Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach
Abstract
1. Introduction
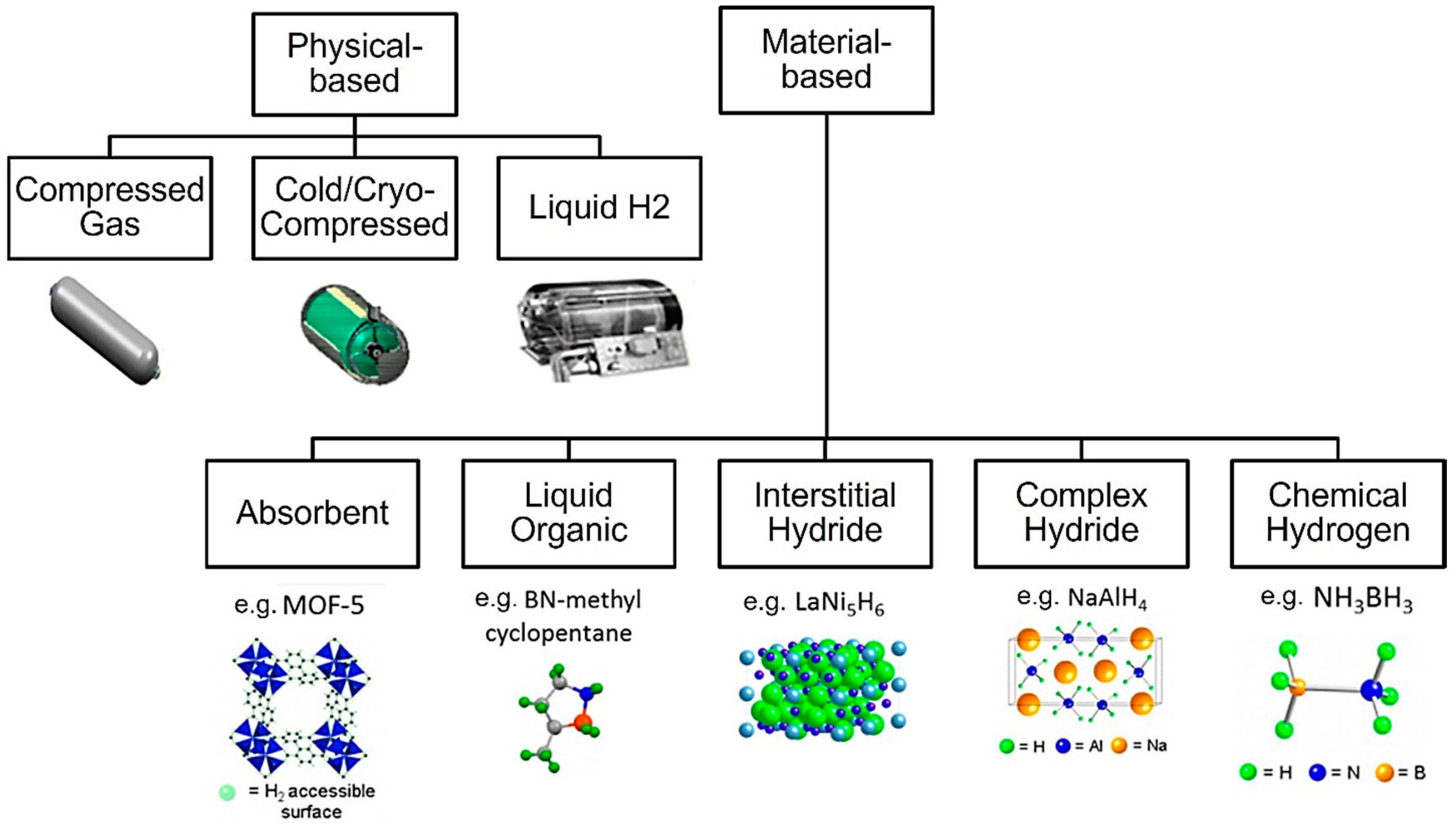
2. Materials and Methods
2.1. Analysis of Existing Hydrogen Storage Solutions
- Compressed Gas—storing hydrogen at high pressures in specially designed tanks. The image shows a cylindrical tank, likely made of reinforced materials to withstand high pressure [16].
- Cold/Cryo-Compressed—combines compression with cooling. The image shows a more complex tank with insulation, likely to maintain the low temperatures required [17].
- Liquid Hydrogen—hydrogen is stored in liquid form at extremely low temperatures (around −253°. The tank shown is larger and more elaborate, reflecting the need for significant insulation and temperature control [18].
- Absorbent—exemplified by MOF-5, showing a crystalline structure with nodes and linkers creating a porous framework where hydrogen molecules can be absorbed [19].
- Liquid Organic—exemplified by BN-methyl cyclopentane, whose molecular structure is made of carbon, nitrogen, and boron, forming a cyclic compound that can reversibly store hydrogen [20].
- Interstitial Hydride—illustrated by LaNi5H6, a structure where hydrogen is integrated within the interstices of a metal alloy composed of lanthanum and nickel [21].
- Complex Hydride—represented by NaAlH4, a complex crystal structure with sodium, aluminum, and hydrogen atoms arranged in a specific pattern [22].
- Chemical Hydrogen—exemplified by NH3BH3, forming a molecular structure with nitrogen, boron, and hydrogen atoms bonded together [23].
2.2. Analysis of Existing Material Types
2.3. Analysis of Existing Glass Types
2.4. Storage Capacity Evaluation
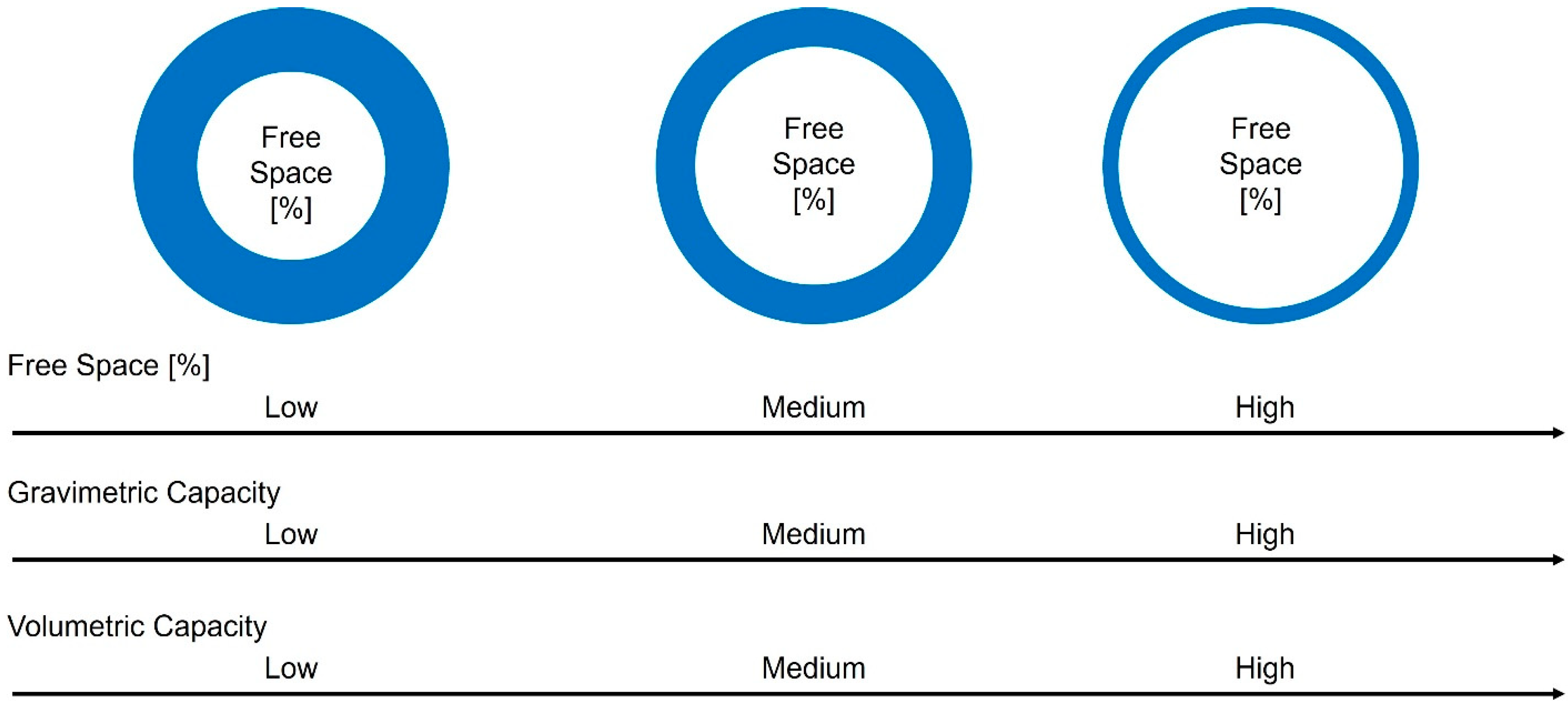
- Gravimetric capacity—represents the amount of hydrogen that can be stored per unit mass of storage. It is a measure of the efficiency of hydrogen storage in terms of weight. Higher gravimetric capacity indicates a greater amount of hydrogen that can be stored while minimizing the weight of the storage system and can be calculated using the following equation:
- mH2—the mass of hydrogen calculated by multiplying the density of hydrogen at 700 bar and the volume of hydrogen inside the capillary tube (VH2 = πRi2L, where L is the length of the capillary tube)
- mstorage—the mass of storage calculated by multiplying the density of storage material and the volume of storage (for a capillary tube Vstorage = Vcapillary = πRe2L, where L is the length of the capillary tube)
- 2.
- Volumetric capacity—represents the amount of hydrogen that can be stored per unit volume of storage. It reflects the ability to store a larger quantity of hydrogen within a given spatial volume. Higher volumetric capacity indicates a more compact storage system capable of storing greater amounts of hydrogen. The parameter can be calculated using the following equation:
- 700 bar inner pressure load for the capillary tube;
- safety factor of 2;
- theoretical tensile strength of the capillary tube—considered only in the tangential direction—without longitudinal stress when having closed-ended cylinders.
3. Results
3.1. Free Space Influence on Capillary Tube Wall Stress
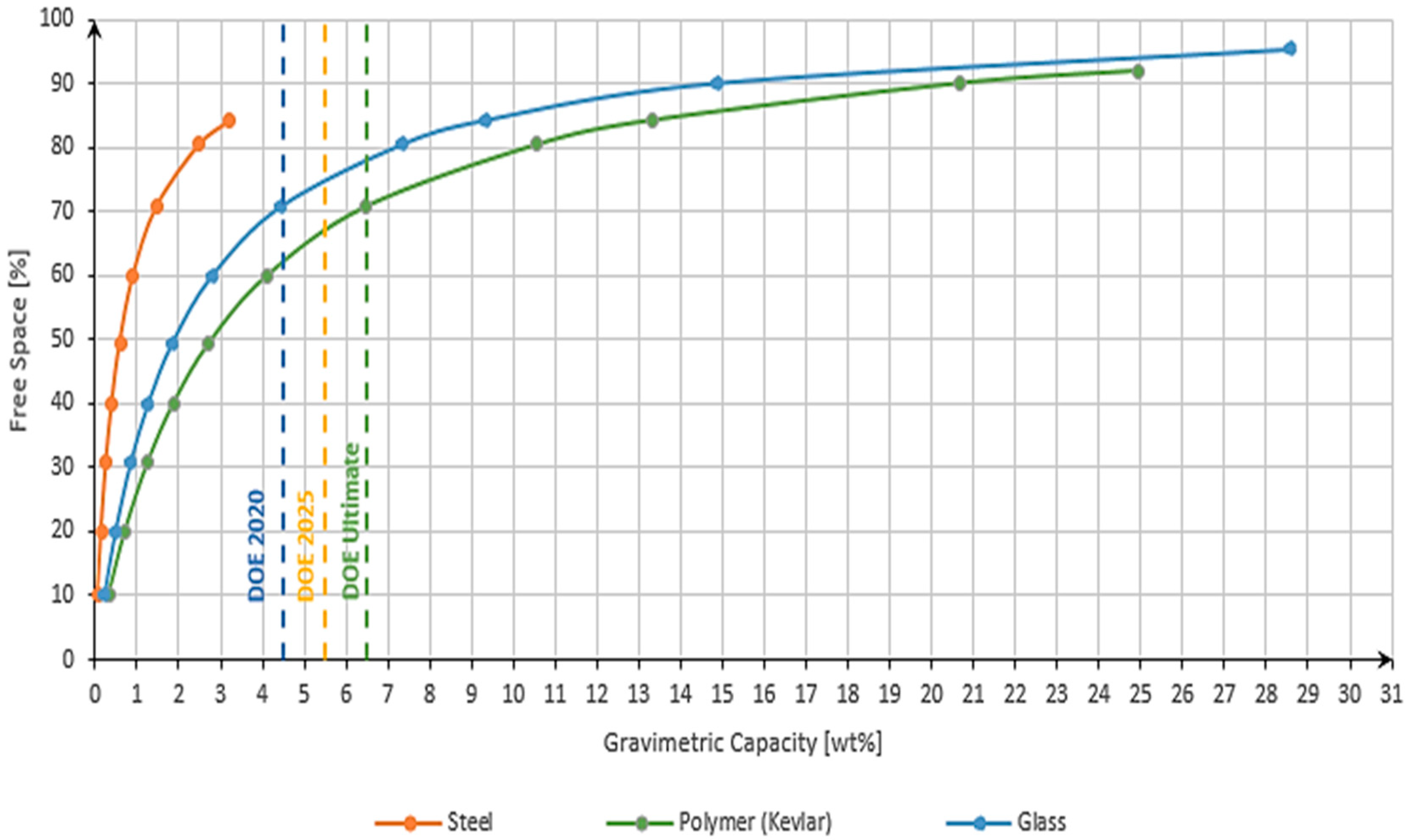
3.2. Gravimetric Capacity
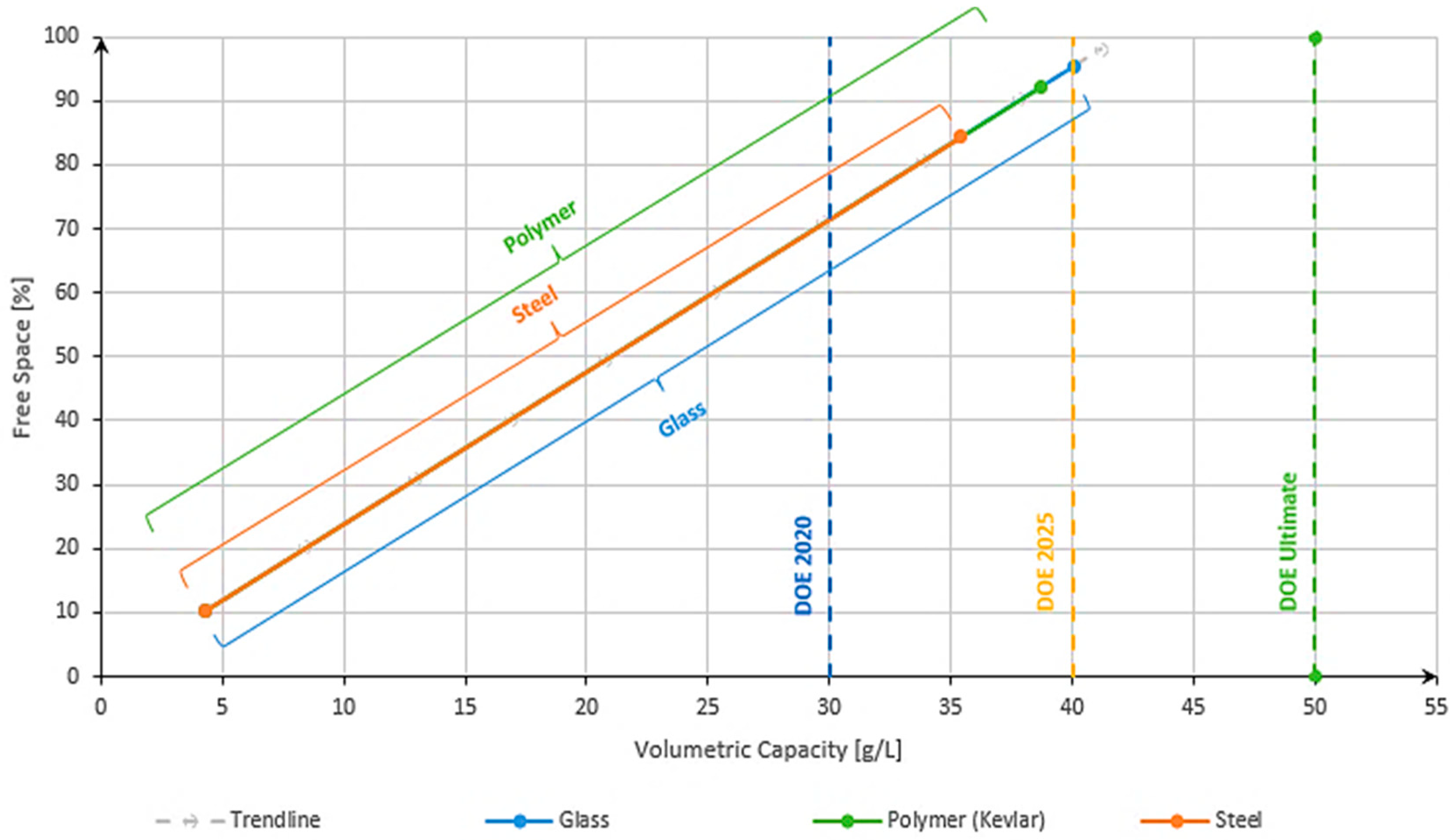
3.3. Volumetric Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Razmi, A.R.; Sharifi, S.; Gholamian, E.; Arabkoohsar, A.; Shahbakhti, M. 15. Green Hydrogen. In Future Grid-Scale Energy Storage Solutions; Academic Press: Cambridge, MA, USA, 2023; pp. 573–619. [Google Scholar]
- Clean Vehicles Directive. Available online: https://web.archive.org/web/20240329095320/https://transport.ec.europa.eu/transport-themes/clean-transport/clean-and-energy-efficient-vehicles/clean-vehicles-directive_en (accessed on 29 March 2024).
- Fuel Cell Electric Vehicle Market. Available online: https://web.archive.org/web/20240329100517/https://www.precedenceresearch.com/fuel-cell-electric-vehicle-market (accessed on 29 March 2024).
- Hydrogen Storage. Available online: https://web.archive.org/web/20240329094758/https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 29 March 2024).
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://web.archive.org/web/20240329104827/https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 29 March 2024).
- Zuettel, A. Materials for Hydrogen Storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Delgado, M. Análisis del entorno externo para explorar el futuro del hidrógeno. Ambiociencias. Rev. Divulg. Cient. E Innov. Docente 2023, 21, 7–25. [Google Scholar] [CrossRef]
- Cryo-Compressed Hydrogen Storage. Available online: https://web.archive.org/web/20240521085440/https://www.yumpu.com/en/document/read/26741293/cryo-compressed-hydrogen-storage (accessed on 21 May 2024).
- Hua, T.; Ahluwalia, R.; Peng, J.-K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical Assessment of Compressed Hydrogen Storage Tank Systems for Automotive Applications; Argonne National Laboratory: Lemont, IL, USA, 2010. [Google Scholar]
- Ahluwalia, R.K.; Hua, T.Q.; Peng, J.-K.; Lasher, S.; McKenney, K.; Sinha, J.; Gardiner, M. Technical Assessment of Cryo-Compressed Hydrogen Storage Tank System for Automotive Applications; Argonne National Laboratory: Lemont, IL, USA, 2009. [Google Scholar]
- Satyapal, S.; Read, C.; Ordaz, G.; Stetson, N.; Thomas, G.; Petrovic, J. The U.S. Department of Energy’s National Hydrogen Storage Project: Goal, Progress, and Future Plans. In Proceedings of the Fourth U.S.-Korea Forum on Nanotechnology: Sustainable Energy, Honolulu, HI, USA, 26–27 April 2007. [Google Scholar]
- Ahmed, A.; Seth, S.; Purewal, J.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat. Commun. 2019, 10, 1568. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Titus, E.; Salimian, M.; Okhay, O.; Rajendran, S.; Rajkumar, A.; Sousa, J.M.G.; Ferreira, A.L.C.; Gil, J.C.; Gracio, J. Hydrogen Storage for Energy Application; Intech Open: London, UK, 2012. [Google Scholar]
- Meyer, R. A New Technology for Hydrogen Safety: Glass Structures as Storage System. In Proceedings of the 4th International Conference on Hydrogen Safety (ICHS), San Francisco, CA, USA, 12–14 September 2011. [Google Scholar]
- Cheng, Q.; Zhang, R.; Shi, Z.; Lin, J. Review of common hydrogen storage tanks and current manufacturing methods for aluminum alloy tank liners. Int. J. Lightweight Mater. Manuf. 2024, 7, 269–284. [Google Scholar]
- Moradi, R.; Growth, K.M. Hydrogen storage and delivery: Review of the state-of-the-art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.; Ju, Y. Review on the key technologies and future development of insulation structure for liquid hydrogen storage tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1315. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Zhang, Y. The application of MOFs for hydrogen storage. Inorganica Chim. Acta 2023, 557, 121683. [Google Scholar] [CrossRef]
- Flekiewicz, M.; Kubica, G. Hydrogen Onboard Storage Technologies for Vehicles. In Diesel Engines—Current Challenges and Future Perspectives; Intech Open: London, UK, 2023. [Google Scholar]
- Keith, A.; Zlotea, C.; Szilágyi, P.Á. Perspective of interstitial hydrides of high-entropy alloys for vehicular hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 531–546. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrogen Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Barthélémy, H. Hydrogen storage—Industrial prospective. Int. J. Hydrogen Energy 2012, 37, 17364–17372. [Google Scholar] [CrossRef]
- Eliezer, D. An Innovative Technology for Hydrogen Storage in Glass Capillary Arrays for Portable and Mobile Systems. In Proceedings of the Hydrogen Conference & Expo, Long Beach, CA, USA, 3–6 May 2010. [Google Scholar]
- Holtappels, K.; Beckmann-Kluge, M.; Gebauer, M.; Grüneberg, M.; Eliezer, D. Hydrogen Storage in Glass Capillary Arrays for portable and mobile systems. In Proceedings of the International Conference on Hydrogen Safety, Ajaccio, France, 16–18 September 2009. [Google Scholar]
- Table of Design Material Properties for Structural Steel. Available online: https://eurocodeapplied.com/design/en1993/steel-design-properties (accessed on 26 March 2021).
- High Performance Steel Alloys for Lightweight Design. Available online: https://www.amt-advanced-materials-technology.com/materials/steel/ (accessed on 26 March 2021).
- The Mechanical Properties of Glass. Available online: https://web.archive.org/web/20230318223501/http://glassproperties.com/references/MechPropHandouts.pdf (accessed on 21 May 2024).
- High Performance Steel Alloys for Lightweight Design. Available online: https://www.amt-advanced-materials-technology.com/materials/aluminum-high-strength/#:~:text=High%20strength%20Aluminum%20alloys,Aluminum%20alloys%20show%20higher%20strength (accessed on 26 March 2021).
- High Performance Steel Alloys for Lightweight Design. Available online: https://www.amt-advanced-materials-technology.com/materials/magnesium-high-strength/ (accessed on 26 March 2021).
- High Performance Steel Alloys for Lightweight Design. Available online: https://www.amt-advanced-materials-technology.com/materials/titanium-high-strength/ (accessed on 26 March 2021).
- Mechanical Properties of Plastics. Available online: https://omnexus.specialchem.com/polymer-properties/properties/strength-at-break-tensile (accessed on 26 March 2021).
- Ceramics. Available online: http://www.keranova.se/Engelsk/Exceltabell_E.htm (accessed on 26 March 2021).
- Ceramic Materials Properties Charts. Available online: https://www.ceramicindustry.com/ceramic-materials-properties-charts/ (accessed on 26 March 2021).
- Glass Fiber Differences and Properties. Available online: https://www.princelund.com/glass-fiber.html#gsc.tab=0 (accessed on 29 March 2024).
- Jewett, R.P.; Walter, R.J.; Chandler, W.T.; Frohmberg, R.P. Hydrogen Environment Embrittlement of Metals; NASA: Washington, DC, USA, 1973. [Google Scholar]
- Fujiwara, H.; Ono, H.; Onoue, K.; Nishimura, S. High-pressure gaseous hydrogen permeation test method—Property of polymeric materials for high-pressure hydrogen devices. Int. J. Hydrogen Energy 2020, 45, 29082–29094. [Google Scholar] [CrossRef]
- Barton, J.L.; Moraine, M. Hydrogen Diffusion in Silicate Glass. J. Bob-Cryst. Solids 1970, 3, 115–126. [Google Scholar] [CrossRef]
- Ried, P.; Gaber, M.; Kluge, M.; Müller, R.; Holtappels, K.; Eliezer, D. H2-permeability and burst pressure of glass capillaries. In Proceedings of the International Congress on Glass 2010, Bahia, Brazil, 20–25 September 2010. [Google Scholar]
- Interglad. Available online: https://www.newglass.jp/interglad_n/gaiyo/outline_e.html (accessed on 24 October 2022).
- Ratoi, A.; Munteanu, C.; Istrate, B.; Eliezer, D. Review of properties, composition and defects of different glasses with potential use for glass hydrogen storage systems. EJMSE 2021, 6, 142–147. [Google Scholar] [CrossRef]
- Yue, Y.; Tuheen, M.I.; Du, J. Borosilicate Glasses. Encycl. Mater. Tech. Ceram. Glas. 2021, 2, 519–539. [Google Scholar]
- Tripa, P. Rezistenta Materialelor (Eng. Strength of Materials); Mirton: Timisoara, Romania, 2001. [Google Scholar]
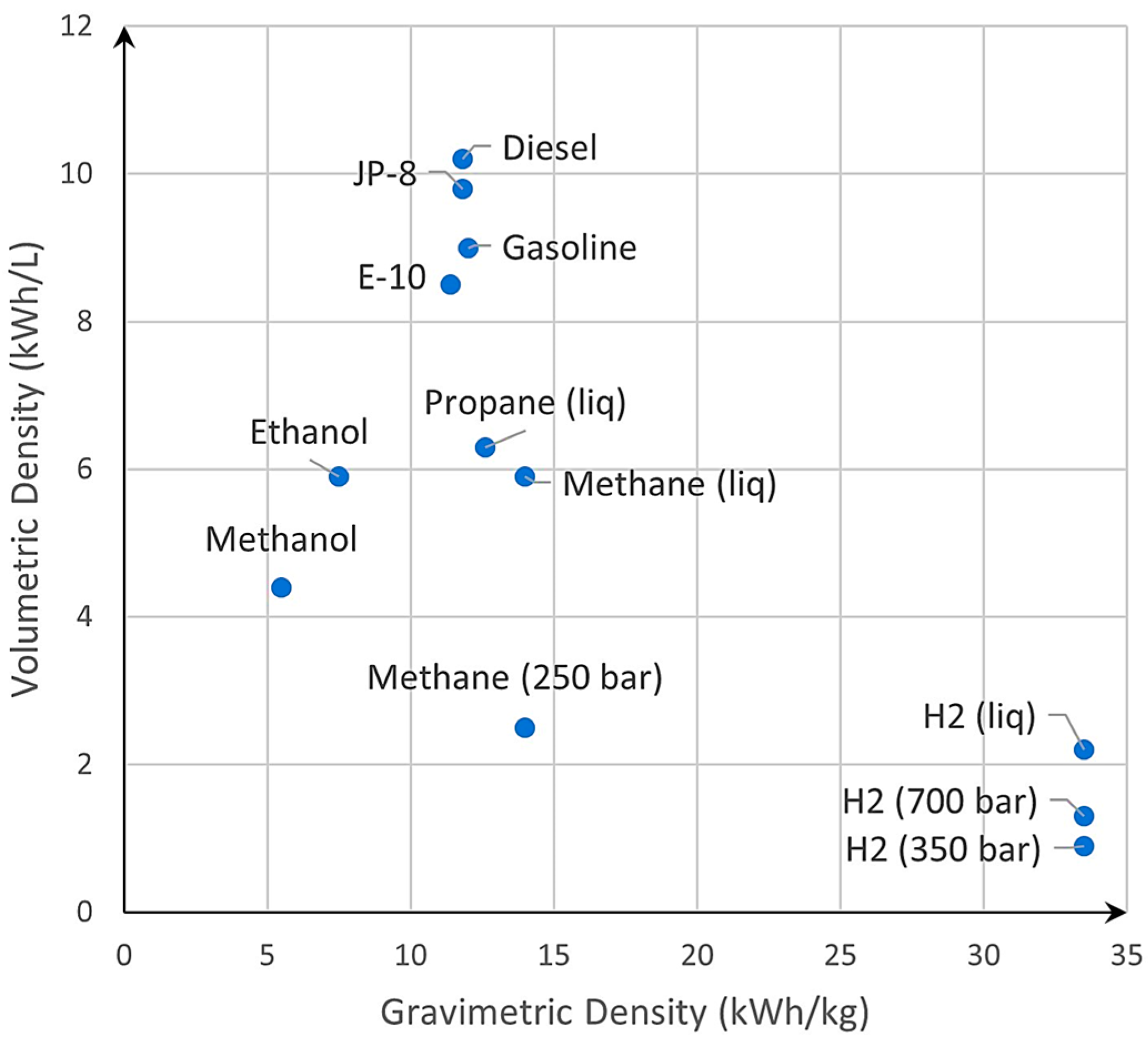

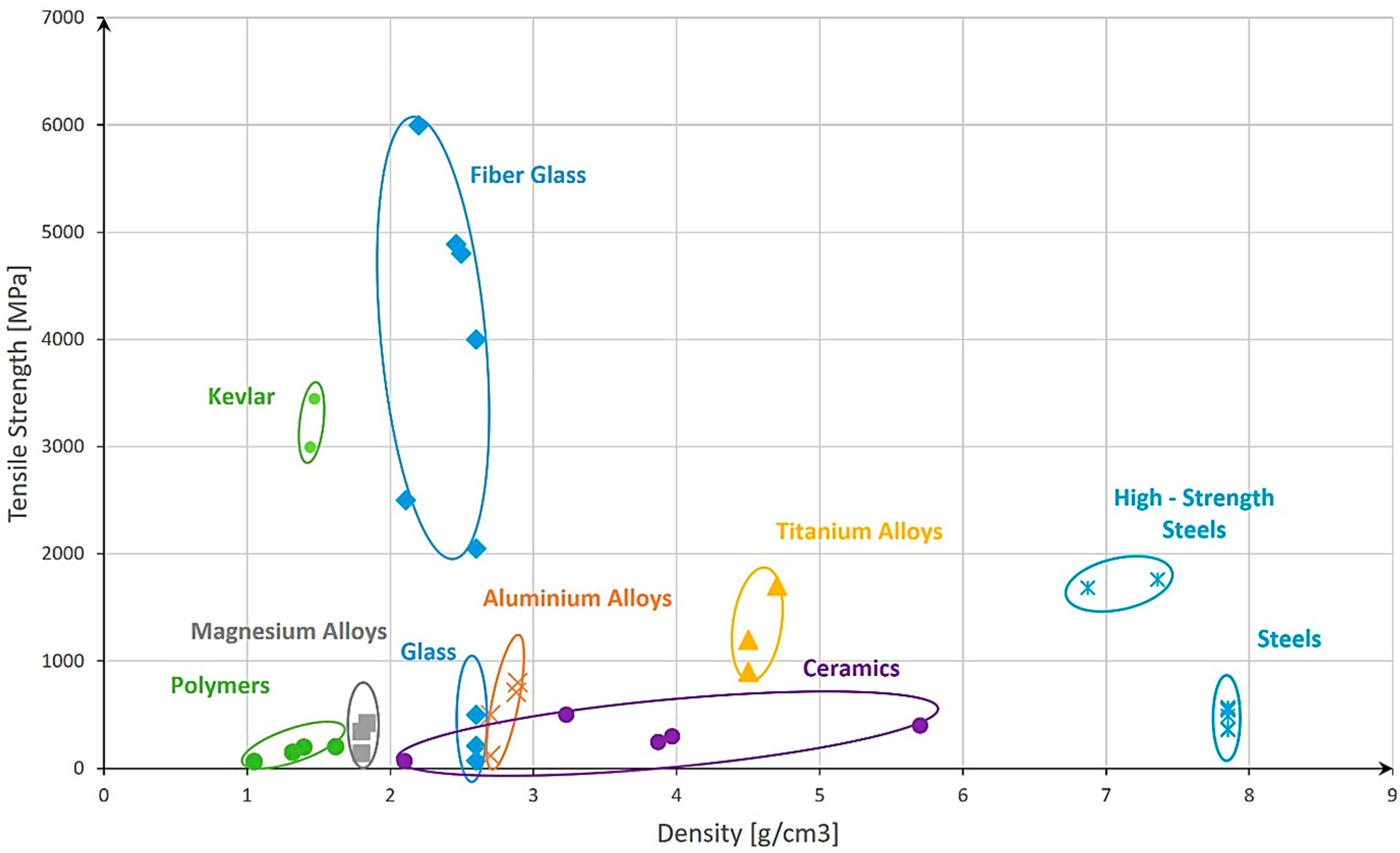

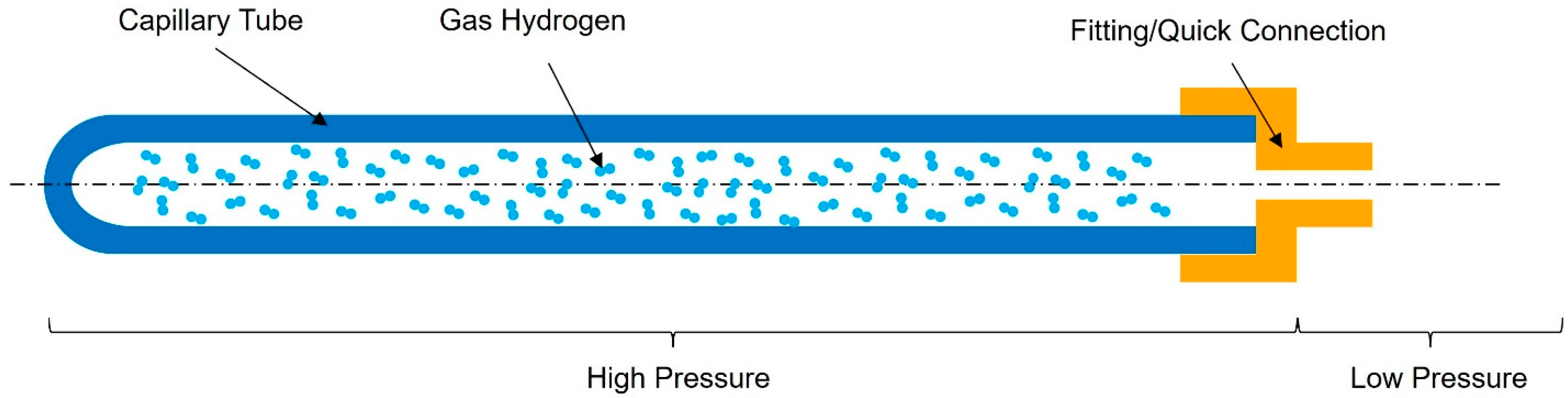



| Material | Density [g/cm3] | UTS [MPa] | σ Admissible [MPa] * |
|---|---|---|---|
| Steel XMP 21 | 6.87 | 1683 | 842 |
| Pure silica quartz fiber | 2.2 | 6000 | 3000 |
| Polymer (Kevlar 149) | 1.47 | 3450 | 1725 |
| Material | Free Space [%] |
|---|---|
| Steel XMP 21 | 84 |
| Polymer (Kevlar 149) | 92 |
| Pure silica quartz fiber | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratoi, A.; Munteanu, C.; Eliezer, D. Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach. Materials 2024, 17, 4288. https://doi.org/10.3390/ma17174288
Ratoi A, Munteanu C, Eliezer D. Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach. Materials. 2024; 17(17):4288. https://doi.org/10.3390/ma17174288
Chicago/Turabian StyleRatoi, Andrei, Corneliu Munteanu, and Dan Eliezer. 2024. "Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach" Materials 17, no. 17: 4288. https://doi.org/10.3390/ma17174288
APA StyleRatoi, A., Munteanu, C., & Eliezer, D. (2024). Maximizing Onboard Hydrogen Storage Capacity by Exploring High-Strength Novel Materials Using a Mathematical Approach. Materials, 17(17), 4288. https://doi.org/10.3390/ma17174288







