Effect of Citric Acid Hard Anodizing on the Mechanical Properties and Corrosion Resistance of Different Aluminum Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hard Anodizing Process
- ○
- Citric acid concentration [1 M];
- ○
- As a reference, use a sulfuric acid solution [1 M];
- ○
- Citric acid concentration [1 M] + 5 mL/L of sulfuric acid; finally
- ○
- Citric acid concentration [1 M] + 10 mL/L of sulfuric acid.
2.3. Microstructural Characterization
2.4. Vickers Hardness Test
2.5. Corrosion Test
3. Results
3.1. Chemical Composition by X-ray Fluorescence (XRF)
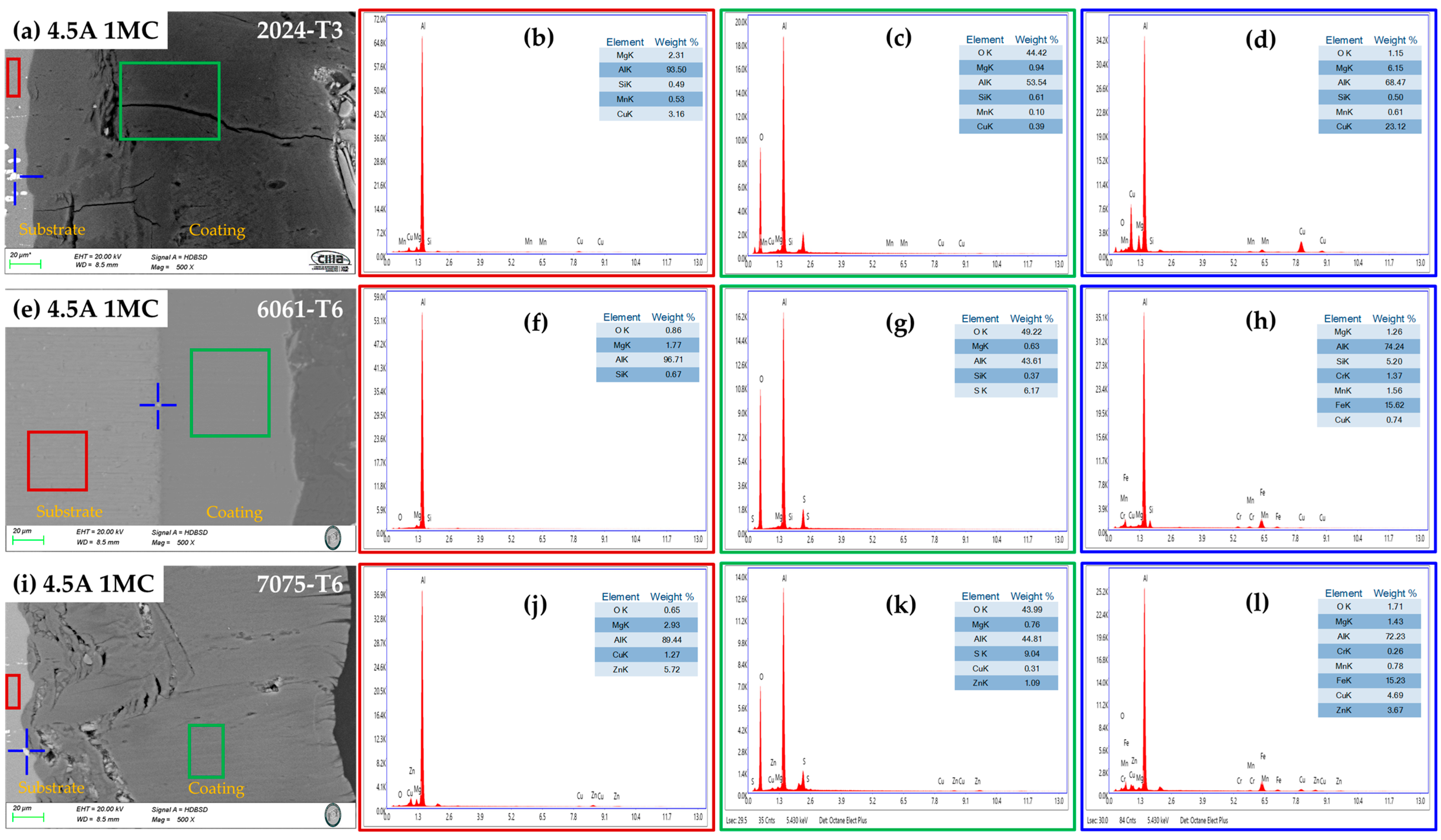
3.2. Microstructural Characterization (SEM)
3.2.1. Surface Morphology
3.2.2. Morphology of Cross-Section
3.2.3. Chemical Composition of the Cross-Section by SEM-EDS
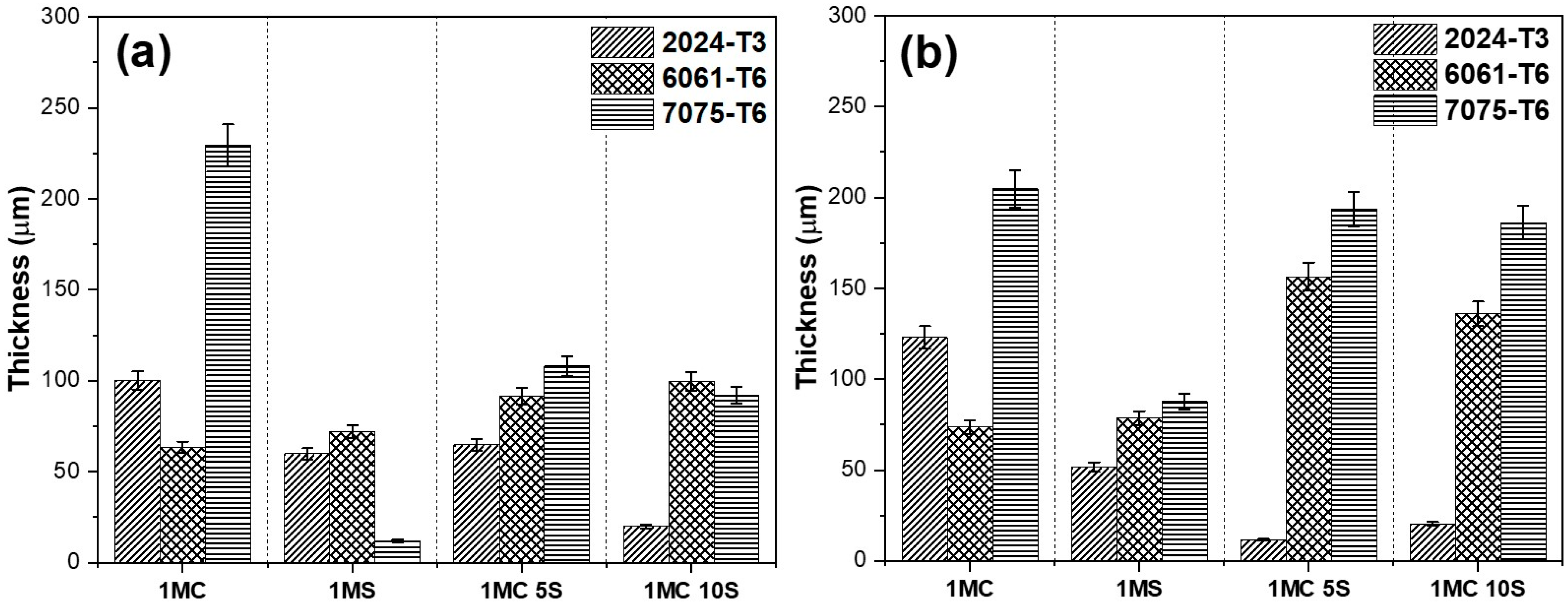
3.3. Thickness of Anodized Aluminum Alloys
3.4. Vickers Microhardness
3.5. Electrochemical Corrosion Test
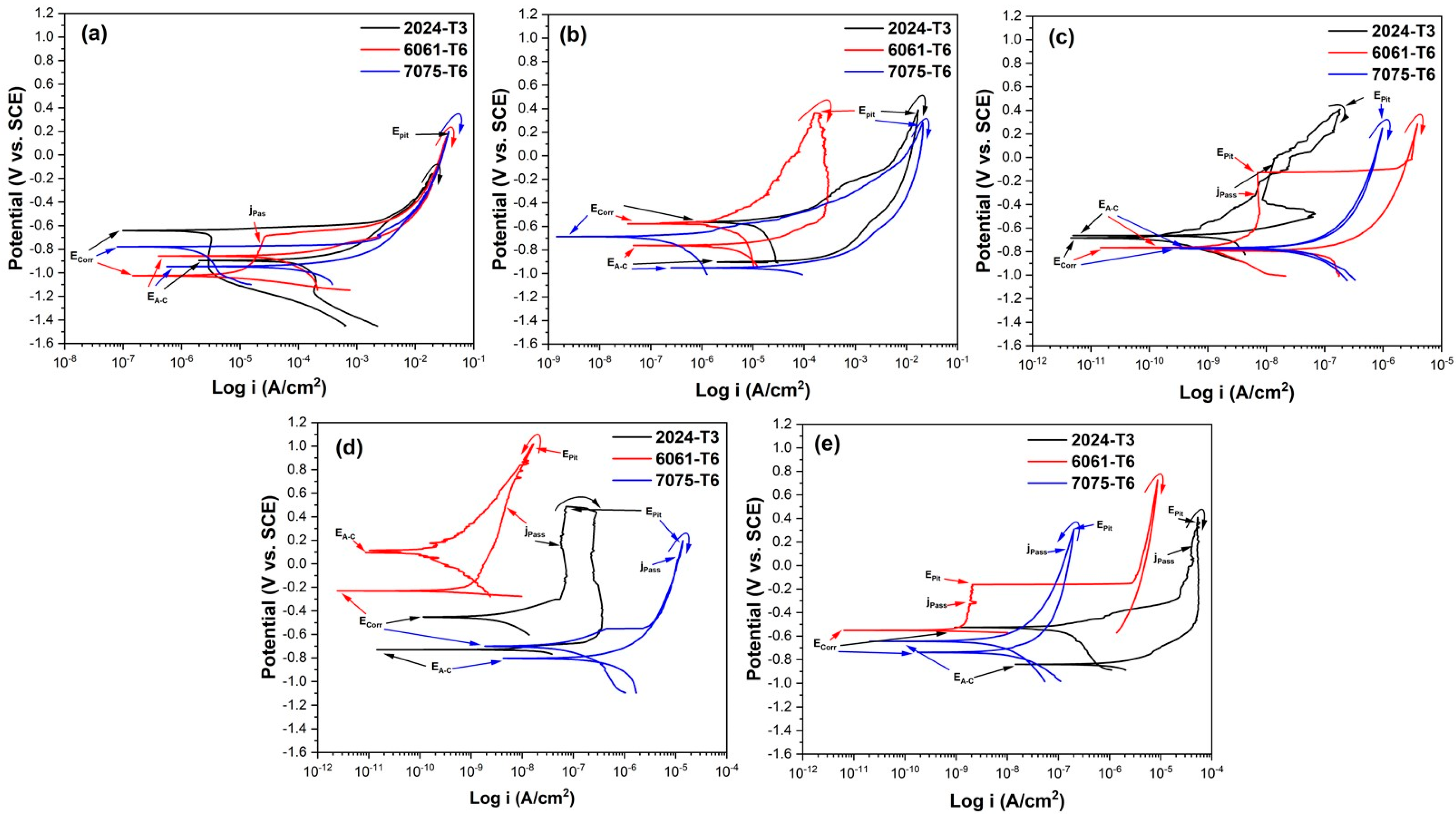
3.5.1. Cyclic Potentiodynamic Polarization Curve (CPPC)
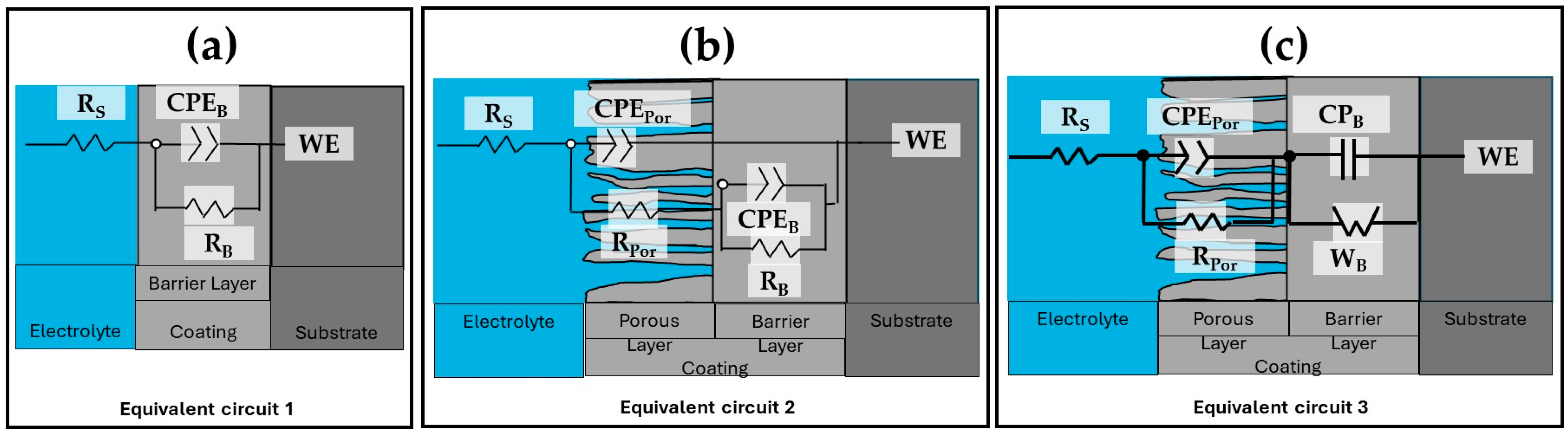
3.5.2. Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl. Surf. Sci. 2014, 321, 364–370. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, B.; Zhang, S. The Advancement of 7XXX Series Aluminum Alloys for Aircraft Structures: A Review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M.; Sánchez-Amaya, J.M.; González-Rovira, L. Behavior of the alloy AA2017 in aqueous solutions of NaCl. Part I: Corrosion mechanisms. Corr. Sci. 2023, 51, 518–524. [Google Scholar] [CrossRef]
- Kwolek, P. Hard Anodic Coatings on Aluminum Alloys. Adv. Manuf. Sci. Technol. 2017, 41, 35–46. [Google Scholar]
- Gloria, A.; Nontanari, R.; Richetta, M.; Varone, A. Alloys for Aeronautic Applications: State of the Art and Perspectives. Metals 2019, 9, 662. [Google Scholar] [CrossRef]
- Rangel, R.d.C.R.; Rangel, A.L.R.; da Silva, K.B.; Escada, A.L.d.A.; Chaves, J.A.M.; Maia, F.R.; Pina, S.; Reis, R.L.; Oliveira, J.M.; Rosifini Alves, A.P. Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies. Materials 2024, 17, 3627. [Google Scholar] [CrossRef] [PubMed]
- Rozenblium, I.; Yuferov, Y.; Borodianskiy, K. A Comprehensive Study of Aluminum Anodization in Transition Modes. Materials 2024, 17, 3438. [Google Scholar] [CrossRef]
- Sitanggang, R.B.; Nur’aini, S.; Susanto, S.; Widiyastuti, W.; Setyawan, H. The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Appl. Sci. 2024, 14, 6263. [Google Scholar] [CrossRef]
- Li, S.S.; Yue, X.; Li, Q.Y.; Peng, H.L.; Dong, B.X.; Liu, T.S.; Yang, H.Y.; Fan, J.; Shu, S.L.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Wen, W.; Carlson, B.; Banu, M. Evaluation of corrosion and its impact on the mechanical performance of Al–Steel joints. Materials 2024, 17, 3542. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, M.; Zhang, Z. The Effect of high-temperature deformation on the mechanical properties and corrosion resistance of the 2024 aluminum alloy joint after friction stir welding. Materials 2024, 17, 2969. [Google Scholar] [CrossRef]
- Guzmán-Flores, I.; Granda-Gutiérrez, E.E.; Cruz-González, C.E.; Hernández-García, H.M.; Díaz-Guillén, J.C.; Flores-González, L.; Praga-Alejo, R.J.; Martínez-Delgado, D.I. Enhancing the mechanical properties of a 6061 aluminum alloy by heat treatment from the perspective of taguchi design-of-experiments. Appl. Sci. 2024, 14, 5407. [Google Scholar] [CrossRef]
- Brace, A.W.; Sheasby, P.G. The Technology of Anodizing Aluminum; Technicopy Limited: Gloucestershire, UK, 1979; ISBN 0-905228-08-1. [Google Scholar]
- Aerts, T. Study of the Influence of Temperature and Heat Transfer during Anodic Oxide Growth on Aluminum. Ph.D. Thesis, Vrije Universiteit Brussel, Ixelles, Belgium, 2009. [Google Scholar]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Film 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Abrahami, S. Cr(VI)-Free Pre-Treatments for Adhesive Bonding of Aerospace Aluminum Alloys. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2016. [Google Scholar]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In Nanostructured Materials in Electrochemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 1–116. ISBN 978-3-527-62150-7. [Google Scholar]
- Xu, Y.; Thompson, G.E.; Wood, G.C.; Bethune, B. Anion incorporation and migration during barrier film formation on aluminum. Corros. Sci. 1987, 27, 83–102. [Google Scholar] [CrossRef]
- Ye, T.; Xia, E.; Qiu, S.; Liu, J.; Yue, H.; Tang, J.; Wu, Y. Deformation behavior of an extruded 7075 aluminum alloy at elevated temperatures. Materials 2024, 17, 1210. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villafañe, A.; Almeraya-Calderón, F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Applications of 2024 Aluminum Alloy. Available online: https://www.howardprecision.com/applications-of-2024-aluminum-alloy/ (accessed on 11 July 2022).
- Huda, Z.; Taib, N.I.; Zaharinie, T. Characterization of 2024-T3: An aerospace aluminum alloy. Mater. Chem. Phys. 2009, 113, 515–517. [Google Scholar] [CrossRef]
- Davis, J.R. Aluminum and Aluminum Alloys; ASM International: Almere, The Netherlands, 2001. [Google Scholar]
- Kummert, R.; Stumm, W. The surface complexation of organic acids on hydrous γ-Al2O3. J. Colloid Interface Sci. 1980, 75, 373–385. [Google Scholar] [CrossRef]
- Stumm, W.; Kummert, R.; Sigg, L. A ligand exchange model for the adsorption of inorganic and organic ligands at hydrous oxide interfaces. Croat. Chem. Acta. 1980, 53, 291–312. [Google Scholar]
- Hidber, P.C.; Graule, T.J.; Gauckler, L.J. Citric Acid—A dispersant for aqueous alumina suspensions. J. Am. Ceram. Soc. 1996, 79, 1857–1867. [Google Scholar] [CrossRef]
- Park, J.; Son, K.; Lee, J.; Kim, D.; Chung, W. Effects of Anodizing conditions on thermal properties of Al 20XX alloys for aircraft. Symmetry 2021, 13, 433. [Google Scholar] [CrossRef]
- Paz Martínez-Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Raffin, F.; Echouard, J.; Volovitch, P. Influence of the Anodizing time on the microstructure and immersion stability of tartaric-sulfuric acid anodized aluminum alloys. Metals 2023, 13, 993. [Google Scholar] [CrossRef]
- Tomczyk, K.; Stępniowski, W.J. Incorporation of anions into anodic alumina—A new track in Cr(VI) anodizing substitution? Materials 2024, 17, 2938. [Google Scholar] [CrossRef] [PubMed]
- Książek, E. Citric acid: Properties, microbial production, and applications in industries. Molecules 2024, 29, 22. [Google Scholar] [CrossRef]
- Mores, S.; Porto de Souza, L.; Irineudo, A.; César, J.; Murawski de Mello, A.; Pandey, A.; Soccol, C. Citric acid bioproduction and downstream processing: Status, opportunities and challenges. Bioresour. Technol. 2020, 320, 124426. [Google Scholar] [CrossRef]
- Cabral Miramontes, J.C.; Gaona Tiburcio, C.; García Mata, E.; Esneider Alcála, M.Á.; Maldonado-Bandala, E.; Lara-Banda, M.; Nieves-Mendoza, D.; Olguín-Coca, J.; Zambrano-Robledo, P.; López-León, L.D.; et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials 2022, 15, 6401. [Google Scholar] [CrossRef]
- Jalal, H.; Hussein, K.; Karabet, F.; Saound, Y. Study of the effect mechanism of organic additives on the aluminum alloy 6066 anodization. J. Min. Met. Mat. Eng. 2019, 5, 73–81. [Google Scholar]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Katsuta, Y.; Yasumori, A. Large-scale fabrication of ordered nanoporous alumina films with arbitrary pore intervals by critical-potential anodization. J. Electrochem. Soc. 2006, 153, B384–B391. [Google Scholar] [CrossRef]
- Chao-lei, B.; Ye-dong, H.; Xin, S. Effect of citric acid on microstructure and electrochemical characteristics of high voltaje anodized alumina film forme don etched Al Foils. Trans. Nonferrous Met. Soc. China 2011, 21, 133–138. [Google Scholar]
- Xiangfeng, M.; Guoying, W.; Hongliang, G.; Yundan, Y.; Ying, C.; Dettinger, H. Anodization for 2024 Al alloy from sulfuric-citric acid and anticorrosión performance of anodization films. Int. J. Electrochem. Sci. 2013, 8, 10660–10671. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, L.; Niu, D.; Liu, Z.; Wang, Y.; Liu, C. Effects of Additive for Anodizing Electrolyte on Anodic Film of High Silicon Aluminum Alloy. Int. J. Electrochem. Sci. 2016, 11, 1549–1557. [Google Scholar] [CrossRef]
- Memerifard, S.A.; Rahimipour, M.; Mobashrpour, I. Investigation of organic additives on voltaje rate in aluminum hard-anodizing process. Int. J. Bio-Inorg. Hybr. Nanomater. 2017, 6, 59–63. [Google Scholar]
- Solmaz, R.; Kardas, G.; Yazici, B.; Erbil, M. Citric acid as a natural corrosión inhibitor for aluminum protection. Corros. Eng. Sci. Technol. 2008, 43, 186–191. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- ASTM E407-07; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2011.
- Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Lara Banda, M.; Olguín-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.Á.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals 2021, 11, 1838. [Google Scholar] [CrossRef]
- Zhou, M. Aluminum room temperature hard anodizing technology. Mechanism of organic additives and bath liquid maintenance. Plating Environ. Protect. 2002, 22, 28–30. [Google Scholar]
- Franco, M.; Krishna, T.H.; Pillai, A.M.; Rajendra, A.; Sharma, A.K. A comparative study on the corrosion behavior of hard anodic coatings on AA 6061 obtained using dc and pulsed dc power sources. Acta Metall. Sin. Engl. Lett. 2013, 26, 647–656. [Google Scholar] [CrossRef]
- Gazapo, J.L.; Gea, J. Anodizing of Aluminium; European Aluminium Association: Alicante, Spain, 1994; pp. 1–27. [Google Scholar]
- Alwitt, R.S.; McClung, R.C.; Jacobs, S. Anodized Aluminum Coatings for Thermal Control, Part I: Coating Process and Stresses. In Proceedings of the AIAA Materials Specialist Conference, Dallas, TX, USA, 16–17 April 1992; pp. 39–45, AIAA-922158-CP; AAIA Technical Papers (A92-31285 12-23). [Google Scholar]
- ASTM E92-17; Standard Test Method for Vickers Hardness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Estupiñan-López, F.; Zambrano-Robledo, P.; Orozco Cruz, R.; Chacón-Nava, J.G.; Baltazar-Zamora, M.Á.; Almeraya-Calderón, F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Chacon-Nava, J.G.; Matutes-Aquino, J.A.; Martinez-Villafañe, A. Electrochemical response of permanent magnets in different solutions. J. Alloys Compd. 2004, 369, 78–80. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.M.; Delgado, A.D.; Flores-De Los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM G106-15; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Setyarini, P.H.; Soenoko, R.; Suprapto, A.; Irawan, Y.S. Effect of low potential anodizing of AA 6061. J. Eng. Appl. Sci. 2017, 12, 6419–6422. [Google Scholar]
- Soffritti, C.; Fortini, A.; Nastruzzi, A.; Sola, R.; Merlin, M.; Garagnani, G.L. Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments. Materials 2021, 14, 3281. [Google Scholar] [CrossRef] [PubMed]
- Guezmil, M.; Bensalah, W.; Khalladi, A.; Elleuch, K.; Depetris-Wery, M.; Ayedi, H.F. Friction coefficient and microhardness of anodized aluminum alloys under different elaboration conditions. Trans. Non-Ferr. Met. Soc. China 2015, 25, 1950–1960. [Google Scholar] [CrossRef]
- Koczera, A.E. The Effects of Carboxylic Acids in Aluminum Anodizing. Ph.D. Thesis, Honors Theses and Capstones, University of New Hampshire, Durham, NH, USA, 2017. [Google Scholar]
- Santiago, G.; Baltazar, M.A.; Galván, R.; López, L.; Zapata, F.; Zambrano, P.; Gaona, C.; Almeraya, F. Electrochemical Evaluation of Reinforcement Concrete Exposed to Soil Type SP Contaminated with Sulphates. Int. J. Electrochem. Sci. 2016, 11, 4850–4864. [Google Scholar] [CrossRef]
- Lerner, L.M. Hard anodizing of aerospace aluminium alloys. Trans. Inst. Met. Finish. 2010, 88, 21–24. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Wang, L.W.; Tian, H.Y.; Gao, H.; Xie, F.Z.; Zhao, K.; Cui, Z.Y. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiCp composite for marine applications. Prot. Met. Phys. Chem. Surf. 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Loto, R.T.; Adeleke, A.J. Corrosion of Aluminum Alloy Metal Matrix Composites in Neutral Chloride Solutions. Failure Anal. Prev. 2016, 16, 874–885. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion Prediction Using Electrochemical Techniques, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1129–1166. [Google Scholar]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Mansouri, K.; Ibrik, K.; Bensalah, N.; Abdel-Wahab, A. Anodic dissolution of pure aluminum during electrocoagulation process: Influence of supporting electrolyte, initial pH and current density. Ind. Eng. Chem. Res. 2011, 50, 13362–13372. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Feliu, S.J. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modifi cation of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Bastidas, D.M.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderón, F.M.; Gaona-Tiburcio, C. Corrosion behavior of Zn-TiO2, and Zn-ZnO Electrodeposited Coating in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Haripriya, A.; Manimekala, T.; Dharmalingam, G.; Manakshi, M.; Sivasubramanian, R. Asymmetric supercapacitors based on ZnCo2O4 nanohexagons and orange peel derived activated carbon electrodes. Chem. Asian J. 2024, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Swathi, R.; Tharani, D.; Manickam, M.; Sivasubramanian, R. A sol-gel derived LaCoO3 perovskite as an electrocatalyst for Al-air batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef]
- Hoar, T.P.; Wood, G.C. The sealing of porous anodic oxide fi lms on aluminium. Electrochim. Acta 1962, 7, 333–353. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multicoatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Kramer, G.R.; Mendez, C.M.; Ares, A.E. Evaluation of corrosion resistance of aluminum-based alloys in bioethanol produced in Misiones. Proc. Mat. Sci. 2015, 9, 341–349. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.W.; Nagao, S.J.; Nogi, M.; Koga, H.; Ma, J.S. The role of Zn precipitates and Cl anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Khadiri, M.; Elyaagoubi, M.; Idouhli, R.; Koumya, Y.; Zakir, O.; Benzakour, J.; Benyaich, A.; Abouelfida, A.; Outzourhit, A. Electrochemical Study of Anodized Titanium in Phosphoric. Acid. Adv. Mater. Sci. Eng. 2020, 2020, 5769071. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical Impedance Spectroscopy. Chapter, I. In Nanostructures and Thin Films for Multifunctional Applications; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Fabris, R.; Masi, G.; Bignozzi, M.C. Corrosion Behavior of Aluminum Alloys in Different Alkaline Environments: Effect of Alloying Elements and Anodization Treatments. Coatings 2024, 14, 240. [Google Scholar] [CrossRef]
- Mopon, M.L., Jr.; Garcia, J.S.; Manguerra, D.M.; Narisma, C.J.C. Corrosion Behavior of AA 1100 Anodized in Gallic-Sulfuric Acid Solution. Coatings 2021, 11, 405. [Google Scholar] [CrossRef]
- Elkilany, H.A.; Shoeib, M.A.; Abdel-Salam, O.E. Infl uence of hard anodizing on the mechanical and corrosion properties of different aluminum alloys. Metallogr. Microstruct. Anal. 2019, 8, 861–879. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Phosphoric acid anodization of Ti–6Al–4V–Structural and corrosion aspects. Corros. Sci. 2007, 49, 542–558. [Google Scholar] [CrossRef]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Nieves-Mendoza, D.; Lopez-Leon, L.D.; Jaquez-Muñoz, J.M.; Estupiñán-López, F.; et al. Use of Electrochemical Noise for the Study of Corrosion by Passivated CUSTOM 450 and AM 350 Stainless Steels. Metals 2024, 14, 341. [Google Scholar] [CrossRef]
- Macdonald, D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Jiang, B.; Liu, C.; Gu, X.; Chen, D. A Comparative Study of Corrosion Behavior of Hard Anodized and Micro-Arc Oxidation Coatings on 7050 Aluminum Alloy. Metals 2018, 8, 165. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical noise measurements of advanced high-strength steels in different solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Franco, M.; Anoop, S.; Uma Rani, R.; Sharma, A.K. Porous Layer Characterization of Anodized and Black-Anodized Aluminum by Electrochemical Studies. Int. Sch. Res. Not. 2012, 2012, 323676. [Google Scholar]
- López, V.; Otero, E.; Bautista, A.; González, J.A. Sealing of anodic films obtained in oxalic acid baths. Surf. Coat. Technol. 2000, 124, 76–84. [Google Scholar] [CrossRef]
- Van Der Linden, B.; Terryn, H.; Vereecken, J. Investigation of anodic aluminium oxide layers by electrochemical impedance spectroscopy. J. Appl. Electrochem. 1990, 20, 798–803. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Paez, M.A.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Xhou, X. Oxidation of copper and mobility of copper ions during anodizing of an Al–1.5 wt.% Cu alloy. Surf. Interface Anal. 1995, 23, 892–898. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Dimitrov, N.; Sieradzki, K. Dealloying and corrosion of Al alloy 2024 T3. J. Electrochem. Soc. 2002, 149, 428–439. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Martinez, M.A.; Montes, L.P. Evidence for Cu ions formation by dissolution and dealloying of Al2CuMg intermetallic compound in rotating disk. Electrochem. Soc. 2000, 147, 119–124. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagettia, J.; Normand, B. An electrochemical approach to the anodic oxidation of Al 2024 alloy in sulfuric acid containing inhibitors. Surf. Coat. Technol. 2002, 161, 267–274. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E. Structure of the Copper–enriched layer introduced by anodic oxidation of copper-containing aluminum Alloy. Electrochim. Acta. 2015, 179, 394–401. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhang, X.; Zhou, X.; Skeldon, P.; Haigh, S.J.; Thompson, G.E. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminum alloy using high-resolution 2D and 3D electron imaging. Corros. Sci. 2016, 103, 157–164. [Google Scholar] [CrossRef]
- Zhuravlyova, E.; Iglesias-Rubianes, L.; Pakes, A.; Skeldon, P.; Thompson, G.E.; Zhou, X.; Quance, T.; Graham, M.J.; Habzaki, H.; Shimizu, K. Oxygen evolution within barrier oxide films. Corros. Sci. 2002, 44, 2153–2159. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.E.; Terryn, H.; Skeldon, P.; Thompson, G.E.; Duszczyk, J.; Katgerman, L. Influence of substrate microstructure on the growth of anodic oxide layers. Electrochim. Acta. 2004, 49, 1127–1140. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. the composition of the alloy/film interface during anodic oxidation of Al-W alloys. J. Electrochem. Soc. 1996, 143, 2465–2470. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X. Effects of alloying elements in anodizing of aluminium. Trans IMF 1997, 75, 18–23. [Google Scholar]
- Noh, K.; Brammer, K.; Kim, H.; Jung, S.; Seong, T.; Jin, S. Highly self-assembled nanotubular aluminum oxide by hard anodization. J. Mater. Res. 2011, 26, 186–193. [Google Scholar] [CrossRef]
- Iglesias-Rubianes, L.; Garcia-Vergara, S.J.; Skeldon, P.; Thompsona, G.E.; Ferguson, J.; Beneke, M. Cyclic oxidation processes during anodizing of Al–Cu alloys. Electrochim. Acta 2007, 52, 7148–7157. [Google Scholar] [CrossRef]
- Arenas, M.A.; Skeldon, P.; Thompson, G.E.; Bailey, P.; Noakes, T.C.Q.; Habazaki, H.; Shimizu, K. Anodic behavior of a model second phase: Al–20 at.% Mg–20 at.% Cu. Corros. Sci. 2006, 48, 1225–1248. [Google Scholar]
- Caliari, D.; Timelli, G.; Salata, T.; Cavagnini, G.; Maestri, S. Surface defects of anodized HPDC Al alloy components. Metall. Ital. 2016, 108, 69–72. [Google Scholar]
- Tsangaraki-Kaplanoglou, I.; Theohari, S.; Dimogerontakis, T.; Wang, Y.M.; Kuo, H.H.H.; Kia, S. Effect of alloy types on the anodizing process of aluminium. Surf. Coat. Technol. 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Zhu, B.; Seifeddine, S.; Persson, P.O.; Jarfors, A.E.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
- Torrescano-Alvarez, J.M.; Curioni, M.; Habazaki, H.; Hashimoto, T.; Skeldon, P.; Zhou, X. Incorporation of alloying elements into porous anodic fi lms on aluminium alloys: The role of cell diameter. Electrochim. Acta 2019, 296, 783–789. [Google Scholar] [CrossRef]
- Konieczny, J.; Dobrza’nski, L.A.; Labisz, K.; Duszczyk, J. The infl uence of cast method and anodizing parameters on structure and layer thickness of aluminium alloys. J. Mater. Process. Technol. 2004, 157, 718–723. [Google Scholar] [CrossRef]
- Kwolek, P.; Krupa, K.; Obłój, A.; Kocurek, P.; Wierzbin’ska, M.; Sieniawski, J. Tribological Properties of the Oxide Coatings Produced onto 6061-T6 Aluminum Alloy in the Hard Anodizing Process. J. Mater. Eng. Perform. 2018, 27, 3268–3275. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Ramirez, A.; Gonzalez-Rodriguez, J.; Campillo, B. An Electrochemical Study of the Corrosion Behavior of a Dual Phase Steel in 0.5m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786. [Google Scholar]
- Thomas, R.W. Hard Anodizing of Aluminum; Brace, A.W., Ed.; Technicopy Ltd.: Stonehouse, UK, 1987; pp. 1–7. [Google Scholar]
- Pellegrini-Cervantes, M.J.; Almeraya-Calderon, F.; Borunda-Terrazas, A.; Bautista-Margulis, R.G.; Chacón-Nava, J.G.; Fajardo-San-Miguel, G.; Almaral-Sanchez, J.L.; Barrios-Durstewitz, C.P.; Martinez-Villafañe, A. Corrosion Resistance, Porosity and Strength of Blended Portland Cement Mortar Containing Rice Husk Ash And Nano-SiO2. Int. J. Electrochem. Sci. 2013, 8, 10697–10710. [Google Scholar] [CrossRef]
- Castorena-González, J.H.; Martin, U.; Gaona-Tiburcio, C.; Núñez-Jáquez, R.E.; Almeraya-Calderón, F.M.; Bastidas, J.M.; Bastidas, D.M. Modeling Steel Corrosion Failure in Reinforced Concrete by Cover Crack Width 3D FEM Analysis. Front. Mater. 2020, 7, 41. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.D.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti-6Al-4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Ariza-Figueroa, H.A.; Bosch, J.; Baltazar-Zamora, M.A.; Croche, R.; Santiago-Hurtado, G.; Landa-Ruis, L.; Mendoza-Rangel, J.M.; Bastidas, J.M.; Almeraya-Calderón, F.; Bastidas, D.M. Corrosion Behavior of AISI 304 Stainless Steel Reinforcements in SCBA-SF Ternary Ecological Concrete Exposed to MgSO4. Materials 2020, 13, 2412. [Google Scholar] [CrossRef]
- Wieland, E.; Stumm, W. Dissolution kinetics of kaolinite in acidic aqueous solutions at 25 °C. Geochim. Acta 1992, 56, 3339–3355. [Google Scholar] [CrossRef]
- Šeruga, M.; Hasenay, D. Electrochemical and surface properties of aluminium in citric acid solutions. J. Appl. Electrochem. 2001, 31, 961–967. [Google Scholar] [CrossRef]
- Huang, Y.; Shih, H.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the Corrosion Resistance of Anodizing Aluminum 6061 using Electrochemical Impedance Spectroscopy. Corr. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Cerezo, H.R.; Tiburcio, C.G.; Miramontes, J.A.C.; Bautista-Margulis, R.G.; Mendoza, D.N.; Bandala, E.M.; Estupiñán-López, F.H.; Calderón, F.A. Electrochemical characterization of Al–Li alloys AA2099 and AA2055 for aeronautical applications: Effect of thermomechanical treatments. J. Solid State Electrochem. 2023, 27, 3101–3117. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Lu, J.; Li, Y.; Yang, Y.; Feng, C.; Hao, C.; Zhang, Z.; Hu, J.; et al. Pore nucleation mechanism of self-ordered alumina with large period in stable anodization in citric acid. J. Electrochem. Soc. 2018, 165, E311–E317. [Google Scholar] [CrossRef]
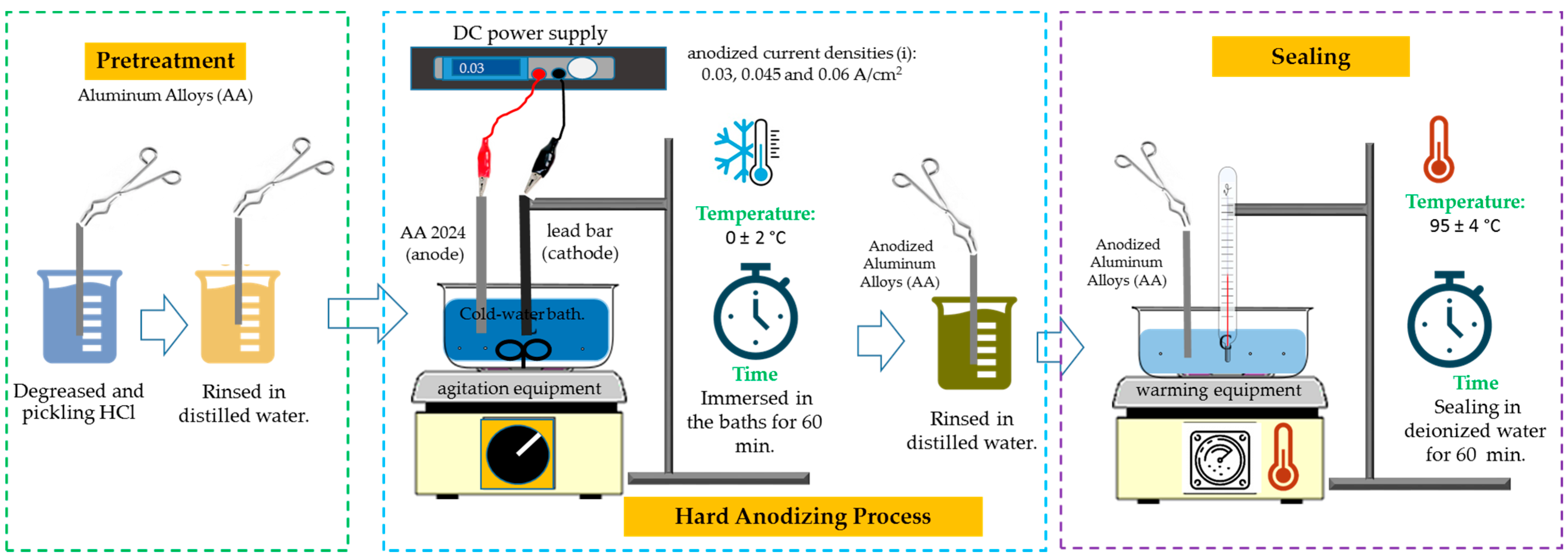
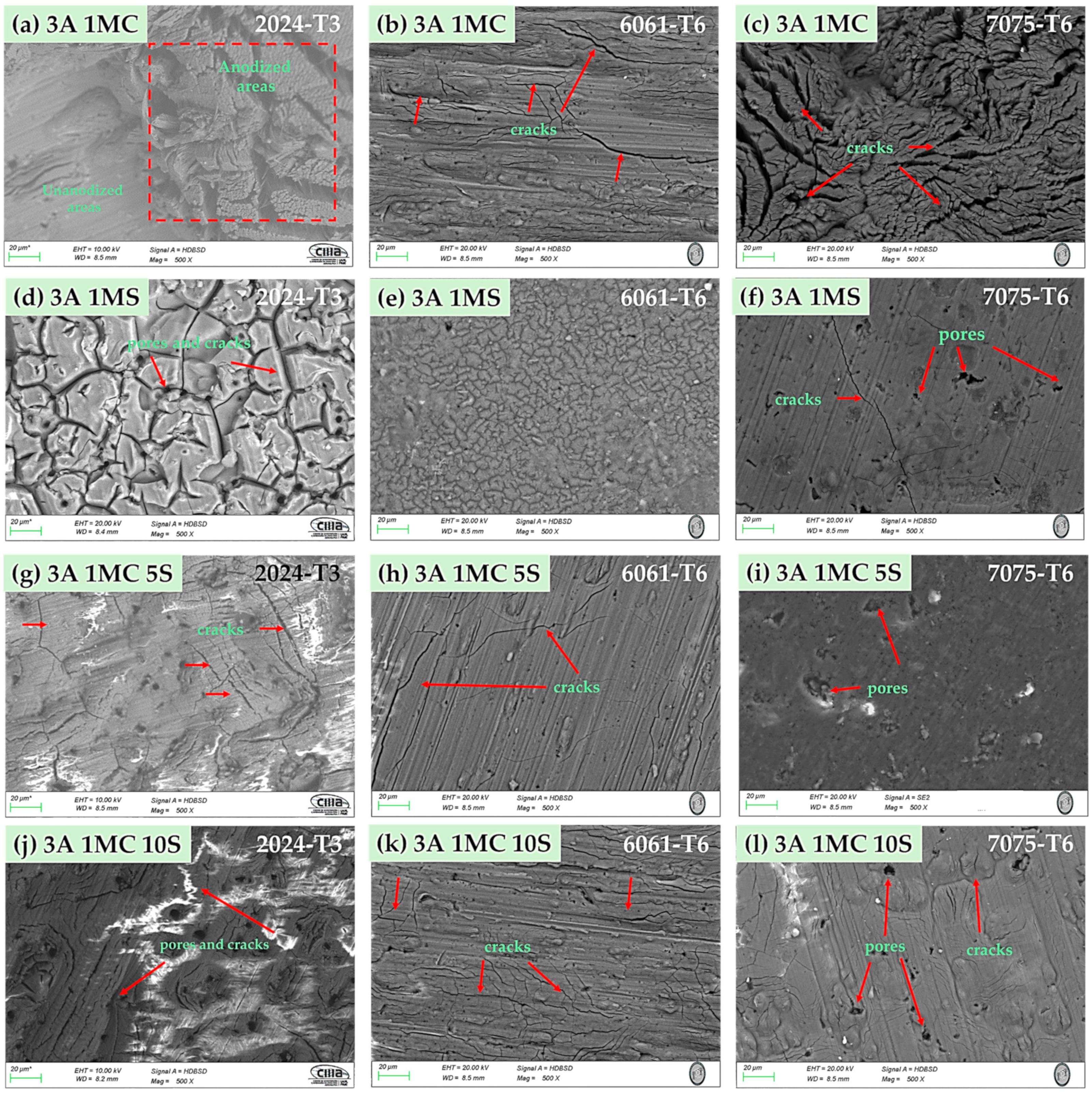


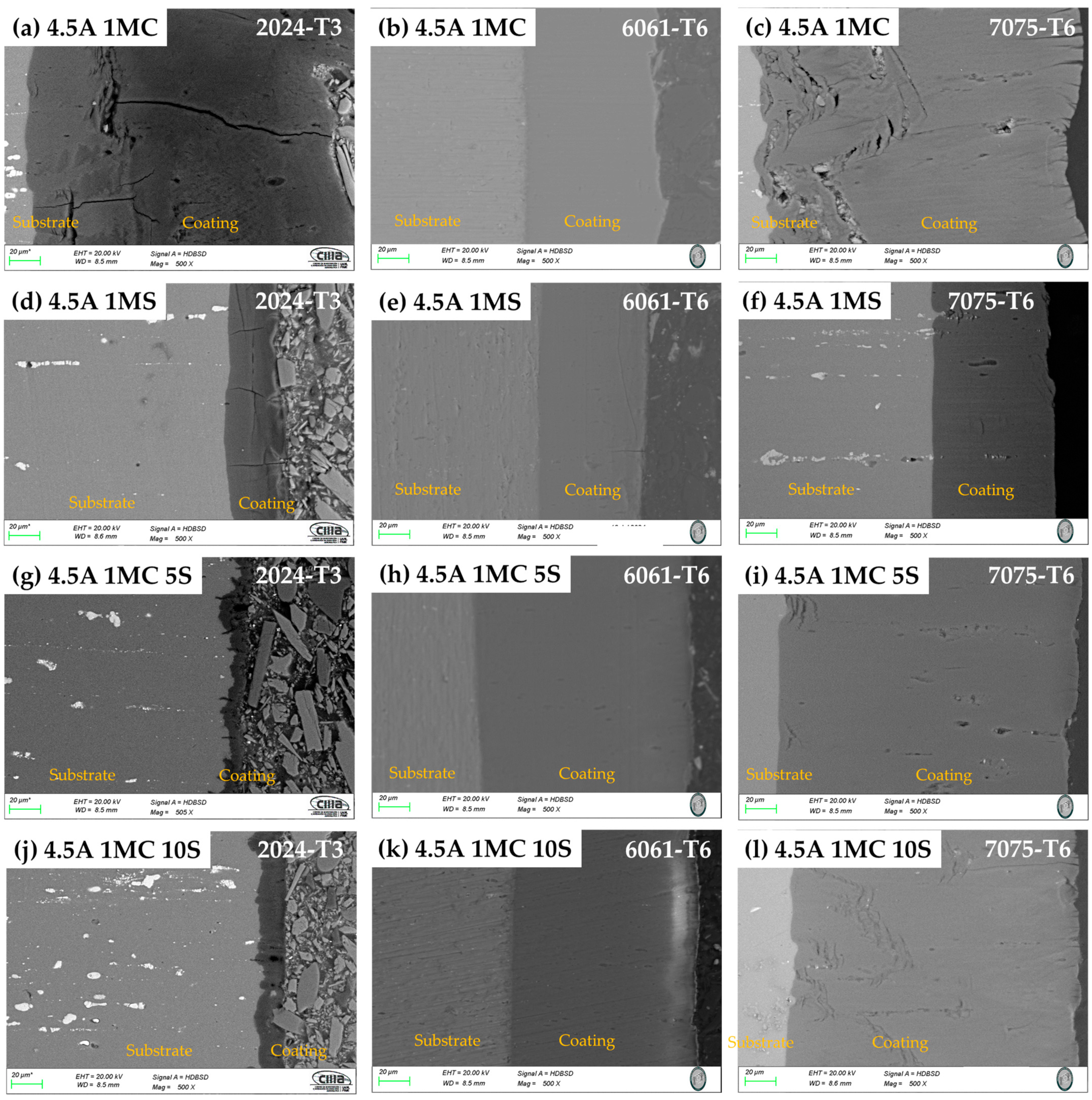
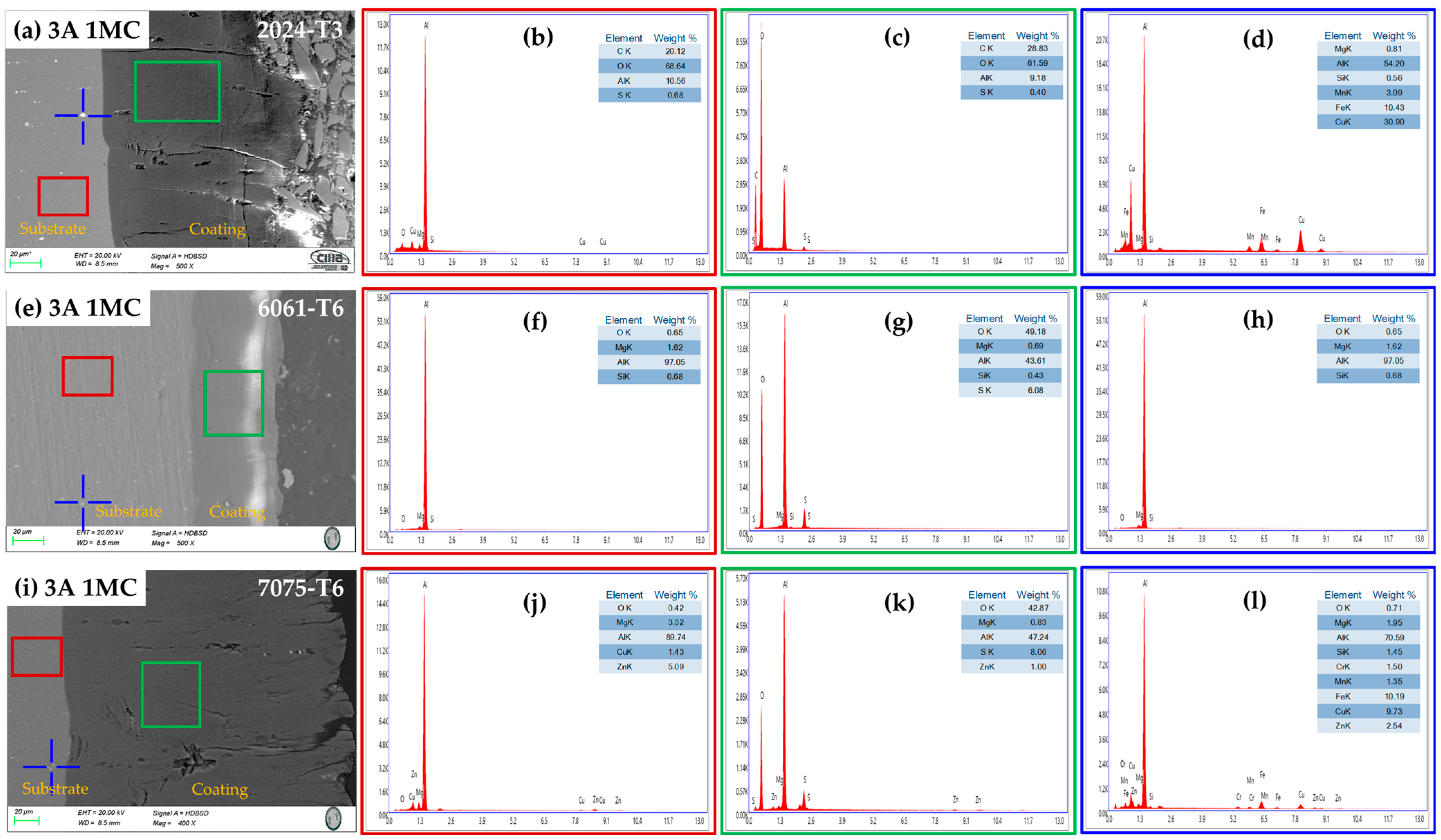




| Alloy | Anodizing | Sealing | Nomenclature | |||
|---|---|---|---|---|---|---|
| Current Density (i) (A/dm2) | Time and Temperature | Solutions Concentration | ||||
| Citric Acid | Sulfuric Acid | |||||
| 2024-T3 | 3 | Time: 1 h Temperature: 0 ± 2 °C | [1 M] | --- | Deionized water Time: 1 h Temperature: 95 ± 4 °C | 3A 1MC |
| --- | [1 M] | 3A 1MS | ||||
| [1 M] | 5 mL/L | 3A 1MC 5S | ||||
| [1 M] | 10 mL/L | 3A 1MC 10S | ||||
| 4.5 | [1 M] | --- | 4.5A 1MC | |||
| --- | [1 M] | 4.5A 1MS | ||||
| [1 M] | 5 mL/L | 4.5A 1MC 5S | ||||
| [1 M] | 10 mL/L | 4.5A 1MC 10S | ||||
| 6061-T6 | 3 | [1 M] | --- | 3A 1MC | ||
| --- | [1 M] | 3A 1MS | ||||
| [1 M] | 5 mL/L | 3A 1MC 5S | ||||
| [1 M] | 10 mL/L | 3A 1MC 10S | ||||
| 4.5 | [1 M] | --- | 4.5A 1MC | |||
| --- | [1 M] | 4.5A 1MS | ||||
| [1 M] | 5 mL/L | 4.5A 1MC 5S | ||||
| [1 M] | 10 mL/L | 4.5A 1MC 10S | ||||
| 7075-T6 | 3 | [1 M] | --- | 3A 1MC | ||
| --- | [1 M] | 3A 1MS | ||||
| [1 M] | 5 mL/L | 3A 1MC 5S | ||||
| [1 M] | 10 mL/L | 3A 1MC 10S | ||||
| 4.5 | [1 M] | --- | 4.5A 1MC | |||
| --- | [1 M] | 4.5A 1MS | ||||
| [1 M] | 5 mL/L | 4.5A 1MC 5S | ||||
| [1 M] | 10 mL/L | 7 4.5A 1MC 10S | ||||
| Alloy | Chemical Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Cu | Zn | Mg | Si | Fe | Mn | Cr | Ti | |
| 2024-T3 | Bal. | 4.273 | 0.201 | 1.012 | 0.367 | 0.268 | 0.494 | 0.043 | 0.047 |
| 6061-T6 | Bal. | 0.226 | 0.195 | 0.932 | 0.723 | 0.454 | 0.086 | 0.200 | 0.338 |
| 7075-T6 | Bal. | 1.641 | 5.657 | 2.531 | 0.224 | 0.348 | 0.078 | 0.166 | 0.129 |
| Aluminum Alloy | Current Density and Solution | ECorr (V vs. SCE) | EA-C (V vs. SCE) | EPit (C vs. SCE) | jPass (A/cm2) | jCorr (A/cm2) | Hysteresis |
|---|---|---|---|---|---|---|---|
| 2024-T3 | * | −0.656 | −0.895 | 0.256 | * | 0.34 × 10−6 | Positive |
| 6061-T6 | * | −1.025 | −0.858 | −0.124 | 2.60 × 10−5 | 9.47 × 10−6 | Positive |
| 7075-T6 | * | −0.808 | −0.945 | 0.198 | * | 0.31 × 10−6 | Positive |
| 2024-T3 | 3A 1MC | −0.571 | −0.900 | −0.411 | * | 1.50 × 10−6 | Positive |
| 6061-T6 | −0.577 | −0.762 | 0.363 | * | 8.02 × 10−6 | Positive | |
| 7075-T6 | −0.688 | −0.951 | 0.283 | * | 0.11 × 10−6 | Positive | |
| 2024-T3 | 3A 1MS | −0.664 | −0.685 | 0.404 | 0.005 × 10−5 | 0.0046 × 10−6 | Positive |
| 6061-T6 | −0.768 | −0.792 | −0.128 | 0.0007 × 10−5 | 0.0040 × 10−6 | Positive | |
| 7075-T6 | −0.768 | −0.768 | 0.248 | 0.063 × 10−5 | 0.0296 × 10−6 | Positive | |
| 2024-T3 | 3A 1MC 5S | −0.453 | −0.730 | 0.511 | 3.97 × 10−5 | 0.0053 × 10−6 | Positive |
| 6061-T6 | −0.230 | 0.112 | 1.006 | 0.0004 × 10−5 | 0.0003 × 10−6 | Negative | |
| 7075-T6 | −0.701 | −0.805 | 0.196 | 0.861 × 10−5 | 0.085 × 10−6 | Positive | |
| 2024-T3 | 3A 1MC 10S | −0.526 | −0.840 | 0.407 | 4.09 × 10−5 | 0.104 × 10−6 | Positive |
| 6061-T6 | −0.551 | * | −0.161 | 0.0002 × 10−5 | 0.0010 × 10−6 | Positive | |
| 7075-T6 | −0.739 | −0.604 | 0.307 | 0.014 × 10−5 | 0.0046 × 10−6 | Negative |
| Aluminum Alloy | Current Density and Solution | ECorr (V vs. SCE) | EA-C (V vs. SCE) | EPit (C vs. SCE) | jPass (A/cm2) | jCorr (A/cm2) | Hysteresis |
|---|---|---|---|---|---|---|---|
| 2024-T3 | * | −0.656 | −0.895 | 0.256 | * | 0.34 × 10−6 | Positive |
| 6061-T6 | * | −1.025 | −0.858 | −0.124 | 2.60 × 10−5 | 9.47 × 10−6 | Positive |
| 7075-T6 | * | −0.808 | −0.945 | 0.198 | * | 0.310 × 10−6 | Positive |
| 2024-T3 | 4.5A 1MC | −0.577 | −0.907 | 0.378 | * | 48.3 × 10−6 | Positive |
| 6061-T6 | −0.560 | −0.792 | 0.232 | * | 0.202 × 10−6 | Positive | |
| 7075-T6 | −0.716 | −0.879 | 0.177 | * | 0.51 × 10−6 | Positive | |
| 2024-T3 | 4.5A 1MS | −0.431 | * | 0.611 | * | 0.016 × 10−6 | Positive |
| 6061-T6 | −0.293 | 0.143 | 0.978 | 0.00059 × 10−5 | 0.0012 × 10−6 | Negative | |
| 7075-T6 | −0.754 | −0.807 | 0.244 | 0.89 × 10−5 | 1.15 × 10−6 | Positive | |
| 2024-T3 | 4.5A 1MC 5S | −0.756 | −0.911 | 0.093 | 0.012 × 10−5 | 2.51 × 10−6 | Positive |
| 6061-T6 | −0.590 | 0.065 | 0.673 | 0.00040 × 10−5 | 0.00047 × 10−6 | Negative | |
| 7075-T6 | −0.679 | −0.870 | −0.288 | 0.059 × 10−5 | 0.025 × 10−6 | Positive | |
| 2024-T3 | 4.5A 1MC 10S | −0.839 | −0.971 | −0.411 | 1.57 × 10−5 | 0.297 × 10−6 | Negative |
| 6061-T6 | −0.230 | 0.150 | 1.048 | 0.00053 × 10−5 | 0.0011 × 10−6 | Negative | |
| 7075-T6 | −0.811 | −0.702 | −0.171 | 1.81 × 10−5 | 0.143 × 10−6 | Negative |
| Aluminum Alloy | Current Density and Solution | RSol (Ω·cm) | CPEPor (µF/cm2) | nPor | RPor (Ω·cm2) | CPEB (F/cm2) | nB | RB (Ω·cm2) | WB (Ω·cm2/s0.5) | Error | χ2 | EEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024-T3 | * | 28.56 | * | * | * | 8.63 × 10−5 | 0.73 | 14.14 × 103 | * | <1.42 | 1 × 10−2 | 1 |
| 6061-T6 | * | 21.61 | * | * | * | 6.84 × 10−6 | 0.94 | 0.17 × 106 | * | <1.33 | 3 × 10−2 | 1 |
| 7075-T6 | * | 25.44 | * | * | * | 2.96 × 10−5 | 0.94 | 19.84 × 103 | * | <1.40 | 1 × 10−2 | 1 |
| 2024-T3 | 3A 1MC | 22.77 | 1.41 × 10−6 | 0.81 | 0.559 × 103 | 4.02 × 10−5 | 0.74 | 6.38 × 103 | * | <1.95 | 3 × 10−3 | 2 |
| 6061-T6 | 21.57 | 1.21 × 10−6 | 0.81 | 1.81 × 103 | 4.65 × 10−6 | 0.75 | 18.63 × 103 | * | <3.28 | 1 × 10−2 | 2 | |
| 7075-T6 | 30.3 | 7.29 × 10−9 | 0.77 | 1.31 × 106 | 9.80 × 10−9 | 1.00 | * | 3.16 × 107 | <1.42 | 1 × 10−2 | 3 | |
| 2024-T3 | 3A 1MS | 24.40 | 6.68 × 10−7 | 0.77 | 152.82 × 103 | 8.41 × 10−7 | 0.94 | 6209 × 103 | * | <1.79 | 1 × 10−2 | 2 |
| 6061-T6 | 27.28 | * | * | * | 1.82 × 10−9 | 0.83 | 3.90 × 106 | <1.33 | 1 × 10−2 | 1 | ||
| 7075-T6 | 23.5 | 1.16 × 10−8 | 0.66 | 1.42 × 106 | 1.07 × 10−6 | 0.69 | 2.72 × 106 | * | <4.14 | 6 × 10−2 | 2 | |
| 2024-T3 | 3A 1MC 5S | 22.77 | 2.28 × 10−7 | 0.78 | 46.016 × 103 | 9.30 × 10−7 | 0.81 | 5.81 × 106 | * | <1.96 | 1 × 10−2 | 2 |
| 6061-T6 | 14.17 | 4.67 × 10−9 | 0.77 | 2.67 × 106 | 1.04 × 10−9 | 1.00 | * | 8.63 × 106 | <2.35 | 8 × 10−3 | 3 | |
| 7075-T6 | 21.19 | 2.16 × 10−7 | 0.68 | 42.58 × 103 | 1.30 × 10−6 | 0.61 | 5.12 × 105 | * | <2.27 | 8 × 10−3 | 2 | |
| 2024-T3 | 3A 1MC 10S | 14.26 | 8.8 × 10−7 | 0.74 | 47.54 × 103 | 3.12 × 10−6 | 0.69 | 362.56 × 103 | * | <1.14 | 1 × 10−2 | 2 |
| 6061-T6 | 26.34 | 9.42 × 10−9 | 0.84 | 2.32 × 106 | 5.49 × 10−9 | 1.00 | * | 2.06 × 107 | <2.01 | 3 × 10−2 | 3 | |
| 7075-T6 | 16.99 | 7.54 × 10−9 | 0.75 | 1.48 × 106 | 9.80 × 10−9 | 1.00 | * | 3.16 × 107 | <1.42 | 1 × 10−2 | 3 |
| Aluminum Alloy | Current Density and Solution | RSol (Ω·cm) | CPEPor (µF/cm2) | nPor | RPor (KΩ·cm2) | CPEB (µF/cm2) | nB | RB (KΩ·cm2) | WB (KΩ·cm2/s0.5) | Error | χ2 | EEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024-T3 | * | 28.56 | * | * | * | 8.63 ×10−5 | 0.73 | 14.14 × 103 | * | <1.42 | 1 × 10−2 | 1 |
| 6061-T6 | * | 21.61 | * | * | * | 6.84 ×10−6 | 0.94 | 0.17 × 106 | * | <1.33 | 3 × 10−2 | 1 |
| 7075-T6 | * | 25.44 | * | * | * | 2.96 × 10−5 | 0.94 | 19.84 × 103 | * | <1.40 | 1 × 10−2 | 1 |
| 2024-T3 | 4.5A 1MC | 25.58 | 5.43 × 10−5 | 0.74 | 6.55 × 103 | 4.81 × 10−4 | 0.71 | 19.47 × 103 | * | <1.95 | 3 × 10−3 | 2 |
| 6061-T6 | 42.86 | 1.33 × 10−6 | 0.72 | 4.55 × 103 | 5.73 × 10−6 | 0.84 | 27.90 × 103 | * | <3.28 | 1 × 10−2 | 2 | |
| 7075-T6 | 43.78 | 4.32 × 10−6 | 0.57 | 457.75 × 103 | 4.60 × 10−6 | 0.64 | 531.22 × 103 | * | <1.42 | 1 × 10−2 | 2 | |
| 2024-T3 | 4.5A 1MS | 25.35 | 6.55 × 10−7 | 0.61 | 89.04 × 103 | 1.26 × 10−6 | 0.91 | 1.02 × 107 | * | <1.79 | 1 × 10−2 | 2 |
| 6061-T6 | 15.20 | 4.49 × 10−9 | 0.79 | 1.55 × 106 | 2.74 × 10−9 | 1.00 | * | 2.40 × 107 | <1.33 | 1 × 10−2 | 3 | |
| 7075-T6 | 38.56 | 2.09 × 10−8 | 0.63 | 41.14 × 103 | 8.68 × 10−7 | 0.95 | 18.78 × 103 | * | <4.14 | 6 × 10−2 | 2 | |
| 2024-T3 | 4.5A 1MC 5S | 63.58 | 6.13 × 10−7 | 0.85 | 2.67 × 103 | 6.11 × 10−6 | 0.54 | 143.83 × 103 | * | <1.96 | 1 × 10−2 | 2 |
| 6061-T6 | 24.73 | 7.18 × 10−8 | 0.68 | 301.01 × 103 | 1.52 × 10−8 | 1.00 | * | 3.32 × 106 | <2.35 | 8 × 10−3 | 3 | |
| 7075-T6 | 51.75 | 1.61 × 10−7 | 0.61 | 75.87 × 103 | 1.11 × 10−8 | 1.00 | * | 9.23 × 106 | <2.27 | 8 × 10−3 | 3 | |
| 2024-T3 | 4.5A 1MC 10S | 16.88 | 1.27 × 10−6 | 0.75 | 15.65 × 103 | 1.26 × 10−6 | 0.90 | 63.23 × 103 | * | <1.14 | 1 × 10−2 | 2 |
| 6061-T6 | 35.38 | 5.03 × 10−8 | 0.72 | 409.58 × 103 | 6.71 × 10−9 | 1.00 | * | 3.00 × 106 | <2.01 | 3 × 10−2 | 3 | |
| 7075-T6 | 17.04 | 2.07 × 10−6 | 0.68 | 13.19 × 103 | 3.12 × 10−7 | 1.00 | * | 3.74 × 106 | <2.70 | 5 × 10−3 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miramontes, J.; Almeraya-Calderón, F.; Méndez-Ramírez, C.T.; Flores-De los Rios, J.P.; Maldonado-Bandala, E.; Baltazar-Zamora, M.Á.; Nieves-Mendoza, D.; Lara-Banda, M.; Pedraza-Basulto, G.; Gaona-Tiburcio, C. Effect of Citric Acid Hard Anodizing on the Mechanical Properties and Corrosion Resistance of Different Aluminum Alloys. Materials 2024, 17, 4285. https://doi.org/10.3390/ma17174285
Cabral-Miramontes J, Almeraya-Calderón F, Méndez-Ramírez CT, Flores-De los Rios JP, Maldonado-Bandala E, Baltazar-Zamora MÁ, Nieves-Mendoza D, Lara-Banda M, Pedraza-Basulto G, Gaona-Tiburcio C. Effect of Citric Acid Hard Anodizing on the Mechanical Properties and Corrosion Resistance of Different Aluminum Alloys. Materials. 2024; 17(17):4285. https://doi.org/10.3390/ma17174285
Chicago/Turabian StyleCabral-Miramontes, José, Facundo Almeraya-Calderón, Ce Tochtli Méndez-Ramírez, Juan Pablo Flores-De los Rios, Erick Maldonado-Bandala, Miguel Ángel Baltazar-Zamora, Demetrio Nieves-Mendoza, María Lara-Banda, Gabriela Pedraza-Basulto, and Citlalli Gaona-Tiburcio. 2024. "Effect of Citric Acid Hard Anodizing on the Mechanical Properties and Corrosion Resistance of Different Aluminum Alloys" Materials 17, no. 17: 4285. https://doi.org/10.3390/ma17174285
APA StyleCabral-Miramontes, J., Almeraya-Calderón, F., Méndez-Ramírez, C. T., Flores-De los Rios, J. P., Maldonado-Bandala, E., Baltazar-Zamora, M. Á., Nieves-Mendoza, D., Lara-Banda, M., Pedraza-Basulto, G., & Gaona-Tiburcio, C. (2024). Effect of Citric Acid Hard Anodizing on the Mechanical Properties and Corrosion Resistance of Different Aluminum Alloys. Materials, 17(17), 4285. https://doi.org/10.3390/ma17174285









