Adsorption Technology for PFAS Removal in Water: Comparison between Novel Carbonaceous Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
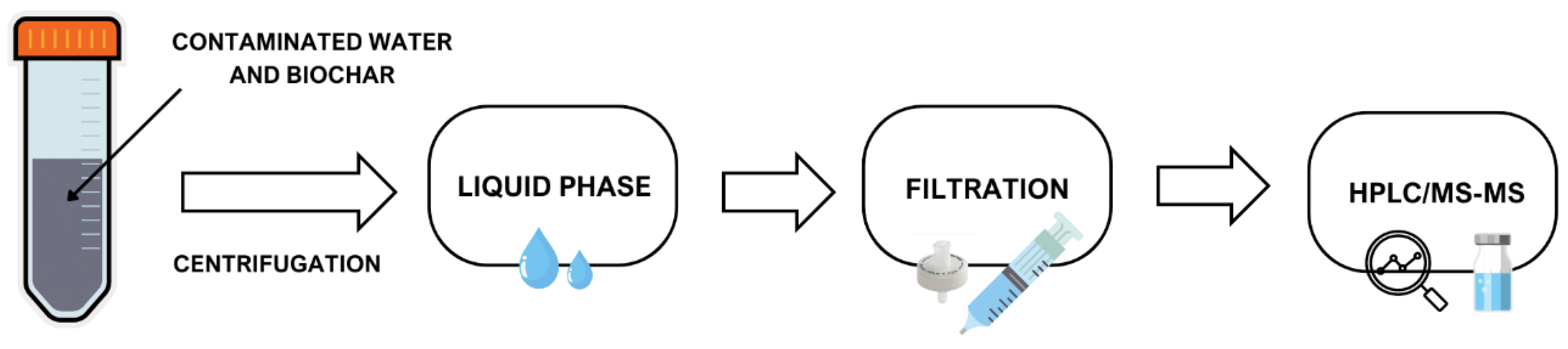
2.2. Batch Adsorption Tests
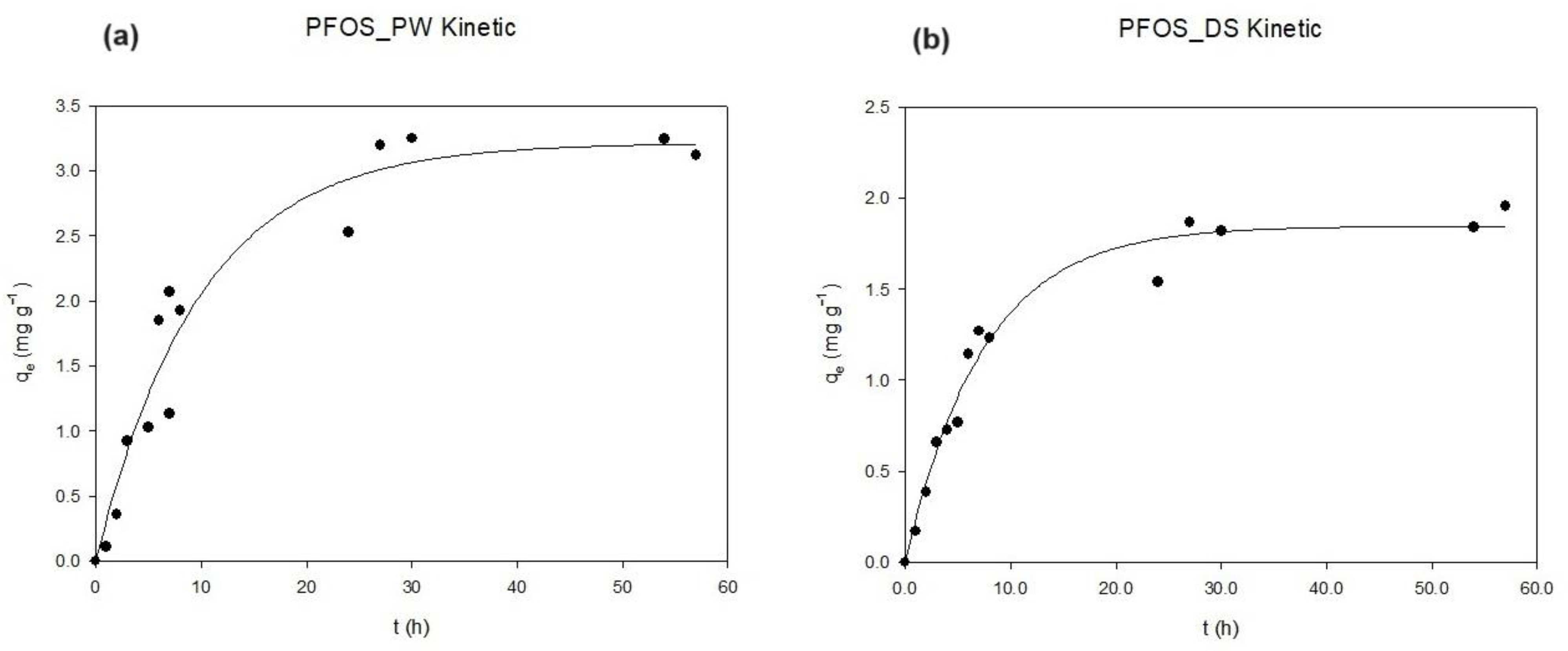
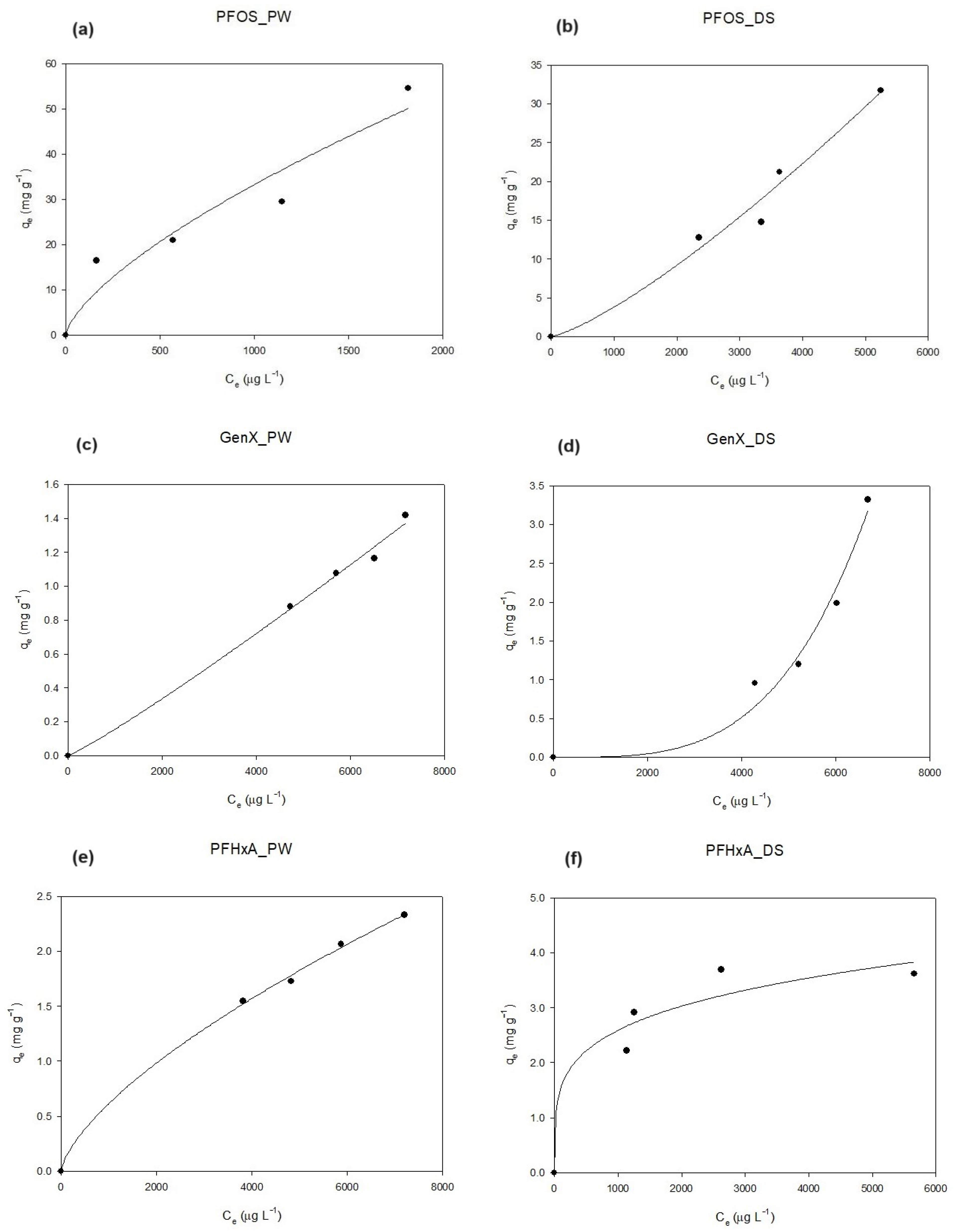
2.3. Equilibrium and Kinetic Modeling
2.4. HPLC/MS-MS
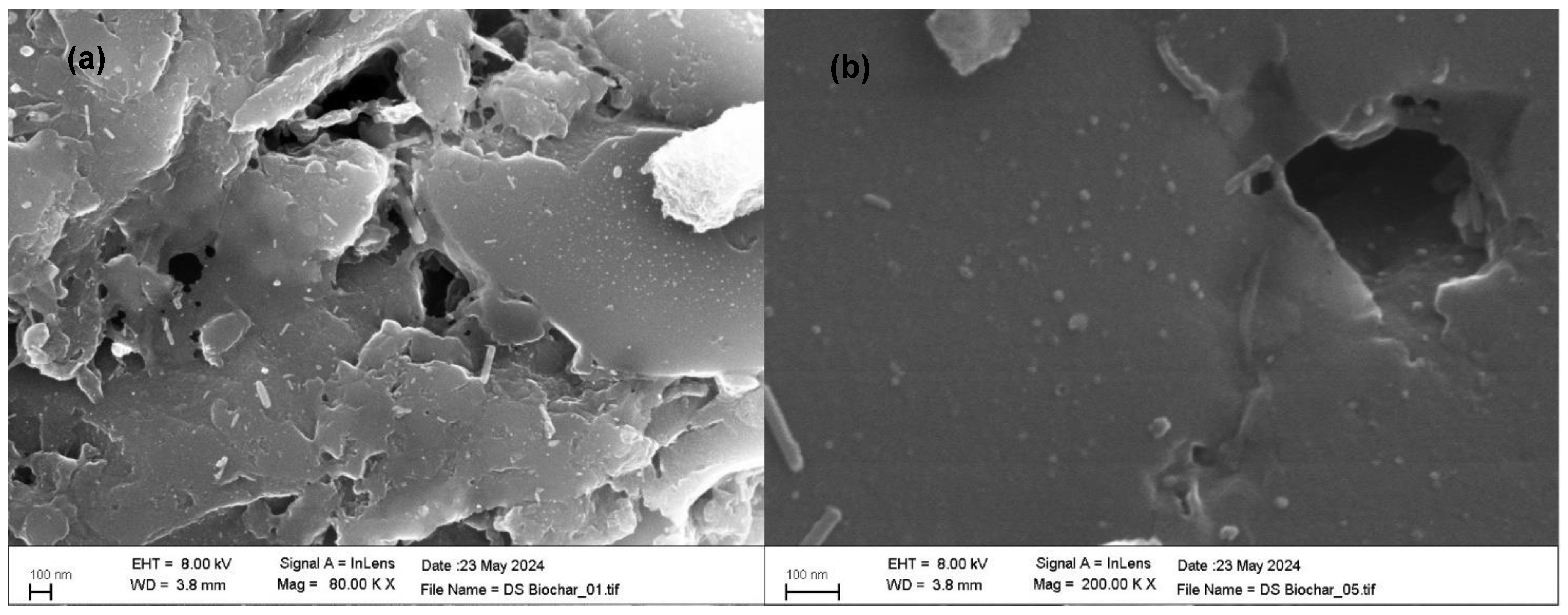
2.5. Textural Characterization and Morphology
3. Results and Discussion
3.1. Adsorbent Characterization of Date Biochar
3.2. Kinetic Tests
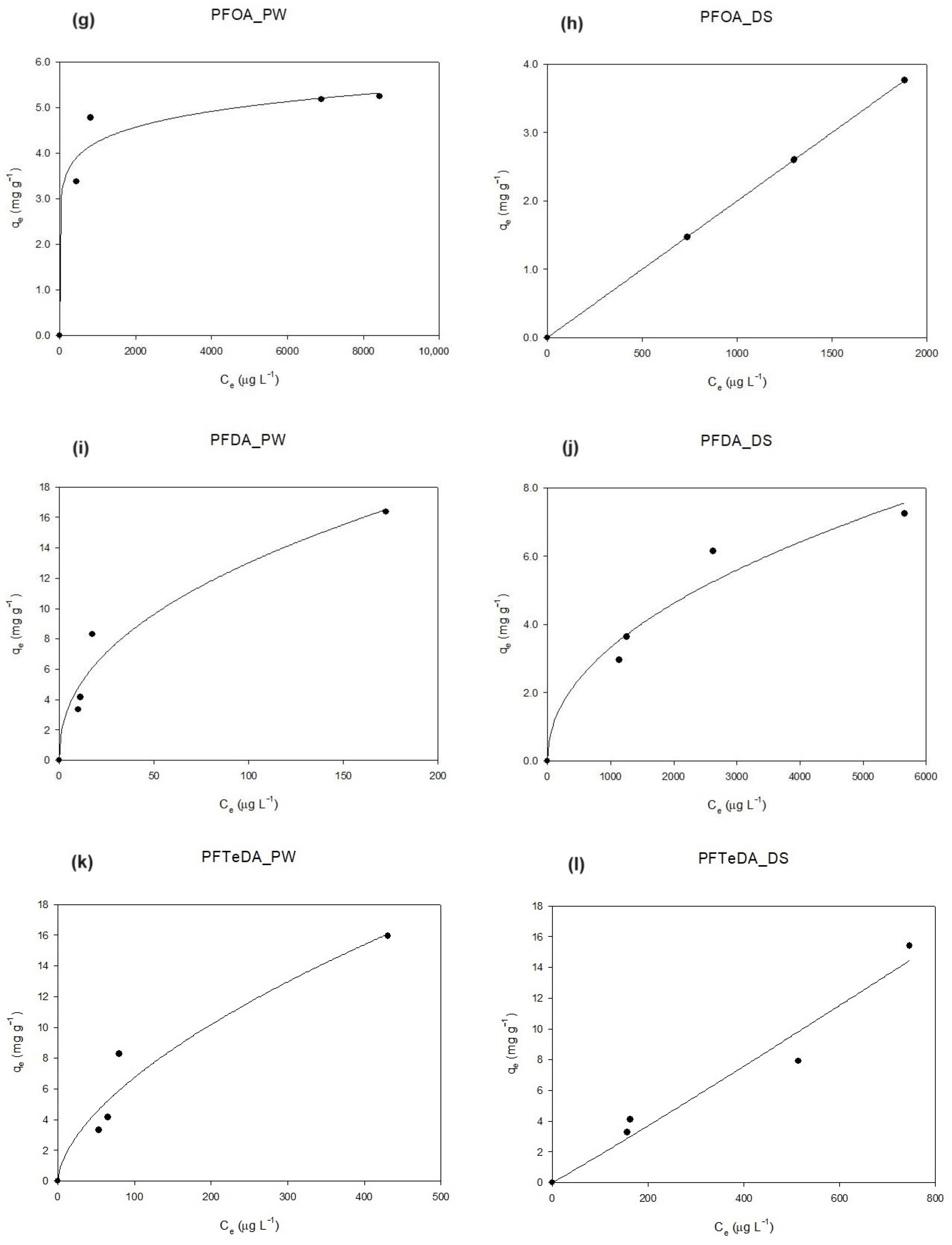
3.3. Isotherm Curves: Pinewood vs. Dates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- European Chemical Agency (ECHA). Available online: https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (accessed on 1 July 2024).
- Ng, C.A.; Hungerbühler, K. Bioconcentration of perfluorinated alkyl acids: How important is specific binding? Environ. Sci. Technol. 2013, 47, 7214–7223. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Sciences NIEHS. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). Available online: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm#footnote (accessed on 4 July 2024).
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination endocrine-mediated effects and disease. Toxicology 2021, 465, 153031. [Google Scholar] [CrossRef]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. Int. J. Environ. Res. Public Health 2015, 12, 6098–6114. [Google Scholar] [CrossRef]
- Liu, G.; Dhana, K.; Furtado, J.D.; Rood, J.; Zong, G.; Liang, L.; Qi, L.; Bray, G.A.; DeJong, L.; Coull, B.; et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018, 15, e1002502. [Google Scholar] [CrossRef]
- Bach, C.C.; Vested, A.; Jorgensen, K.; Bonde, J.P.; Henriksen, T.B.; Toft, G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: A systematic review. Crit. Rev. Toxicol. 2016, 46, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Ji, Y.; Zhang, B.; Zheng, X.; Brusseau, M.L. Transport of GenX in Saturated and Unsaturated Porous Media. Environ. Sci. Technol. 2020, 54, 11876–11885. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Sun, Y.; Guo, Y.; Dai, J. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ. Sci. Technol. 2018, 52, 7621–7629. [Google Scholar] [CrossRef]
- Brandsma, S.H.; Koekkoek, J.C.; van Velzen, M.J.M.; de Boer, J. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands. Chemosphere 2019, 220, 493–500. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Fact Sheet: Draft Toxicity Assessments for GenX Chemicals and PFBS; Environmental Protection Agency (EPA): Washington, DC, USA. Available online: https://www.epa.gov/chemical-research/human-health-toxicity-assessments-genx-chemicals (accessed on 1 July 2024).
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. Environ. Monit. Assess. 2011, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency (EPA). Drinking Water. Standards and Health Advisories; EPA 822-F-18-001; Environmental Protection Agency (EPA): Washington, DC, USA. Available online: https://www.epa.gov/sdwa/drinking-water-health-advisories-has (accessed on 1 July 2024).
- Environmental Protection Agency (EPA). Fact Sheet: PFAS National Primary Drinking Water Regulation. Available online: https://dep.nj.gov/pfas/epa-pfas-rule/ (accessed on 1 July 2024).
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediation 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Kewalramani, J.A.; Li, B.; Marsh, R.W. A Review of the Applications Environmental Release and Remediation Technologies of Per- and Polyfluoroalkyl Substances. Int. J. Environ. Res. Public Health 2020, 17, 8117. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.M.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Ostlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE). Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Liu, C.J.; David, W.; Bellona, C. Removal of per- and polyfluoroalkyl substances (PFASs) from contaminated groundwater using granular activated carbon: A pilot-scale study with breakthrough modeling. Environ. Sci. Water Res. Technol. 2019, 5, 1844–1853. [Google Scholar] [CrossRef]
- Najm, I.; Gallagher, B.; Vishwanath, N.; Blute, N.; Gorzalski, A.; Feffer, A.; Richardson, S. Per- and polyfluoroalkyl substances removal with granular activated carbon and a specialty adsorbent: A case study. AWWA Water Sci. 2021, 3, e1245. [Google Scholar] [CrossRef]
- Zhang, D.; He, Q.; Wang, M.; Zhang, W.; Liang, Y. Sorption of perfluoroalkylated substances (PFASs) onto granular activated carbon and biochar. Environ. Technol. 2019, 42, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Aboughaly, M.; Fattah, I. Production of Biochar from Biomass Pyrolysis for Removal of PFAS from Wastewater and Biosolids: A Critical Review. Preprints 2023, 2023040309. [Google Scholar] [CrossRef]
- Rossi, M.M.; Silvani, L.; Amanat, N.; Petrangeli Papini, M. Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal. Materials 2021, 14, 1776. [Google Scholar] [CrossRef]
- Silvani, L.; Vrchotova, B.; Kastanek, P.; Demnerova, K.; Pettiti, I.; Papini, M.P. Characterizing Biochar as Alternative Sorbent for Oil Spill Remediation. Sci. Rep. 2017, 7, 43912. [Google Scholar] [CrossRef]
- Sonego, E.; Simonetti, G.; Di Filippo, P.; Riccardi, C.; Buiarelli, F.; Fresta, A.; Olivastri, M.; Pomata, D. Characterization of organophosphate esters (OPEs) and polyfluoralkyl substances (PFASs) in settled dust in specific workplaces. Environ. Sci. Pollut. Res. 2022, 29, 52302–52316. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances—I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. T Method J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Gurvitsch, L. Physicochemical attractive force. Russ. J. Phys. Chem. 1915, 47, 805–827. [Google Scholar]
- Maziarka, P.; Sommersacher, P.; Wang, X.; Kienzl, N.; Retschitzegger, S.; Prins, W.; Hedin, N.; Ronsse, F. Tailoring of the pore structures of wood pyrolysis chars for potential use in energy storage applications. Appl. Energy 2021, 286, 116431. [Google Scholar] [CrossRef]
- Libby, B.; Monson, P.A. Adsorption/desorption hysteresis in inkbottle pores: A density functional theory and Monte Carlo simulation study. Langmuir 2004, 20, 4289–4294. [Google Scholar] [CrossRef]
- Fan, C.; Do, D.D.; Nicholson, D. On the Cavitation and Pore Blocking in Slit-Shaped Ink-Bottle Pores. Langmuir 2011, 27, 3511–3526. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches engineering applications challenges and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Q.; Gao, B.; Chiang, S.Y.; Woodward, D.; Huang, Q. Sorption of perfluorooctanoic acid; perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 2015, 144, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, G.A. Coordinated trifluoromethanesulfonate and fluorosulfate. Chem. Rev. 1986, 86, 17–33. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Ziwen, D.; Shubo, D.; Yue, B.; Qian, H.; Bin, W.; Jun, H.; Gang, Y. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Luft, C.M.; Schutt, T.C.; Shukla, M.K. Properties and Mechanisms for PFAS Adsorption to Aqueous Clay and Humic Soil Components. Environ. Sci. Technol. 2022, 56, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Dickenson, E.R.V. The use of carbon adsorbents for the removal of perfluoroalkyl acids from potable reuse systems. Chemosphere 2017, 184, 168–175. [Google Scholar] [CrossRef]
- Hansen, M.C.; Børresen, M.H.; Schlabach, M.; Cornelissen, G. Sorption of perfluorinated compounds from contaminated water to activated carbon. J. Soil Sediments 2010, 10, 179–185. [Google Scholar] [CrossRef]

| Pinewood | ||
| Surface Area (m2 g−1) | Volume Pore (m3 g−1) | |
| Total | 343 ± 2 | 0.383 |
| Micropores | 224 | 0.136 |
| Mesopores | 119 | 0.247 |
| Date Seeds | ||
| Surface Area (m2 g−1) | Volume Pore (m3 g−1) | |
| Total | 290 ± 4 | 0.136 |
| Micropores | 270 | 0.110 |
| Mesopores | 20 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrangeli Papini, M.; Senofonte, M.; Cuzzola, R.A.; Remmani, R.; Pettiti, I.; Riccardi, C.; Simonetti, G. Adsorption Technology for PFAS Removal in Water: Comparison between Novel Carbonaceous Materials. Materials 2024, 17, 4169. https://doi.org/10.3390/ma17174169
Petrangeli Papini M, Senofonte M, Cuzzola RA, Remmani R, Pettiti I, Riccardi C, Simonetti G. Adsorption Technology for PFAS Removal in Water: Comparison between Novel Carbonaceous Materials. Materials. 2024; 17(17):4169. https://doi.org/10.3390/ma17174169
Chicago/Turabian StylePetrangeli Papini, Marco, Marta Senofonte, Riccardo Antonino Cuzzola, Rania Remmani, Ida Pettiti, Carmela Riccardi, and Giulia Simonetti. 2024. "Adsorption Technology for PFAS Removal in Water: Comparison between Novel Carbonaceous Materials" Materials 17, no. 17: 4169. https://doi.org/10.3390/ma17174169
APA StylePetrangeli Papini, M., Senofonte, M., Cuzzola, R. A., Remmani, R., Pettiti, I., Riccardi, C., & Simonetti, G. (2024). Adsorption Technology for PFAS Removal in Water: Comparison between Novel Carbonaceous Materials. Materials, 17(17), 4169. https://doi.org/10.3390/ma17174169








