Structure and Corrosion Behavior of Multiphase Intermetallic ZrCu-Based Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Ingots and Ribbons
2.2. Structural Investigations and Thermal Analysis
2.3. Corrosion Studies
2.4. Hardness and Tribological Measurements
3. Results and Discussion
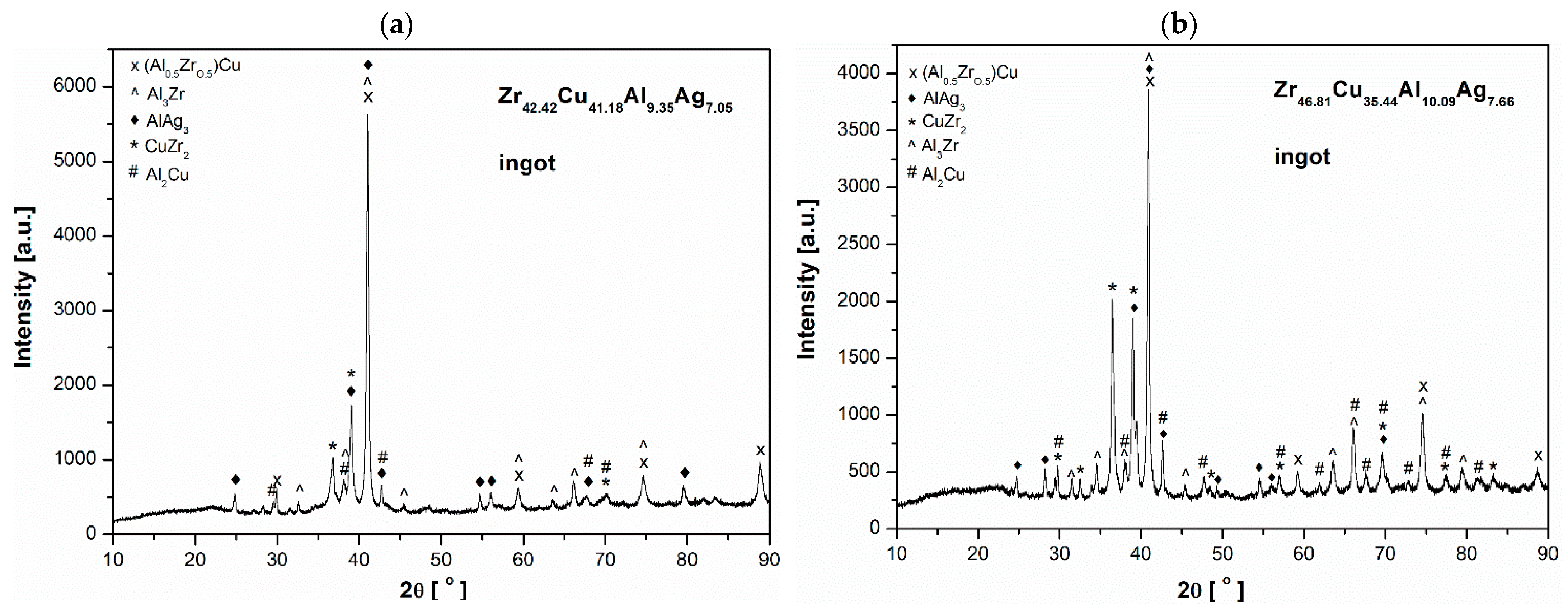
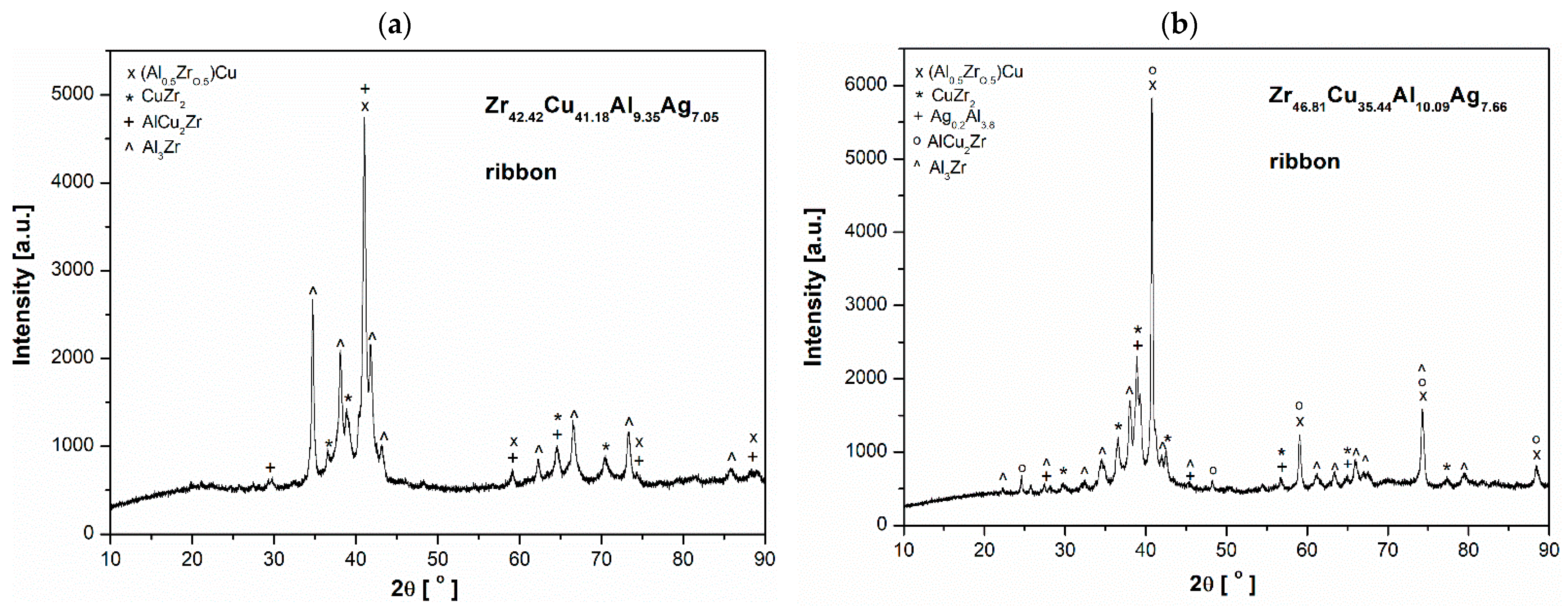
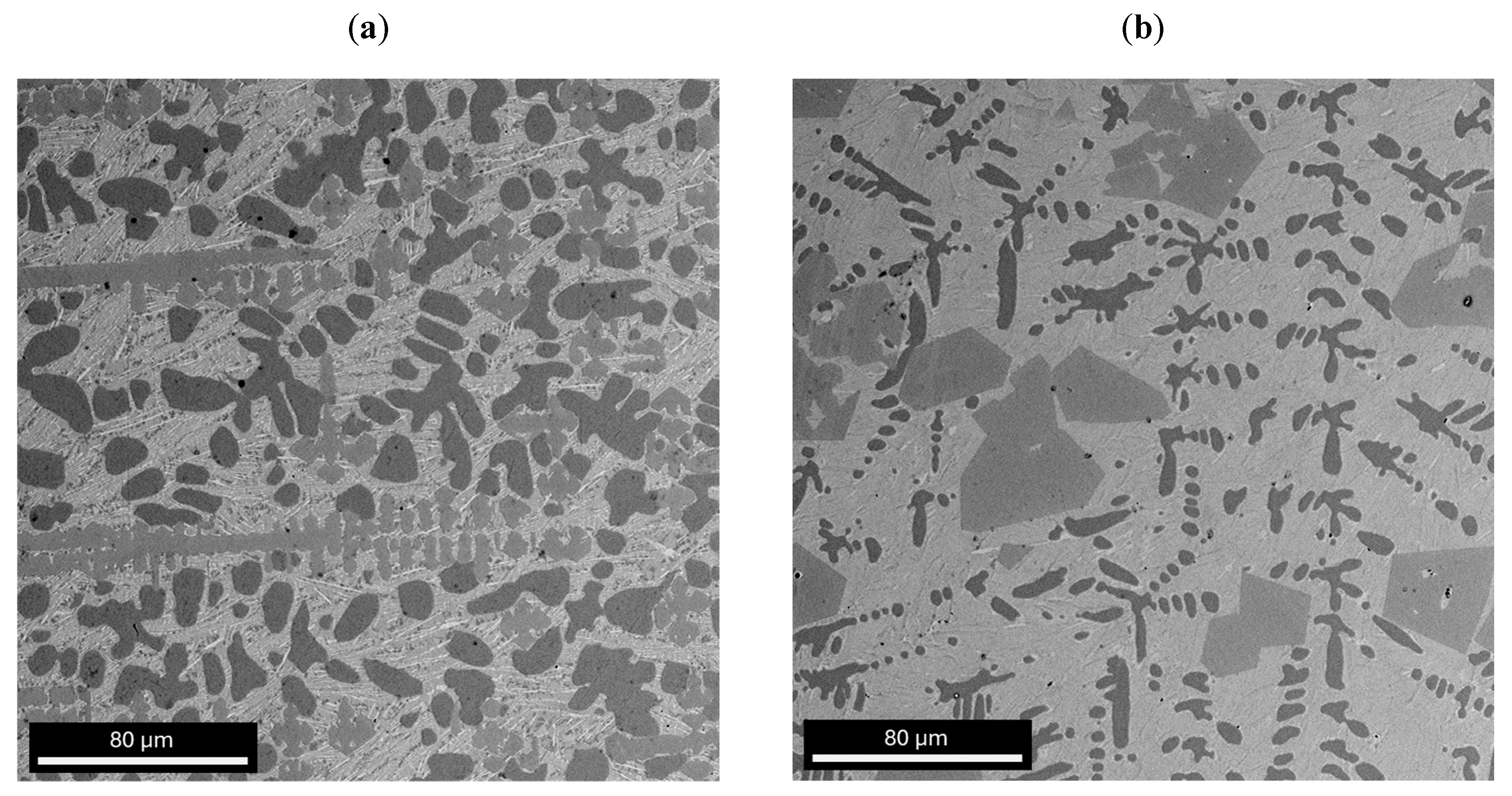
3.1. Structural Analysis
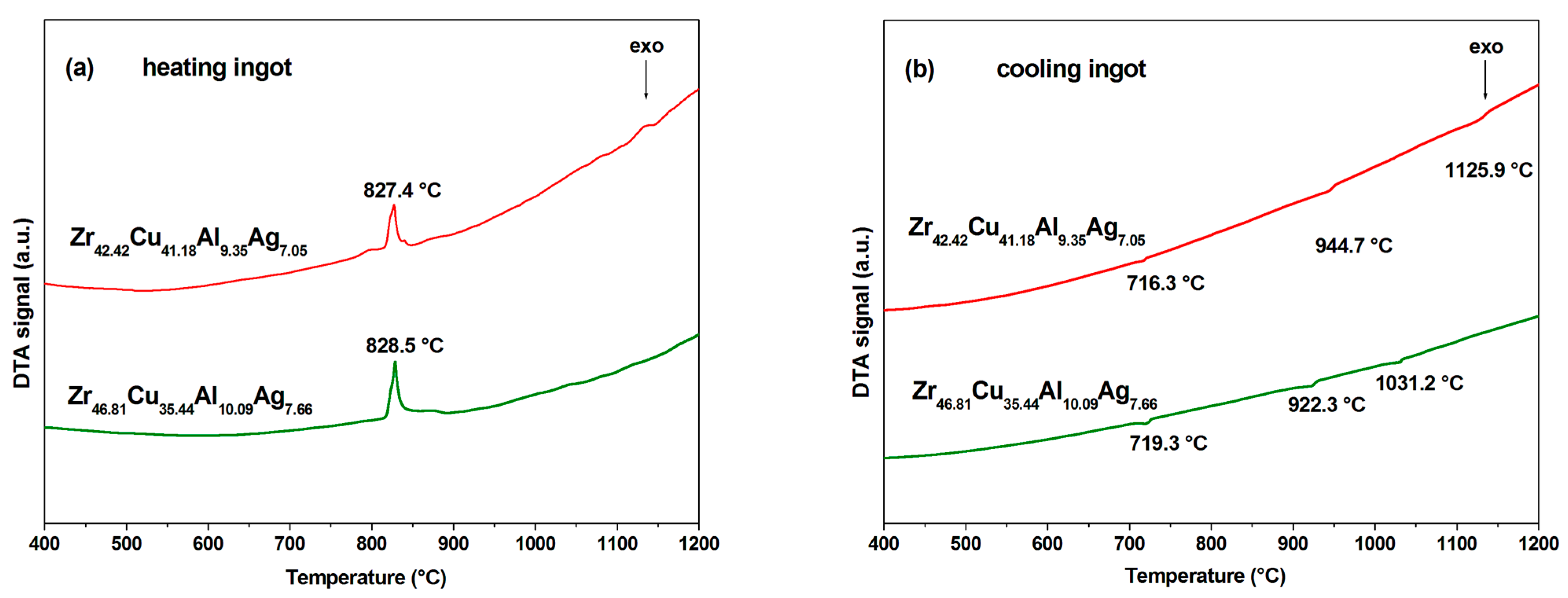
3.2. Thermal Analysis and Glass-Forming Ability
3.3. Corrosion Behavior
3.4. Mechanical Properties
4. Conclusions
- The samples of the alloys Zr42.42Cu41.18Al9.35Ag7.05 and Zr46.81Cu35.44Al10.09Ag7.66, both ingot and ribbon forms, exhibited a crystalline structure.
- In both alloy ingots of ZrCu-based alloys, the characteristic peaks of (Al0.5Zr0.5)Cu, Al3Zr, AlAg3, CuZr2, and Al2Cu were identified. The ribbon samples exhibited the following phases: (Al0.5Zr0.5)Cu, CuZr2, and Al3Zr. Furthermore, the Al0.2Ag3.8 phase was identified in the Zr46.81Cu35.44Al10.09Ag7.66 alloy.
- The DTA curves for the two alloy ingots exhibit a comparable shape. During the heating process, the temperature of the analyzed alloy ingots increased in a uniform manner. Exothermic effects were observed at comparable temperatures, reaching a maximum at 827.4 and 828.5 °C for the alloy ingots Zr42.42Cu41.18Al9.35Ag7.05 and Zr46.81Cu35.44Al10.09Ag7.66, respectively. These temperatures are likely associated with the formation of the CuZr2 phase.
- Ingot samples of both ZrCu-based alloys were characterized by higher corrosion activity compared to that of the ribbon form. Ribbons with a higher Ag content have a higher corrosion resistance. This is confirmed by both the results of open-circuit potential and polarization measurements. For the Zr46.81Cu35.44Al10.09Ag7.66 ribbon the corrosion potential (Ecorr) was found to be equal to −0.317 V, the corrosion current density (jcorr) was 1.09 μA·cm−2, and polarization resistance (Rp) was 5.31 kΩ·cm2.
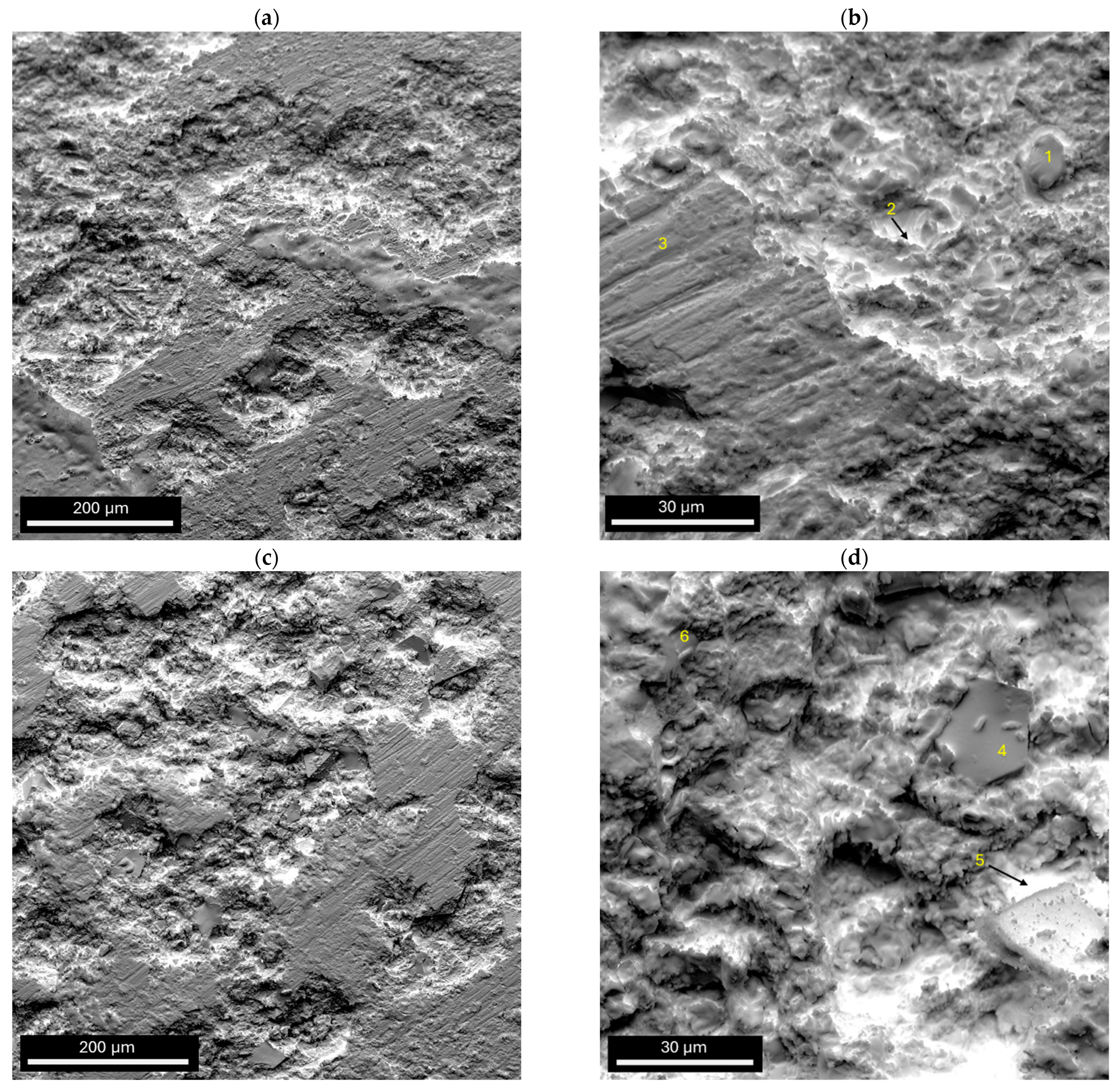
- The surface damage observed in both ingot samples after corrosion studies was consistent with pitting corrosion. It was found to be less severe in the Zr42.42Cu41.18Al9.35Ag7.05 alloy. Microscopic observations of the corrosion products confirmed the corrosion test results, which indicated that the ingot with a higher Cu and lower Zr content exhibited greater resistance to corrosion.
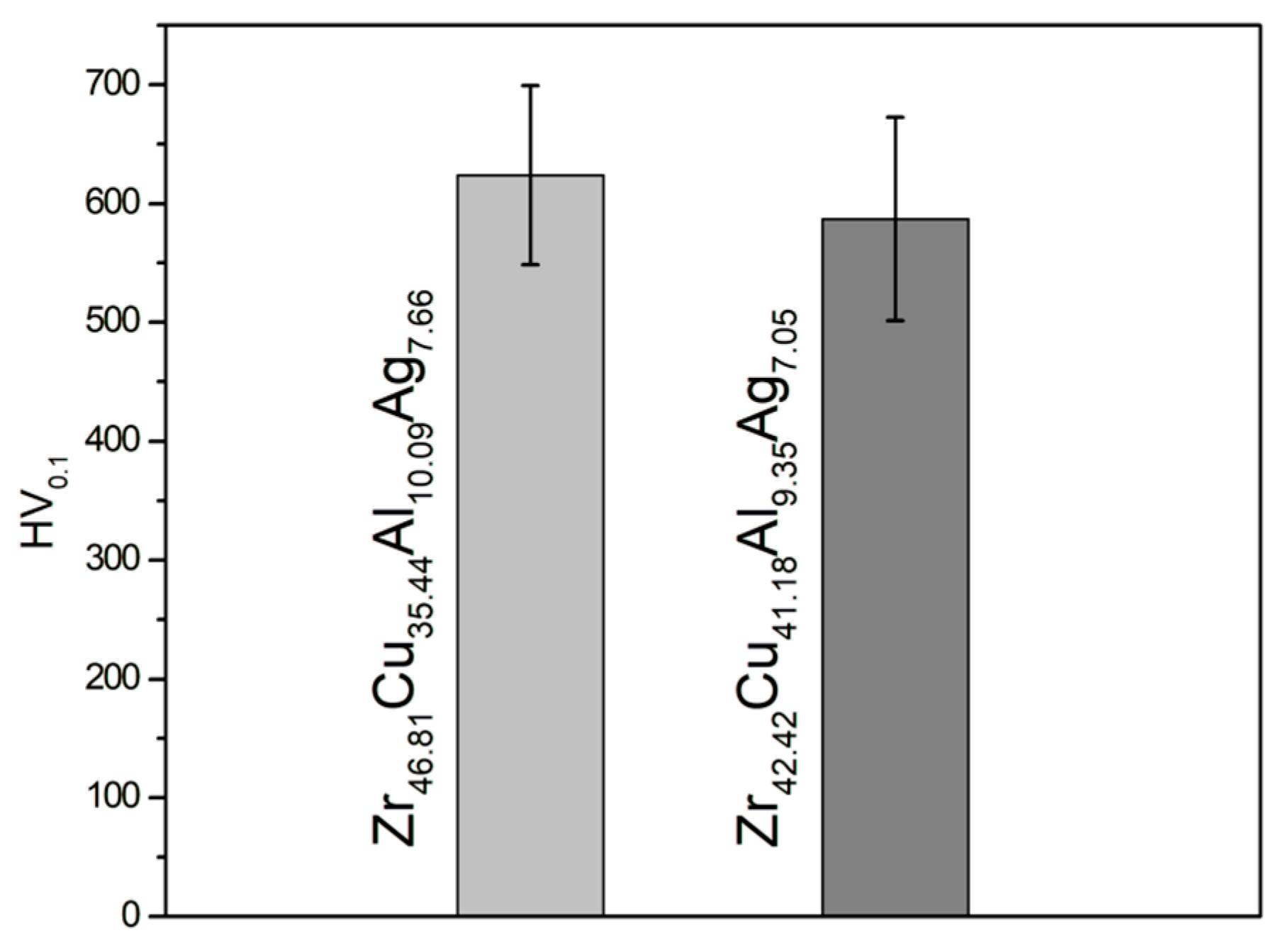
- The results of the microhardness tests showed that the alloy Zr46.81Cu35.44Al10.09Ag7.66 with a lower Cu content exhibited an average microhardness of 623.8 (±75.3) HV, which is indicative of its mechanical durability. Zr42.42Cu41.18Al9.35Ag7.05 exhibited a lower value of 587.0 (±85.7) HV.
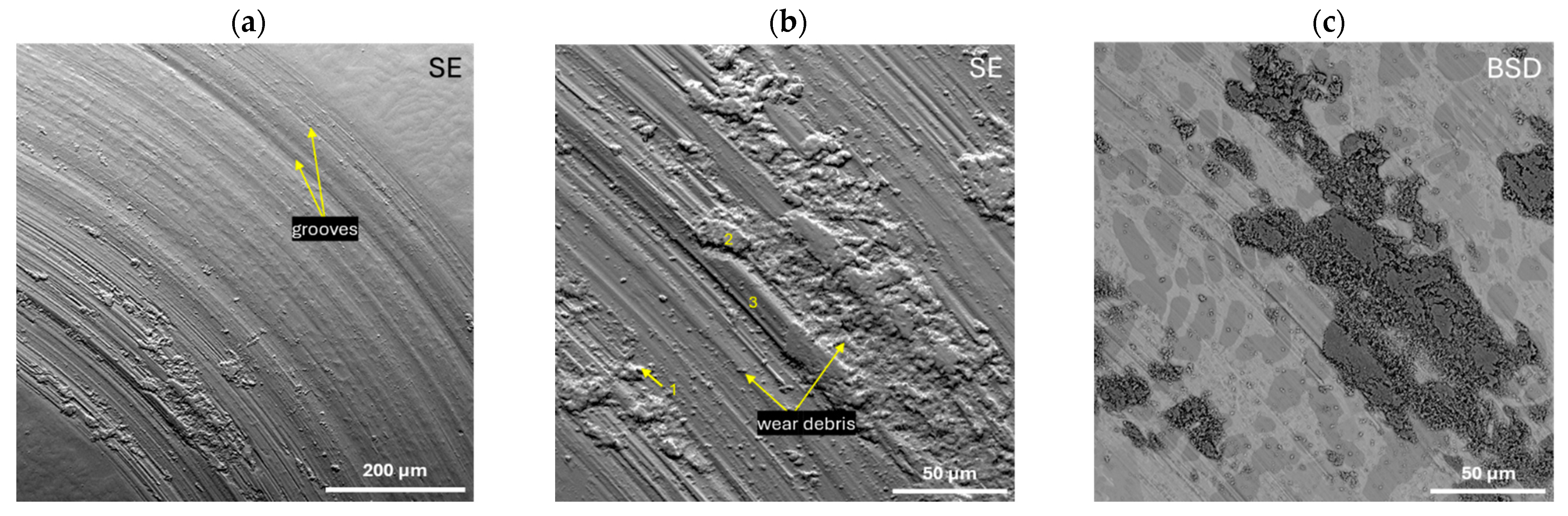
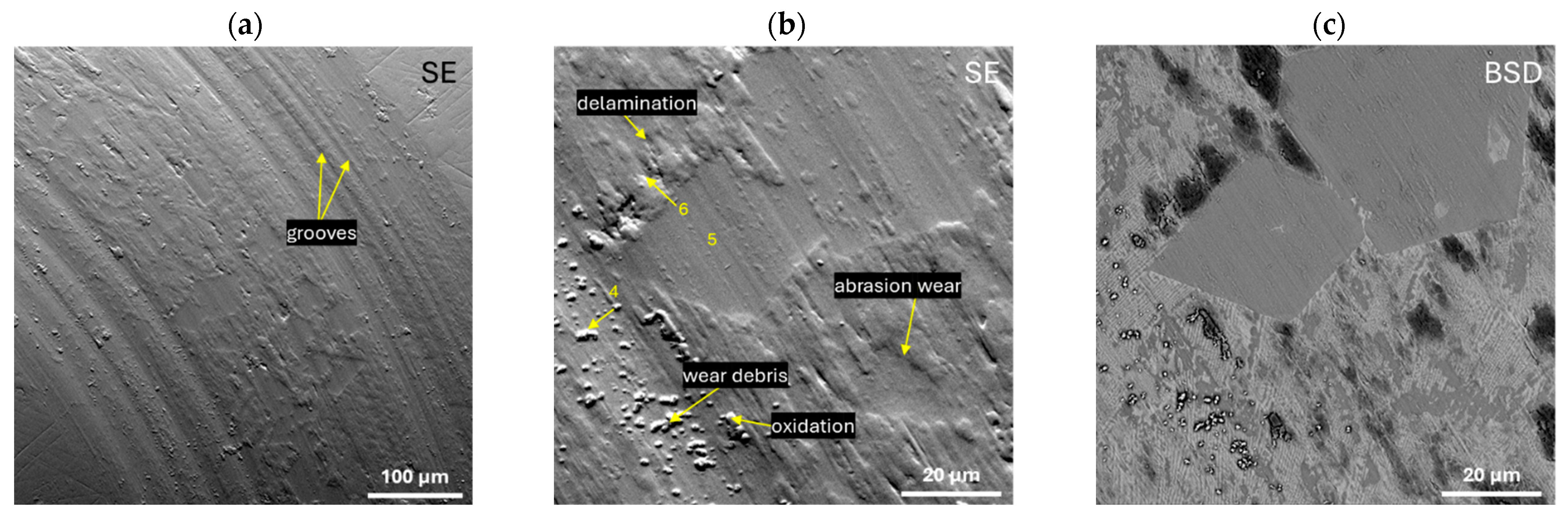
- The results of the abrasive wear resistance tests demonstrated that the ingots of both studied alloys exhibited a comparable average friction coefficient; however, the alloy SEM images showed that Zr46.81Cu35.44Al10.09Ag7.66 was characterized by more uniform wear compared to an alloy with a higher copper content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motta, A.T.; Capolungo, L.; Chen, L.Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.-C.A.; Tonks, M.R.; et al. Hydrogen in Zirconium Alloys: A Review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]
- Prabhu, Y.; Jain, A.; Vincent, S.; Ryu, W.H.; Park, E.S.; Kumar, R.; Bagde, A.D.; Bhatt, J. Compositional Design and in Vitro Investigation on Novel Zr–Co–Cu–Ti Metallic Glass for Biomedical Applications. Intermetallics 2022, 150, 107692. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Qiang, J.; Jiang, H.; Gu, L. Low-Cost Zr-Based Bulk Metallic Glasses for Biomedical Devices Applications. J. Non. Cryst. Solids 2019, 520, 119442. [Google Scholar] [CrossRef]
- Haratian, S.; Grumsen, F.B.; Villa, M.; Christiansen, T.L.; Somers, M.A.J. Self-Repair by Stress-Induced Diffusion of Noble Elements during Oxidation of Zr48Cu36Al8Ag8 Bulk Metallic Glass. Scr. Mater. 2019, 164, 126–129. [Google Scholar] [CrossRef]
- Saini, S.; Srivastava, A.P.; Neogy, S. Crystallization Kinetics and Mechanical Property of Yttrium-Modified Zr-Cu-Ag-Al Bulk Metallic Glasses. Mater. Lett. 2024, 355, 135454. [Google Scholar] [CrossRef]
- Xiong, Z.; Tao, P.; Long, Z.; Huang, Z.; Long, K.; Zhu, X.; Xu, X.; Deng, H.; Lin, H.; Li, W. The Effect of Ta Addition on Mechanical Properties of Zr-Based Bulk Metallic Glasses. Intermetallics 2023, 153, 107779. [Google Scholar] [CrossRef]
- Peng, L.; Li, J.; Zhang, M.; Lin, H.; Li, Z.; Li, W. Effects of Normal Load and Reciprocating Frequency on the Tribological Behaviors of a Zr-Based Bulk Metallic Glass. Wear 2023, 520–521, 204732. [Google Scholar] [CrossRef]
- Ouyang, D.L.; Yan, Y.H.; Chen, S.S.; Huang, D.; Wang, Z.R.; Cui, X.; Hu, Q.; Guo, S. Influence of Casting Temperature on the Castability and Glass-Forming Ability of Zr-Based Bulk Metallic Glasses. J. Non. Cryst. Solids 2023, 603, 122118. [Google Scholar] [CrossRef]
- Prabhu, Y.; Srivastav, A.K.; Gunderov, D.V.; Bhatt, J. Thermodynamic Model to Predict Bulk Metallic Glass Forming Composition in Zr-Cu-Fe-Al System and Understanding the Role of Dy Addition. Phys. B Phys. Condens. Matter 2022, 624, 413416. [Google Scholar] [CrossRef]
- Prabhu, Y.; Vincent, S.; Bhatt, J. Thermodynamic Modelling to Optimize Glass Forming Composition in Multicomponent Zr-Cu-Co-Al System. Mater. Today Proc. 2020, 28, 1239–1244. [Google Scholar] [CrossRef]
- Long, Z.; Tao, P.; Kong, L.; Wang, G.; Huang, S.; Wen, S.; He, H.; Huang, Z.; Zhu, X.; Xu, X.; et al. Effect of Cryogenic Thermal Cycling on the Microstructure and Mechanical Properties of Zr-Based Bulk Metallic Glasses. Mater. Sci. Eng. A 2023, 863, 144513. [Google Scholar] [CrossRef]
- Liu, Y.; Padmanabhan, J.; Cheung, B.; Liu, J.; Chen, Z.; Scanley, B.E.; Wesolowski, D.; Pressley, M.; Broadbridge, C.C.; Altman, S.; et al. Combinatorial Development of Antibacterial Zr-Cu-Al-Ag Thin Film Metallic Glasses. Sci. Rep. 2016, 6, 26950. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Hsu, K.C.; Chan, Y.C.; Duh, J.G.; Lee, J.W.; Jang, J.S.C.; Chen, G.J. Antimicrobial Properties of Zr-Cu-Al-Ag Thin Film Metallic Glass. Thin Solid Films 2014, 561, 98–101. [Google Scholar] [CrossRef]
- Qin, L.; Du, W.; Cipiccia, S.; Bodey, A.J.; Rau, C.; Mi, J. Synchrotron X-ray Operando Study and Multiphysics Modelling of the Solidification Dynamics of Intermetallic Phases under Electromagnetic Pulses. Acta Mater. 2024, 265, 119593. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Long, C.; Wu, L.; Zhou, C.; Zhang, X. Synthesis of Nano Zirconium Oxide and Its Application in Dentistry. Nanotechnol. Rev. 2019, 8, 396–404. [Google Scholar] [CrossRef]
- Zhang, N.; Xia, C.; Qin, J.; Li, Q.; Zhang, X.; Liu, R. Research Progress of Novel Zirconium Alloys with High Strength and Toughness. J. Met. Mater. Miner. 2022, 32, 23–36. [Google Scholar] [CrossRef]
- Zhang, E.L.; Fu, S.; Wang, R.X.; Li, H.X.; Liu, Y.; Ma, Z.Q.; Liu, G.K.; Zhu, C.S.; Qin, G.W.; Chen, D.F. Role of Cu Element in Biomedical Metal Alloy Design. Rare Met. 2019, 38, 476–494. [Google Scholar] [CrossRef]
- Lacerda, D.; Barbosa De Souza, J.; Bueno, E.V.; Meridiana, S.; Ramos, F.; Medeiros, S.; Dillion, I.; Cavalcanti, L.; Macário, I.; Cavalcanti, F. Antibacterial and Antibiofilm Potential of Silver Nanoparticles against Antibiotic-Sensitive and Multidrug-Resistant Pseudomonas Aeruginosa Strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar]
- Rosalbino, F.; Macciò, D.; Scavino, G. Corrosion Behaviour of Zr-Ag Alloys for Dental Implant Application. Mater. Sci. Appl. 2023, 14, 501–514. [Google Scholar] [CrossRef]
- Bosetti, M.; Massè, A.; Tobin, E.; Cannas, M. Silver Coated Materials for External Fixation Devices: In Vitro Biocompatibility and Genotoxicity. Biomaterials 2002, 23, 887–892. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of New Metallic Alloys for Biomedical Applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Kisnieriene, V.; Lapeikaite, I. When Chemistry Meets Biology: The Case of Aluminium—A Review. Chemija 2015, 26, 148–158. [Google Scholar]
- Jiang, X.J.; Zhang, Y.Y.; Li, C.L.; Liang, G.D.; Han, R.H.; Zhang, X.Y. Microstructure and Mechanical Properties of ZrAl Binary Alloys. J. Alloys Compd. 2019, 811, 152068. [Google Scholar] [CrossRef]
- Prabhu, Y.; Vincent, S.; Manulal, S.; Nair, A.; Bhatt, J. Cu-Zr-Ti-Al Metallic Glass: Thermodynamic Prediction, Synthesis, and Biocorrosion Studies. Phys. B Condens. Matter 2021, 609, 412918. [Google Scholar] [CrossRef]
- Roman, A.-M.; Voiculescu, I.; Cimpoesu, R.; Istrate, B.; Chelariu, R.; Cimpoesu, N.; Zegan, G.; Panaghie, C.; Lohan, N.M.; Axinte, M.; et al. Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy. Crystals 2023, 13, 109. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, W.; Inoue, A. Influence of Al and Ag on the Devitrification Behavior of a Cu-Zr Glassy Alloy. Mater. Trans. 2007, 48, 2128–2132. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Li, S.; Zhang, Q.; Zhang, W.; Suryanarayana, C.; Inoue, A. Glass-Forming Ability and Differences in the Crystallization Behavior of Ribbons and Rods of Cu36Zr48Al8Ag8 Bulk Glass-Forming Alloy. J. Mater. Res. 2009, 24, 1886–1895. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, Q.; Suryanarayana, C.; Inoue, A. Formation, Structure, and Crystallization Behavior of Cu-Based Bulk Glass-Forming Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 1664–1669. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Kou, H.; Li, J.; Zhang, P. Phase Separation and Microstructure Evolution of Zr48Cu36Ag8Al8 Bulk Metallic Glass in the Supercooled Liquid Region. Rare Met. Mater. Eng. 2016, 45, 567–570. [Google Scholar] [CrossRef]
- Altounian, Z.; Guo-hua, T.; Ström-Olsen, J.O.; Muir, W.B. Crystallization of Amorphous CuZr2. Jpn. J. Appl. Phys. 1981, 24, 505–509. [Google Scholar] [CrossRef]
- Nie, L.; Zhan, Y.; Liu, H.; Tang, C. In Situ Synthesized Low Modulus Biomedical Zr-4Cu-XNb Alloys. Mater. Sci. Eng. C 2013, 33, 5105–5108. [Google Scholar] [CrossRef]
- Jia, Z.H.; Couzinie, J.P.; Cherdoudi, N.; Guillot, I.; Arnberg, L.; Asholt, P.; Brusethaug, S.; Barlas, B.; Massinon, D. Precipitation Behaviour of Al3Zr Precipitate in Al-Cu-Zr and Al-Cu-Zr-Ti-V Alloys. Trans. Nonferrous Met. Soc. China 2012, 22, 1860–1865. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Dispersoid Precipitation and Process Modelling in Zirconium Containing Commercial Aluminum Alloys. Acta Mater. 2001, 49, 599–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Glass Forming Properties of Zr-Based Bulk Metallic Alloys. J. Non. Cryst. Solids 2003, 315, 206–210. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Formation and Mechanical Strength of Bulk Glassy Alloys in Zr-Al-Co-Cu System. Mater. Trans. 2002, 43, 1250–1253. [Google Scholar] [CrossRef][Green Version]
- Lyubenova, L.; Rangelova, V.; Spassova, M.; Spassov, T. Glass Forming Ability of Zr-Based Zr–Cu–Ni–Al–(Ag) Alloys. J. Therm. Anal. Calorim. 2023, 148, 3975–3980. [Google Scholar] [CrossRef]
- Gallego, L.J.; Somoza, J.A.; Alonso, J.A. Glass Formation in Ternary Transition Metal Alloys. J. Phys. Condens. Matter 1990, 2, 6245–6250. [Google Scholar] [CrossRef]
- Miedema, A.R.; de Châtel, P.F.; de Boer, F.R. Cohesion in Alloys-Fundamentals of a Semi-Empirical Model. Phys. B+C 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Mansori, G.A.; Carnahan, N.F.; Starling, K.E.; Leland, T. W, Jr. Equilibrium Thermodynamic Properties of the Mixture of Hard Spheres. J. Chem. Phys. 1971, 54, 1523–1525. [Google Scholar] [CrossRef]
- Vincent, S.; Peshwe, D.R.; Murty, B.S.; Bhatt, J. Thermodynamic Prediction of Bulk Metallic Glass Forming Alloys in Ternary Zr-Cu-X (X = Ag, Al, Ti, Ga) Systems. J. Non. Cryst. Solids 2011, 357, 3495–3499. [Google Scholar] [CrossRef]
- Bhatt, J.; Dey, G.K.; Murty, B.S. Thermodynamic and Topological Modeling and Synthesis of Cu-Zr-Ti-Ni-Based Bulk Metallic Glasses by Mechanical Alloying. Metall. Mater. Trans. A 2008, 39, 1543–1551. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Khond, A.A.; Bhatt, J.G.; Palikundwar, U.A. Thermodynamic and Kinetic Studies of Glass-Forming Compositions in Ca–Mg–Cu Ternary Metallic Glasses. Glass Phys. Chem. 2023, 49, 604–616. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Xie, G.; Inoue, A. Glass-Forming Ability and Mechanical Properties of the Ternary Cu-Zr-Al and Quaternary Cu-Zr-Al-Ag Bulk Metallic Glasses. Mater. Trans. 2007, 48, 1626–1630. [Google Scholar] [CrossRef]
- Jiang, Q.K.; Wang, X.D.; Nie, X.P.; Zhang, G.Q.; Ma, H.; Fecht, H.J.; Bendnarcik, J.; Franz, H.; Liu, Y.G.; Cao, Q.P.; et al. Zr-(Cu,Ag)-Al Bulk Metallic Glasses. Acta Mater. 2008, 56, 1785–1796. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Fan, H.; Wang, Y.; Ning, Z.; Liu, F.; Feng, D.; Jin, X.; Shen, J.; Sun, J.; et al. In Vitro and in Vivo Biocompatibility of an Ag-Bearing Zr-Based Bulk Metallic Glass for Potential Medical Use. J. Non. Cryst. Solids 2015, 419, 82–91. [Google Scholar] [CrossRef]
- Tian, F.; Qu, J.W.; Shi, M.H.; Li, B.S.; Li, J. Study on Effects of Cu Content on Microstructure and Corrosion Resistance of Zr-Nb Alloys. J. Phys. Conf. Ser. 2023, 2539, 012010. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Cheng, X.; Fan, H.; Sun, Y.; Ning, Z.; Cao, F.; Sun, J. Biocompatibility of a Micro-Arc Oxidized ZrCuAlAg Bulk Metallic Glass. J. Mater. Res. Technol. 2021, 13, 486–497. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Kou, L.; Zhang, P.; Du, J.; Liu, H.; Shang, X. Corrosion pit-induced stress concentration in 7005 aluminium alloy: Mechanical degradation and pit parameter analysis. Eng. Fract. Mech. 2024, 301, 110024. [Google Scholar] [CrossRef]
- Mudali, U.K.; Baunack, S.; Eckert, J.; Schultz, L.; Gebert, A. Pitting Corrosion of Bulk Glass-Forming Zirconium-Based Alloys. J. Alloys Compd. 2004, 377, 290–297. [Google Scholar] [CrossRef]
- Kawashima, A.; Ohmura, K.; Yokoyama, Y.; Inoue, A. The corrosion behaviour of Zr-based bulk metallic glasses in 0.5 M NaCl solution. Corros. Sci. 2011, 53, 2778–2784. [Google Scholar] [CrossRef]
- Green, B.A.; Meyer, H.M.; Benson, R.S.; Yokoyama, Y.; Liaw, P.K.; Liu, C.T. A study of the corrosion behaviour of Zr50Cu(40−X)Al10PdX bulk metallic glasses with scanning Auger microanalysis. Corros. Sci. 2008, 50, 1825–1832. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Schultz, L. Effect of surface finishing of a Zr-based bulk metallic glass on its corrosion behaviour. Corros. Sci. 2010, 52, 1711–1720. [Google Scholar] [CrossRef]
- Adamson, R.; Garzarolli, F.; Cox, B.; Strasser, A.; Rudling, P. Corrosion Mechanisms in Zirconium Alloys. In ZIRAT12 Special Topic Report; A.N.T. International: Mölnlycke, Sweden, 2007. [Google Scholar]
- Bell, B.D.C.; Murphy, S.T.; Burr, P.A.; Comstock, R.J.; Partezana, J.M.; Grimes, R.W.; Wenman, M.R. The Influence of Alloying Elements on the Corrosion of Zr Alloys. Corros. Sci. 2016, 105, 36–43. [Google Scholar] [CrossRef]
- Wen, S.; Dai, C.; Mao, W.; Zhao, Y.; Han, G.; Wang, X. Effects of Ag and Co Microalloying on Glass-Forming Abilities and Plasticity of Cu-Zr-Al Based Bulk Metallic Glasses. Mater. Des. 2022, 220, 110896. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, X.J.; Shao, M.; Wang, L.N.; Yang, J.L.; Gao, L.P.; Chen, L.Y.; Liu, C.X. Wear Behavior of a Series of Zr-Based Bulk Metallic Glasses. Mater. Sci. Eng. A 2008, 475, 124–127. [Google Scholar] [CrossRef]
- Umeda, J.; Nishimura, N.; Fujii, H.; Jia, L.; Kondoh, K. In-Situ Formed Al3Zr Compounds Reinforced Al Composites and Tribological Application. Crystals 2021, 11, 227. [Google Scholar] [CrossRef]




| Alloy | Zr | Cu | Al | Ag |
|---|---|---|---|---|
| Zr42.42Cu41.18Al9.35Ag7.05 | 44.02 | 35.49 | 12.28 | 8.22 |
| Zr46.81Cu35.44Al10.09Ag7.66 | 46.87 | 30.71 | 12.86 | 9.55 |
| Alloy | Sample | EOCP [V] (±0.01) | Ecorr [V] (±0.01) | Rp [kΩ·cm2] (±0.1) | jcorr [μA·cm−2] (±0.1) |
|---|---|---|---|---|---|
| Zr42.42Cu41.18Al9.35Ag7.05 | ingot | −0.427 | −0.390 | 1.49 | 3.52 |
| ribbon | −0.396 | −0.350 | 2.18 | 1.79 | |
| Zr46.81Cu35.44Al10.09Ag7.66 | ingot | −0.432 | −0.391 | 0.97 | 3.81 |
| ribbon | −0.353 | −0.317 | 5.31 | 1.09 |
| Point Number | Figure | Zr | Cu | Al | Ag | Na | Cl | K | Ca | O |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8b | 7.45 | 13.48 | 6.05 | 1.93 | 0.87 | 2.34 | 0.08 | 0.07 | 67.72 |
| 2 | 8b | 8.34 | 13.41 | 6.92 | 2.02 | 1.71 | 0.45 | - | - | 67.15 |
| 3 | 8b | 36.77 | 17.86 | 16.02 | 1.96 | 1.22 | - | - | - | 26.17 |
| 4 | 8d | 34.6 | 16.49 | 14.96 | 2.09 | 0.95 | - | - | - | 30.90 |
| 5 | 8d | 13.85 | 58.27 | 1.62 | 3.05 | 0.48 | 0.67 | - | - | 22.06 |
| 6 | 8d | 25.97 | 33.00 | 8.85 | 2.33 | 0.59 | - | - | - | 29.26 |
| Point Number | Figure | Zr | Cu | Al | Ag | O |
|---|---|---|---|---|---|---|
| 1 | 11b | 10.14 | 8.03 | 2.89 | 2.12 | 76.82 |
| 2 | 11b | 9.85 | 10.23 | 3.06 | 2.31 | 74.56 |
| 3 | 11b | 35.76 | 27.60 | 11.58 | 1.82 | 23.24 |
| 4 | 12b | 14.06 | 8.60 | 3.52 | 3.01 | 70.81 |
| 5 | 12b | 38.72 | 19.69 | 14.56 | 3.33 | 23.69 |
| 6 | 12b | 32.43 | 23.32 | 7.67 | 7.85 | 28.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babilas, R.; Młynarek-Żak, K.; Kania, A.; Deshmukh, A.A.; Warski, T.; Hawełek, Ł. Structure and Corrosion Behavior of Multiphase Intermetallic ZrCu-Based Alloys. Materials 2024, 17, 4182. https://doi.org/10.3390/ma17174182
Babilas R, Młynarek-Żak K, Kania A, Deshmukh AA, Warski T, Hawełek Ł. Structure and Corrosion Behavior of Multiphase Intermetallic ZrCu-Based Alloys. Materials. 2024; 17(17):4182. https://doi.org/10.3390/ma17174182
Chicago/Turabian StyleBabilas, Rafał, Katarzyna Młynarek-Żak, Aneta Kania, Akash A. Deshmukh, Tymon Warski, and Łukasz Hawełek. 2024. "Structure and Corrosion Behavior of Multiphase Intermetallic ZrCu-Based Alloys" Materials 17, no. 17: 4182. https://doi.org/10.3390/ma17174182
APA StyleBabilas, R., Młynarek-Żak, K., Kania, A., Deshmukh, A. A., Warski, T., & Hawełek, Ł. (2024). Structure and Corrosion Behavior of Multiphase Intermetallic ZrCu-Based Alloys. Materials, 17(17), 4182. https://doi.org/10.3390/ma17174182







