Binary Alloys. Metals 2021, 11, 372. [Google Scholar] [CrossRef]
- Kozakevich, J.R.; Stroh, J.; Sediako, D.; Weiss, D. Solidification Kinetics of an Al-Ce Alloy with Additions of Ni and Mn. Metals 2023, 13, 955. [Google Scholar] [CrossRef]
- Kozakevich, J.; Stroh, J.; Mallouhi, V.; Sediako, D.; Weiss, D. Interplay Between Cooling Rate, Microstructure, and Mechanical Properties of an Al–Ce–Ni–Mn Alloy. In Proceedings of the Light Metals 2022; Eskin, D., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 83–91. [Google Scholar]
- Zhang, H.; Wu, M.; Li, Z.; Xiao, D.; Huang, Y.; Huang, L.; Liu, W. Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys. Materials 2024, 17, 3999. [Google Scholar] [CrossRef]
- Shen, S.; Wu, C.; Li, Y.; Huang, Y.; Huang, W.; Zhang, P.; Zhong, S.; Lu, Y.; Luo, G.; Gan, Z.; et al. Refining Mechanism and Elevated-Temperature Mechanical Properties of Al-Ce Alloys Solidified under Super Gravity Field. Mater. Sci. Eng. A 2023, 879, 145191. [Google Scholar] [CrossRef]
- Perrin, A.E.; Michi, R.A.; Leonard, D.N.; Sisco, K.D.; Plotkowski, A.J.; Shyam, A.; Poplawsky, J.D.; Allard, L.F.; Yang, Y. Effect of Mn on Eutectic Phase Equilibria in Al-Rich Al-Ce-Ni Alloys. J. Alloys Compd. 2023, 965, 171455. [Google Scholar] [CrossRef]
- Aghaie, E.; Stroh, J.; Sediako, D.; Rashidi, A.; Milani, A.S. Improving the Mechanical Properties of the B319 Aluminum Alloy by Addition of Cerium. Mater. Sci. Eng. A 2020, 793, 139899. [Google Scholar] [CrossRef]
- Sims, Z.C.; Weiss, D.; Rios, O.; Henderson, H.B.; Kesler, M.S.; McCall, S.K.; Thompson, M.J.; Perron, A.; Moore, E.E. The Efficacy of Replacing Metallic Cerium in Aluminum-Cerium Alloys with LREE Mischmetal. In Proceedings of the Light Metals 2020; The Minerals, Metals & Material Series; Springer International Publishing: Cham, Switzerland, 2020; pp. 216–221. [Google Scholar]
- Stroh, J.; Sediako, D.; Weiss, D. Development of Cerium-Reinforced Specialty Aluminum Alloy with Application of X-ray and Neutron Diffraction. Int. J. Met. 2021, 15, 29–39. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Chankitmunkong, S.; Wang, S.; Eskin, D.G.; Patakham, U.; Limmaneevichitr, C.; Pandee, P. Enhancing Ambient and Elevated Temperature Performance of Hypoeutectic Al–Ce Cast Alloys by Al3(Sc,Zr) Precipitate. J. Mater. Res. Technol. 2024, 28, 1188–1197. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum–Cerium Binary Alloys Containing the Al–Al11Ce3 Eutectic. Mater. Sci. Eng. A 2021, 809, 140973. [Google Scholar] [CrossRef]
- Czerwinski, F.; Amirkhiz, B.S. On the Al–Al11Ce3 Eutectic Transformation in Ce3 Eutectic Transformation in in Alloys. Materials 2020, 13, 4549. [Google Scholar] [CrossRef] [PubMed]
- Czerwinski, F. A Search for the Eutectic System of High-Temperature Cast Aluminium Alloys. Mater. Sci. Technol. UK 2021, 37, 683–692. [Google Scholar] [CrossRef]
- Sims, Z.C.; Weiss, D.; McCall, S.K.; McGuire, M.A.; Ott, R.T.; Geer, T.; Rios, O.; Turchi, P.A.E. Cerium-Based, Intermetallic-Strengthened Aluminum Casting Alloy: High-Volume Co-Product Development. J. Miner. Met. Mater. Soc. 2016, 68, 1940–1947. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, J.; Han, Q.; Sun, B.; Wang, J.; Liu, C. Microstructure Diversity Dominated by the Interplay between Primary Intermetallics and Eutectics for Al-Ce Heat-Resistant Alloys. J. Alloys Compd. 2022, 899, 162914. [Google Scholar] [CrossRef]
- Weiss, D.; Rios, O.; Sims, Z.; Mccall, S.; Ott, R. Casting Characteristics of High Cerium Content Aluminum Alloys. In Light Metals 2017; Springer International Publishing: Cham, Switzerland, 2017; pp. 205–211. [Google Scholar]
- Jin, L.; Liu, K.; Chen, X.G. Evolution of Dispersoids and Their Effects on Elevated-Temperature Strength and Creep Resistance in Al-Si-Cu 319 Cast Alloys with Mn and Mo Additions. Mater. Sci. Eng. A 2020, 770, 138554. [Google Scholar] [CrossRef]
- Meyer, M.D.; Vanderschueren, D. The Characterization of Retained Austenite in TRIP Steels by X-ray Diffraction. In Proceedings of the Mechanical Working and Steel Processing Conference Proceedings, Baltimore, MD, USA, 24–27 October 1999; Volume 37, pp. 265–276. [Google Scholar]
- Onuki, Y.; Hoshikawa, A.; Sato, S.; Ishigaki, T.; Tomida, T. Quantitative Phase Fraction Analysis of Steel Combined with Texture Analysis Using Time-of-Flight Neutron Diffraction. J. Mater. Sci. 2017, 52, 11643–11658. [Google Scholar] [CrossRef]
- Lisowski, P.W.; Schoenberg, K.F. The Los Alamos Neutron Science Center. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2006, 562, 910–914. [Google Scholar] [CrossRef]
- Wenk, H.-R.; Lutterotti, L.; Vogel, S.C. Rietveld Texture Analysis from TOF Neutron Diffraction Data. Powder Diffr. 2010, 25, 283–296. [Google Scholar] [CrossRef]
- Losko, A.S.; Vogel, S.C.; Reiche, H.M.; Nakotte, H. A Six-Axis Robotic Sample Changer for High-Throughput Neutron Powder Diffraction and Texture Measurements. J. Appl. Crystallogr. 2014, 47, 2109–2112. [Google Scholar] [CrossRef]
- Takajo, S.; Vogel, S.C. Determination of Pole Figure Coverage for Texture Measurements with Neutron Time-of-Flight Diffractometers. J. Appl. Crystallogr. 2018, 51, 895–900. [Google Scholar] [CrossRef]
- Wenk, H.-R.; Lutterotti, L.; Vogel, S. Texture Analysis with the New HIPPO TOF Diffractometer. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2003, 515, 575–588. [Google Scholar] [CrossRef]
- Sims, Z.C. Development of a Novel Casting Alloy Composed of Aluminum and Cerium with Other Minor Additions. Ph.D. Thesis, The University of Tennessee Knoxville, Knoxville, TN, USA, 2020. [Google Scholar]
- Easton, M.A.; StJohn, D.H. A Model of Grain Refinement Incorporating Alloy Constitution and Potency of Heterogeneous Nucleant Particles. Acta Mater. 2001, 49, 1867–1878. [Google Scholar] [CrossRef]
- Dong, G.; Li, S.; Ma, S.; Zhang, D.; Bi, J.; Wang, J.; Starostenkov, M.D.; Xu, Z. Process Optimization of A356 Aluminum Alloy Wheel Hub Fabricated by Low-Pressure Die Casting with Simulation and Experimental Coupling Methods. J. Mater. Res. Technol. 2023, 24, 3118–3132. [Google Scholar] [CrossRef]
- Henderson, H.B.; Weiss, D.; Sims, Z.C.; Thompson, M.J.; Moore, E.E.; Perron, A.; Meng, F.; Ott, R.T.; Rios, O. Ternary Interactions and Implications for Third Element Alloying Potency in Al–Ce-Based Alloys. In Light Metals 2020; Tomsett, A., Ed.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2020; pp. 227–232. ISBN 978-3-030-36407-6. [Google Scholar]
- Weiss, D. Improved High-Temperature Aluminum Alloys Containing Cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Samuel, E.; Samuel, A.M.; Songmene, V.; Samuel, F.H. A Review on the Analysis of Thermal and Thermodynamic Aspects of Grain Refinement of Aluminum-Silicon-Based Alloys. Materials 2023, 16, 5639. [Google Scholar] [CrossRef]
- Bi, Q.; Luo, X.; Guo, L.; Zuo, X.; Huang, B.; Yi, J.; Zhou, Y. Effects of In-Situ Reaction, Extrusion Ratio and CeO2 on the Performance of Al-Ti-C-(Ce) Grain Refiners for Refining Pure Aluminum Grains. Materials 2023, 16, 4481. [Google Scholar] [CrossRef]
- Lane, R.J.; Momen, A.M.; Kesler, M.S.; Brechtl, J.; Rios, O.; Nawaz, K.; Mirzaeifar, R. Developing an Experimental-Computational Framework to Investigate the Deformation Mechanisms and Mechanical Properties of Al-8Ce-10Mg Alloys at Micro and Macroscales. Mater. Today Commun. 2021, 28, 102674. [Google Scholar] [CrossRef]
- Ng, D.S.; Dunand, D.C. Aging- and Creep-Resistance of a Cast Hypoeutectic Al-6.9Ce-9.3Mg (Wt.%) Alloy. Mater. Sci. Eng. A 2020, 786, 139398. [Google Scholar] [CrossRef]
- Saville, A.I.; Creuziger, A.; Mitchell, E.B.; Vogel, S.C.; Benzing, J.T.; Klemm-Toole, J.; Clarke, K.D.; Clarke, A.J. MAUD Rietveld Refinement Software for Neutron Diffraction Texture Studies of Single- and Dual-Phase Materials. Integr. Mater. Manuf. Innov. 2021, 10, 461–487. [Google Scholar] [CrossRef]
- Lutterotti, L. MAUD Tutorial—Instrumental Broadening Determination; Dipartimento di Ingegneria dei Materiali, Università di Trento: Trento, Italy, 2006. [Google Scholar]
- Crystallography Open Database: Browse the COD. Available online: https://www.crystallography.net/cod/browse.html (accessed on 26 May 2024).
- Ding, W.-J.; Yi, J.-X.; Chen, P.; Li, D.-L.; Peng, L.-M.; Tang, B.-Y. Elastic Properties and Electronic Structures of Typical Al–Ce Structures from First-Principles Calculations. Solid State Sci. 2012, 14, 555–561. [Google Scholar] [CrossRef]
- Shin, E.J.; Seong, B.S.; Joung, T.W.; Hong, K.P.; Lee, C.H.; Kim, Y.J. Quantitative Phase Analysis of the Multi-Phase Alloys Using Neutron Diffraction. Key Eng. Mater. 2004, 270–273, 1285–1290. [Google Scholar] [CrossRef]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Toby, B.H. A Simple Solution to the Rietveld Refinement Recipe Problem. J. Appl. Crystallogr. 2024, 57, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.G.; Davidson, C.J. Solidification and Precipitation Behaviour of Al-Si-Mg Casting Alloys. J. Mater. Sci. 2001, 36, 739–750. [Google Scholar] [CrossRef]
- Wang, Q.G.; Caceres, C.H.; Griffiths, J.R. Damage by Eutectic Particle Cracking in Aluminum Casting Alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2901–2912. [Google Scholar] [CrossRef]
- Taylor, J.A.; St John, D.H.; Barresi, J.; Couper, M.J. Influence of Mg Content on the Microstructure and Solid Solution Chemistry of Al-7%Si-Mg Casting Alloys during Solution Treatment; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2000; Volume 331–337, ISBN 978-3-0357-0550-8. [Google Scholar]
- Nie, J.F.; Sridhara, S.; Muddle, B.C. Characterization of Dispersed Phases in Rapidly Quenched Intermetallic AI-Ti-Ce Alloys. Metall. Trans. A 1994, 179–180, 619–624. [Google Scholar] [CrossRef]
- Aghaie, E.; Stroh, J.; Sediako, D.; Smith, M. In-Situ Fitness-for-Service Assessment of Aluminum Alloys Developed for Automotive Powertrain Lightweighting. In Light Metals 2018; Martin, O., Ed.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 397–400. ISBN 978-3-319-72283-2. [Google Scholar]
- Golovin, I.S.; Bychkov, A.S.; Medvedeva, S.V.; Hu, X.S.; Zheng, M.Y. Mechanical Spectroscopy of Al-Mg Alloys. Phys. Met. Metallogr. 2013, 114, 327–338. [Google Scholar] [CrossRef]


| Alloys | Al | Si | Mg | Ce | Cu | Fe | Mn | Ti | La | Nd | Pr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A356 | Bal. | 7.28 | 0.49 | - | 0.03 | 0.13 | 0.10 | 0.20 | - | - | - |
| A356 + 3.5RE | Bal. | 7.28 | 0.49 | 1.83 | 0.03 | 0.13 | 0.10 | 0.20 | 0.92 | 0.58 | 0.19 |
| Al-8Ce-10Mg | Bal. | 0.15 | 9.5–10.00 | 7.84–8.16 | 0.03 | 0.15 | 0.25 | 0.25 | - | - | - |
| Alloy | Conditioning | Sigma | Rwp (%) | Rexp (%) |
|---|---|---|---|---|
| A356 | T6 | 1.09 | 5.92 | 5.43 |
| A356 | T6 + 250 °C-200 h | 1.01 | 5.54 | 5.49 |
| A356 | T6 + 300 °C-200 h | 1.06 | 5.74 | 5.42 |
| A356 + 3.5RE | As-cast | 1.11 | 6.00 | 5.41 |
| A356 + 3.5RE | 250 °C-200 h | 1.06 | 5.83 | 5.50 |
| A356 + 3.5RE | 300 °C-200 h | 1.03 | 5.51 | 5.37 |
| A356 + 3.5RE | T6 | 1.02 | 5.45 | 5.36 |
| A356 + 3.5RE | T6 + 250 °C-200 h | 1.00 | 5.52 | 5.54 |
| A356 + 3.5RE | T6 + 300 °C-200 h | 1.04 | 5.70 | 5.48 |
| Al-8Ce-10Mg | As-cast | 0.95 | 5.33 | 5.61 |
| Al-8Ce-10Mg | 250 °C-200 h | 0.94 | 5.16 | 5.49 |
| Al-8Ce-10Mg | 300 °C-200 h | 0.99 | 5.51 | 5.57 |
| Alloy | Conditioning | Volume Percentage of Phases | ||||
|---|---|---|---|---|---|---|
| Al | Si | ᴨ-Al9FeSi3Mg5 | Mg2Si | β-Al5FeSi | ||
| A356 | T6 | 89.14 | 6.95 ± 0.02 | 3.17 ± 0.05 | 0.23 ± 0.02 | 0.51 ± 0.02 |
| A356 | T6 + 250 °C-200 h | 88.75 | 7.90 ± 0.02 | 2.96 ± 0.04 | 0.17 ± 0.03 | 0.22 ± 0.03 |
| A356 | T6 + 300 °C-200 h | 88.68 | 7.88 ± 0.03 | 3.02 ± 0.05 | 0.21 ± 0.02 | 0.22 ± 0.02 |
| Alloy | Conditioning | Volume Percentage of Phases | |||||
|---|---|---|---|---|---|---|---|
| Al | Si | Al4Ce3Si6 | ᴨ-Al9FeSi3Mg5 | Al20Ti2Ce | β-Al5FeSi | ||
| A356 + 3.5RE | As-Cast | 86.66 | 4.33 ± 0.03 | 2.46 ± 0.09 | 2.91 ± 0.05 | 3.29 ± 0.07 | 0.35 ± 0.05 |
| A356 + 3.5RE | 250 °C-200 h | 85.80 | 5.32 ± 0.02 | 2.07 ± 0.08 | 2.97 ± 0.05 | 3.62 ± 0.06 | 0.23 ± 0.04 |
| A356 + 3.5RE | 300 °C-200 h | 85.84 | 5.39 ± 0.02 | 2.37 ± 0.08 | 2.49 ±0.05 | 3.60 ± 0.07 | 0.31 ± 0.04 |
| A356 + 3.5RE | T6 | 86.76 | 4.95 ± 0.02 | 2.47 ± 0.02 | 2.25 ± 0.04 | 2.85 ± 0.02 | 0.73 ± 0.01 |
| A356 + 3.5RE | T6 + 250 °C-200 h | 86.32 | 5.59 ± 0.01 | 2.48 ± 0.02 | 1.99 ± 0.04 | 2.91 ±0.02 | 0.71 ± 0.01 |
| A356 + 3.5RE | T6 + 300 °C-200 h | 86.39 | 5.76 ± 0.03 | 2.40 ± 0.04 | 1.86 ± 0.03 | 2.88 ± 0.01 | 0.71 ± 0.02 |
| Alloy | Conditioning | Volume Percentage of Phase | ||
|---|---|---|---|---|
| Al | Al11Ce3 | β-Al3Mg2 | ||
| Al-8Ce-10Mg | As-Cast | 82.4 | 9.6 ± 0.07 | 8.0 ± 0.03 |
| Al-8Ce-10Mg | 250 °C-200 h | 76.5 | 9.0 ± 0.05 | 14.6 ± 0.04 |
| Al-8Ce-10Mg | 300 °C-200 h | 75.0 | 8.9 ± 0.06 | 16.1 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakevich, J.R.; Sediako, D.; Weiss, D.; Vogel, S.C. A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications. Materials 2024, 17, 4311. https://doi.org/10.3390/ma17174311
Kozakevich JR, Sediako D, Weiss D, Vogel SC. A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications. Materials. 2024; 17(17):4311. https://doi.org/10.3390/ma17174311
Chicago/Turabian StyleKozakevich, Jordan Roger, Dimitry Sediako, David Weiss, and Sven C. Vogel. 2024. "A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications" Materials 17, no. 17: 4311. https://doi.org/10.3390/ma17174311
APA StyleKozakevich, J. R., Sediako, D., Weiss, D., & Vogel, S. C. (2024). A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications. Materials, 17(17), 4311. https://doi.org/10.3390/ma17174311







