Electron Beam Irradiation on the Production of a Si- and Zr-Based Hybrid Material: A Study by FTIR and WDXRF
Abstract
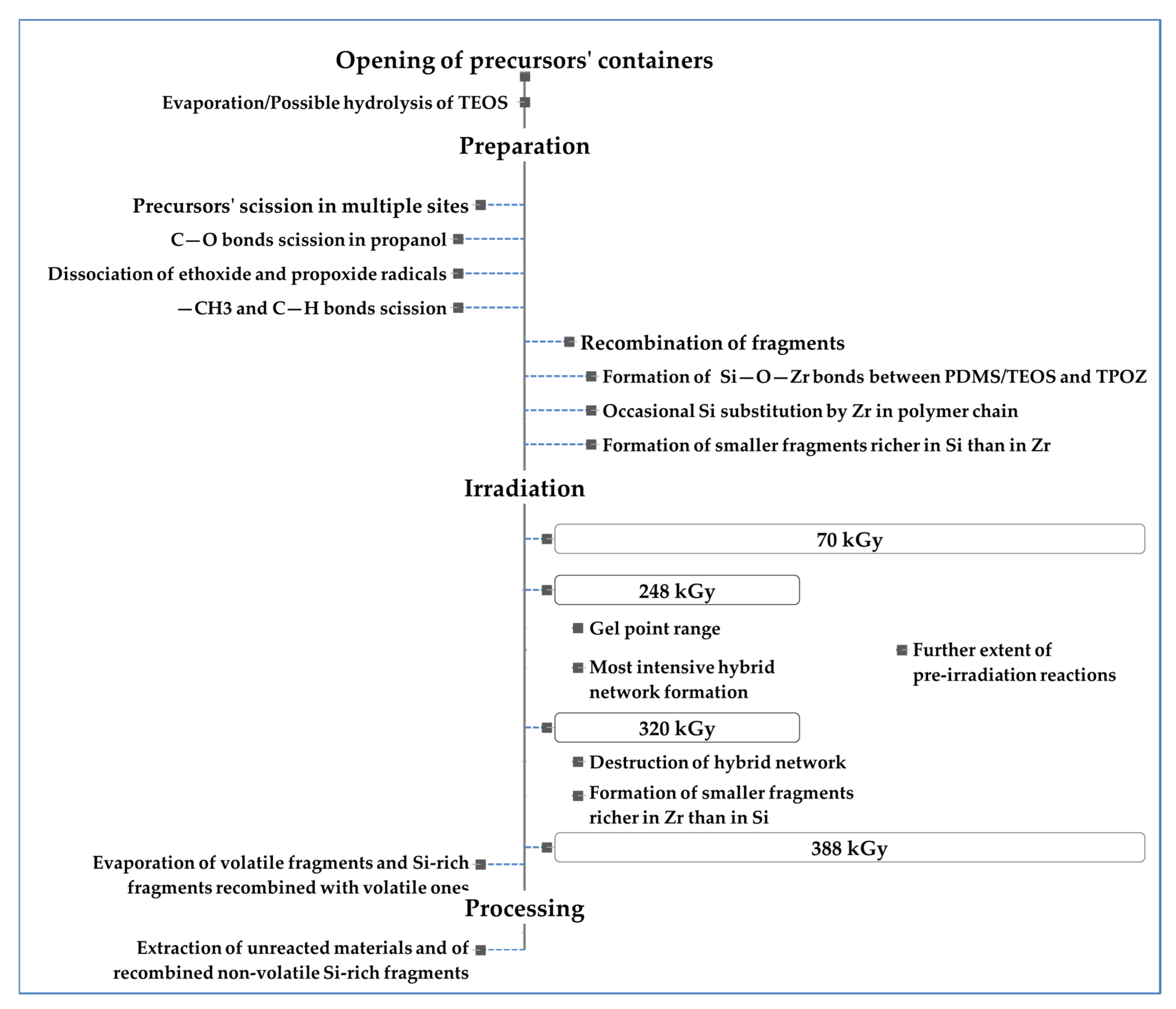
1. Introduction
- catalysts not needed to promote cross-linking;
- no solvents or water required;
- residues after hybrid network formation are reduced;
- higher cross-linking degree due to presence of more activated sites on precursors structures generated by radiolysis.
2. Materials and Methods
2.1. Materials
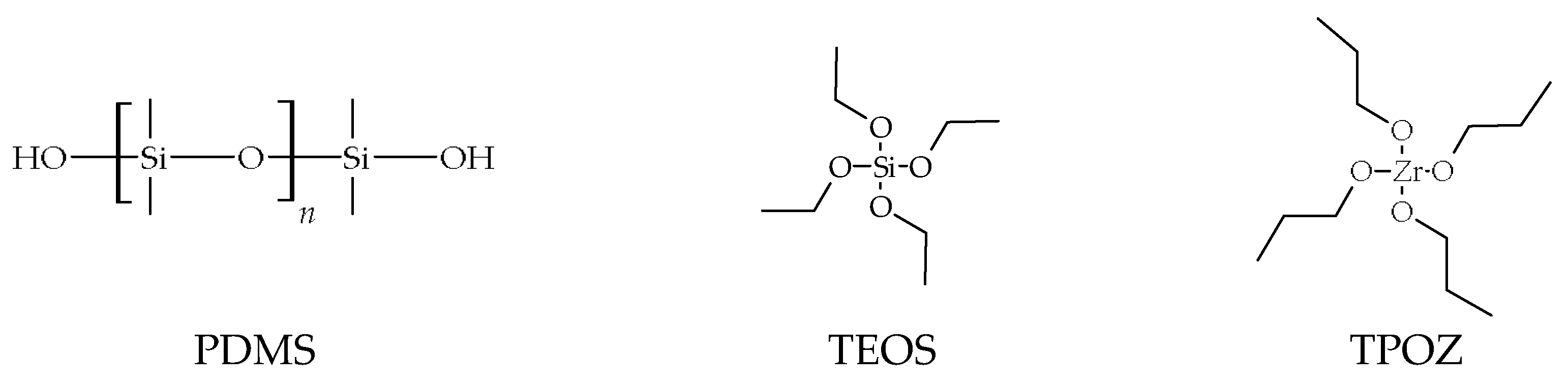
- Silanol-terminated PDMS, with 0.8 wt% in OH, with molar mass 43,500 g·mol−1 and 3500 cSt viscosity (S33) (ABCR GmbH, Karlsruhe, Germany)
- TEOS (Si(OCH2CH3)4) (Aldrich, St. Louis, MO, USA)
- TPOZ (Zr(O(CH2)2CH3)4), 70 wt% solution in 1-propanol (Aldrich)
2.2. Choice of Formulation
2.3. Samples Preparation and Conditioning
2.4. Irradiation Parameters
2.5. e-Mix Characterisation
2.5.1. Gel mass Fraction Determination
- Extraction of unreacted materials and precursors’ fragments by immersion in tetrahydrofuran for 72 h;
- Evaporation of extraction solvent and extracted substances by drying in air for 6 days;
- Drying at 80 °C for 12 h in a laboratory oven, to guarantee non-matrix materials’ evaporation.
2.5.2. Wavelength Dispersive X-ray Fluorescence
2.5.3. Fourier Transform Infrared Spectroscopy
3. Results
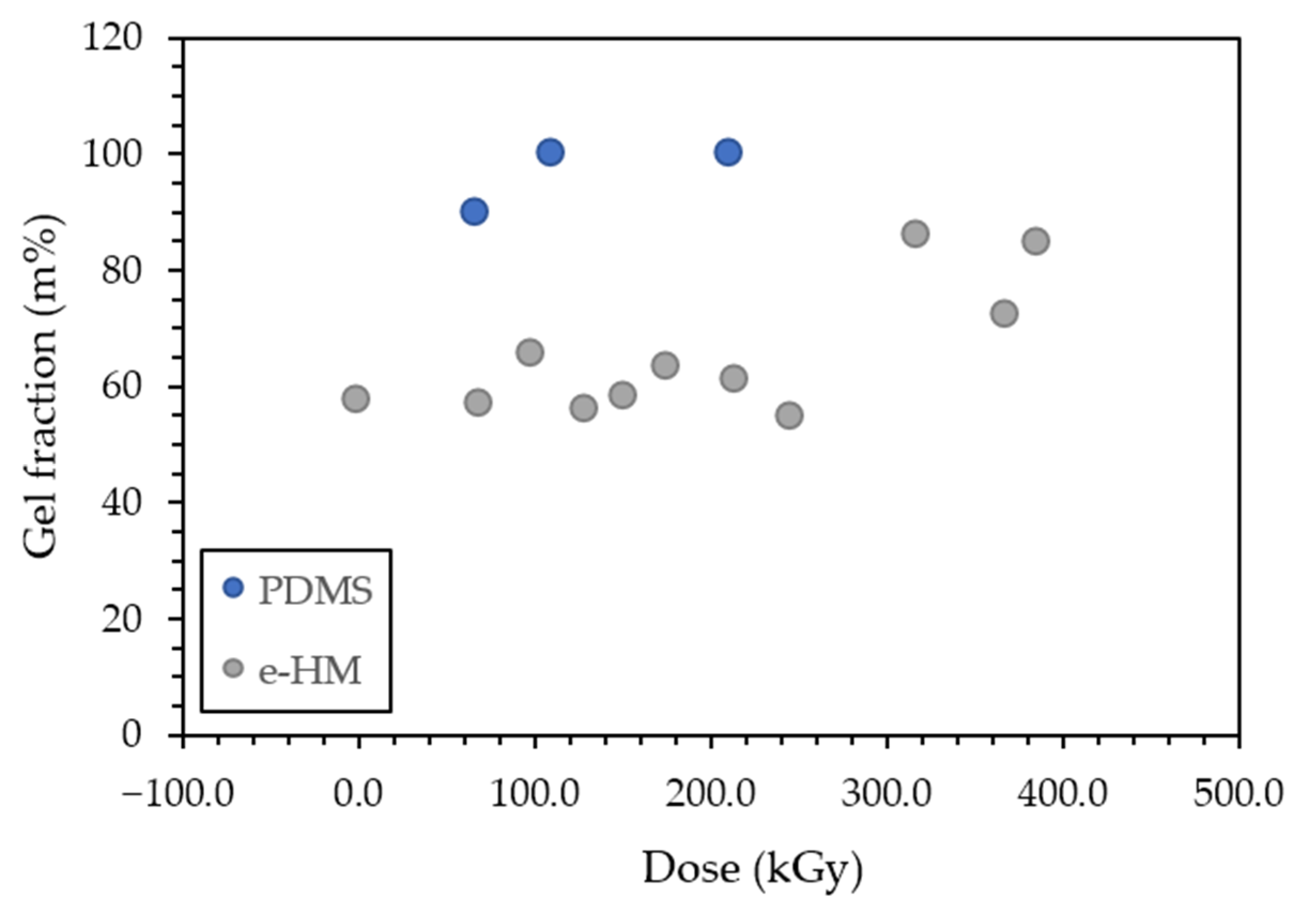
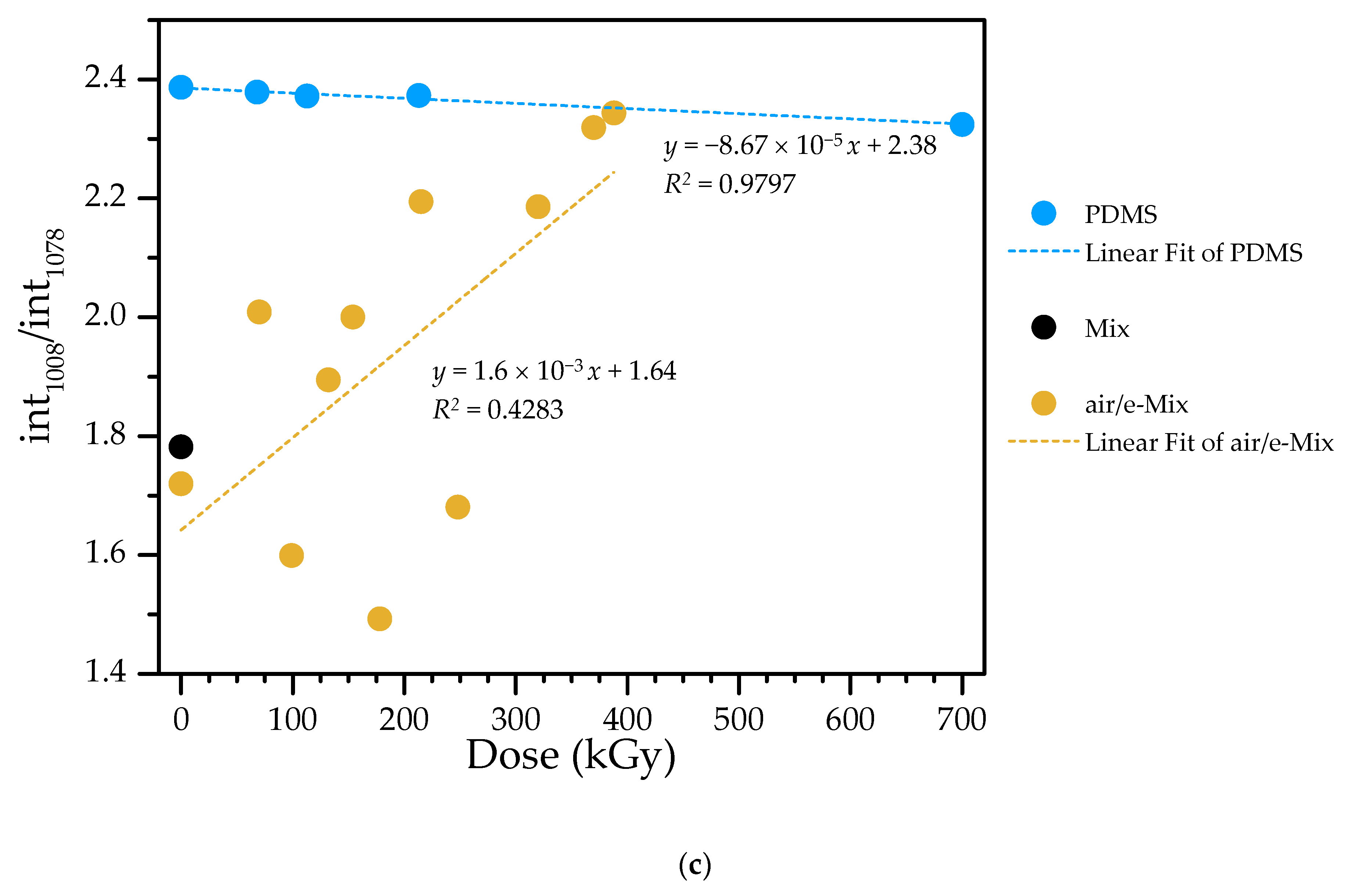
3.1. Gel Fraction and Gel Point Determination
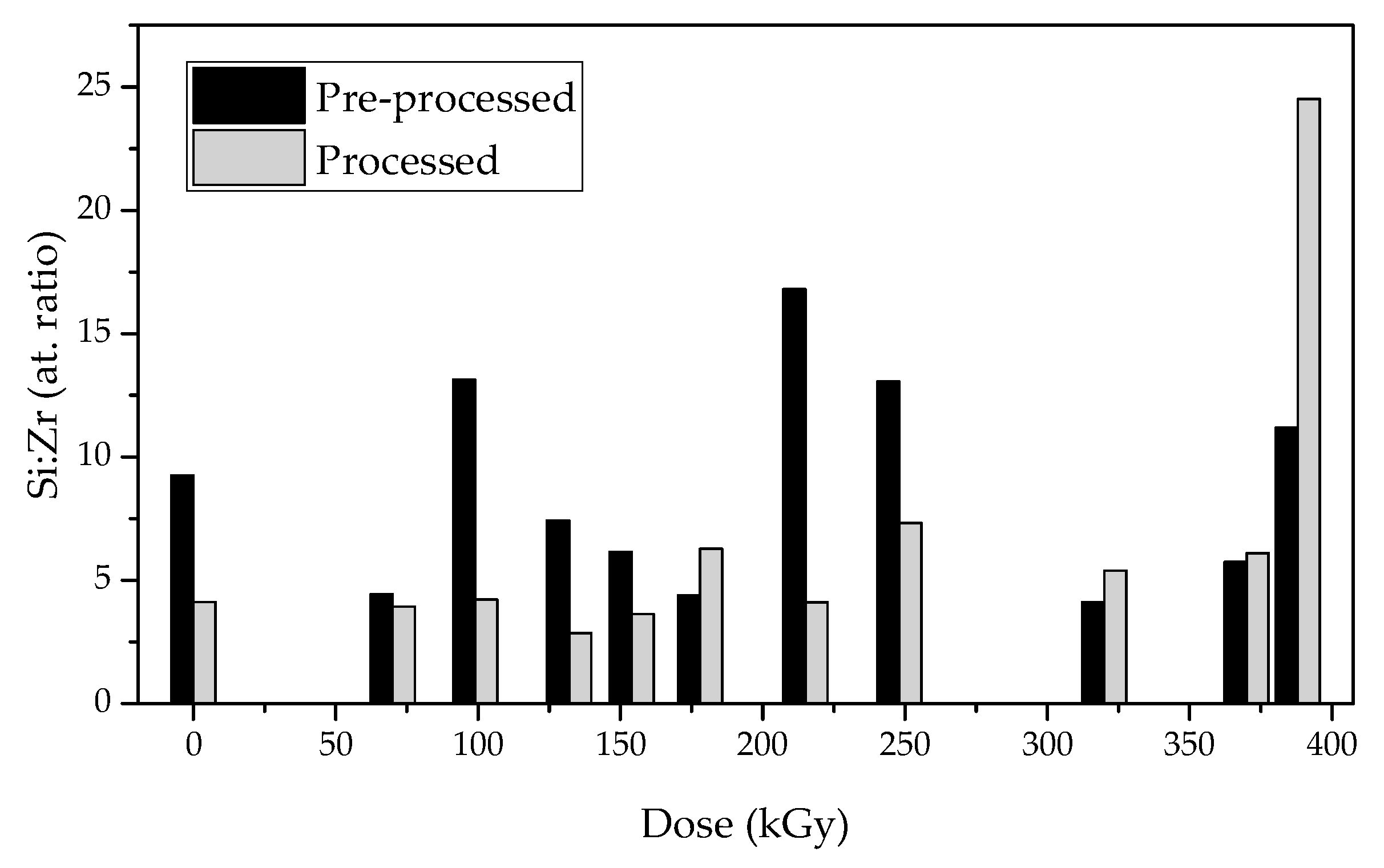
3.2. WDXRF Spectroscopy
3.3. FTIR-ATR Spectroscopy
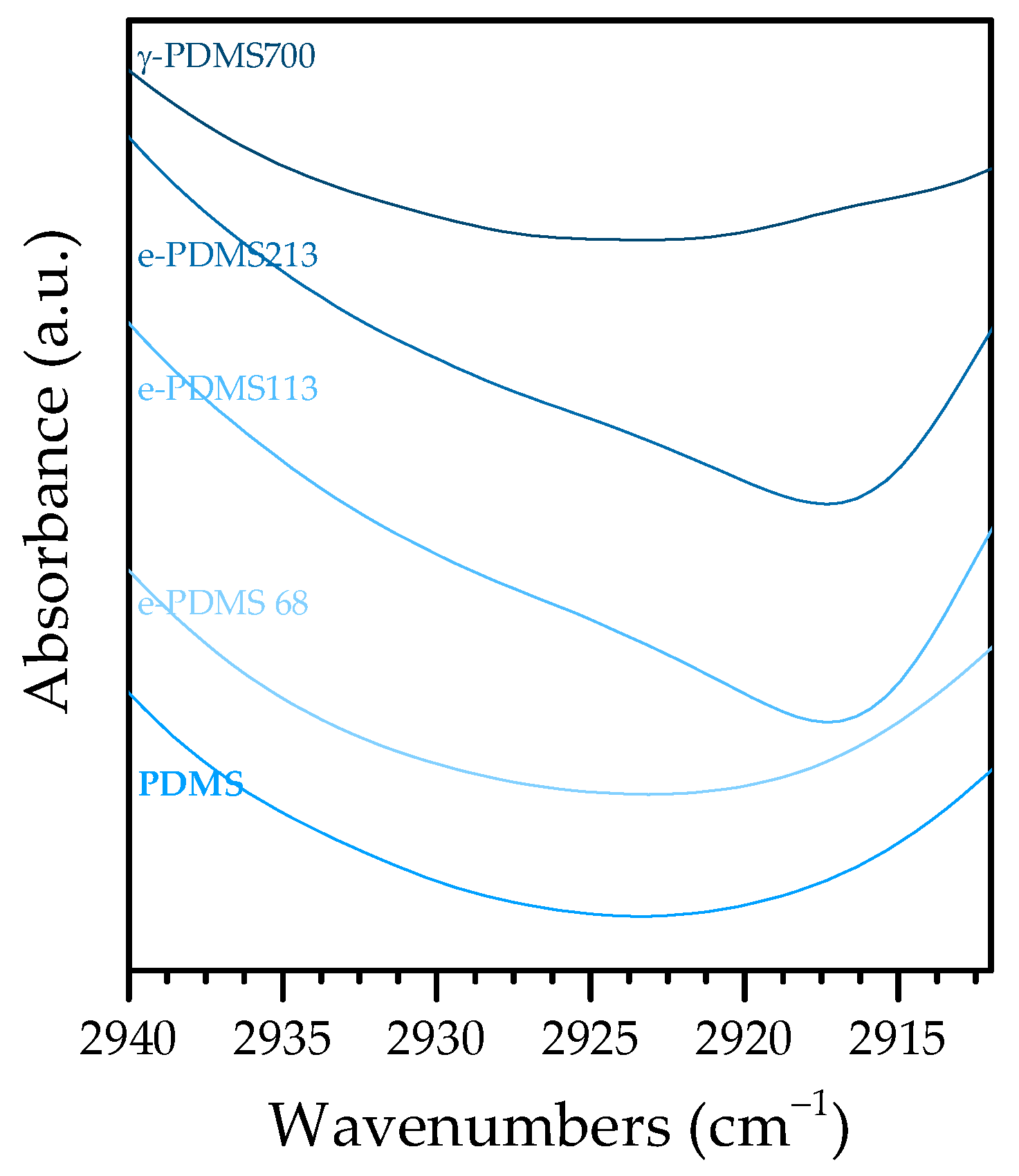
3.3.1. PDMS
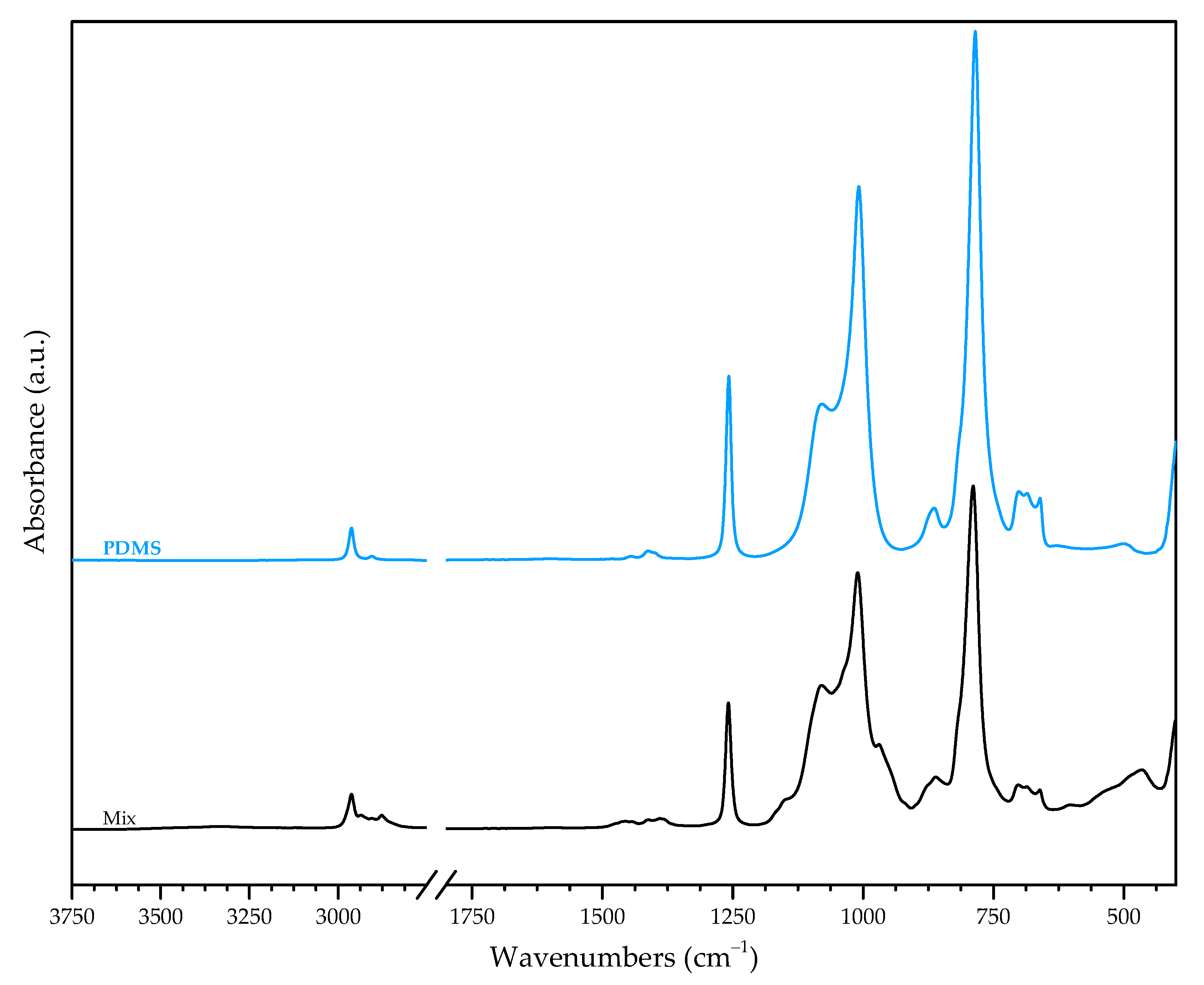
3.3.2. Precursors vs. Non-Irradiated Mix
- 2936, 2877, 1161, 953, 864 and 789 cm−1 from methyl groups;
- 1150 cm−1 from C—H vibrations in propoxide;
- 1010 cm−1, 482 cm−1 from vibrations of PDMS’s backbone, Si—O—Si, as well as of Si—O—Zr.
- 2975 cm−1 (TEOS) and 2958 cm−1 (TPOZ), associated with methyl groups;
- 1296 cm−1 (TEOS) and 1297 cm−1 (TPOZ), associated with C—H bonds;
- 1275 cm−1 and 1252 cm−1 (TPOZ), 1100 cm−1 (TEOS), 1128, 1107 and 1002 cm−1 (TPOZ), associated to C—O bonds (first and second in propanol, third and sixth in (C—O)Zr).
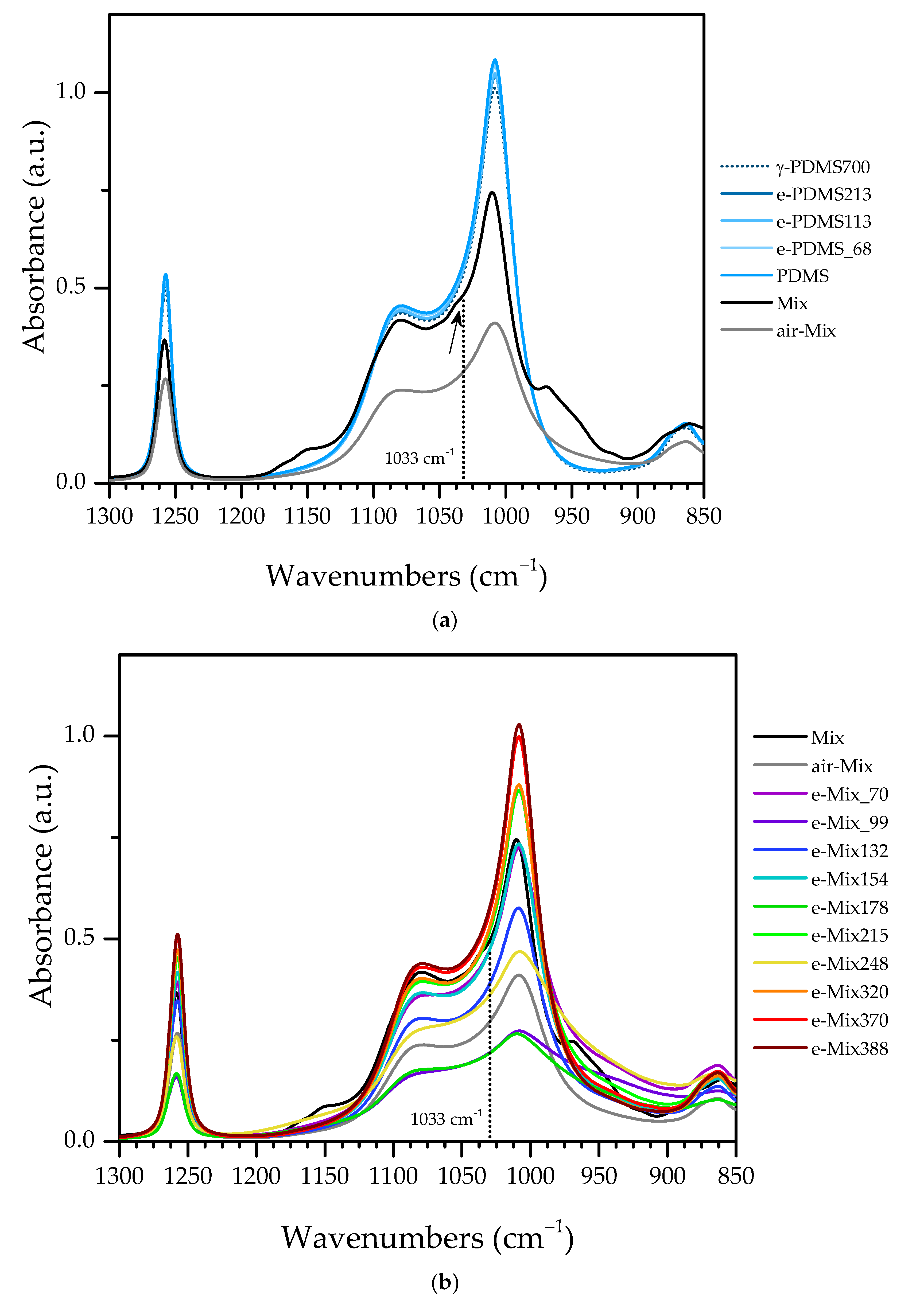
3.3.3. e-Mix
- 2936, 2877, 1471, 1456, 1365 and 1161 cm−1, associated to C—H in methyl and methylene groups;
- 1044 cm−1 and 970 cm−1, associated with C—O in propanol;
- 1390, 1382, 1150 cm−1, associated to C—H, the latter in propoxide;
- 1390 cm−1 and 1382 cm−1, associated with C—O—Si and C—O—Zr, respectively;
- 1033 cm−1, associated with Si—O—Zr;
- 603, 534 and 482 cm−1, associated with (Zr—O)C;
- 466 cm−1, associated with O—C—C and/or O—H.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Preparation of the Mixture to Be Irradiated
Appendix A.1. Transfer Material and Method
Appendix A.2. Reagent Addition Sequence
Appendix A.2.1. Method A
Appendix A.2.2. Method B
Appendix B
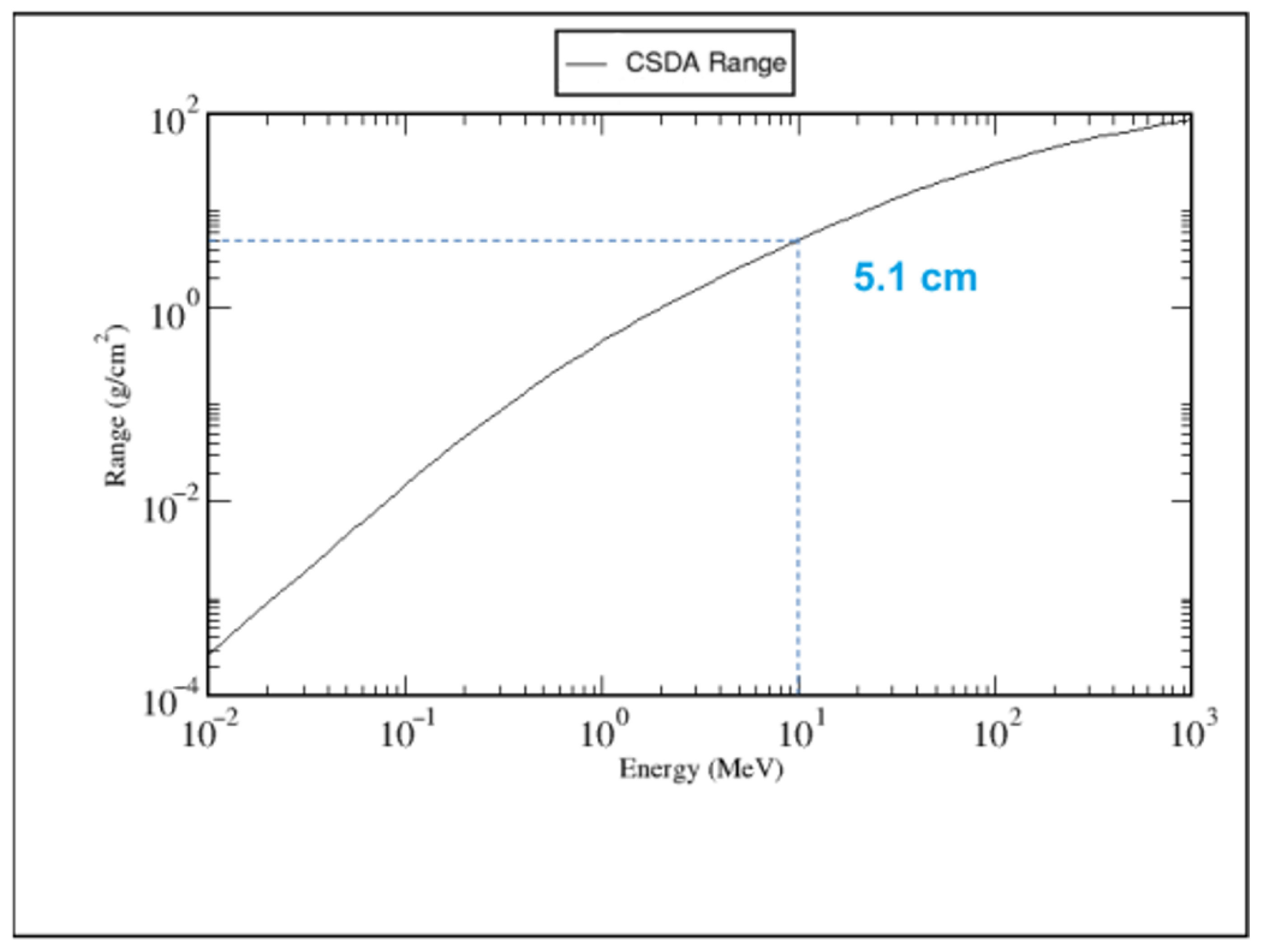
Sample Positioning and Dosimetry
References
- Currie, H.A.; Patwardhan, S.V.; Perry, C.C.; Roach, P.; Shirtcliffe, N.J. Natural and Artificial Hybrid Biomaterials. In Hybrid Materials–Synthesis, Characterization and Applications; Kickelbick, G., Ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 255–299. ISBN 9783527312993. [Google Scholar]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sanchez, C. Hybrid Materials, Functional Applications. An Introduction. In Functional Hybrid Materials; Gómez-Romero, P., Sanchez, C., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 1–14. [Google Scholar]
- Castro, A.G.B.; Bastos, A.C.; Galstyan, V.; Faglia, G.; Sberveglieri, G.; Miranda Salvado, I.M. Synthesis and electrochemical study of a hybrid structure based on PDMS-TEOS and titania nanotubes for biomedical applications. Nanotechnology 2014, 25, 365701. [Google Scholar] [CrossRef] [PubMed]
- Suzana, A.F.; Ferreira, E.A.; Benedetti, A.V.; Carvalho, H.W.P.; Santilli, C.V.; Pulcinelli, S.H. Corrosion protection of chromium-coated steel by hybrid sol-gel coatings. Surf. Coat. Technol. 2016, 299, 71–80. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Moliner-Martínez, Y.; Herráez-Hernández, R.; Molins-Legua, C.; Verdú-Andrés, J.; Campíns-Falcó, P. Designing solid optical sensors for in situ passive discrimination of volatile amines based on a new one-step hydrophilic PDMS preparation. Sens. Actuators B Chem. 2016, 223, 333–342. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, H.; Wang, L.; Li, W.; Zhou, T.; Rong, B. TEOS/PDMS-OH hybrid material for the consolidation of damaged pottery. Herit. Sci. 2013, 1, 1. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Cervantes, J.; Puy-Alquiza, M.J.; Miranda, R. Conservation of building materials of historic monuments using a hybrid formulation. J. Cult Herit. 2015, 16, 185–191. [Google Scholar] [CrossRef]
- Lu, Q. Synthesis of PDMS-Metal Oxide Hybrid Nanocomposites Using an In Situ Sol-Gel Route. Ph.D. thesis, Michigan Technological University, Houghton, MI, USA, 2012. [Google Scholar]
- Gomes, S.R.; Margaça, F.M.A.; Ferreira, L.M.; Salvado, I.M.M.; Falcão, A.N. Hybrid PDMS-Silica-Zirconia materials prepared by γ-irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2007, 265, 114–117. [Google Scholar] [CrossRef]
- Gomes, S.R.; Margaça, F.M.A.; Miranda Salvado, I.M.; Leal, J.P.; Marques, C.; Alves, E.; Ferreira, L.M.; Falcão, A.N. Elemental and RBS analysis of hybrid materials prepared by gamma-irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mate.r Atoms. 2008, 266, 288–294. [Google Scholar] [CrossRef]
- Nanko, M. Definitions and Categories of Hybrid Materials. AZojomo 2009, 6, 1–8. [Google Scholar] [CrossRef]
- Serbin, J.; Egbert, A.; Ostendorf, A.; Chichkov, B.N.; Houbertz, R.; Domann, G.; Schulz, J.; Cronauer, C.; Fröhlich, L.; Popall, M. Femtosecond laser-induced two-photon polymerization of inorganic–organic hybrid materials for applications in photonics. Opt. Lett. 2003, 28, 301–303. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Gaidukeviciute, A.; Chichkov, B.N.; Oubaha, M.; MacCraith, B.D.; Sakellari, I.; Giakoumaki, A.; Gray, D.; Vamvakaki, M.; Farsari, M.; et al. Two-Photon Polymerization of Hybrid Sol-Gel Materials for Photonics Applications. Laser Chem. 2008, 2008, 493059. [Google Scholar] [CrossRef]
- Farsari, M.; Chichkov, B.N. Two-photon fabrication. Nat. Photonics. 2009, 3, 450–452. [Google Scholar] [CrossRef]
- Falcão, A.N.; Carrapiço, M.; Santos Sousa, J.; Margaça, F.M.A.; Ferreira, L.M.; Carvalho, F.G.; Miranda Salvado, I.M.; Teixeira, J. Investigation of organic-inorganic hybrid materials prepared by irradiation. J. Sol.-Gel. Sci. Technol. 2003, 26, 349–352. [Google Scholar] [CrossRef]
- Gomes, S.R.; Margaça, F.M.A.; Miranda Salvado, I.M.; Ferreira, L.M.; Falcão, A.N.; Salvado, I.M.M. Preparation of silica-based hybrid materials by gamma irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2006, 248, 291–296. [Google Scholar] [CrossRef]
- Lancastre, J.J.H.; Margaça, F.M.A.; Ferreira, L.M.; Falcão, A.N.; Salvado, I.M.M.; Nabiça, M.S.M.S.; Fernandes, M.H.V.; Almasy, L. Thermal analysis and SANS characterisation of hybrid materials for biomedical applications. J. Therm. Anal. Calorim. 2012, 109, 413–418. [Google Scholar] [CrossRef]
- Almeida, J.C.; Lancastre, J.; Vaz Fernandes, M.H.; Margaça, F.M.A.; Ferreira, L.; Miranda Salvado, I.M. Evaluating structural and microstructural changes of PDMS-SiO2 hybrid materials after sterilization by gamma irradiation. Mater. Sci. Eng. C 2015, 48, 354–358. [Google Scholar] [CrossRef]
- Lancastre, J.J.H.; Falcão, A.N.; Margaça, F.M.A.; Ferreira, L.M.; Salvado, I.M.M.; Casimiro, M.H.; Almásy, L.; Meiszterics, A. Influence of the polymer molecular weight on the microstructure of hybrid materials prepared by γ-irradiation. Radiat. Phys. Chem. 2015, 106, 126–129. [Google Scholar] [CrossRef][Green Version]
- Torraca, G. Lectures on Materials Science for Achitectural Conservation; Escobar, A., Ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2009; ISBN 9780982766835. [Google Scholar]
- Marques, R.d.S. Síntese, Caracterização e Aplicações de Membranas Poliméricas Catalíticas à Base de PDMS e PVA. Master’s Thesis, Universidade Federal de São João Del Rei, São João del-Rei, Brazil, 2009. [Google Scholar]
- International Atomic Energy Agency. Radiation Synthesis and Modification of Polymers for Biomedical Application—Final Results of a Co-Ordinated Research Project 1996–2000; International Atomic Energy Agency: Vienna, Austria, 2002. [Google Scholar]
- Price, C.A. Stone Conservation: An Overview of Current Research, 1st ed.; Berland, D., Ed.; The Getty Conservation Institute: Santa Monica, CA, USA, 1996; ISBN 0892363894. [Google Scholar]
- Horie, V. Materials for Conservation. Organic Consolidant and Coating, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 9780750669054. [Google Scholar]
- de Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Vezzalini, G. Hybrid sol-gel based protective coatings for historical window glasses. Sci. Technol. Conserv. Cult Herit. 2013, 3, 231–234. [Google Scholar] [CrossRef]
- Angulo-Olais, R.; Illescas, J.F.; Aguilar-Pliego, J.; Vargas, C.A.; Haro-Pérez, C. Gel Point Determination of TEOS-Based Polymeric Materials with Application on Conservation of Cultural Heritage Buildings. Adv. Condens. Matter Phys. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Khan, M.; Shaik, M.R.; Khan, S.T.; Adil, S.F.; Kuniyil, M.; Khan, M.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Nawaz Tahir, M. Enhanced Antimicrobial Activity of Biofunctionalized Zirconia Nanoparticles. ACS Omega 2020, 5, 1987–1996. [Google Scholar] [CrossRef]
- Jangra, S.L.; Stalin, K.; Dilbaghi, N.; Kumar, S.; Tawale, J.; Singh, S.P.; Pasricha, R. Antimicrobial activity of zirconia (ZrO2) nanoparticles and zirconium complexes. J. Nanosci. Nanotechnol. 2012, 12, 7105–7112. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-based nanomaterials: Recent developments in synthesis and applications. Nanoscale Adv. 2022, 4, 4210–4236. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.R.R. Preparação e Caracterização de Materiais Híbridos. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2007. [Google Scholar]
- Berger, M.J.; Coursey, J.S.; Zucker, M.A.; Chang, J. ESTAR, PSTAR, and ASTAR: Computer Programs for Calculating Stopping-Power and Range Tables for Electrons, Protons, and Helium Ions (Version 1.2.3); Online; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005.
- Craciun, G.; Manaila, E.; Stelescu, M.D. New elastomeric materials based on natural rubber obtained by electron beam irradiation for food and pharmaceutical use. Materials 2016, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Chowdhury, S.R.; Mahanwar, P.A.; Sarma, K.S.S. Influence of Electron Beam Treatment on the Crystallization and Thermal Stability of Ldpe/Epdm Blends. Am. J. Eng. Appl. Sci. 2014, 7, 338–352. [Google Scholar] [CrossRef]
- IUPAC. Quantities, Units and Symbols in Physical Chemistry, 3rd ed.; Cvitas, T., Frey, J.G., Holström, B., Kuchitsu, K., Marquardt, R., Mills, I., Pavese, F., Quack, M., Stohner, J., Strauss, H.L., Eds.; RSC Publishing: Cambridge, UK, 2007. [Google Scholar]
- Hofmann, J. IR Spectroscopic Method for Determination of Silicone Cross-Linking; Pressure Sensitive Tape Council: Chicago, IL, USA, 2016; pp. 1–9. [Google Scholar]
- Chen, D.; Yi, S.; Wu, W.; Zhong, Y.; Liao, J.; Huang, C.; Shi, W. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using Vinyl-POSS derivatives as cross linking agents. Polymer 2010, 51, 3867–3878. [Google Scholar] [CrossRef]
- Stafie, N.; Stamatialis, D.F.; Wessling, M. Effect of PDMS cross-linking degree on the permeation performance of PAN/PDMS composite nanofiltration membranes. Sep. Purif. Technol. 2005, 45, 220–231. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. [Google Scholar]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Smith, B.C. A process for successful infrared spectral interpretation. Spectrosc 2016, 31, 14–21. [Google Scholar]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A. Characterization of the oxides present in a polydimethylsiloxane layer obtained by polymerisation of its liquid precursor in corona discharge. J. Nanomater. 2015, 2015, 204296. [Google Scholar] [CrossRef]
- Philip, J.L.; Barry, A. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations. In Silicon Compounds: Silanes and Silicones—A Survey of Properties and Chemistry; Arkles, B., Larson, G.L., Eds.; Gelest, Inc.: Morrisville, PA, USA, 2013; pp. 177–180. ISBN 978-0-578-12235-9. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Sadtler. The Infrared Spectra Atlas of Monomers and Polymers; Sadtler Research Laboratories: Philadelphia, PA, USA, 1980; ISBN 0-08456-0064-8. [Google Scholar]
- Mayerhöfer, T.G.; Shen, Z.; Leonova, E.; Edén, M.; Kriltz, A.; Popp, J. Consolidated silica glass from nanoparticles. J. Solid State Chem. 2008, 181, 2442–2447. [Google Scholar] [CrossRef]
- Colomer, M.T. Straightforward synthesis of Ti-doped YSZ gels by chemical modification of the precursors alkoxides. J. Sol.-Gel. Sci. Technol. 2013, 67, 135–144. [Google Scholar] [CrossRef]
- Mendoza-Serna, R.; Bosch, P.; Padilla, J.; Lara, V.H.; Méndez-Vivar, J. Homogeneous Si-Ti and Si-Ti-Zr polymeric systems obtained from monomeric precursors. J. Non. Cryst. Solids. 1997, 217, 30–40. [Google Scholar] [CrossRef]
- Kongwudthiti, S.; Praserthdam, P.; Tanakulrungsank, W.; Inoue, M. The influence of Si-O-Zr bonds on the crystal-growth inhibition of zirconia prepared by the glycothermal method. J. Mater. Process Technol. 2003, 136, 186–189. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Hybrid polymer-inorganic nanocomposites. Russ. Chem. Rev. 2000, 69, 53–80. [Google Scholar] [CrossRef]

| Field | Information | Code | Description |
|---|---|---|---|
| Prefix | Curing method | [none] | Not cured |
| air | In-air curing | ||
| e | Irradiation with electron beam | ||
| γ | Irradiation with gamma photons | ||
| Root | Composition | PDMS | Polydimethilsiloxane as received |
| Mix | Non-irradiated PDMS:TEOS:TPOZ with 67:13:20 m% concentration, as of preparation | ||
| Suffix | Accumulated dose (kGy) | [numeral representing dose] | Accumulated dose in kGy |
| Material | Dose (kGy) | Gel Fraction (m%) |
|---|---|---|
| e-PDMS | 68 | 89.5 |
| 113 | 99.5 | |
| 213 | 99.4 | |
| air-Mix | 0 | 57.3 |
| e-Mix | 70 | 56.4 |
| 99 | 65.3 | |
| 132 | 55.3 | |
| 154 | 57.5 | |
| 178 | 62.7 | |
| 215 | 60.8 | |
| 248 | 53.8 | |
| 320 | 85.5 | |
| 370 | 71.5 | |
| 388 | 83.8 |
| Dose (kGy) | Si (wt%) | Zr (wt%) | Si:Zr (χm) | Si:Zr (χat) |
|---|---|---|---|---|
| Mix | * 82.87 | * 17.13 | 4.84 | 15.71 |
| air-Mix | 74.0 | 26.0 | 2.85 | 9.24 |
| e-Mix70 | 57.2 | 41.9 | 1.37 | 4.43 |
| e-Mix99 | 79.7 | 19.7 | 4.05 | 13.14 |
| e-Mix132 | 69.1 | 30.2 | 2.29 | 7.43 |
| e-Mix154 | 65.0 | 34.3 | 1.90 | 6.16 |
| e-Mix178 | 56.9 | 42.1 | 1.35 | 4.39 |
| e-Mix215 | 83.3 | 16.1 | 5.17 | 16.80 |
| e-Mix248 | 79.6 | 19.8 | 4.02 | 13.06 |
| e-Mix320 | 55.4 | 43.7 | 1.27 | 4.12 |
| e-Mix370 | 63.4 | 35.9 | 1.77 | 5.74 |
| e-Mix388 | 77.5 | 22.5 | 3.44 | 11.19 |
| Dose (kGy) | Si (wt%) | Zr (wt%) | Si:Zr (χm) | Si:Zr (χat) |
|---|---|---|---|---|
| Mix | * 82.87 | * 17.13 | 4.84 | 15.71 |
| air-Mixp | 55.4 | 43.7 | 1.27 | 4.12 |
| e-Mix70p | 57.2 | 47.3 | 1.21 | 3.93 |
| e-Mix99p | 68.5 | 52.7 | 1.30 | 4.22 |
| e-Mix132p | 46.4 | 52.7 | 0.88 | 2.86 |
| e-Mix154p | 52.3 | 46.8 | 1.12 | 3.63 |
| e-Mix178p | 65.5 | 33.9 | 1.93 | 6.28 |
| e-Mix215p | 55.2 | 43.7 | 1.26 | 4.10 |
| e-Mix248p | 68.8 | 30.5 | 2.26 | 7.33 |
| e-Mix320p | 61.9 | 37.3 | 1.66 | 5.39 |
| e-Mix370p | 64.8 | 34.5 | 1.88 | 6.10 |
| e-Mix388p | 88.3 | 11.7 | 7.55 | 24.51 |
| Frequency (cm−1) | Assignment | References | ||
|---|---|---|---|---|
| PDMS | e-PDMS | γ-PDMS | ||
| 2962/2905 | 2962/2905 | 2962/2906 | C—H asym/sym stretch in Si—CH3 | [36,37,38] |
| Background overlap | 2927–2917 (113 kGy, 213 kGy) Background overlap (68 kGy) | 2933 | C—H asym stretch in —CH2— | [39,40] |
| 1600 | 1600 | 1607 | adsorb H2O bend | [41,42] |
| 1480/1373 | 1481 (68 kGy) 1484 (113 kGy, 213 kGy) | 1480 | C—H asym/sym bend in —CH3 and/or —CH2— | [39] |
| 1445 | 1445 (68 kGy) 1446 (113 kGy, 213 kGy) | 1445 | C—H asym bend in —CH3 | [39,40] |
| 1412 | 1412 | 1412 | —CH3 asym bend in Si—CH3 | [43] |
| 1402/1258 | 1402/1258 | 1402/1258 | —CH3 asym/sym bend in Si—CH3 | [41,42] |
| 1258 | 1258 | 1258 | —CH3 sym bend in Si—CH3 | [38,43] |
| 1078/1008 | 1080/1008 | 1078–1080/1008 | Si—O—Si asym stretch in linear structures | [40,44,45] |
| 864 | 864 | 864 | Si—CH3 rock in PDMS | [43] |
| 785 | 785 | 785 | SiO4 asym vibration, Si—O—Si bend in SiO2, Si—CH3 rock, CH3 rock | [40,44,46] |
| 500 | 500 | 502 | Si—O—Si bend, phonons from M—M and M—O—M′ (M=Si, M′=Zr) | [47] |
| Frequency (cm−1) | Assignment | References | ||||
|---|---|---|---|---|---|---|
| PDMS | TEOS | TPOZ | Mix | e-Mix | ||
| 3344 | 3345 | 3500 | O—H stretch in H-bonded OH/propOH | [39,40] | ||
| 2962/2905 | 2962/2905 | 2962/2905 | C—H asym/sym stretch in Si—CH3 | [36,37,38] | ||
| 2975 | 2958 | - | - | C—H asym stretch in —CH3 | [39,40,48] | |
| 2929 | 2933 | 2936 | - | C—H asym stretch in —CH2— | [39,40] | |
| 2889 | 2873 | 2877 | - | C—H sym stretch in —CH2—/—CH3 | [39,40,48] | |
| 1600 | 1603 | 1600–1634 | adsorb H2O bend | [41,42] | ||
| 1480 | 1484 | 1470 | 1471 | - | C—H asym bend in —CH3 and —CH2— | [39] |
| 1458 | 1456 | - | C—H asym bend in —CH2—/—CH3 (in propOx from TPOZ) | [39,48] | ||
| 1445 | 1444 | 1438 | 1443 | 1445 | C—H asym bend in —CH3 | [39,40] |
| 1412 | 1412 | 1412 | —CH3 asym bend in Si—CH3 | [43] | ||
| 1402/1258 | 1/1258 | 1404 | —CH3 asym/sym bend in Si—CH3 | [41,42] | ||
| 1373(?) 2 | 1391 | 1380 | 1390 1382 | - | C—H sym bend in —CH3 and/or in propOx and propOH | [39,40] |
| 1391 | 1380 | 1390 1382 | C—O—M stretch, with M=Si in TEOS and M=Zr in TPOZ | [49] | ||
| 1366 | 1364 | 1365 | - | C—H wag in —CH2— | [40] | |
| 1296 | 1297 | - | C—H wag/twist | [40] | ||
| 1275/1252 | - | C—O asym/sym stretch in propOH | [48] | |||
| 1258 | 1258 | 1258 | —CH3 sym bend in Si—CH3 | [38,43] | ||
| 1168 1100/1073 959 | 1161 1096/1080 953 | - -/1078 - | Si—OCH2CH3 | [44] | ||
| 1168 | 1161 | - | C—H rock in —CH3 | [40] | ||
| 1154 | 1150 | - | C—H wag/twist in propOx | [48] | ||
| 1100/1073 | 1096/1080 | -/1078 | Si—O—Si asym/sym stretch in linear structures | [40,44,45] | ||
| 1100 | 1107 | - | - | C—O asym stretch | [40] | |
| 1128 | - | - | (C—O)Zr asym stretch and «skeletal stretches» combination | [48] | ||
| 1080/1008 | 1080/1010 | 1078/ 1010–1008 | Si—O—Si asym stretch in linear structures | [40,44,45] | ||
| 1045 1012 968 | 1044 1010 970 | - 1008 - | C—O stretch in propOH | [48] | ||
| 1033 | - | Si—O—Zr | [50] | |||
| 1002 | - | - | (C—O)Zr sym stretch | [48] | ||
| 968 | 970 | - | C—O stretch in propOH | [48] | ||
| 959 | 953 | - | C—H rock in —CH3, Si—O(H) stretch | [40,48] | ||
| 864 | 862 | 861 | 864 | Si—CH3 rock in PDMS, C—H twist in propOx | [43,48] | |
| 811 | 813 | 813 | —CH2— rock | [40] | ||
| 785 | 785 | 782 | 789 | 789–785 | SiO4 asym vibration, Si—O—Si bend in SiO2, Si—CH3 rock, CH3 rock | [40,44,46] |
| 596 545 498 | 603 534 482 | - - - | (Zr—O)C stretch | [48] | ||
| 500 | 482 | 493 | Si—O—Si bend, phonons from M—O—M and M—O—M′ (M=Si, M′=Zr) | [47] | ||
| 472 | 466 | - | O—C—C bend | [40] | ||
| 459 | 466 | - | O—H wag/twist | [51] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.P.; Santos, P.M.P.; Veiga, J.P.; Casimiro, M.H.; Ferreira, L.M. Electron Beam Irradiation on the Production of a Si- and Zr-Based Hybrid Material: A Study by FTIR and WDXRF. Materials 2023, 16, 489. https://doi.org/10.3390/ma16020489
Rodrigues AP, Santos PMP, Veiga JP, Casimiro MH, Ferreira LM. Electron Beam Irradiation on the Production of a Si- and Zr-Based Hybrid Material: A Study by FTIR and WDXRF. Materials. 2023; 16(2):489. https://doi.org/10.3390/ma16020489
Chicago/Turabian StyleRodrigues, Alexandra P., Pedro M. P. Santos, João Pedro Veiga, Maria Helena Casimiro, and Luís M. Ferreira. 2023. "Electron Beam Irradiation on the Production of a Si- and Zr-Based Hybrid Material: A Study by FTIR and WDXRF" Materials 16, no. 2: 489. https://doi.org/10.3390/ma16020489
APA StyleRodrigues, A. P., Santos, P. M. P., Veiga, J. P., Casimiro, M. H., & Ferreira, L. M. (2023). Electron Beam Irradiation on the Production of a Si- and Zr-Based Hybrid Material: A Study by FTIR and WDXRF. Materials, 16(2), 489. https://doi.org/10.3390/ma16020489









