Progress of Metal Chalcogenides as Catalysts for Efficient Electrosynthesis of Hydrogen Peroxide
Abstract
1. Introduction
2. Electrochemical H2O2 Synthesis through 2e− ORR
2.1. Mechanism of Electrocatalytic 2e− ORR
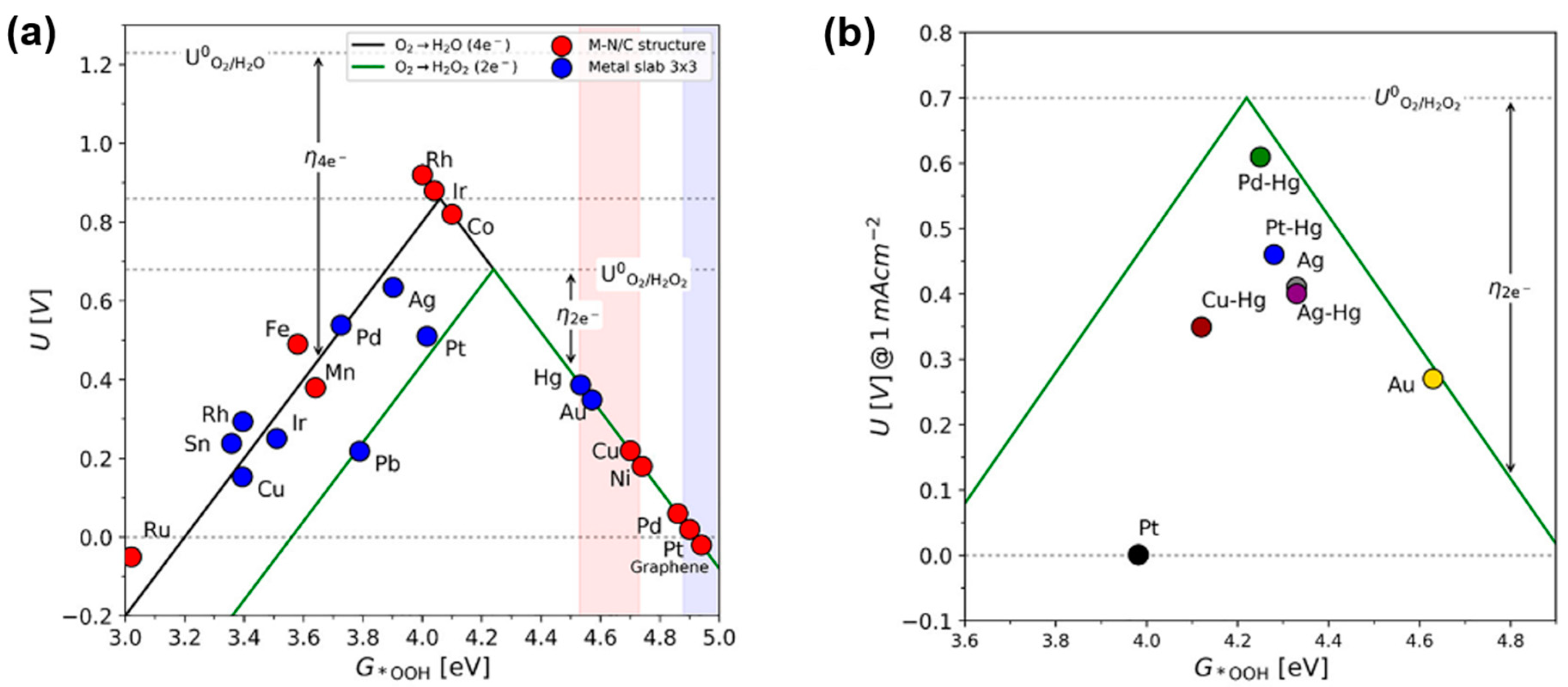
2.2. Reaction Kinetics and Volcano Plot Analysis for 2e− ORR
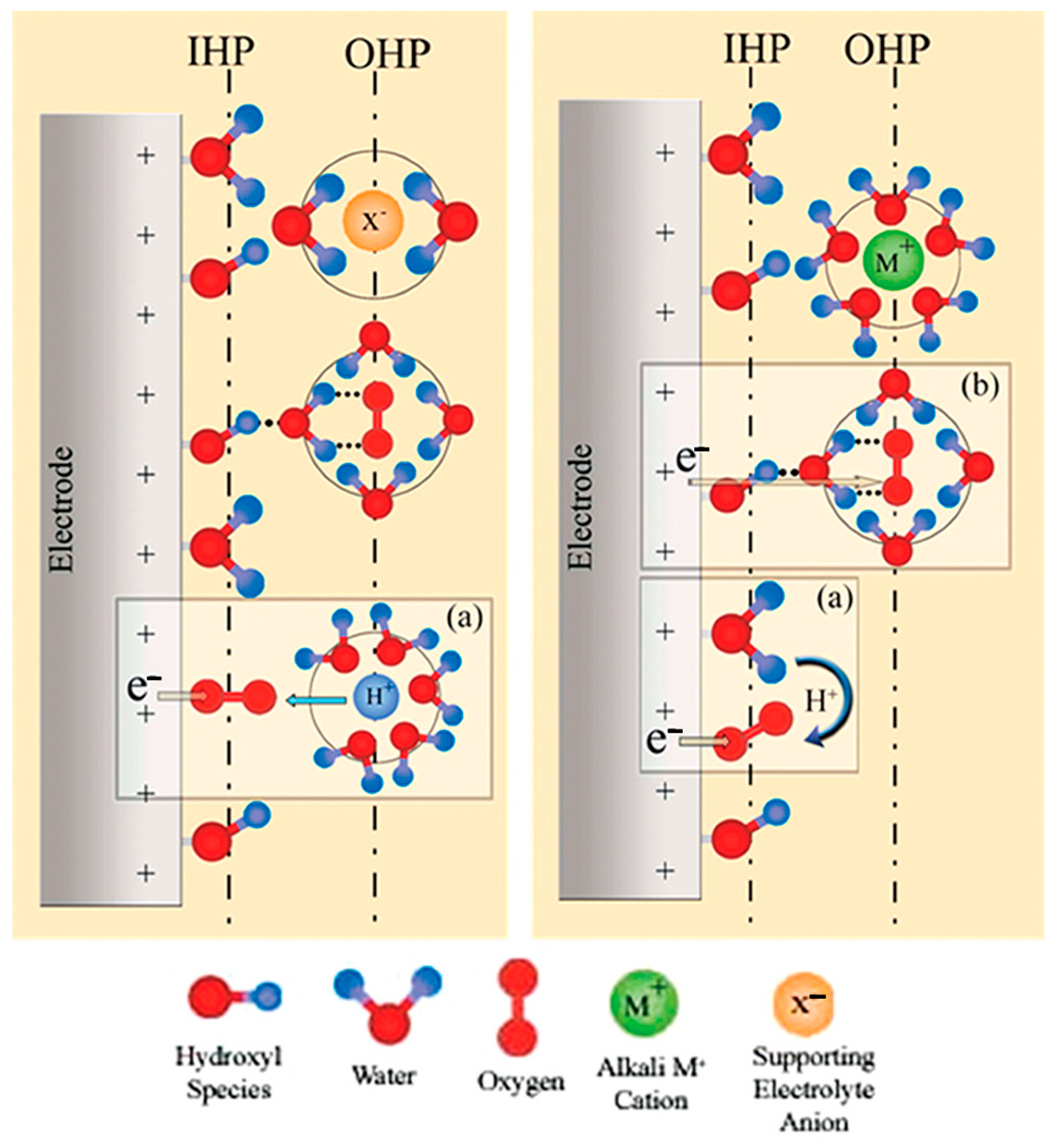
2.3. Effects of Electrolyte pH on ORR Mechanism
3. Metal Chalcogenide Catalysts for H2O2 Electrosynthesis
3.1. Material Design for Efficient Electrocatalysts
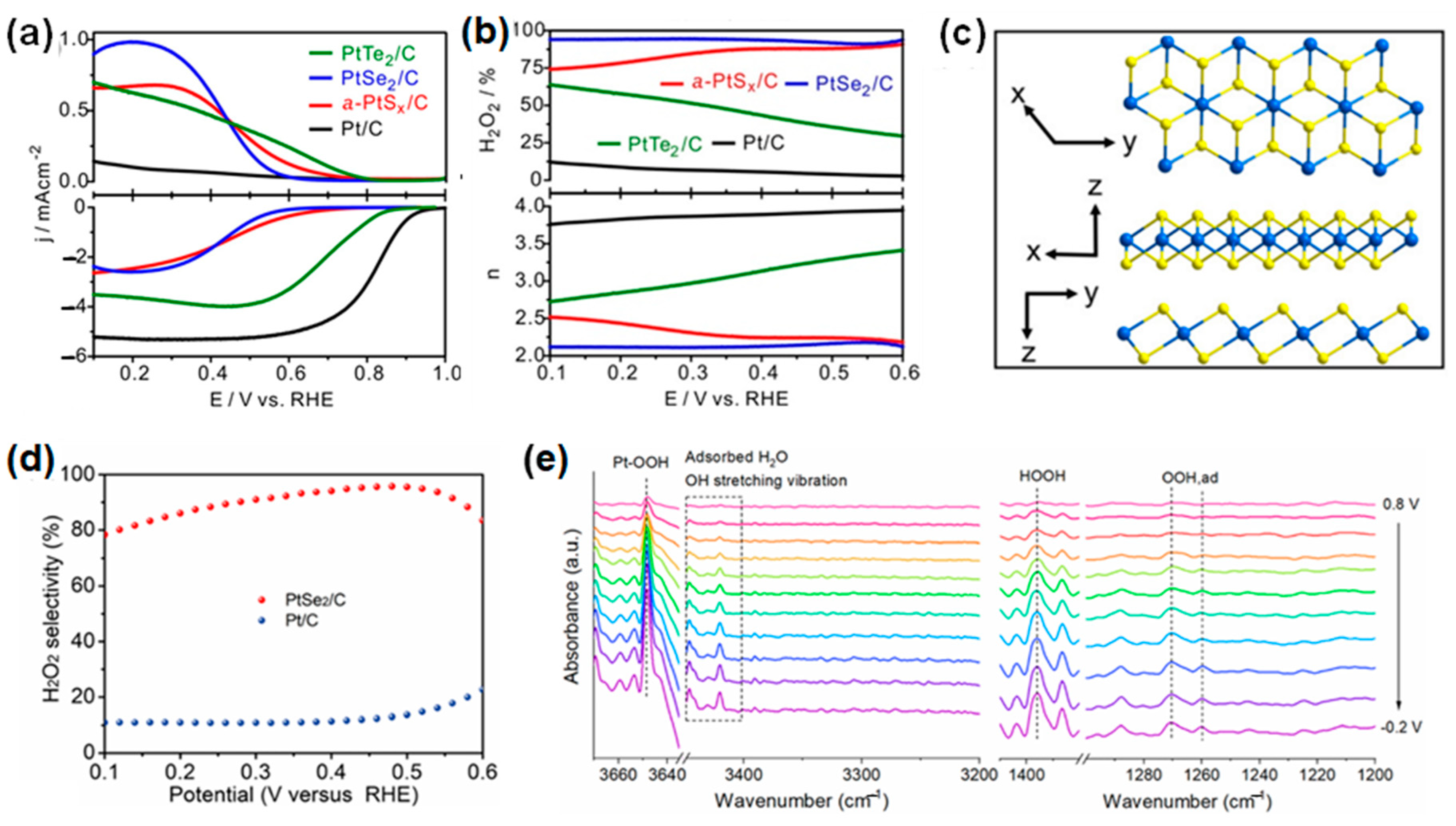
3.2. Noble-Metal Chalcogenides
3.3. Non-Noble-Metal Chalcogenides
3.3.1. Cobalt Chalcogenides
3.3.2. Nickel Chalcogenides
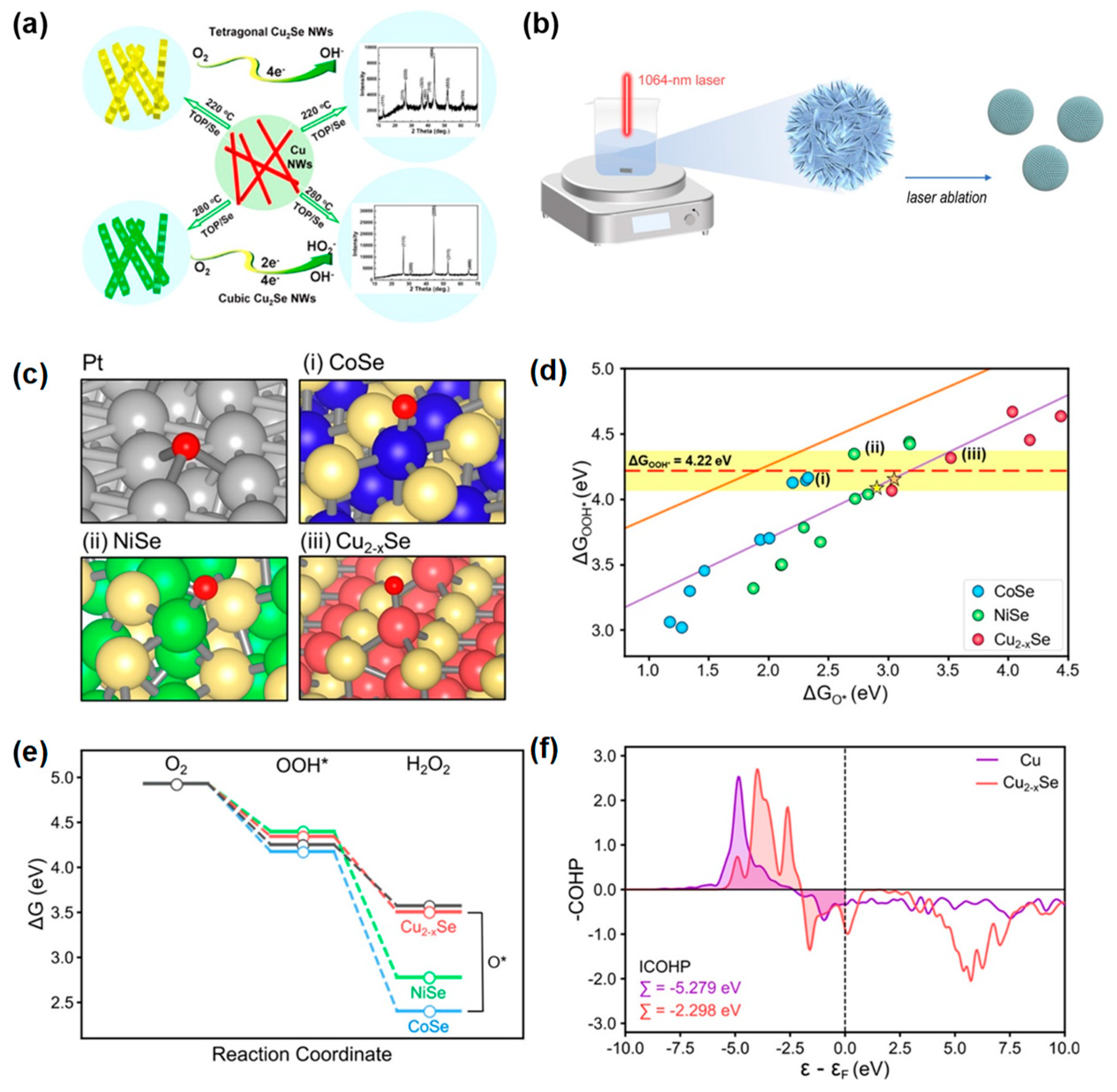
3.3.3. Copper Chalcogenides
3.3.4. Other Metal Chalcogenides
4. Conclusions
- (1)
- Development of high-performing electrocatalysts: TMCs have demonstrated superior catalytic efficiency in acidic media, outperforming carbon-based materials, largely due to their electronic structure. Key strategies such as doping and defect engineering, which modulate electron density, are essential for further performance enhancement. Among TMCs, cobalt chalcogenides have garnered significant interest, owing to the favorable adsorption energies associated with cobalt’s electron density. Future research should prioritize the exploration of charge redistribution on cobalt chalcogenides’ surfaces, employing advanced strategies to drive further performance improvements.
- (2)
- H2O2 stability and catalyst durability: Hydrogen peroxide is slightly acidic and highly stable in such conditions, allowing it to be maintained without the need for stabilizers. TMCs like BP/CoSe2 and NiS2 show excellent selectivity and activity in acidic electrolytes, making them strong candidates for 2e− ORR electrocatalysts in practical applications. However, the acidic environment also brings heightened risks of corrosion and degradation, which must be addressed. To ensure these catalysts perform reliably in real-world conditions, future research should focus on improving their long-term stability and durability, such as through the development of carbon shells.
- (3)
- Mechanistic insights: In-depth mechanistic studies using advanced characterization techniques like in situ ATR-IR, in situ Raman, XANES, and EXAFS are necessary to gain a better understanding of the ORR processes at the atomic level. Such insights will aid in the rational design of more effective catalysts.
- (4)
- Scale-up and operational costs: To transition from laboratory-scale experiments to practical large-scale applications, several key challenges must be addressed. These challenges include optimizing reactor design, increasing current density, and ensuring high Faradaic efficiency during prolonged electrolysis. For practical implementation, maintaining high Faradaic efficiency at current densities above 300 mA cm−2 is essential. Additionally, reducing stabilizer use in acidic electrolytes, lowering production costs by using affordable non-precious-metal catalysts, and innovating electrochemical reactor designs—such as gas diffusion electrodes (GDEs) and membrane electrode assemblies (MEAs)—are critical for scaling up the ORR process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Sa, Y.J.; Joo, S.H. Catalyst design, measurement guidelines, and device integration for H2O2 electrosynthesis from oxygen reduction. Cell Rep. Phys. Sci. 2022, 3, 100987. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Cheng, M.J.; Lu, Q. Tailoring the electrochemical production of H2O2: Strategies for the rational design of high-performance electrocatalysts. Small 2020, 16, 1902845. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Lienke, A. Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew. Chem. Int. Ed. 2006, 45, 206–222. [Google Scholar] [CrossRef]
- De Leon, C.P.; Pletcher, D. Removal of formaldehyde from aqueous solutions via oxygen reduction using a reticulated vitreous carbon cathode cell. J. Appl. Electrochem. 1995, 25, 307–314. [Google Scholar] [CrossRef]
- Kosaka, K.; Yamada, H.; Shishida, K.; Echigo, S.; Minear, R.A.; Tsuno, H.; Matsui, S. Evaluation of the treatment performance of a multistage ozone/hydrogen peroxide process by decomposition by-products. Water Res. 2001, 35, 3587–3594. [Google Scholar] [CrossRef]
- Puértolas, B.; Hill, A.; García, T.; Solsona, B.; Torrente-Murciano, L. In-situ synthesis of hydrogen peroxide in tandem with selective oxidation reactions: A mini-review. Catal. Today 2015, 248, 115–127. [Google Scholar] [CrossRef]
- Merchant Research & Consulting, Ltd. Hydrogen Peroxide (HP): 2024 World Market Outlook Up to 2033; Merchant Research & Consulting, Ltd.: Birmingham, UK, 2024. [Google Scholar]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, L.; Lü, S.; Wang, Y.; Mi, Z. Gas−liquid−liquid three-phase reactive extraction for the hydrogen peroxide preparation by anthraquinone process. Ind. Eng. Chem. Res. 2008, 47, 7414–7418. [Google Scholar] [CrossRef]
- Lewis, R.J.; Hutchings, G.J. Recent advances in the direct synthesis of H2O2. ChemCatChem 2019, 11, 298–308. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.-T.; Joo, S.H. Electrocatalyst design for promoting two-electron oxygen reduction reaction: Isolation of active site atoms. Curr. Opin. Electrochem. 2020, 21, 109–116. [Google Scholar] [CrossRef]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Siahrostami, S.; Villegas, S.J.; Bagherzadeh Mostaghimi, A.H.; Back, S.; Farimani, A.B.; Wang, H.; Persson, K.A.; Montoya, J. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 2020, 10, 7495–7511. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Colic, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E. Toward the decentralized electrochemical production of H2O2: A focus on the catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tang, C.; Wang, H.F.; Chen, C.M.; Zhang, X.; Huang, X.; Zhang, Q. Oxygen Reduction Reaction on Graphene in an Electro-Fenton System: In Situ Generation of H2O2 for the Oxidation of Organic Compounds. ChemSusChem 2016, 9, 1194–1199. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of nitrogen moieties in N-doped 3D-graphene nanosheets for oxygen electroreduction in acidic and alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, H.; Liu, Y.; Yan, C.; Hong, S.; Masa, J.; Robertson, A.W.; Liu, S.; Qiu, J.; Sun, Z. Ultrasound-assisted nitrogen and boron codoping of graphene oxide for efficient oxygen reduction reaction. ACS Sustain. Chem. Eng. 2019, 7, 3434–3442. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Cumpson, P.; Joseph, P.; Papakonstantinou, P. Oxygen reduction reaction by electrochemically reduced graphene oxide. Faraday Discuss. 2014, 173, 415–428. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, Y.; Han, G.F.; Zhao, Y.; Ge, X.; Li, F.; Zhang, Z.; Zhong, Q.; Baek, J.B. Carbon-based electrocatalysts for efficient hydrogen peroxide production. Adv. Mater. 2021, 33, 2103266. [Google Scholar] [CrossRef]
- Xia, F.; Li, B.; Liu, Y.; Liu, Y.; Gao, S.; Lu, K.; Kaelin, J.; Wang, R.; Marks, T.J.; Cheng, Y. Carbon free and noble metal free Ni2Mo6S8 electrocatalyst for selective electrosynthesis of H2O2. Adv. Funct. Mater. 2021, 31, 2104716. [Google Scholar] [CrossRef]
- Back, S.; Na, J.; Ulissi, Z.W. Efficient discovery of active, selective, and stable catalysts for electrochemical H2O2 synthesis through active motif screening. ACS Catal. 2021, 11, 2483–2491. [Google Scholar] [CrossRef]
- Sheng, H.; Ross, R.D.; Schmidt, J.; Jin, S. Metal-compound-based electrocatalysts for hydrogen peroxide electrosynthesis and the electro-Fenton process. ACS Energy Lett. 2022, 8, 196–212. [Google Scholar] [CrossRef]
- Dong, K.; Xu, Z.; He, X.; Zhao, D.; Chen, H.; Liang, J.; Luo, Y.; Sun, S.; Zheng, D.; Liu, Q. Ultrathin single-crystal PtSe2 nanosheets for high-efficiency O2 electroreduction to H2O2. Chem. Commun. 2022, 58, 10683–10686. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chen, M.; Zhang, C.; Zhang, J.; Liu, W.; Huang, X.; Li, J.; Feng, G.; Wang, D. Modulating the oxygen reduction selectivity in Pt or Pd chalcogenides via the ensemble effect and electronic effect. ACS Appl. Mater. Interfaces 2023, 15, 31375–31383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, J.; Yue, L.; Xu, Z.; Dong, K.; Liu, Q.; Luo, Y.; Li, T.; Cheng, X.; Cui, G. N-doped carbon nanotubes supported CoSe2 nanoparticles: A highly efficient and stable catalyst for H2O2 electrosynthesis in acidic media. Nano Res. 2022, 15, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ding, H.; Chen, L.; Dong, J.; Yu, H.; Yan, S.; Wang, H. Modification of NiSe2 Nanoparticles by ZIF-8-Derived NC for Boosting H2O2 Production from Electrochemical Oxygen Reduction in Acidic Media. Catalysts 2024, 14, 364. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Bao, J.; Lan, Y.; Tu, W.; Han, M.; Dai, Z. Controllable synthesis of tetragonal and cubic phase Cu2Se nanowires assembled by small nanocubes and their electrocatalytic performance for oxygen reduction reaction. J. Phys. Chem. C 2013, 117, 15164–15173. [Google Scholar] [CrossRef]
- Bonakdarpour, A.; Esau, D.; Cheng, H.; Wang, A.; Gyenge, E.; Wilkinson, D.P. Preparation and electrochemical studies of metal–carbon composite catalysts for small-scale electrosynthesis of H2O2. Electrochim. Acta 2011, 56, 9074–9081. [Google Scholar] [CrossRef]
- Chai, G.-L.; Hou, Z.; Ikeda, T.; Terakura, K. Two-electron oxygen reduction on carbon materials catalysts: Mechanisms and active sites. J. Phys. Chem. C 2017, 121, 14524–14533. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, F.T.; Hor, T.A.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, Y.; Lu, L.; Zhu, C.; Fang, Q.; Wang, J.; Song, S. Insights into dual effect of missing linker-cluster domain defects for photocatalytic 2e− ORR: Radical reaction and electron behavior. Chemosphere 2023, 324, 138220. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Jia, L.; Xiao, Z. Origin of the catalytic activity of graphite nitride for the electrochemical reduction of oxygen: Geometric factors vs. electronic factors. Phys. Chem. Chem. Phys. 2009, 11, 2730–2740. [Google Scholar] [CrossRef]
- Mayrhofer, K.; Blizanac, B.; Arenz, M.; Stamenkovic, V.; Ross, P.; Markovic, N. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.; Zhu, J.; Zhang, H.; Zhao, X.; Gao, L.; Wang, X.; Zhao, J.; Ge, J.; Jiang, Z. Climbing the apex of the ORR volcano plot via binuclear site construction: Electronic and geometric engineering. J. Am. Chem. Soc. 2019, 141, 17763–17770. [Google Scholar] [CrossRef]
- Hornberger, E.; Mastronardi, V.; Brescia, R.; Pompa, P.P.; Klingenhof, M.; Dionigi, F.; Moglianetti, M.; Strasser, P. Seed-mediated synthesis and catalytic ORR reactivity of facet-stable, monodisperse platinum nano-octahedra. ACS Appl. Energy Mater. 2021, 4, 9542–9552. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; Chen, Y.; Yuan, H.; Qiao, Y. Crystal facet-dependent activity of α-Mn2O3 for oxygen reduction and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 22744–22751. [Google Scholar] [CrossRef]
- Blizanac, B.; Ross, P.N.; Marković, N. Oxygen Reduction on Silver Low-Index Single-Crystal Surfaces in Alkaline Solution: Rotating Ring DiskAg (h kl) Studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef]
- Kondo, S.; Nakamura, M.; Maki, N.; Hoshi, N. Active sites for the oxygen reduction reaction on the low and high index planes of palladium. J. Phys. Chem. C 2009, 113, 12625–12628. [Google Scholar] [CrossRef]
- Wang, Q.; Xiaoqiang, C.; Guan, W.; Zhang, L.; Fan, X.; Shi, Z.; Zheng, W. Shape-dependent catalytic activity of oxygen reduction reaction (ORR) on silver nanodecahedra and nanocubes. J. Power Sources 2014, 269, 152–157. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Zhou, Y.; Liu, J.; Li, R.; Xu, Z.; Xiao, L.; Fan, J. Ligand-regulated ORR activity of Au nanoparticles in alkaline medium: The importance of surface coverage of ligands. Catal. Sci. Technol. 2018, 8, 746–754. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Banis, M.N.; Adair, K.; Song, Z.; Yang, L.; Markiewicz, M.; Li, J.; Wang, S.; Li, R. Rational design of porous structures via molecular layer deposition as an effective stabilizer for enhancing Pt ORR performance. Nano Energy 2019, 60, 111–118. [Google Scholar]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H.Y. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, X.; Yan, X.; Brown, C.L.; Chen, Z.; Zhu, G.; Yao, X. Defect-driven oxygen reduction reaction (ORR) of carbon without any element doping. Inorg. Chem. Front. 2016, 3, 417–421. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, J.; Xue, C.; Li, X.; Wang, S.; Han, B.; Yang, M.; Li, L. A novel core–double shell heterostructure derived from a metal–organic framework for efficient HER, OER and ORR electrocatalysis. Inorg. Chem. Front. 2020, 7, 191–197. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, J.; Jiang, Q.; Wang, G. Boosting the OER/ORR/HER activity of Ru-doped Ni/Co oxides heterostructure. Chem. Eng. J. 2022, 439, 135634. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Ma, L.; Duan, T.; Du, Y.; Wu, J.; Zou, J.J.; Gao, J.; Zhu, X.D.; Zhang, Y.C. Recent Progress of Transition Metal Selenides for Electrochemical Oxygen Reduction to Hydrogen Peroxide: From Catalyst Design to Electrolyzers Application. Small 2024, 20, 2309448. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, Z.Y. Design Strategies of Non-Noble Metal-Based Electrocatalysts for Two-Electron Oxygen Reduction to Hydrogen Peroxide. ChemSusChem 2021, 14, 1616–1633. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.-J.; Feng, X.; Ma, Z.; Ma, Z.-F.; Xue, Y. Progress of Pt and iron-group transition metal alloy catalysts with high ORR activity for PEMFCs. J. Electroanal. Chem. 2024, 959, 118165. [Google Scholar] [CrossRef]
- Goswami, C.; Hazarika, K.K.; Bharali, P. Transition metal oxide nanocatalysts for oxygen reduction reaction. Mater. Sci. Energy Technol. 2018, 1, 117–128. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer-Casadevall, A.; Deiana, D.; Karamad, M.; Siahrostami, S.; Malacrida, P.; Hansen, T.W.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E. Trends in the electrochemical synthesis of H2O2: Enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett. 2014, 14, 1603–1608. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent progress of electrochemical production of hydrogen peroxide by two-electron oxygen reduction reaction. Adv. Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Ma, Z.; Zhao, E.; Du, K.; Guo, J.; Ling, T. Spin effect on oxygen electrocatalysis. Adv. Energy Sustain. Res. 2021, 2, 2100034. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, S.; Yang, H.; Xi, S.; Gracia, J.; Xu, Z.J. Spin-related electron transfer and orbital interactions in oxygen electrocatalysis. Adv. Mater. 2020, 32, 2003297. [Google Scholar] [CrossRef]
- Chen, S.; Luo, T.; Li, X.; Chen, K.; Fu, J.; Liu, K.; Cai, C.; Wang, Q.; Li, H.; Chen, Y. Identification of the highly active Co–N4 coordination motif for selective oxygen reduction to hydrogen peroxide. J. Am. Chem. Soc. 2022, 144, 14505–14516. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Wang, Y. The Exploration of Less Expensive Materials for the Direct Synthesis of Hydrogen Peroxide. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2014. [Google Scholar]
- Mishra, S.; Chowdhary, P.; Bharagava, R.N. Conventional methods for the removal of industrial pollutants, their merits and demerits. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–31. [Google Scholar]
- Gao, M.R.; Jiang, J.; Yu, S.H. Solution-based synthesis and design of late transition metal chalcogenide materials for oxygen reduction reaction (ORR). Small 2012, 8, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Zhang, J. Optimizing catalyst loading in non-noble metal electrocatalyst layer to improve oxygen reduction reaction activity. Electrochem. Commun. 2011, 13, 447–449. [Google Scholar] [CrossRef]
- Biddinger, E.J.; Von Deak, D.; Singh, D.; Marsh, H.; Tan, B.; Knapke, D.S.; Ozkan, U.S. Examination of catalyst loading effects on the selectivity of CNx and Pt/VC ORR catalysts using RRDE. J. Electrochem. Soc. 2011, 158, B402. [Google Scholar] [CrossRef]
- Bonakdarpour, A.; Lefevre, M.; Yang, R.; Jaouen, F.; Dahn, T.; Dodelet, J.-P.; Dahn, J. Impact of loading in RRDE experiments on Fe–N–C catalysts: Two-or four-electron oxygen reduction? Electrochem. Solid-State Lett. 2008, 11, B105. [Google Scholar] [CrossRef]
- Chen, S.; Kucernak, A. Electrocatalysis under conditions of high mass transport rate: Oxygen reduction on single submicrometer-sized Pt particles supported on carbon. J. Phys. Chem. B 2004, 108, 3262–3276. [Google Scholar] [CrossRef]
- Saikawa, K.; Nakamura, M.; Hoshi, N. Structural effects on the enhancement of ORR activity on Pt single-crystal electrodes modified with alkylamines. Electrochem. Commun. 2018, 87, 5–8. [Google Scholar] [CrossRef]
- Dan, M.; Zhong, R.; Hu, S.; Wu, H.; Zhou, Y.; Liu, Z.-Q. Strategies and challenges on selective electrochemical hydrogen peroxide production: Catalyst and reaction medium design. Chem Catal. 2022, 2, 1919–1960. [Google Scholar] [CrossRef]
- Tarasevich, M.; Korchagin, O. Electrocatalysis and pH (a review). Russ. J. Electrochem. 2013, 49, 600–618. [Google Scholar] [CrossRef]
- Fóti, G.; Bolzonella, I.; Comninellis, C. Electrochemical promotion of catalysis. In Modern Aspects of Electrochemistry No. 36; Springer: Berlin/Heidelberg, Germany, 2002; pp. 191–254. [Google Scholar]
- Kelly, S.R.; Kirk, C.; Chan, K.; Nørskov, J.K. Electric field effects in oxygen reduction kinetics: Rationalizing pH dependence at the Pt (111), Au (111), and Au (100) electrodes. J. Phys. Chem. C 2020, 124, 14581–14591. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Influence of inner-and outer-sphere electron transfer mechanisms during electrocatalysis of oxygen reduction in alkaline media. J. Phys. Chem. C 2011, 115, 18015–18026. [Google Scholar] [CrossRef]
- Anjana, J.; Muthukrishnan, A. Effect of local pH change on non-PGM catalysts–a potential-dependent mechanistic analysis of the oxygen reduction reaction. Catal. Sci. Technol. 2022, 12, 6246–6255. [Google Scholar] [CrossRef]
- Lu, X.; Chang, Y.; Wang, S.; Li, X.; Bao, J.; Liu, Y. Hydrogen peroxide electrosynthesis via two-electron oxygen reduction: From pH effect to device engineering. Chin. Chem. Lett. 2024, 110277. [Google Scholar] [CrossRef]
- He, H.; Liu, S.; Liu, Y.; Zhou, L.; Wen, H.; Shen, R.; Zhang, H.; Guo, X.; Jiang, J.; Li, B. Review and Perspectives on Carbon-based Electrocatalysts for Production of H2O2 via Two-electron Oxygen Reduction. Green Chem. 2023. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, X. Mechanism investigation of enhanced electrochemical H2O2 production performance on oxygen-rich hollow porous carbon spheres. Nano Res. 2022, 15, 4599–4605. [Google Scholar] [CrossRef]
- Ding, Y.; Xie, L.; Zhou, W.; Sun, F.; Gao, J.; Yang, C.; Zhao, G.; Qin, Y.; Ma, J. Pulsed electrocatalysis enables the stabilization and activation of carbon-based catalysts towards H2O2 production. Appl. Catal. B Environ. 2022, 316, 121688. [Google Scholar] [CrossRef]
- Koh, K.H.; Bagherzadeh Mostaghimi, A.H.; Chang, Q.; Kim, Y.J.; Siahrostami, S.; Han, T.H.; Chen, Z. Elucidation and modulation of active sites in holey graphene electrocatalysts for H2O2 production. EcoMat 2023, 5, e12266. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M.-M.; Chai, G.-L. Mesoporous carbon hollow spheres as efficient electrocatalysts for oxygen reduction to hydrogen peroxide in neutral electrolytes. Acs Catal. 2020, 10, 7434–7442. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Wang, M.; Chai, G.; Guan, L. Pyrimidine-assisted synthesis of S, N-codoped few-layered graphene for highly efficient hydrogen peroxide production in acid. Chem Catal. 2022, 2, 1450–1466. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, Y.; Xia, D.; Chai, G. Regulable pyrrolic-N-doped carbon materials as an efficient electrocatalyst for selective O2 reduction to H2O2. New J. Chem. 2022, 46, 14510–14516. [Google Scholar] [CrossRef]
- Ricciardulli, T.; Gorthy, S.; Adams, J.S.; Thompson, C.; Karim, A.M.; Neurock, M.; Flaherty, D.W. Effect of Pd coordination and isolation on the catalytic reduction of O2 to H2O2 over PdAu bimetallic nanoparticles. J. Am. Chem. Soc. 2021, 143, 5445–5464. [Google Scholar] [CrossRef]
- Zheng, Z.; Ng, Y.H.; Wang, D.W.; Amal, R. Epitaxial growth of Au–Pt–Ni nanorods for direct high selectivity H2O2 production. Adv. Mater. 2016, 28, 9949–9955. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, C.; Pan, L.; Huang, Z.-F.; Zhang, X.; Zou, J.-J. Manipulating the spin state to activate the atomically dispersed Fe–N–C catalyst for oxygen reduction. EES Catal. 2023, 1, 562–570. [Google Scholar] [CrossRef]
- Liu, J.; Gong, Z.; Yan, M.; He, G.; Gong, H.; Ye, G.; Fei, H. Electronic structure regulation of single-atom catalysts for electrochemical oxygen reduction to H2O2. Small 2022, 18, 2103824. [Google Scholar] [CrossRef]
- Xiao, C.; Cheng, L.; Zhu, Y.; Wang, G.; Chen, L.; Wang, Y.; Chen, R.; Li, Y.; Li, C. Super-coordinated Nickel N4Ni1O2 site single-atom catalyst for selective H2O2 electrosynthesis at high current densities. Angew. Chem. 2022, 134, e202206544. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, Y.; Alnoush, W.; Hou, Y.; Higgins, D.; Wu, G. Tuning two-electron oxygen-reduction pathways for H2O2 electrosynthesis via engineering atomically dispersed single metal site catalysts. Adv. Mater. 2022, 34, 2107954. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Siahrostami, S.; Kim, T.R.; Nordlund, D.; Sokaras, D.; Nowak, S.; To, J.W.; Higgins, D.; Sinclair, R. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Eng. 2018, 6, 311–317. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.-P.; Kim, S.-W.; Kim, S.-J.; Bu, Y.; Fu, Z.; Lu, Y. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.b.; Huang, X.; Hung, S.-F.; Cai, W.; Jia, C.; Miao, S.; Chen, H.M.; Yang, X.; Huang, Y. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Zhu, P.; Adler, Z.; Wu, Z.-Y.; Liu, Y.; Wang, H. Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 2022, 13, 2880. [Google Scholar] [CrossRef]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, J.W.; Kim, S.; Le, T.T.; Nandy, S.; Hwang, C.-K.; Paek, S.Y.; Byeon, A.; Chae, K.H.; Lee, S.Y. Penta nitrogen coordinated cobalt single atom catalysts with oxygenated carbon black for electrochemical H2O2 production. Appl. Catal. B Environ. 2023, 331, 122712. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Huang, Y.-X.; Jin, R.-C.; Huang, B.-C. Single atom catalysts for use in the selective production of hydrogen peroxide via two-electron oxygen reduction reaction: Mechanism, activity, and structure optimization. Appl. Catal. B Environ. 2023, 337, 122987. [Google Scholar] [CrossRef]
- Song, M.; Liu, W.; Zhang, J.; Zhang, C.; Huang, X.; Wang, D. Single-atom catalysts for H2O2 electrosynthesis via two-electron oxygen reduction reaction. Adv. Funct. Mater. 2023, 33, 2212087. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, Z.; Xu, M.; Kan, L.; Guo, C.; Liu, J.; He, L.; Du, M.; Zhang, Z. Single-Atom Co–O4 Sites Embedded in a Defective-Rich Porous Carbon Layer for Efficient H2O2 Electrosynthesis. Small 2024, 20, 2310468. [Google Scholar] [CrossRef]
- Su, H.; Jiang, J.; Song, S.; An, B.; Li, N.; Gao, Y.; Ge, L. Recent progress on design and applications of transition metal chalcogenide-associated electrocatalysts for the overall water splitting. Chin. J. Catal. 2023, 44, 7–49. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Kim, D.; Kim, Y.; Prakash, O.; Lee, H. Highly active and stable layered ternary transition metal chalcogenide for hydrogen evolution reaction. Nano Energy 2016, 28, 366–372. [Google Scholar] [CrossRef]
- Ingsel, T.; Gupta, R.K. Transition metal chalcogenides-based electrocatalysts for ORR, OER, and HER. In Nanomaterials for Electrocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–111. [Google Scholar]
- Sheng, H.; Hermes, E.D.; Yang, X.; Ying, D.; Janes, A.N.; Li, W.; Schmidt, J.; Jin, S. Electrocatalytic production of H2O2 by selective oxygen reduction using earth-abundant cobalt pyrite (CoS2). ACS Catal. 2019, 9, 8433–8442. [Google Scholar] [CrossRef]
- Feng, Y.; He, T.; Alonso-Vante, N. Oxygen reduction reaction on carbon-supported CoSe2 nanoparticles in an acidic medium. Electrochim. Acta 2009, 54, 5252–5256. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Hu, S.; Zhang, X.L.; Ju, H.; Wang, Z.; Tan, P.J.; Wu, R.; Gao, F.Y.; Zhuang, T.; Zheng, X. Black phosphorous mediates surface charge redistribution of CoSe2 for electrochemical H2O2 production in acidic electrolytes. Adv. Mater. 2022, 34, 2205414. [Google Scholar] [CrossRef]
- Liu, K.; Li, F.; Zhan, H.; Zhan, S. Recent progress in two-dimensional materials for generation of hydrogen peroxide by two-electron oxygen reduction reaction. Mater. Today Energy 2024, 40, 101500. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Liu, Q.; Luo, Y.; Li, T.; Zhao, H.; Lu, S.; Zhang, F.; Asiri, A.M.; Liu, F. Electrocatalytic hydrogen peroxide production in acidic media enabled by NiS2 nanosheets. J. Mater. Chem. A 2021, 9, 6117–6122. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, G.; Xiong, B.; Chen, L.; Shi, J. Anion-tuned nickel chalcogenides electrocatalysts for efficient 2e− ORR towards H2O2 production in acidic media. Nano Res. 2023, 16, 4729–4735. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, J.; Li, C.; Han, X.; Hu, X.; Cheng, F.; Chen, J. The anion effect on the oxygen reduction of MnX (X= O, S, and Se) catalysts. J. Mater. Chem. A 2015, 3, 3425–3431. [Google Scholar] [CrossRef]
- Pang, Y.; Xie, H.; Sun, Y.; Titirici, M.-M.; Chai, G.-L. Electrochemical oxygen reduction for H2O2 production: Catalysts, pH effects and mechanisms. J. Mater. Chem. A 2020, 8, 24996–25016. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wu, J.; Yang, H.; Kang, Z.; Liu, Y.; Wang, Z.; Menezes, P.W.; Chen, Z. Charge-Polarized Selenium Vacancy in Nickel Diselenide Enabling Efficient and Stable Electrocatalytic Conversion of Oxygen to Hydrogen Peroxide. Adv. Sci. 2023, 10, 2205347. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, J.; Mok, D.H.; Zheng, Z.; Ye, Y.; Liang, C.; Zhou, L.; Back, S.; Jiang, K. Electrochemical hydrogen peroxide synthesis from selective oxygen reduction over metal selenide catalysts. Nano Lett. 2021, 22, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Da, Y.; Wang, X.; Wang, T.; Xu, M.; He, X.; Zhou, W.; Li, Y.; Coleman, J.N. Selective electrochemical production of hydrogen peroxide at zigzag edges of exfoliated molybdenum telluride nanoflakes. Natl. Sci. Rev. 2020, 7, 1360–1366. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, R.; Liu, C.; Lu, J.; Zou, Y.; Yuan, L.; Wang, J.; Wang, G.; Zhao, Y.; Yu, C. Trimetallic sulfide hollow superstructures with engineered D-band center for oxygen reduction to hydrogen peroxide in alkaline solution. Adv. Sci. 2022, 9, 2104768. [Google Scholar] [CrossRef]
- Yang, H.; An, N.; Kang, Z.; Menezes, P.W.; Chen, Z. Understanding Advanced Transition Metal-Based Two Electron Oxygen Reduction Electrocatalysts from the Perspective of Phase Engineering. Adv. Mater. 2024, 36, 2400140. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Jin, W.; Jia, B.; Wang, J.; Ma, T. Recent progress of vacancy engineering for electrochemical energy conversion related applications. Adv. Funct. Mater. 2021, 31, 2009070. [Google Scholar] [CrossRef]
- Guo, N.; Xue, H.; Bao, A.; Wang, Z.; Sun, J.; Song, T.; Ge, X.; Zhang, W.; Huang, K.; He, F. Achieving superior electrocatalytic performance by surface copper vacancy defects during electrochemical etching process. Angew. Chem. 2020, 132, 13882–13888. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Liao, T.; Ma, C.; Chen, B.; Li, Y.; Fan, X.; Peng, W. Fe doping induced selenium vacancy on cobalt selenide for enhanced hydrogen peroxides production. Appl. Catal. B Environ. 2024, 341, 123344. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhao, Y.; Wang, Y.; Zhang, Z.; Wu, T.; Qin, W.; Liu, S.; Jia, B.; Wu, H. Ultrahigh Pt-mass-activity hydrogen evolution catalyst electrodeposited from bulk Pt. Adv. Funct. Mater. 2022, 32, 2112207. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef]
- Moreno-Grijalva, G.I.; Alonso, G.; Oropeza-Guzman, M.T.; Gochi-Ponce, Y. Pt-Based Chalcogenide As Cathodic Electrocatalysts for Proton Exchange Membrane FUEL CELL (PEMFC). In Proceedings of the Electrochemical Society Meeting Abstracts 235, Dallas, TX, USA, 26–30 May 2019; p. 1476. [Google Scholar]
- Eisa, T.; Abdelkareem, M.A.; Jadhav, D.A.; Mohamed, H.O.; Sayed, E.T.; Olabi, A.G.; Castaño, P.; Chae, K.-J. Critical review on the synthesis, characterization, and application of highly efficient metal chalcogenide catalysts for fuel cells. Prog. Energy Combust. Sci. 2023, 94, 101044. [Google Scholar] [CrossRef]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z. 2D transition metal dichalcogenides: Design, modulation, and challenges in electrocatalysis. Adv. Mater. 2021, 33, 1907818. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, N.; Feng, Y.; Hu, Z.; Shao, Q.; Huang, X. Partially pyrolyzed binary metal–organic framework nanosheets for efficient electrochemical hydrogen peroxide synthesis. Angew. Chem. Int. Ed. 2020, 59, 14373–14377. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Pan, L.; Li, Z.; Shi, C.; Yao, Y.; Zhang, X.; Zou, J.J. Engineering facets and oxygen vacancies over hematite single crystal for intensified electrocatalytic H2O2 production. Adv. Funct. Mater. 2020, 30, 1910539. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, Y.; Tan, H.; Huang, Q.; Wang, C.; Pei, Z.; Wang, H.; Liu, Y.; Wang, Y.; Li, S. Cation-vacancy-enriched nickel phosphide for efficient electrosynthesis of hydrogen peroxides. Adv. Mater. 2022, 34, 2106541. [Google Scholar] [CrossRef]
- Singh, H.; Marley-Hines, M.; Chakravarty, S.; Nath, M. Multi-walled carbon nanotube supported manganese selenide as a highly active bifunctional OER and ORR electrocatalyst. J. Mater. Chem. A 2022, 10, 6772–6784. [Google Scholar] [CrossRef]
- Lv, L.-P.; Du, P.; Liu, P.; Li, X.; Wang, Y. Integrating mixed metallic selenides/nitrogen-doped carbon heterostructures in one-dimensional carbon fibers for efficient oxygen reduction electrocatalysis. ACS Sustain. Chem. Eng. 2020, 8, 8391–8401. [Google Scholar] [CrossRef]
- Tan, S.M.; Chua, C.K.; Sedmidubský, D.; Sofer, Z.; Pumera, M. Electrochemistry of layered GaSe and GeS: Applications to ORR, OER and HER. Phys. Chem. Chem. Phys. 2016, 18, 1699–1711. [Google Scholar] [CrossRef]




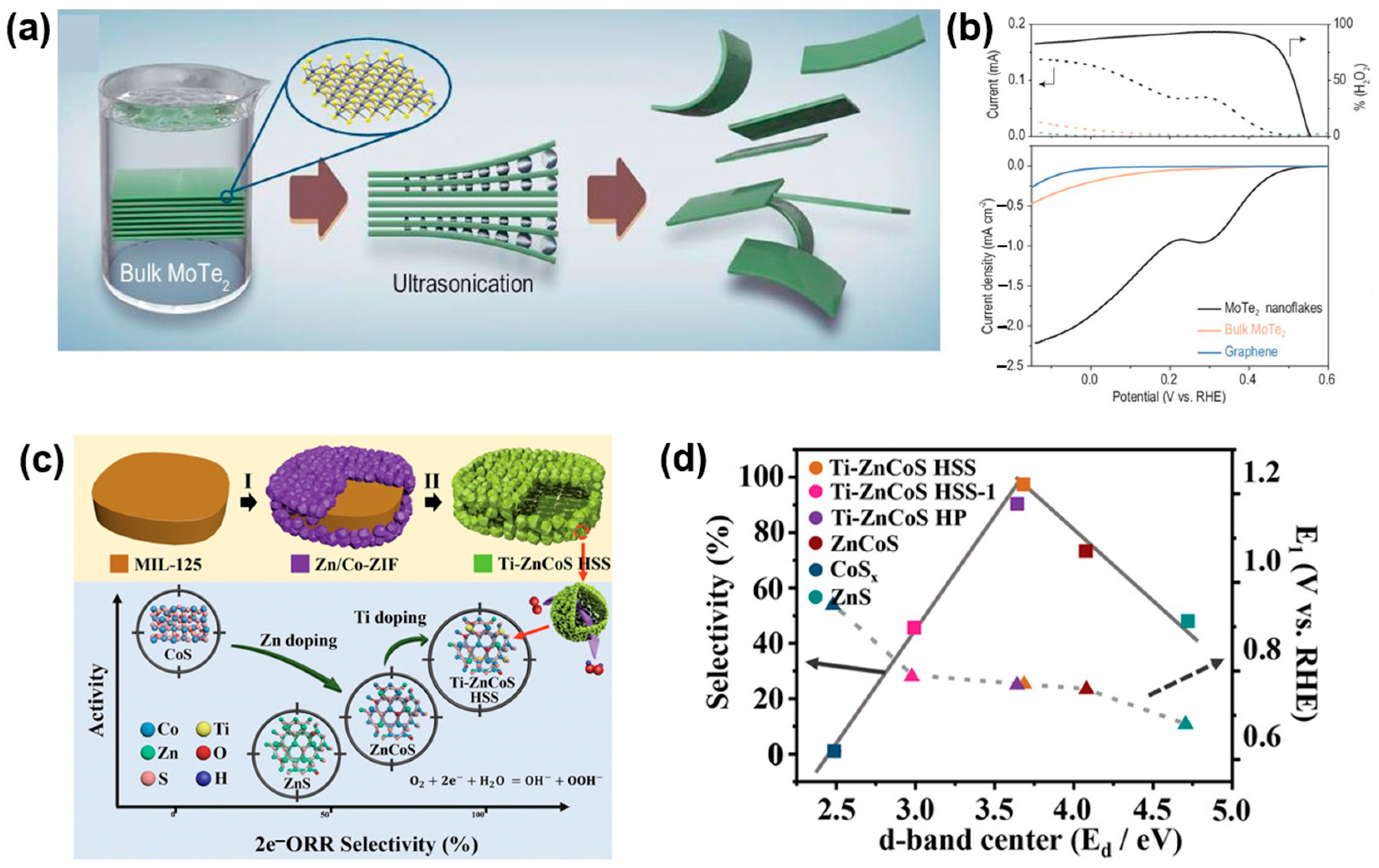
| Classification | Catalyst | Synthesis Method | Electrolyte | Onset Potential (0.1 mA cm−1) [V vs.RHE] | Selectivity [%] | Reference |
|---|---|---|---|---|---|---|
| Noble-metal chalcogenides | PtSe2/C | Chemical Vapor Deposition | 0.1 M HClO4 | 0.6 | ~94 | [27] |
| PtSe2/C | Chemical Vapor Deposition | 0.1 M HClO4 | 0.7 | ~94 | [26] | |
| Non-noble-metal chalcogenides | CoS2 | Hydrothermal | 0.05 M H2SO4 | 0.69 | ~70 | [103] |
| BP/CoSe2 | Hydrothermal | 0.5 M H2SO4 | 0.68 | ~90 | [105] | |
| NiS2 | Hydrothermal–Chemical Vapor Deposition | 0.05 M H2SO4 | 0.56 | ~99 | [107] | |
| NiSe2-Vse | Hydrothermal–Calcination-Annealing | 0.1 M KOH | 0.6 | ~96 | [111] | |
| Cu7.2Se4 | Chemical Vapor Deposition | 0.1 M KOH | 0.64 | ~94 | [112] | |
| 2H-MoTe2 | Liquid Phase Exfoliation | 0.5 M H2SO4 | 0.56 | ~93 | [113] | |
| Ti-ZnCoS HSS | Hydrothermal | 0.1 M KOH | 0.78 | ~98 | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, J.-G.; Choi, M.-J. Progress of Metal Chalcogenides as Catalysts for Efficient Electrosynthesis of Hydrogen Peroxide. Materials 2024, 17, 4277. https://doi.org/10.3390/ma17174277
Kim J-H, Lee J-G, Choi M-J. Progress of Metal Chalcogenides as Catalysts for Efficient Electrosynthesis of Hydrogen Peroxide. Materials. 2024; 17(17):4277. https://doi.org/10.3390/ma17174277
Chicago/Turabian StyleKim, Jeong-Hyun, Jeong-Gyu Lee, and Min-Jae Choi. 2024. "Progress of Metal Chalcogenides as Catalysts for Efficient Electrosynthesis of Hydrogen Peroxide" Materials 17, no. 17: 4277. https://doi.org/10.3390/ma17174277
APA StyleKim, J.-H., Lee, J.-G., & Choi, M.-J. (2024). Progress of Metal Chalcogenides as Catalysts for Efficient Electrosynthesis of Hydrogen Peroxide. Materials, 17(17), 4277. https://doi.org/10.3390/ma17174277






