Preparation of Cu/Cu2O/BC and Its Performance in Adsorption–Photocatalytic Degradation of Methyl Orange in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Cu/Cu2O/BC
2.3. Adsorption and Photocatalysis Experiments
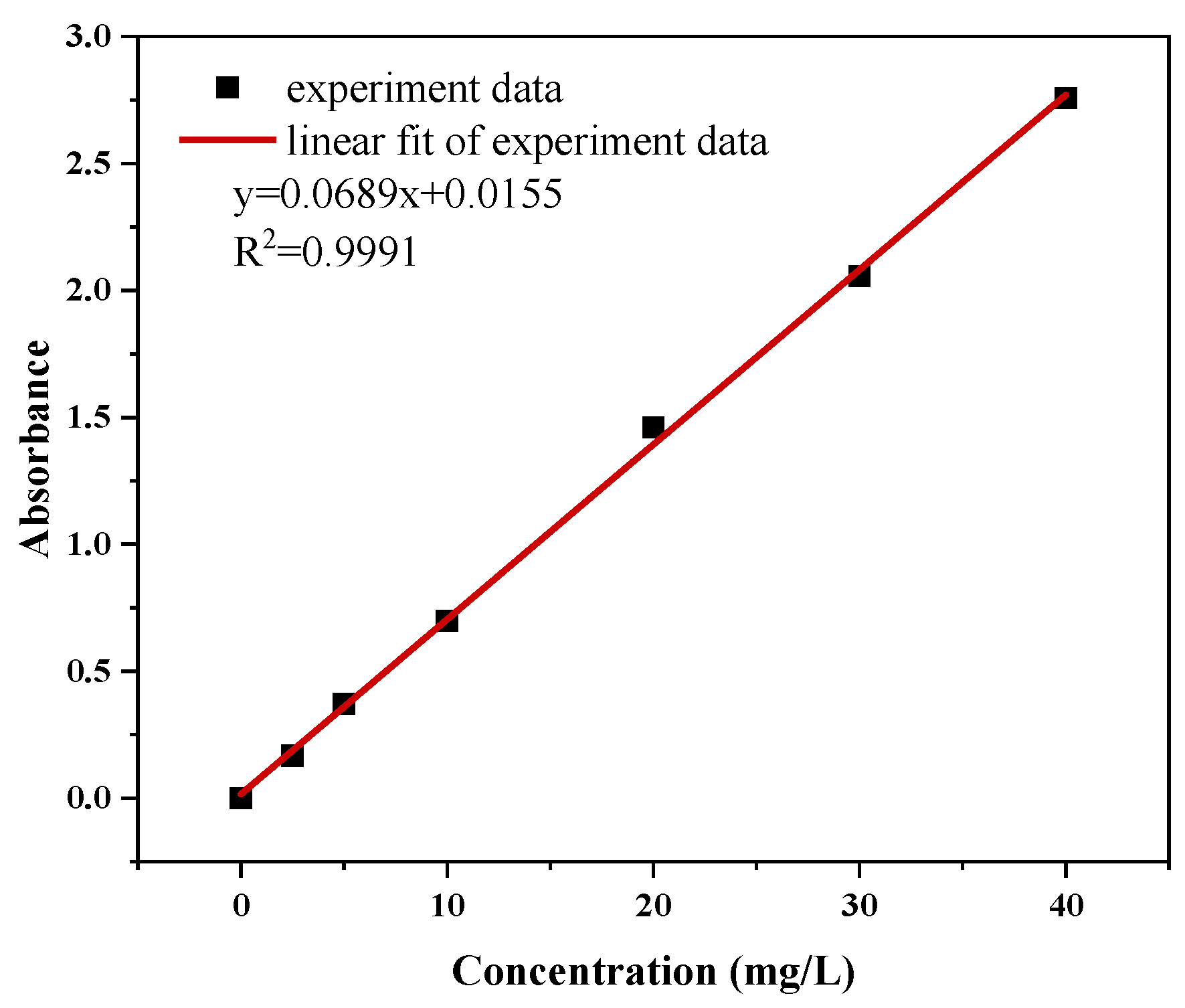
2.4. Test and Characterization
3. Results and Discussion
3.1. Material Characterization
3.1.1. XRD and Raman Analyses
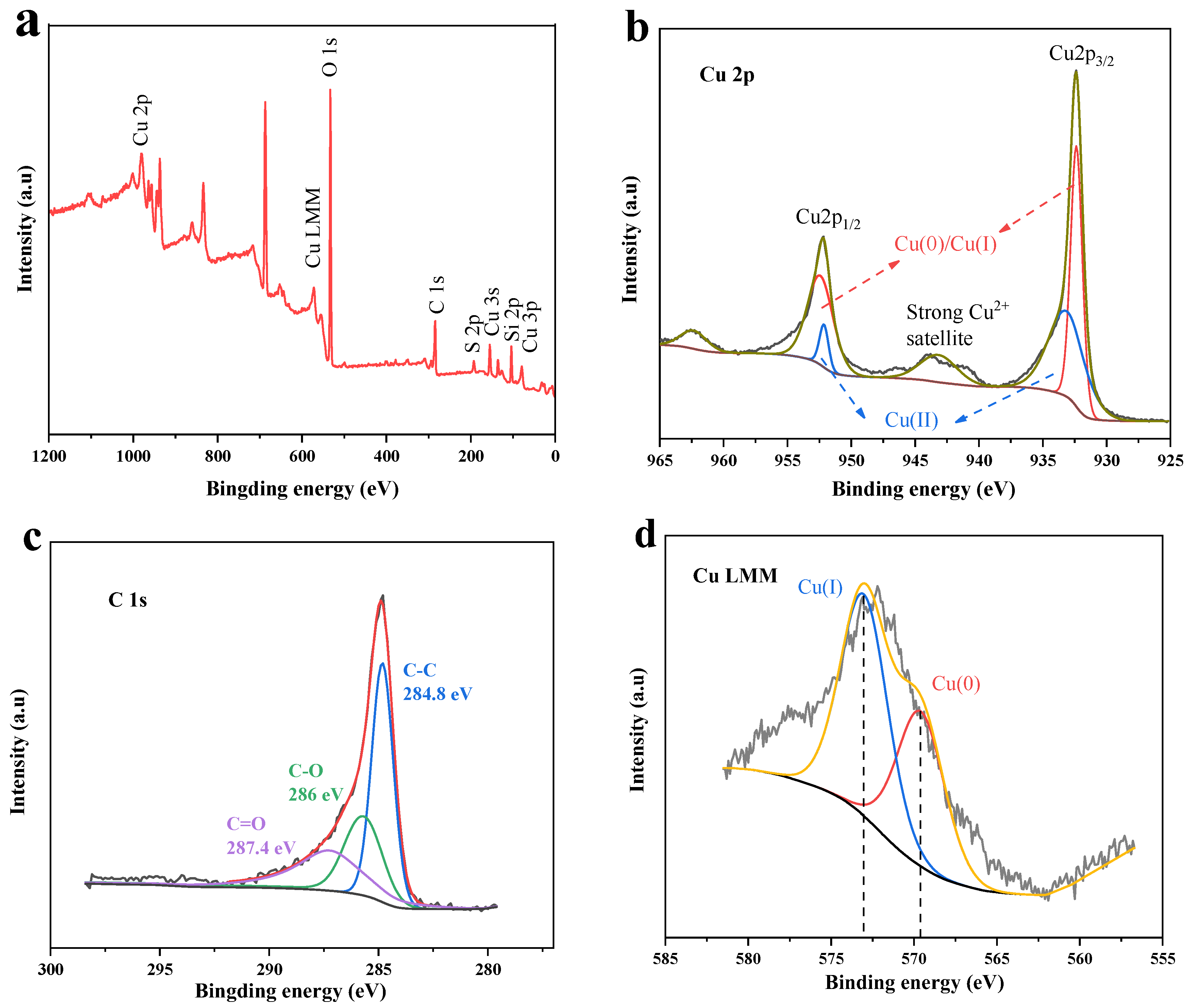
3.1.2. XPS Analysis
3.1.3. SEM-EDS Analysis
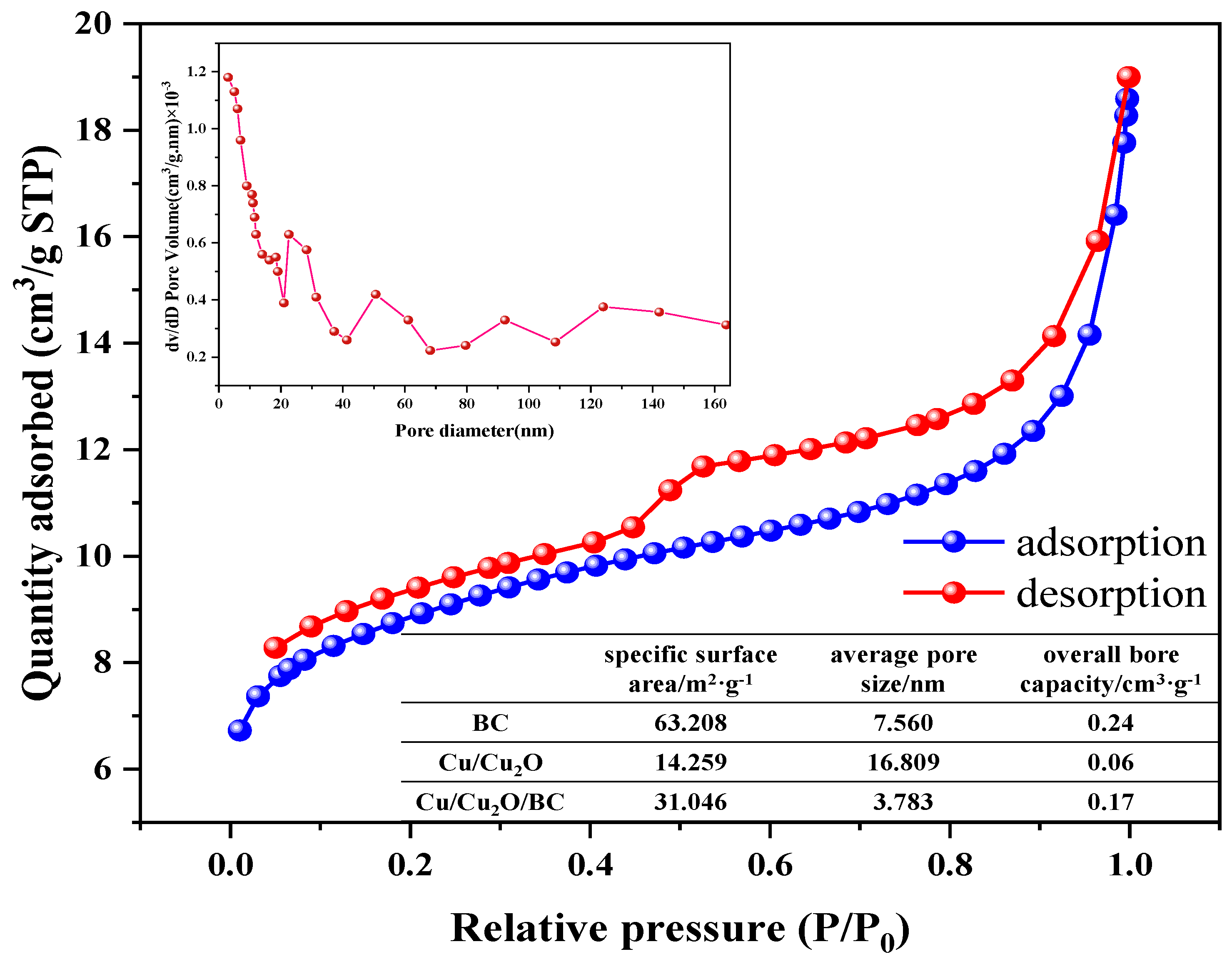
3.1.4. BET Analysis
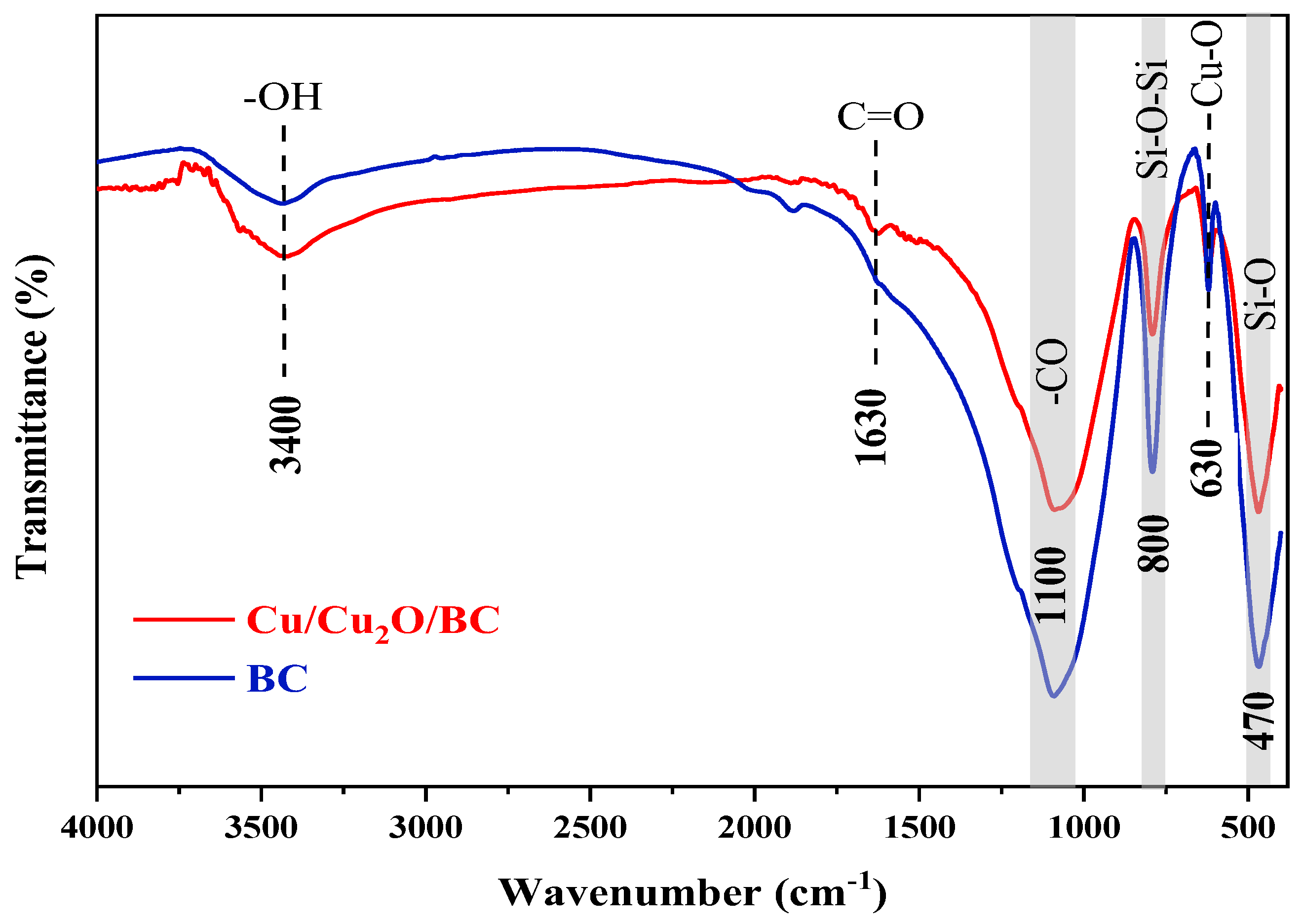
3.1.5. FTIR Analysis
3.1.6. UV-Vis DRS Analysis
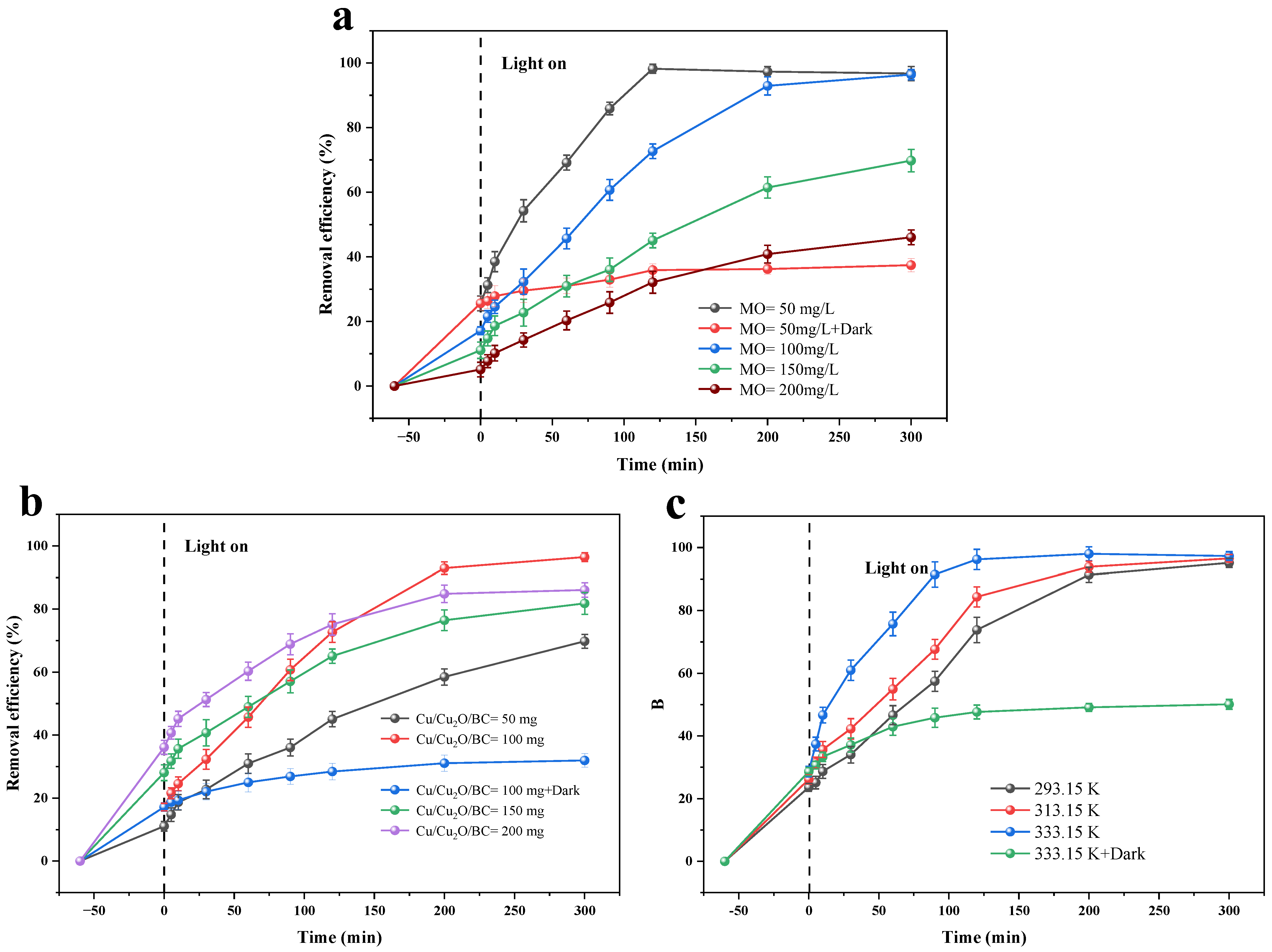
3.2. Adsorption and Photocatalysis
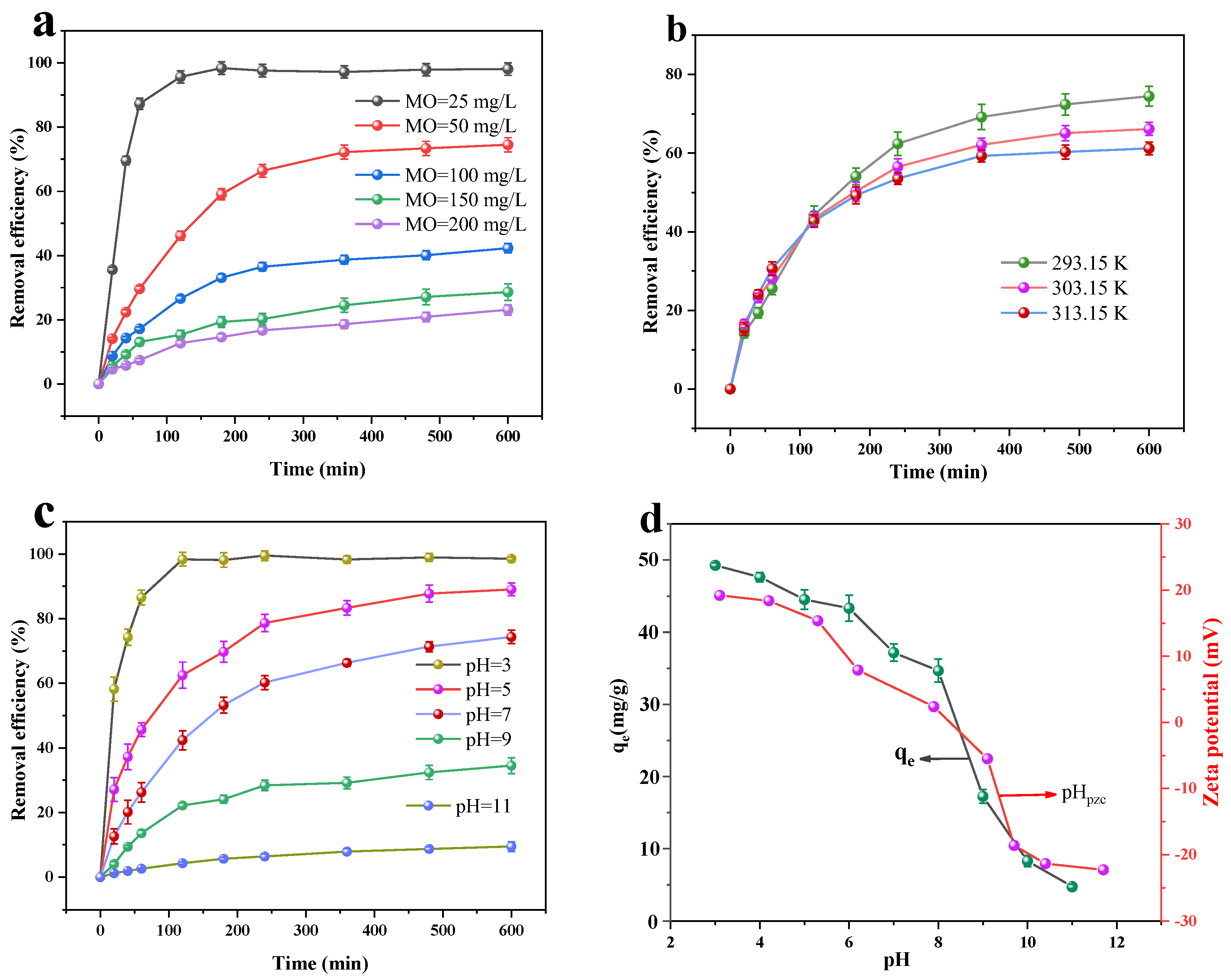
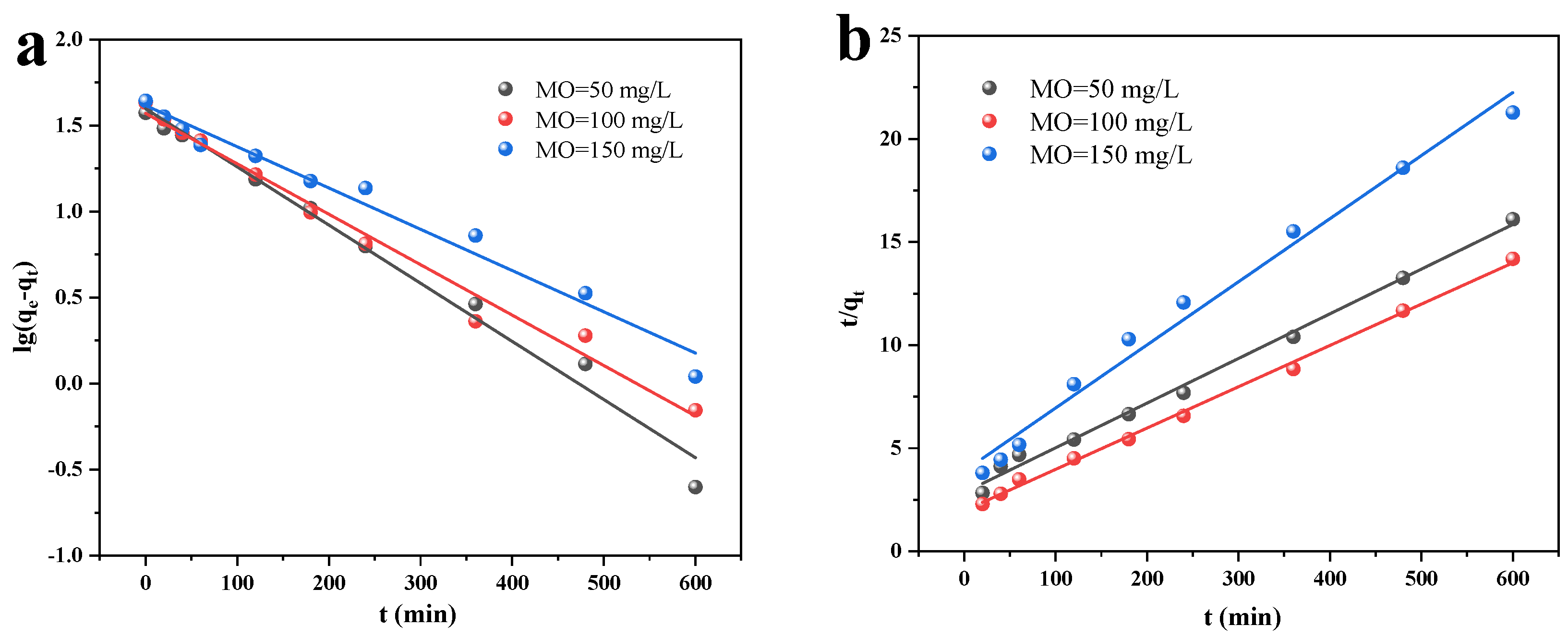
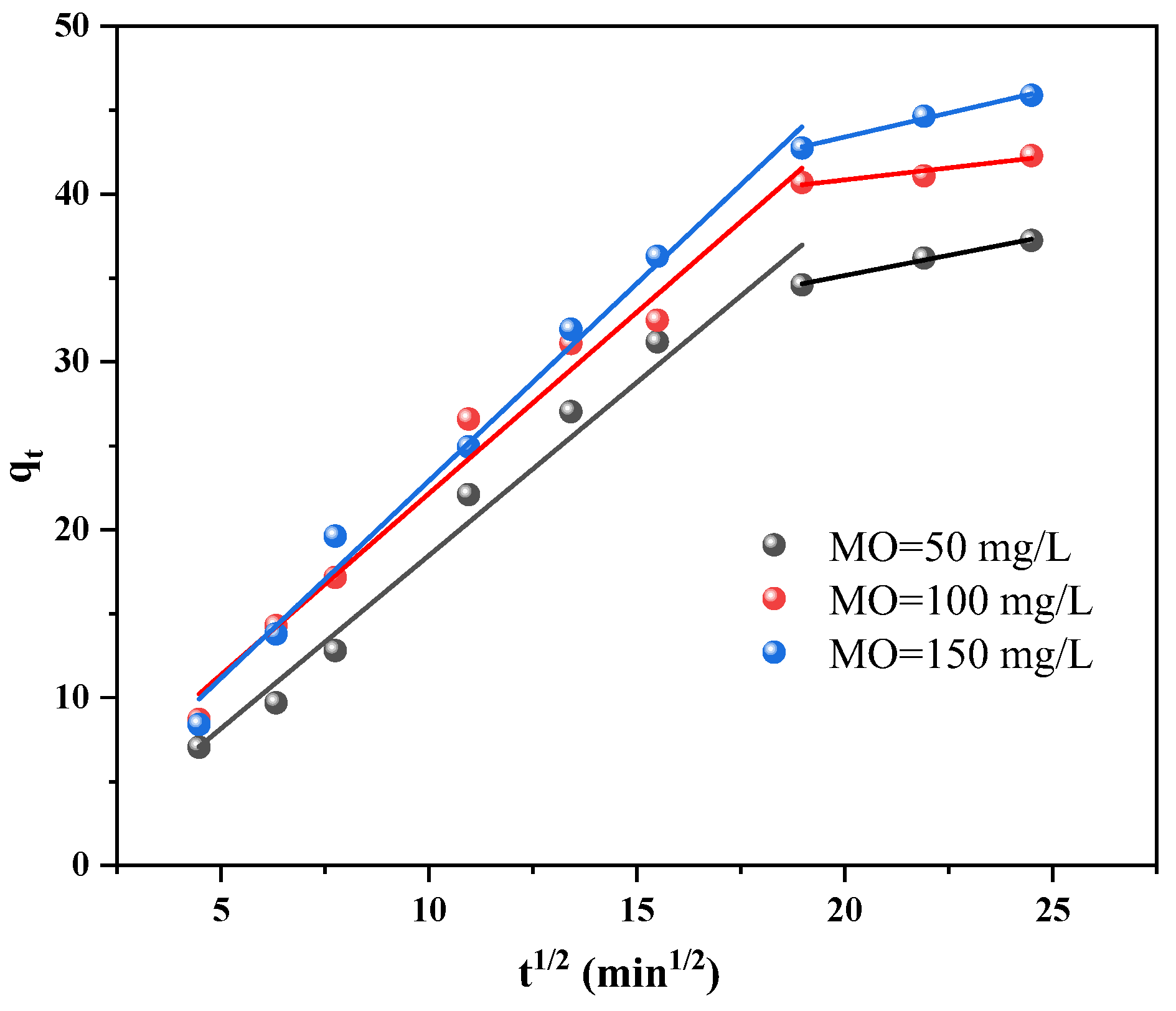
3.2.1. Dark Adsorption and Kinetics
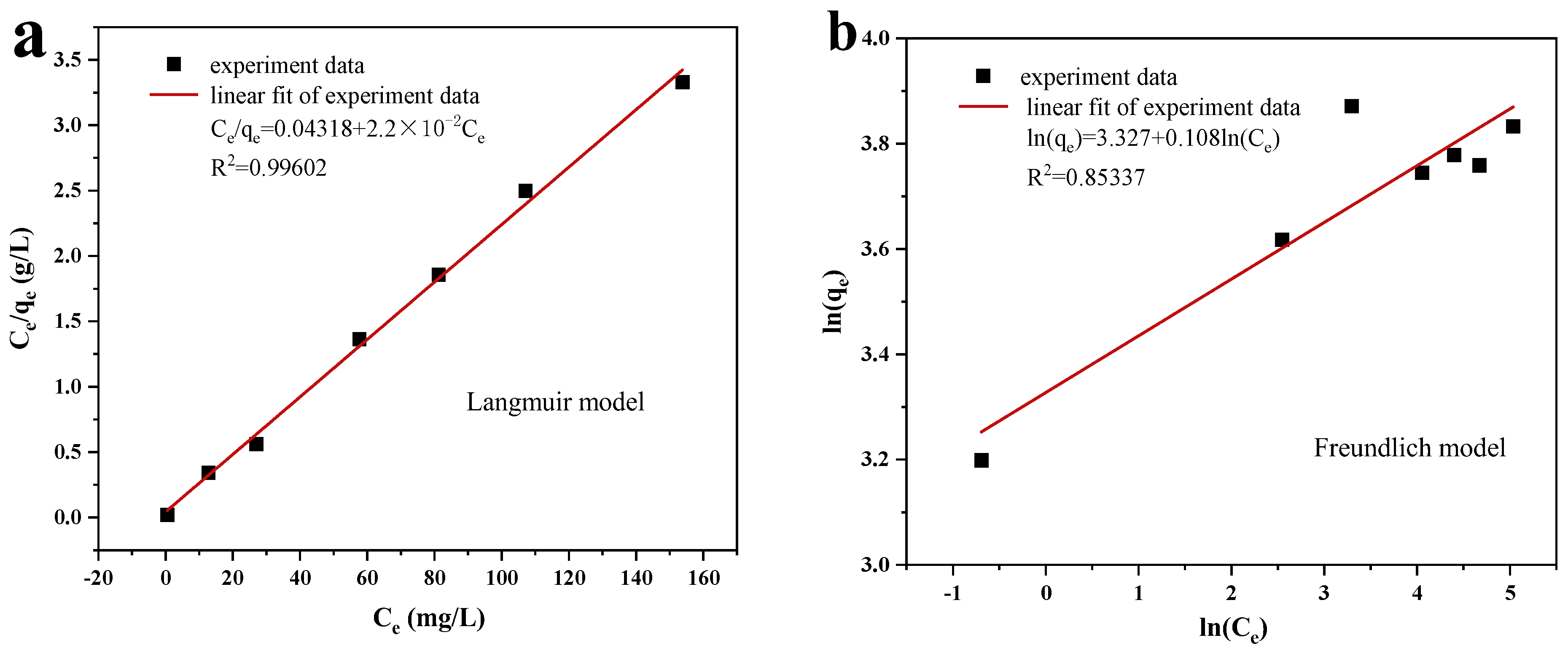
3.2.2. Adsorption Isotherms and Thermodynamic Analyses
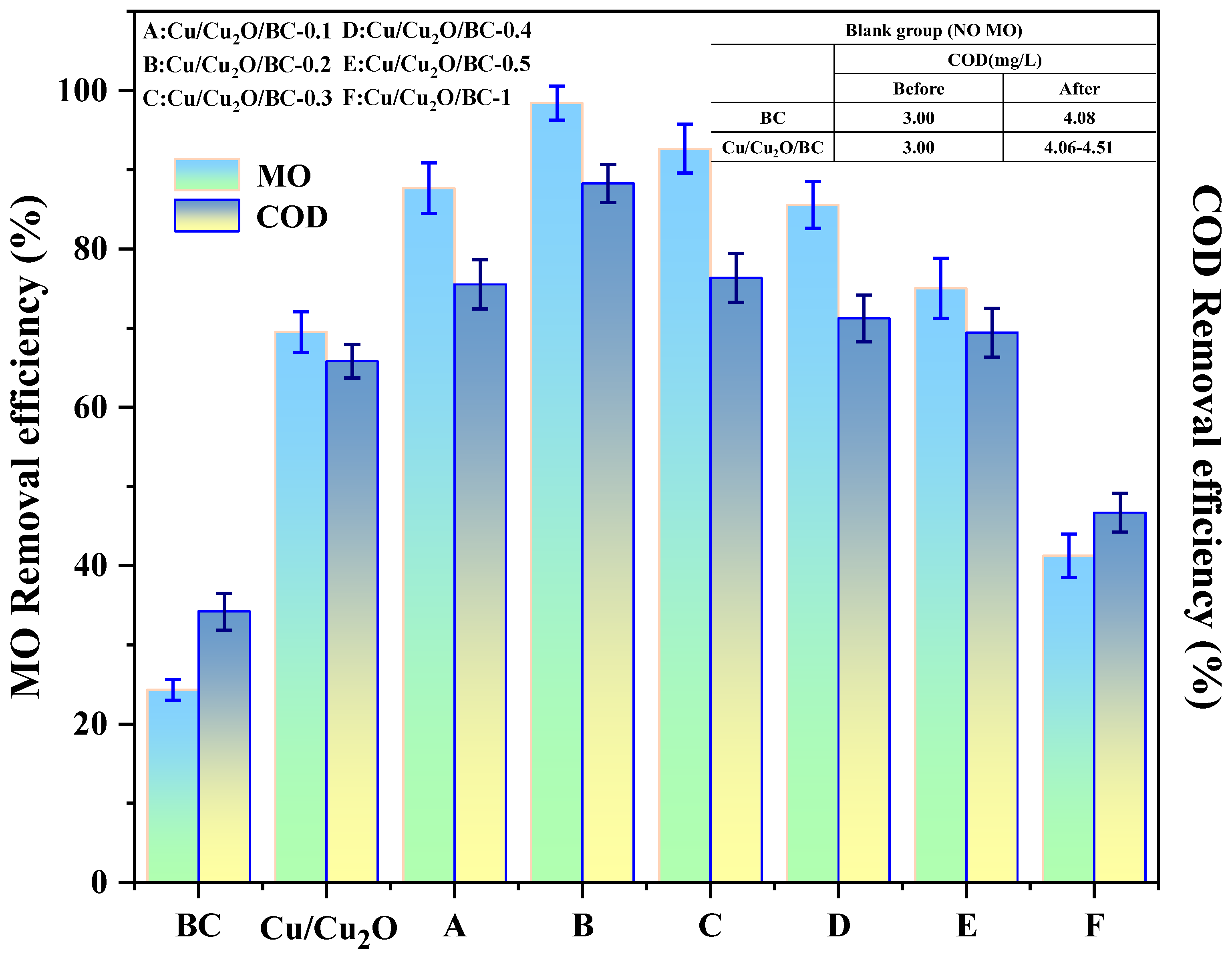
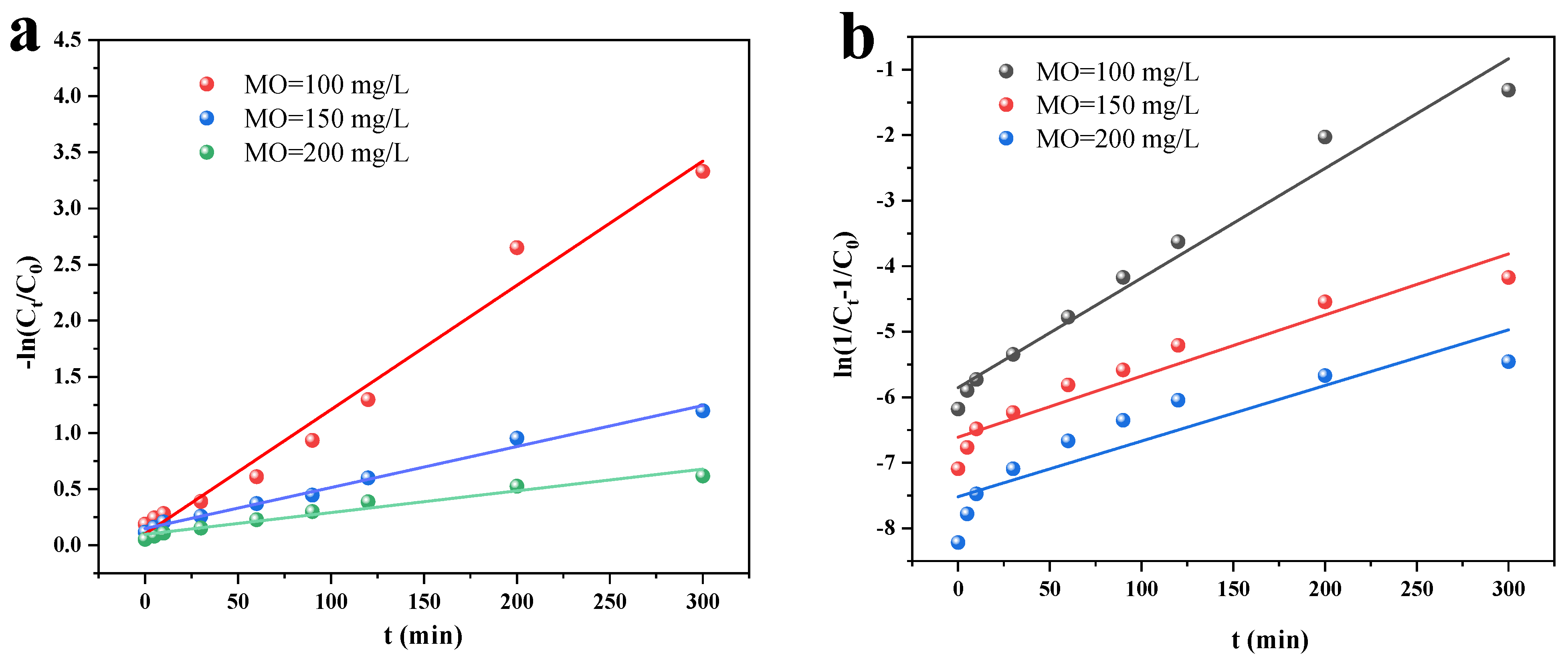
3.2.3. Photocatalysis and Kinetics
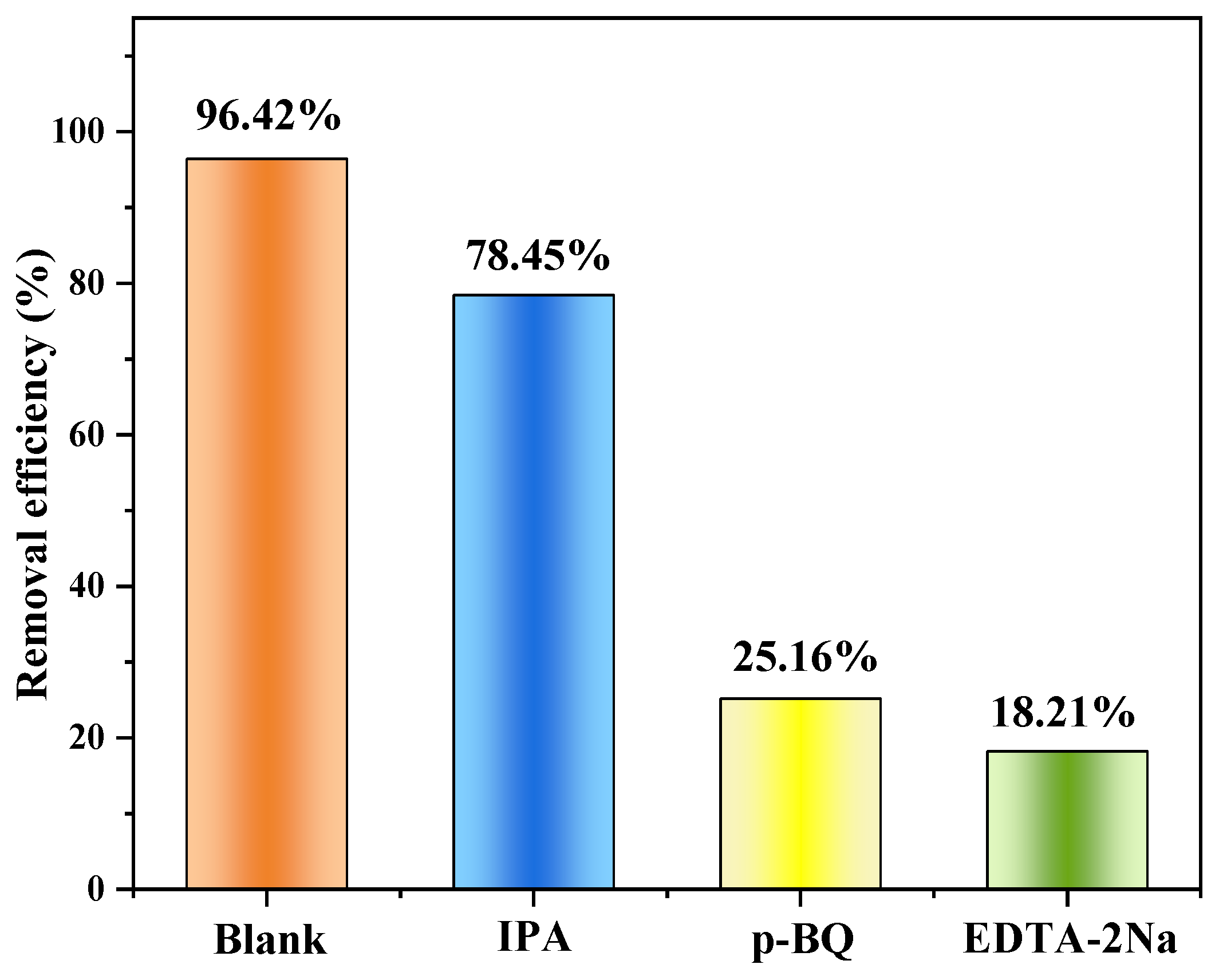
3.2.4. MO Removal Mechanisms
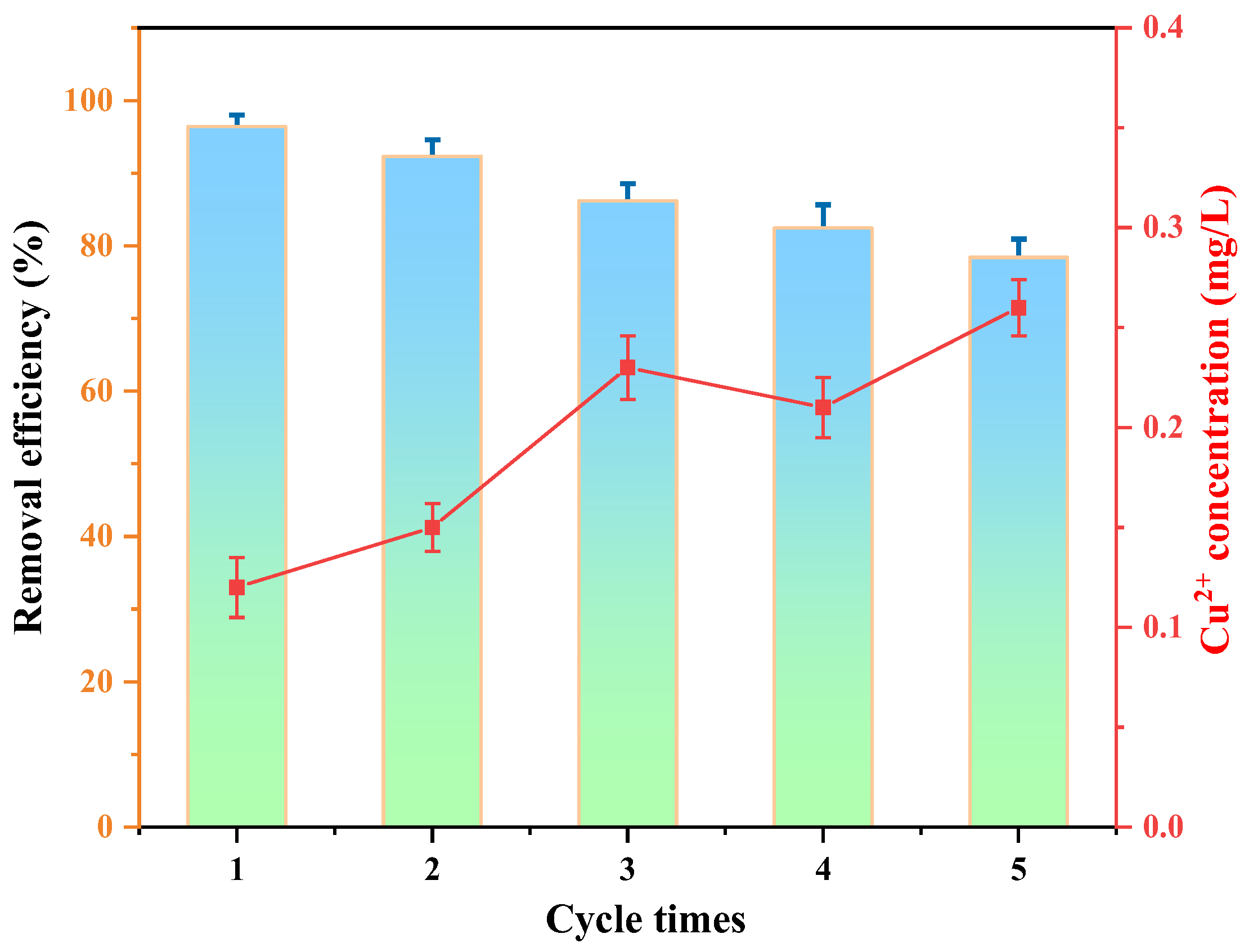
3.3. Reusability and Stability
4. Conclusions
- (1)
- At a mass ratio of BC to Cu/Cu2O of 0.2, the synthesized Cu/Cu2O/BC photocatalyst exhibits the highest adsorption–photocatalytic action. At pH = 6 and T = 25 °C, 100 mg of Cu/Cu2O/BC can remove 95% of the MO and 88.26% of the COD from the aqueous solution at an initial concentration of 100 mg/L.
- (2)
- The maximal removal rate in the dark adsorption method reduces slightly as the temperature rises. The removal rate rises with increasing temperature in the adsorption–photocatalysis synergistic treatment of MO, indicating that the adsorption is an exothermic reaction and that the photocatalysis is an adsorptive one, with the photocatalysis process being more affected than the adsorption process. The greatest removal effect occurs at pH = 3.0, while the adsorption of MO by Cu/Cu2O/BC is inhibited when the pH is greater than 7. The MO removal rate in the adsorption process rises when decreasing the pH of the solution.
- (3)
- The quasi-primary kinetic model and the quasi-secondary kinetic model both suitably describe the adsorption and photocatalytic processes, respectively. Additionally, the adsorption isotherm more closely resembles the Langmuir model, indicating that MO is mostly chemisorbable by Cu/Cu2O/BC. The Langmuir model determines the qmax of MO to be 45.45 mg/g. According to the thermodynamic analysis, entropy, not enthalpy, is primarily responsible for controlling the spontaneous adsorption process during removal.
- (4)
- When MO is adsorbed on Cu/Cu2O/BC, and when Cu2O is exposed to light radiation, photogenerated electrons move to produce holes, with the electrons transferred to Cu and biochar to react with O2 to produce the active substance of •O2−, and MO is then oxidized and reduced by h+ and •O2− to H2O and CO2. A high MO removal rate of 78.41% can remain after five cycles of treatment, indicating the excellent recyclability of the Cu/Cu2O/BC.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zango, Z.U.; Binzowaimil, A.M.; Aldaghri, O.A.; Eisa, M.H.; Garba, A.; Ahmed, N.M.; Lim, J.W.; Ng, H.-S.; Daud, H.; Jumbri, K.; et al. Applications of covalent organic frameworks for the elimination of dyes from wastewater: A state-of-the-arts review. Chemosphere 2023, 343, 140223. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Chandanshive, V.; Kadam, S.; Rane, N.; Jeon, B.-H.; Jadhav, J.; Govindwar, S. In situ textile wastewater treatment in high rate transpiration system furrows planted with aquatic macrophytes and floating phytobeds. Chemosphere 2020, 252, 126513. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Mhamad, S.A.; Aziz, M. Review of various strategies to boost the photocatalytic activity of the cuprous oxide-based photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105138. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Wang, Y.; Li, Y.; Wang, B.; Wang, Y.; Wei, S. Preparation of Cu2O crystals by glucose liquid phase reduction: Morphology evolution and size control. Solid State Sci. 2022, 133, 107006. [Google Scholar] [CrossRef]
- Ali, N.; Hussain, S.; Waqas, M.; Faheem, M.; Ahmad, N.; Ali, A.; Ali, M.Y.; Mahmood, K.; Schmidt-Mende, L. Charge transfer in copper oxide thin films deposited at different electrodeposition potential. Phys. B Condens. Matter 2023, 659, 414881. [Google Scholar] [CrossRef]
- Feng, S.H.; Li, G.H. Chapter 4—Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Gaashani, R.; Hamoudi, H.; Al Marri, M.J.; Hussein, I.A.; Belaidi, A.; Merzougui, B.A.; Alharbi, F.H.; Tabet, N. Controlled growth of Cu2O thin films by electrodeposition approach. Mater. Sci. Semicond. Process. 2017, 63, 203–211. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic sensitive Hericium erinaceus residue chitin/Cu hydrogel nanocomposites for H2 generation by catalyzing NaBH4 hydrolysis. Carbohydr. Polym. 2020, 229, 115426. [Google Scholar] [CrossRef]
- Pech-Pech, I.; Verde-Gómez, Y.; Valenzuela-Muñiz, A. Effect of sodium oxalate on the intensity of surface resonance plasmon of the copper nanoparticles used as substrates for the synthesis of ultrasmall Cu-Pt nanoparticles and the study of their catalytic activity on the oxygen reduction in acid electrolyte. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 615, 126283. [Google Scholar] [CrossRef]
- Ejaz, A.; Jeon, S. Electrooxidation of N2H4 through CuCuO electronic oscillation on a nitrogen-doped GO surface. Sensors Actuators B Chem. 2019, 284, 494–504. [Google Scholar] [CrossRef]
- Nisar, S.; Tariq, M.; Muhammad, S.; Saqib, M.; Akbar, F. Electrocatalytic efficacy of Ni-Cu@VC-72: Non-enzymatic electrochemical detection of glucose using Ni-Cu nanoparticles loaded on carbon black. Synth. Met. 2020, 269, 116578. [Google Scholar] [CrossRef]
- Ding, Y.R.; Li, C.H.; Zhang, L.Y.; Li, J.M.; He, C.; Li, M.H.; Zhu, W.C.; Wei, S.X. Study on Cu(II) Removal Properties of Sulfide Nano Zero-Valent Iron Composites. Mater. Rep. 2024, 1–17. Available online: https://kns.cnki.net/kcms2/article/abstract?v=xpM8-w1VMS83hW6fg3fwSVC3lcTXIe3fkM4gu2zfmRxleQKwDIxR9tW3DyTdIabmdh1BcMc_a5haajx7vliQxHZyD42F6bwNUZ8ncoihnqB6oewTUMqLsxIC2w8-iJuIcFrz_abeHzI7BTaqwLrwQM6X5sqkMcJ2stHpS5u0QbbAXqjfmfV2d205xANgtW_T&uniplatform=NZKPT&language=CHS (accessed on 25 August 2024). (In Chinese).
- Amer, O.A.; Ali, S.S.; Azab, M.; El-Shouny, W.A.; Sun, J.; Mahmoud, Y.A.-G. Exploring new marine bacterial species, Alcaligenes faecalis Alca F2018 valued for bioconversion of shrimp chitin to chitosan for concomitant biotechnological applications. Int. J. Biol. Macromol. 2022, 196, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.; Rónavári, A.; Kónya, Z.; Kukovecz, A. Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanoparticles. ACS Sustain. Chem. Eng. 2016, 4, 291–297. [Google Scholar] [CrossRef]
- Gallo, A.; Bianco, C.; Tosco, T.; Tiraferri, A.; Sethi, R. Synthesis of eco-compatible bimetallic silver/iron nanoparticles for water remediation and reactivity assessment on bromophenol blue. J. Clean. Prod. 2019, 211, 1367–1374. [Google Scholar] [CrossRef]
- Li, D.; Tian, X.; Wang, Z.; Guan, Z.; Li, X.; Qiao, H.; Ke, H.; Luo, L.; Wei, Q.; Huang, J. Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chem. Eng. J. 2020, 383, 123127. [Google Scholar] [CrossRef]
- Sodha, V.; Koshti, H.; Gaur, R.; Bandyopadhyay, R.; Shahabuddin, S. Investigation on the adsorption of cationic dyes and heavy metal ions on zeolite HY from aqueous solution. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Li, Y.; Deng, N.; Wang, H.; Jin, Y.; Feng, Y.; Kang, W. Hierarchically porous carbon nanofibers loaded with Cu2O and Cu nanoparticles modified separator with strong adsorption and excellent catalysis of lithium polysulfide for lithium-sulfur batteries. Electrochim. Acta 2024, 475, 143330. [Google Scholar] [CrossRef]
- Tanos, F.; Makhoul, E.; Nada, A.A.; Bekheet, M.F.; Riedel, W.; Kawrani, S.; Belaid, H.; Petit, E.; Viter, R.; Fedorenko, V.; et al. Graphene oxide-induced CuO reduction in TiO2/CaTiO3/Cu2O/Cu composites for photocatalytic degradation of drugs via peroxymonosulfate activation. Appl. Surf. Sci. 2024, 656, 159698. [Google Scholar] [CrossRef]
- Hou, P.; Xing, G.; Tian, L.; Zhang, G.; Wang, H.; Yu, C.; Li, Y.; Wu, Z. Hollow carbon spheres/graphene hybrid aerogels as high-performance adsorbents for organic pollution. Sep. Purif. Technol. 2019, 213, 524–532. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Y.; Zheng, W.; Chang, C.; Dong, J.; Wen, J.; Zheng, X. FeS/MgO-biochar composites for boosting the adsorption-photocatalytic degradation of RhB. Mater. Sci. Semicond. Process. 2024, 174, 108178. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Yu, L.; Li, Z.; Xie, D.; Zhao, Y.; Yu, Y. Facile in situ fabrication of Cu2O@Cu metal-semiconductor heterostructured nanorods for efficient visible-light driven CO2 reduction. Chem. Eng. J. 2020, 385, 123940. [Google Scholar] [CrossRef]
- Ma, A.; Wu, X.; Li, X.; Susanti, Y.D.; Liu, D.; Li, H.; Kuvarega, A.T.; Mamba, B.B.; Gui, J. Electrocatalytic nitrate reduction to ammonia over Cu/Cu2O catalysts with controllable ratios. Appl. Surf. Sci. 2024, 653, 159397. [Google Scholar] [CrossRef]
- Wang, A.D.; Feng, Z.D.; Qin, D.Y.; Zhang, L.; Zhu, L.; Zhang, S.; Tao, H. Study on the Catalytic Oxidation of Rhodamine B by Nanoporous Carbon Materials Loaded with Zero Valence Copper. Acta Sci. Nat. Univ. Pekin. 2020, 56, 703–709. (In Chinese) [Google Scholar] [CrossRef]
- Feng, H.; Schyns, J.F.; Krol, M.S.; Yang, M.; Su, H.; Liu, Y.; Lv, Y.; Zhang, X.; Yang, K.; Che, Y. Water pollution scenarios and response options for China. Sci. Total. Environ. 2024, 914, 169807. [Google Scholar] [CrossRef]
- Song, D.-Y.; Lee, J.-W. Valorization of biochar and lignin derived from the NaOH pretreatment of lignocellulosic biomass for applications as an adsorbent and antioxidant. Biomass Bioenergy 2024, 182, 107103. [Google Scholar] [CrossRef]
- Yang, W.; Wang, G.X.; Chen, J.N.; Guo, Q. Degradation of tetracycline in water by biochar-based rice husk and persulfate. Appl. Chem. Ind. 2024, 53, 341–345. (In Chinese) [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Zhu, L. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar] [CrossRef]
- Aziz, S.; Uzair, B.; Ali, M.I.; Anbreen, S.; Umber, F.; Khalid, M.; Aljabali, A.A.; Mishra, Y.; Mishra, V.; Serrano-Aroca, Á.; et al. Synthesis and characterization of nanobiochar from rice husk biochar for the removal of safranin and malachite green from water. Environ. Res. 2023, 238, 116909. [Google Scholar] [CrossRef]
- Guo, R.; Chang, J.; Li, H.; He, J.; Pan, P.; Yang, Z.; Wei, J. Preparation of 26-facet Cu2O@Cu composite and its methyl orange adsorption behavior. J. Alloys Compd. 2022, 895, 162630. [Google Scholar] [CrossRef]
- Jiku, A.S.; Singha, A.; Faruquee, M.; Rahaman, A.; Alam, A.; Ehsanullah, M. Toxic wastewater status for irrigation usage at Gazipur and Savar industrial vicinity of Bangladesh. Ecol. Front. 2021, 41, 358–364. [Google Scholar] [CrossRef]
- Sojobi, A.O.; Zayed, T. Impact of sewer overflow on public health: A comprehensive scientometric analysis and systematic review. Environ. Res. 2022, 203, 111609. [Google Scholar] [CrossRef]
- Jin, X.; Wu, C.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. A magnetic-void-porous MnFe2O4/carbon microspheres nano-catalyst for catalytic ozonation: Preparation, performance and mechanism. Environ. Sci. Ecotechnol. 2021, 7, 100110. [Google Scholar] [CrossRef]
- Köktürk, M. In vivo toxicity assessment of Remazol Gelb-GR (RG-GR) textile dye in zebrafish embryos/larvae (Danio rerio): Teratogenic effects, biochemical changes, immunohistochemical changes. Sci. Total Environ. 2022, 852, 158473. [Google Scholar] [CrossRef]
- Vilhena, A.O.d.A.; Lima, K.M.M.; de Azevedo, L.F.C.; Rissino, J.D.; de Souza, A.C.P.; Nagamachi, C.Y.; Pieczarka, J.C. The synthetic dye malachite green found in food induces cytotoxicity and genotoxicity in four different mammalian cell lines from distinct tissuesw. Toxicol. Res. 2023, 12, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Thangaraj, S.; Bankole, P.O.; Sadasivam, S.K. Microbial degradation of azo dyes by textile effluent adapted, Enterobacter hormaechei under microaerophilic condition. Microbiol. Res. 2021, 250, 126805. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gupta, A.B.; Gupta, A.; Pandey, A. Introduction to water remediation: Importance and methods. In Water Remediation; Springer: Singapore, 2018; pp. 3–8. [Google Scholar] [CrossRef]
- Khanum, R.; Ali, R.S.; Rangaswamy, H.; Kumar, S.S.; Prashantha, A.; Jagadisha, A. Recent review on Synthesis, spectral Studies, versatile applications of azo dyes and its metal complexes. Results Chem. 2023, 5, 100890. [Google Scholar] [CrossRef]
- Khan, S.B.; Ali, F.; Kamal, T.; Anwar, Y.; Asiri, A.M.; Seo, J. CuO embedded chitosan spheres as antibacterial adsorbent for dyes. Int. J. Biol. Macromol. 2016, 88, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Jiang, R.; Xiao, L.; Li, W. A novel magnetically separable γ-Fe2O3/crosslinked chitosan adsorbent: Preparation, characterization and adsorption application for removal of hazardous azo dye. J. Hazard. Mater. 2010, 179, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Mahdhi, A.; Cheref, A.; Bakhrouf, A. Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization. Desalination 2011, 274, 272–277. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Chen, J.; Wang, Y.; Cheng, Z.; Chen, X.; Gao, X.; Guo, M. Porous spherical Cu2O supported by wood-based biochar skeleton for the adsorption-photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 611, 155744. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Wang, W.; Cai, Y.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Enhanced dark adsorption and visible-light-driven photocatalytic properties of narrower-band-gap Cu2S decorated Cu2O nanocomposites for efficient removal of organic pollutants. J. Hazard. Mater. 2020, 384, 121302. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848-2017; Standard for Groundwater Quality. National Standard of the People’s Republic of China: Beijing, China, 2017.





| C0 | Quasi-First-Order Kinetics Model | Quasi-Second-Order Kinetics Model | ||||
|---|---|---|---|---|---|---|
| q1 | k1 | R2 | q2 | k2 | R2 | |
| 50 | 39.201 | 0.00338 | 0.986 | 37.74 | 0.00215 | 0.990 |
| 100 | 41.134 | 0.00292 | 0.983 | 45.93 | 0.00182 | 0.996 |
| 150 | 51.516 | 0.00239 | 0.977 | 54.88 | 0.00158 | 0.998 |
| C0 | Internal Diffusion Model | |||||
|---|---|---|---|---|---|---|
| kd1 | I1 | R2 | kd2 | I2 | R2 | |
| 50 | 2.062 | −2.153 | 0.979 | 0.481 | 25.525 | 0.993 |
| 100 | 2.160 | 0.555 | 0.983 | 0.286 | 35.126 | 0.950 |
| 150 | 2.351 | −0.594 | 0.990 | 0.572 | 31.963 | 0.993 |
| Langmuir Model Constant | Freundlich Model Constant | |||||
|---|---|---|---|---|---|---|
| qmax | kL | r2 | RL | 1/n | kF | r2 |
| 45.454 | 0.5095 | 0.996 | 0.01–0.455 | 0.108 | 27.855 | 0.853 |
| T (K) | Kc | ΔG0 (kJ/mol) | ΔS0 (J/mol·K) | ΔH0 (kJ/mol) |
|---|---|---|---|---|
| 293.15 | 2.926 | −2.616 | −71.733 | −23.578 |
| 303.15 | 1.955 | −1.689 | ||
| 313.15 | 1.578 | −1.187 |
| C0 (mg/L) | Quasi-Primary Kinetic Model | Quasi-Secondary Kinetic Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (min−1) | R2 | |
| 100 | 1.11 × 10−2 | 0.980 | 1.67 × 10−2 | 0.972 |
| 150 | 3.66 × 10−3 | 0.991 | 9.32 × 10−3 | 0.921 |
| 200 | 1.94 × 10−3 | 0.962 | 8.49 × 10−3 | 0.789 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, G.; Ding, Y.; Li, C.; Zhang, L.; Li, J.; Li, M.; Zhu, W.; He, C. Preparation of Cu/Cu2O/BC and Its Performance in Adsorption–Photocatalytic Degradation of Methyl Orange in Water. Materials 2024, 17, 4306. https://doi.org/10.3390/ma17174306
Du G, Ding Y, Li C, Zhang L, Li J, Li M, Zhu W, He C. Preparation of Cu/Cu2O/BC and Its Performance in Adsorption–Photocatalytic Degradation of Methyl Orange in Water. Materials. 2024; 17(17):4306. https://doi.org/10.3390/ma17174306
Chicago/Turabian StyleDu, Gang, Yarong Ding, Canhua Li, Lanyue Zhang, Jiamao Li, Minghui Li, Weichang Zhu, and Chuan He. 2024. "Preparation of Cu/Cu2O/BC and Its Performance in Adsorption–Photocatalytic Degradation of Methyl Orange in Water" Materials 17, no. 17: 4306. https://doi.org/10.3390/ma17174306
APA StyleDu, G., Ding, Y., Li, C., Zhang, L., Li, J., Li, M., Zhu, W., & He, C. (2024). Preparation of Cu/Cu2O/BC and Its Performance in Adsorption–Photocatalytic Degradation of Methyl Orange in Water. Materials, 17(17), 4306. https://doi.org/10.3390/ma17174306







