Kinetics and Mechanism of SiO2 Extraction from Acid-Leached Coal Gangue Residue by Alkaline Hydrothermal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization Techniques
3. Results and Discussion
3.1. XRF Analysis of Acid-Leached Coal Residue
3.2. XRD Analysis of Acid-Leached Coal Gangue Residue
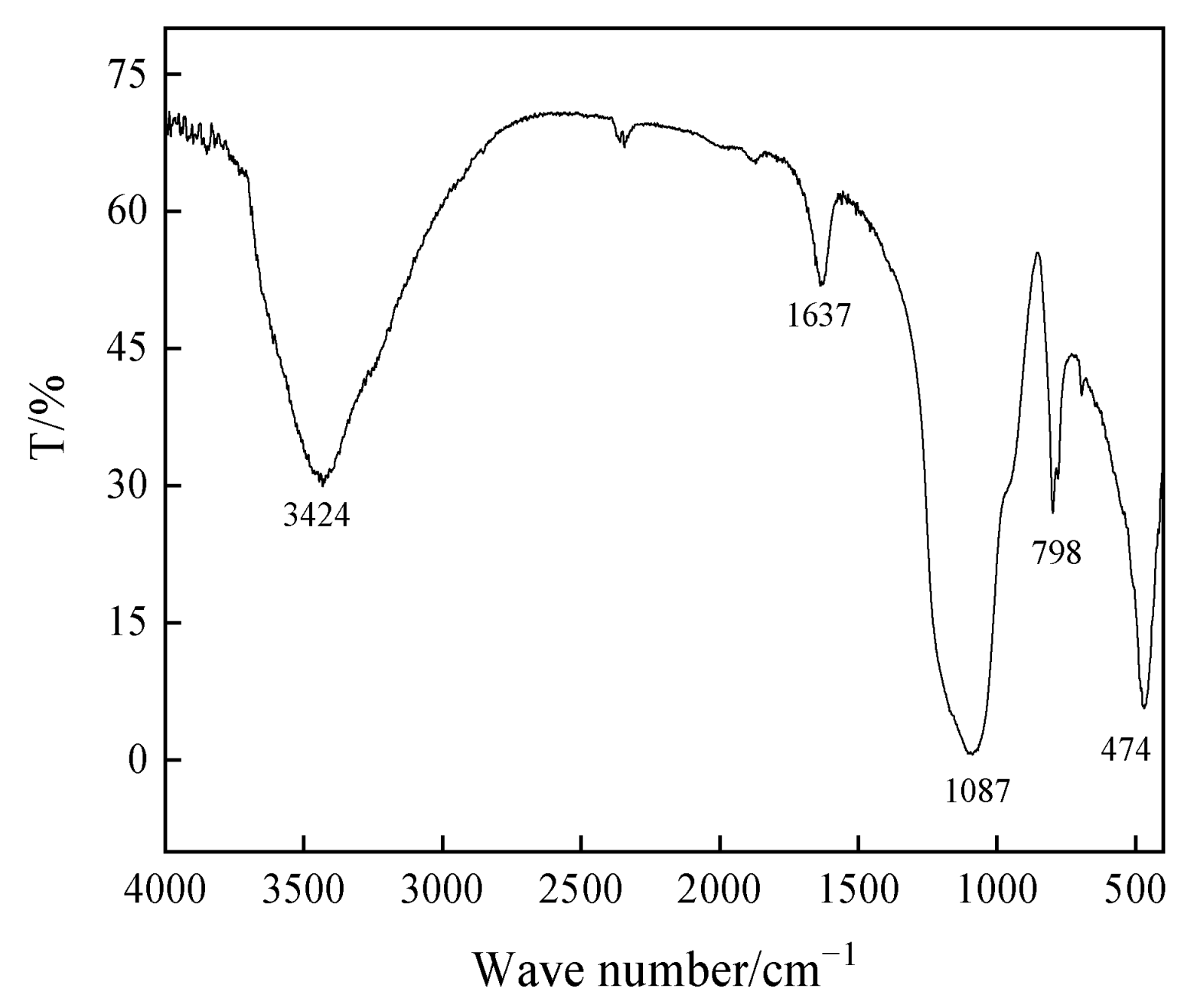
3.3. FT-IR Analysis of Acid-Leached Coal Gangue Residue
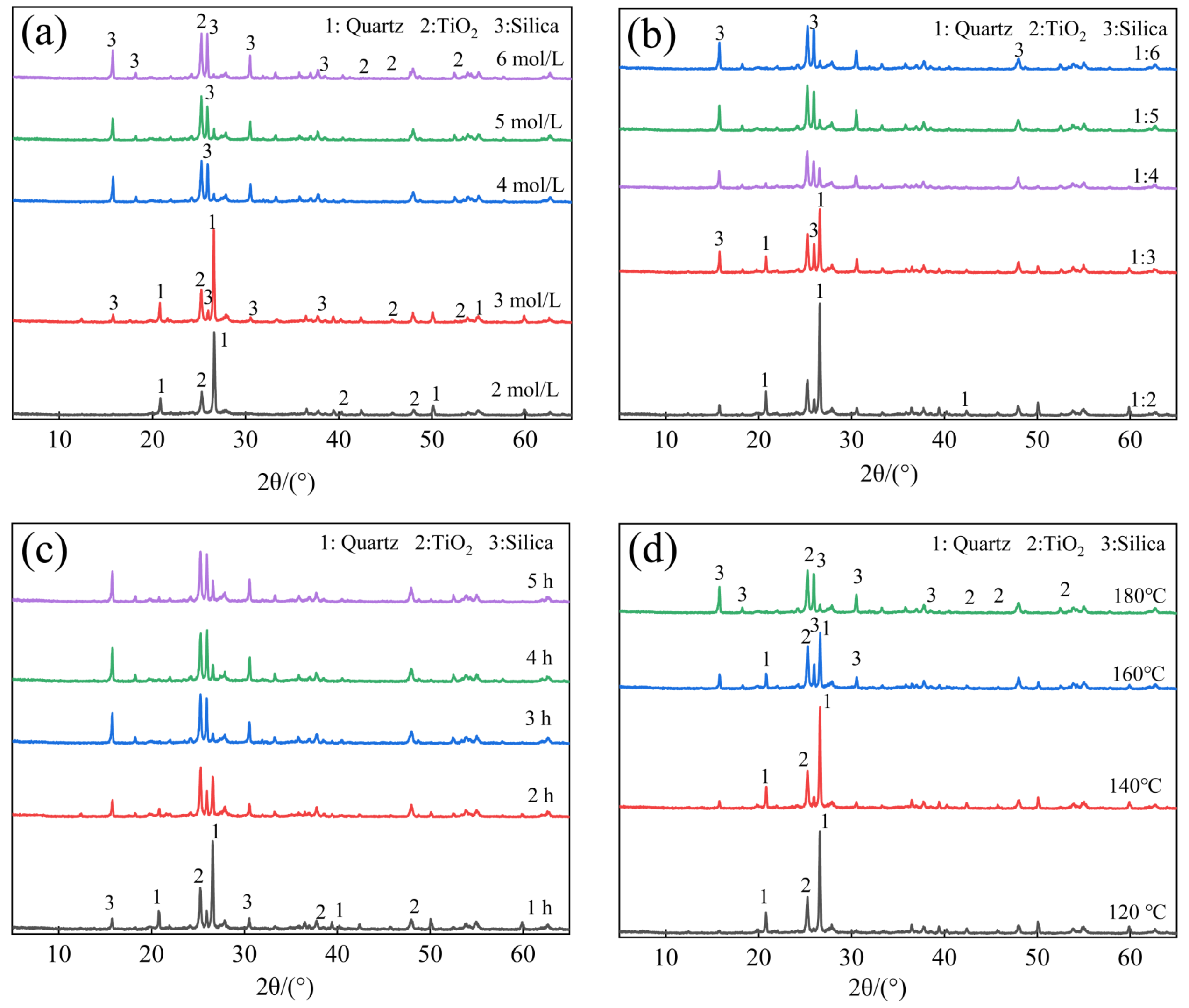
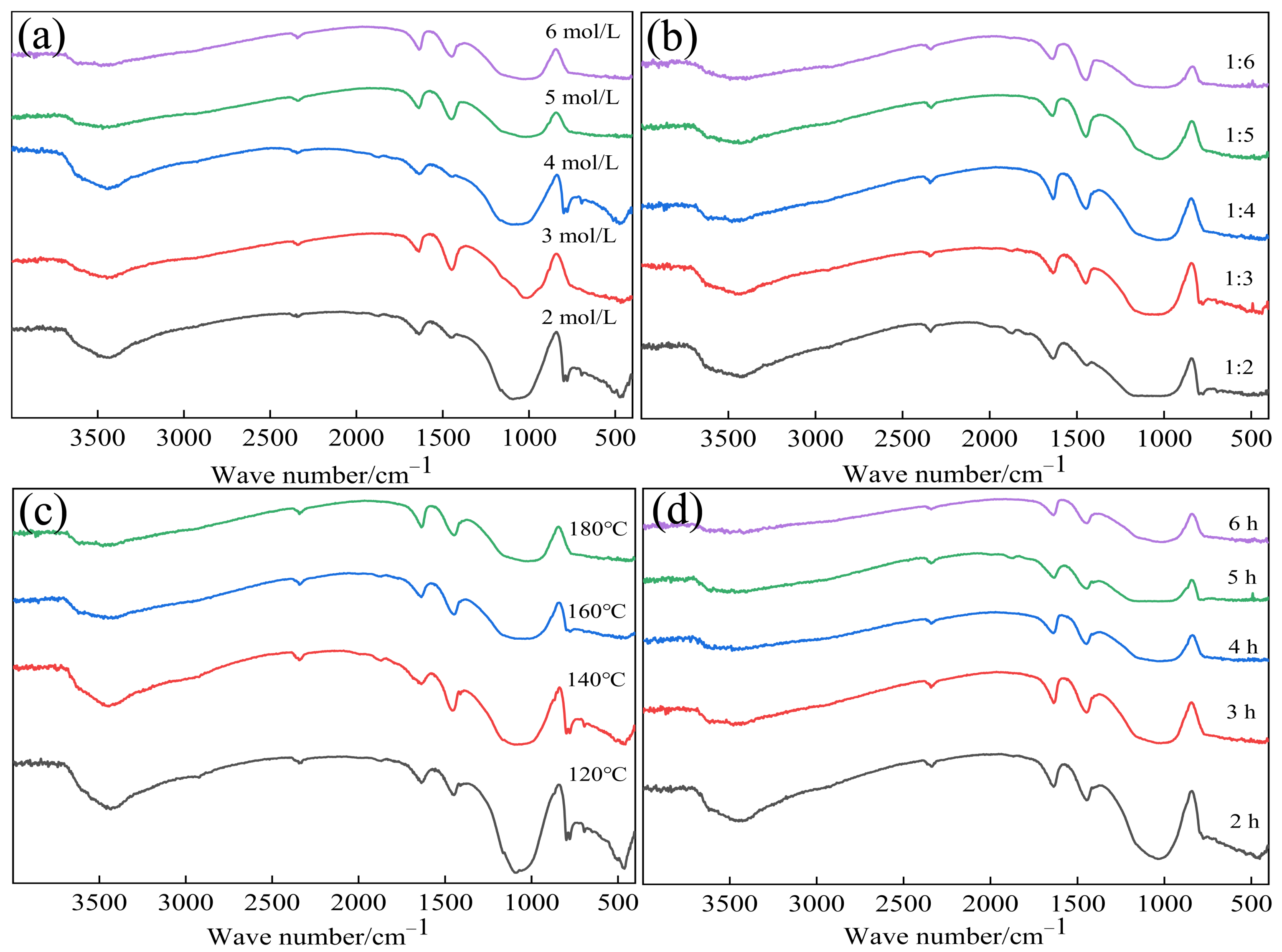
3.4. Effect of Reaction Conditions on SiO2 Extraction
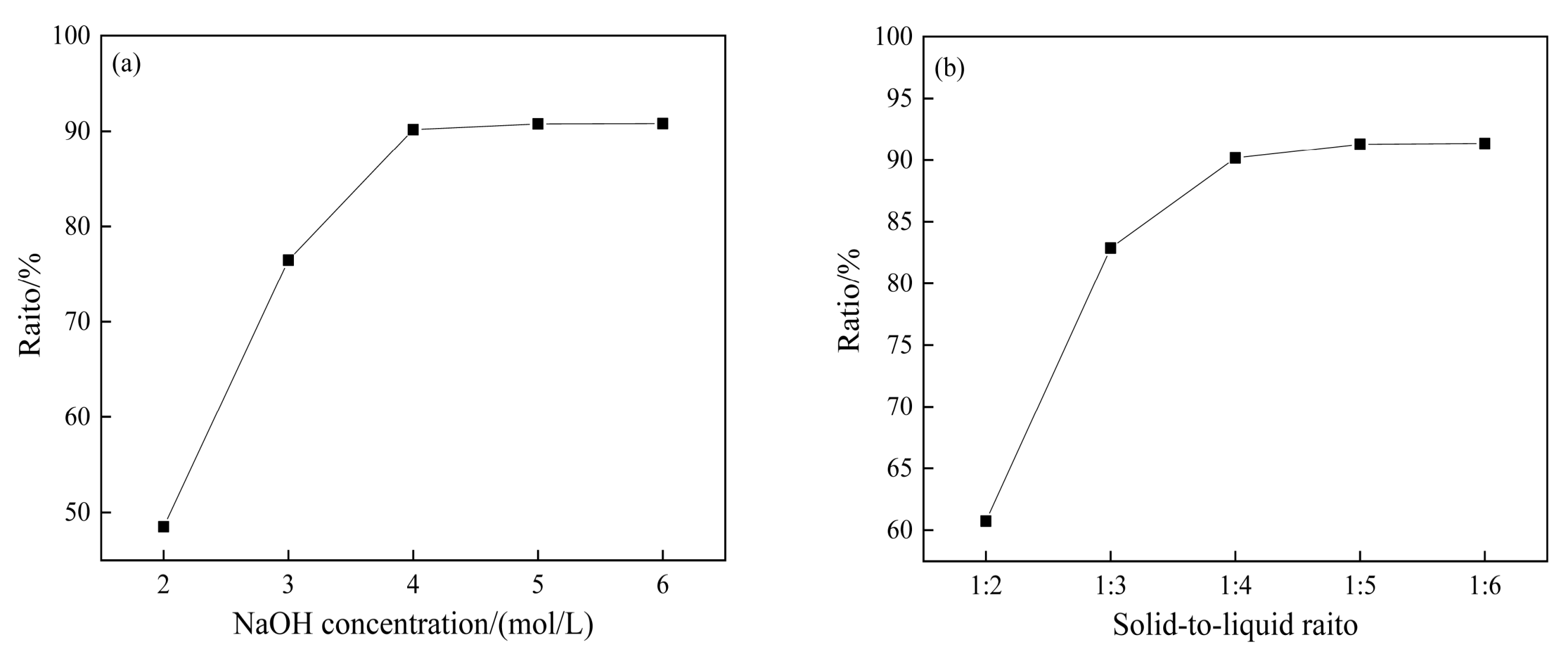
3.4.1. Effect of NaOH Concentration and Solid-to-Liquid Ratio
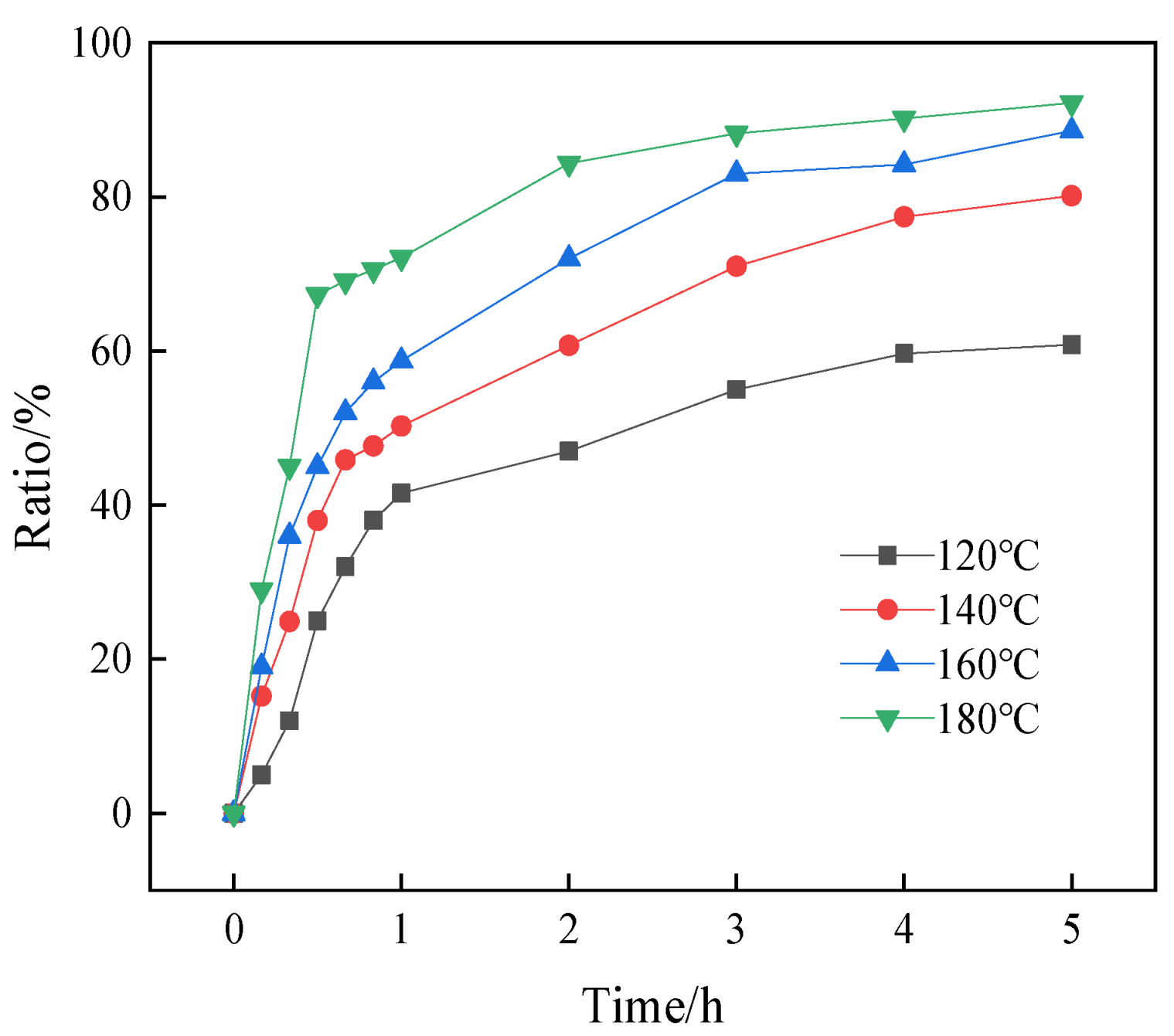
3.4.2. Effect of Reaction Temperature and Time
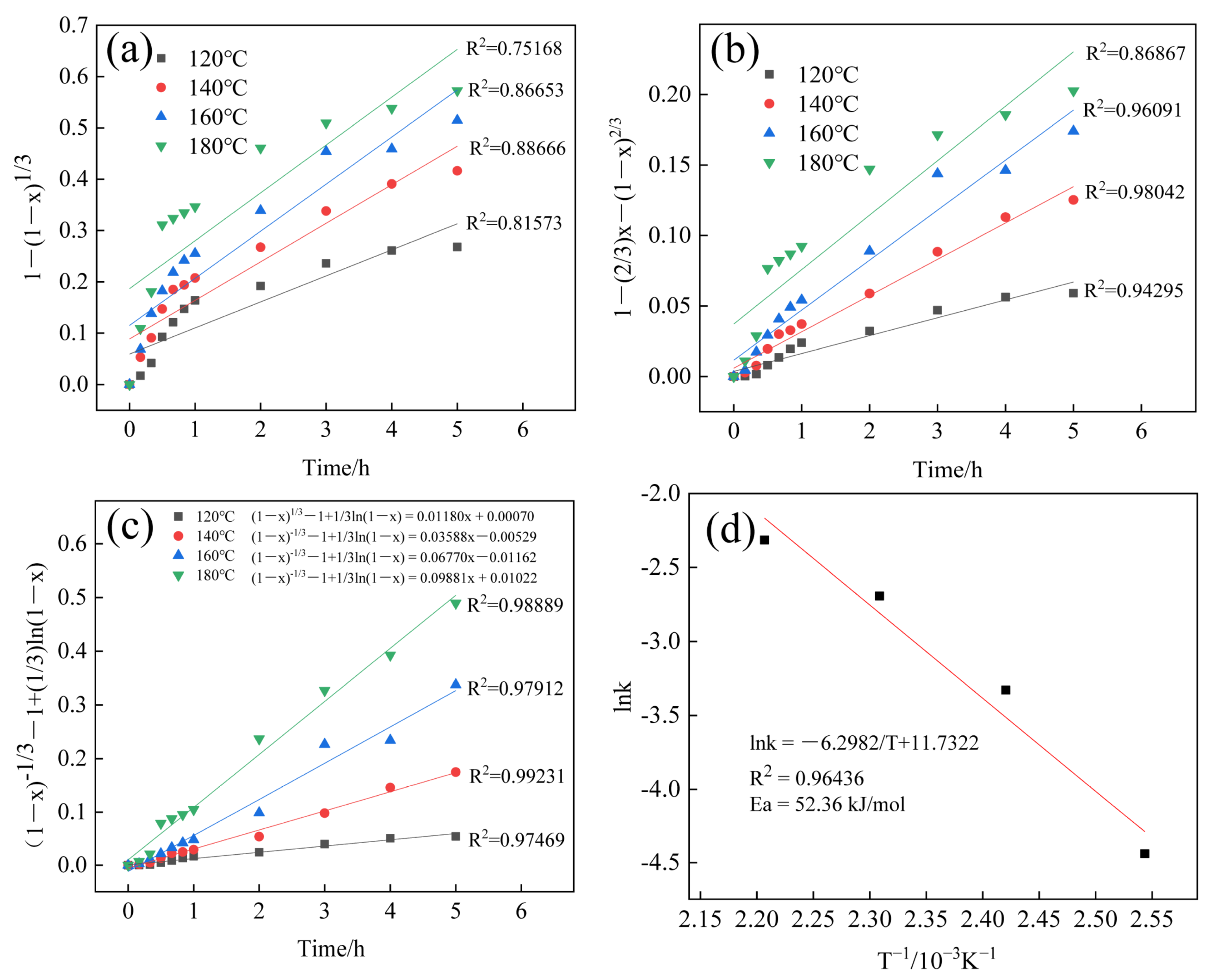
3.5. Kinetics of SiO2 Extraction
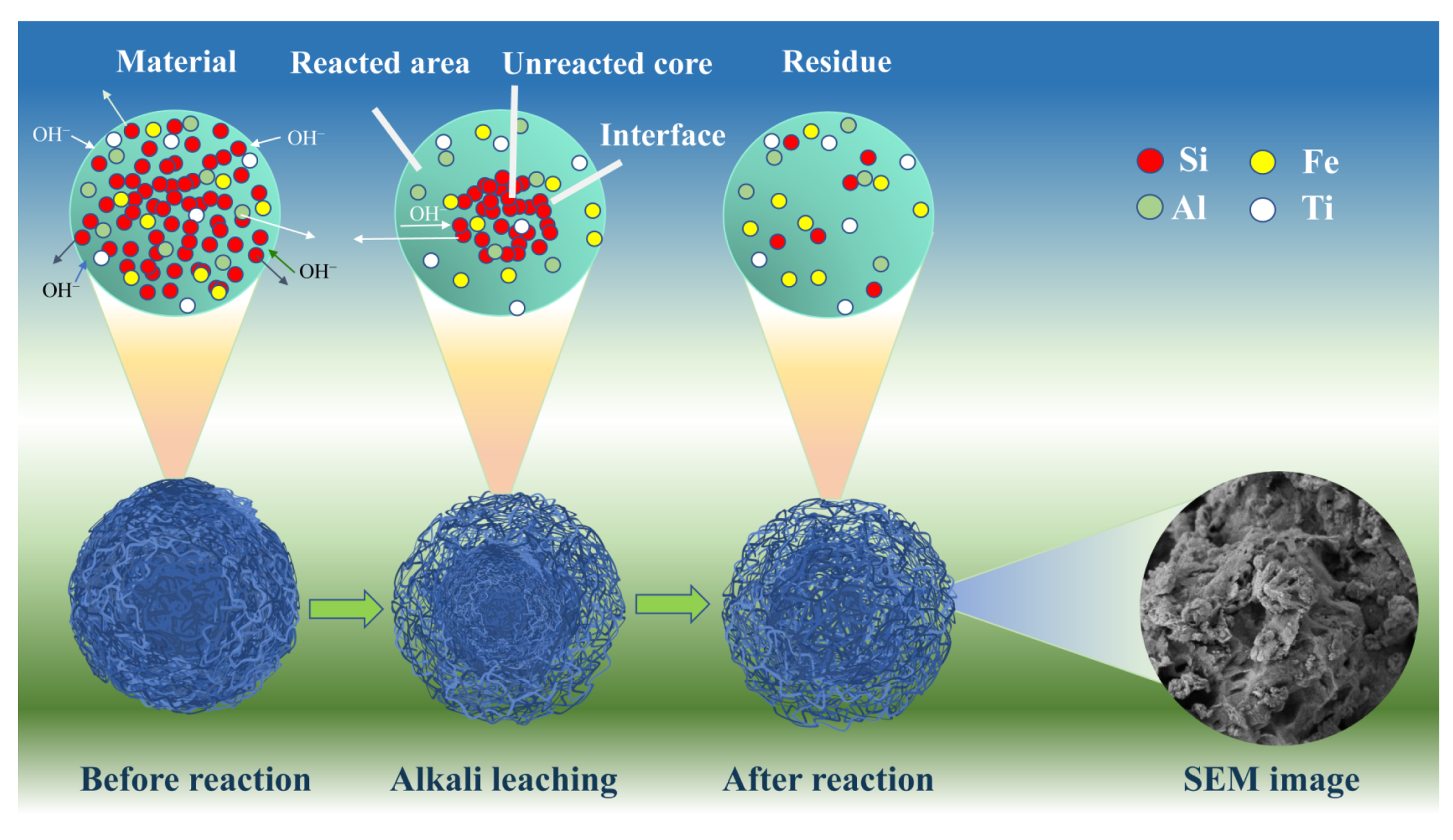
3.6. Reaction Mechanism
3.6.1. XRD Patterns of Acid Residues after Silica Extraction
3.6.2. FT-IR Analyses of Silica Extraction Residues
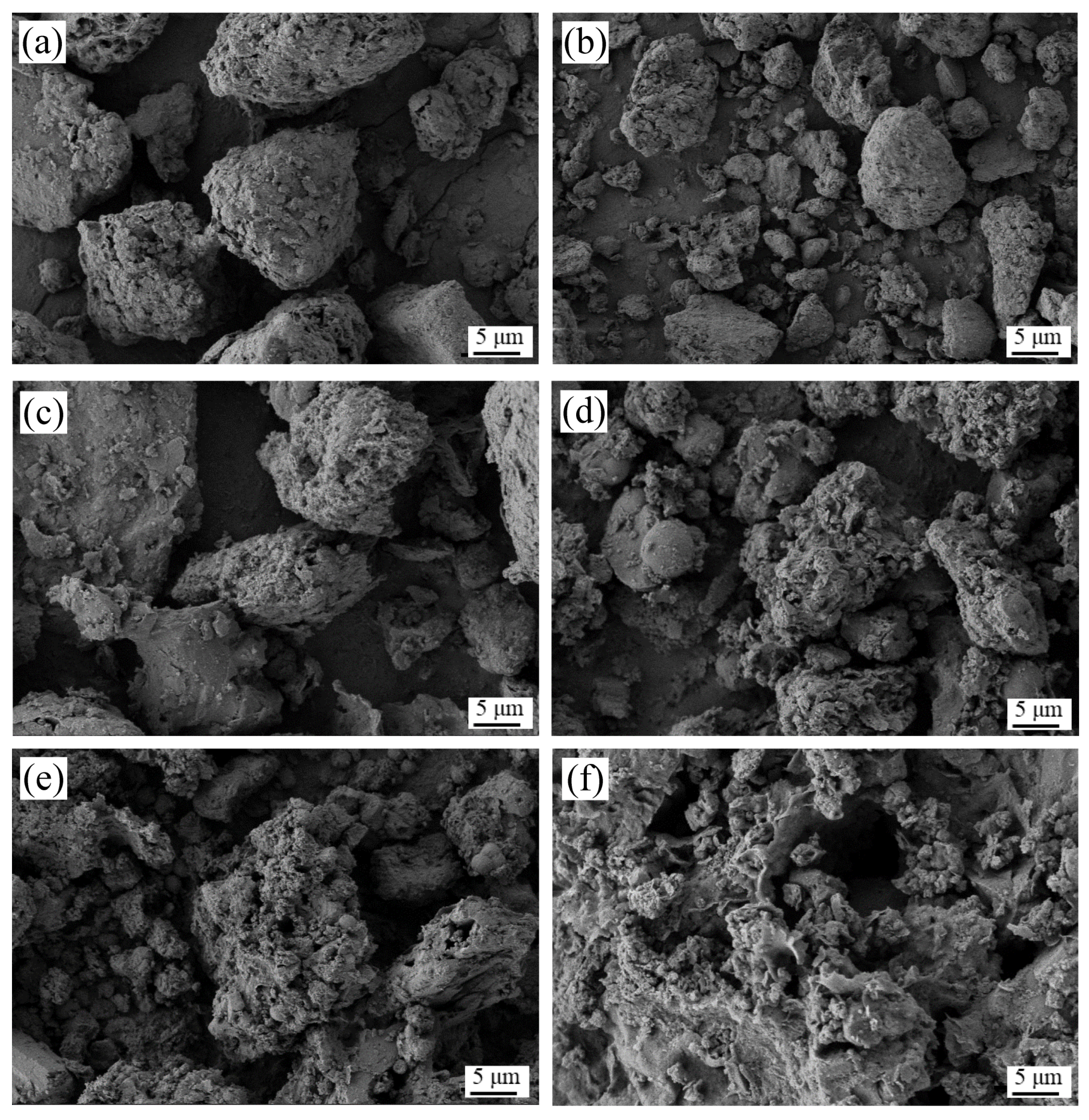
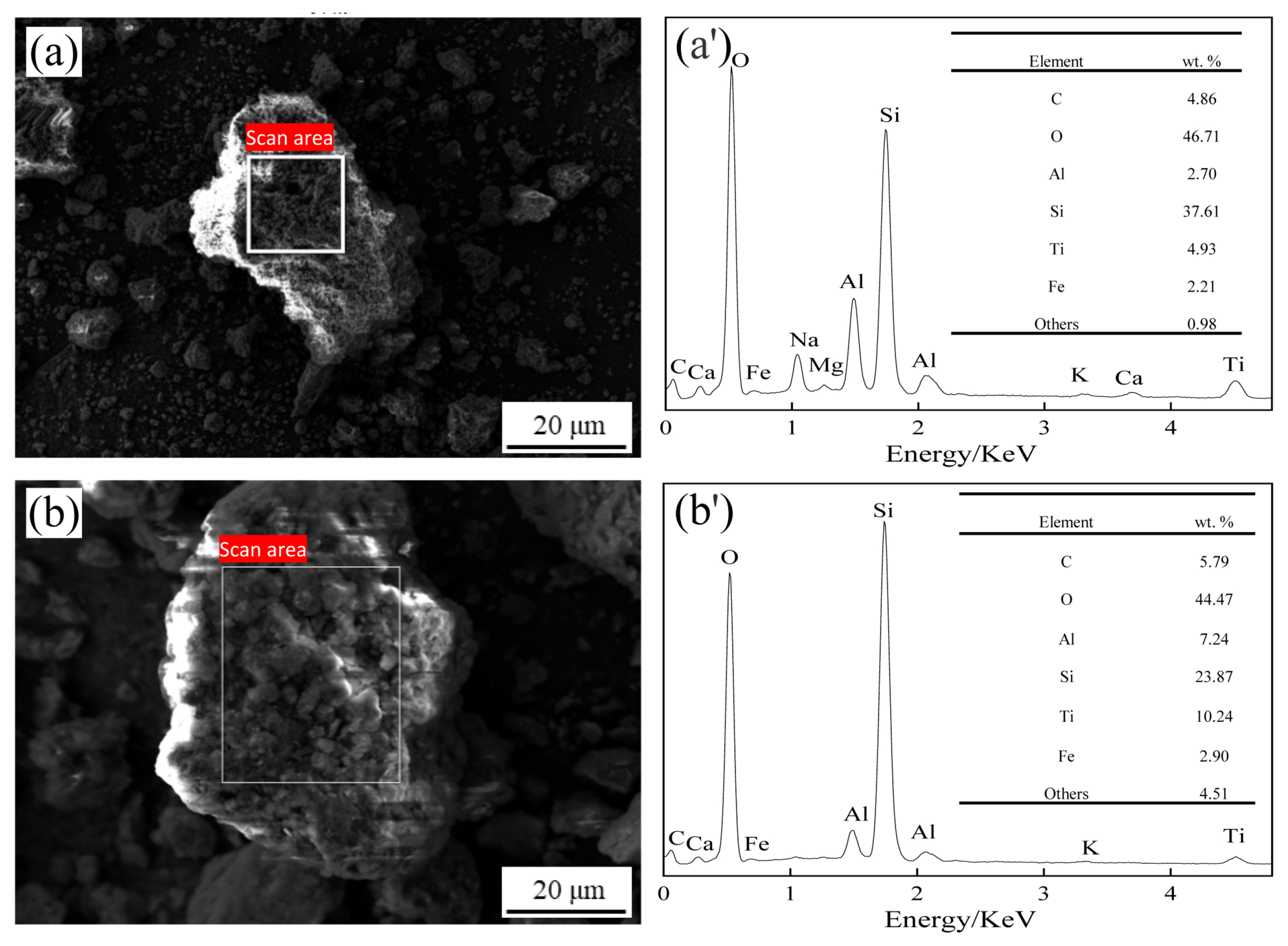
3.6.3. SEM and EDS Analyses of Residues before and after Reactions
3.6.4. Kinetic Modeling Process Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Xiao, J.; Yao, Z.; Yuan, J.; Ye, S.; Zhong, Q. Complementary advantages of spent pot lining and coal gangue in the detoxification and valuable components recovery process. Chemosphere 2022, 307, 136064. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, H.; Zhang, H.; Zhang, P.; Bei, R. Aluminum extraction from activated coal gangue with carbide slag. J. Anal. Appl. Pyrolysis 2022, 163, 105504. [Google Scholar]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Extraction of valuable components from coal gangue through thermal activation and HNO3 leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Pan, J.; Yang, F.; Liu, H.; Zhou, C.; Zhang, N. Extraction of lithium from coal gangue by a roasting-leaching process. Int. J. Coal Prep. Util. 2023, 43, 863–878. [Google Scholar] [CrossRef]
- Liu, C.; Xia, J.; Fan, H.; Li, W.; Zheng, G.; Ma, G.; Liang, Y. Ti leaching differences during acid leaching of coal gangue based on different thermal fields. Waste Manag. 2020, 101, 66–73. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, Z.; Ruan, M. Characteristics of sulfate-reducing bacteria and organic bactericides and their potential to mitigate pollution caused by coal gangue acidification. Environ. Technol. Innov. 2020, 20, 101142. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.; Cao, P.; Li, C.; Lai, S.; Wang, X. Process mineralogy characteristics of acid leaching residue produced in low-temperature roasting-acid leaching pretreatment process of refractory gold concentrates. Int. J. Miner. Metall. Mater. 2018, 25, 1132–1139. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Zhao, W.; Yang, L. Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 2018, 217, 427–433. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Qin, Q.; Geng, H.; Deng, J.; Su, X.; Chen, M.; Park, C.B. Al and other critical metals co-extraction from coal gangue through delamination pretreatment and recycling strategies. Chem. Eng. J. 2023, 477, 147036–147045. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Wang, Y. Efficient separation of alumina and silica in reduction-roasted kaolin by alkali leaching. Trans. Nonferrous Met. Soc. China 2019, 29, 416–423. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Qi, T.; Peng, Z.; Liu, G.; Zhou, Q. Dynamic expansion experiment of efficient separation of aluminum and silicon from coal fly ash. J. Nonferrous Met. 2022, 32, 173–182. [Google Scholar]
- Li, X.; Wang, P.; Wang, H.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z.; Wang, Y.; Shen, L. The alkaline leaching behavior of silica solid solutions in the product obtained by roasting the mixture of high-alumina coal gangue and hematite. J. Sustain. Metall. 2022, 8, 1853–1865. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.; Kontori, E.; Perraki, T.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, F.; Deng, X.; Guo, H.; Zhang, C.; Shi, C.; Zeng, M. Extraction of alumina from alumina rich coal gangue by a hydro-chemical process. R. Soc. Open Sci. 2020, 7, 192132. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Liu, C.; Shen, L. Comprehensive extraction of silica and alumina from High-Alumina Coal Gangue (HACG): Hematite involved roasting-alkaline leaching-bayer digestion process. J. Sustain. Metall. 2021, 7, 1686–1698. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Yan, J.; Shu, X. Efficient extraction of SiO2 and Al2O3 from coal gangue by means of acidic leaching. Adv. Mater. Res. 2014, 878, 149–156. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Z.; Wang, J.; Wang, Z.; Chen, J.; Shen, L. Alkali methods for alumina extraction from the by-products of high alumina coal: A review. Soc. Min. Eng. 2023, 40, 1681–1694. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Chen, K.; Fang, M.; Huang, J.; Liu, Y. Phase and microstructure evolution of mullite synthesized from coal gangue and γ-Al2O3. Adv. Mater. Res. 2012, 624, 17–21. [Google Scholar] [CrossRef]
- Beddiaf, S.; Chihi, S.; Leghrieb, Y. The determination of some crystallographic parameters of quartz, in the sand dunes of Ouargla, Algeria. J. Afr. Earth Sci. 2015, 106, 129–133. [Google Scholar] [CrossRef]
- Yan, K.; Guo, Y.; Zhang, Y.; Guo, Y.; Cheng, F. Comparative study of the isothermal solid-state reaction systems of kaolinite-Na2CO3 and kaolinite-quartz-Na2CO3 for coal gangue activation. Powder Diffr. 2022, 37, 190–199. [Google Scholar] [CrossRef]
- Pasabeyoglu, P.; Moumin, G.; De Oliveira, L.; Roeb, M.; Akata, B. Solarization of the zeolite production: Calcination of kaolin as proof-of-concept. J. Clean. Prod. 2023, 414, 137611. [Google Scholar] [CrossRef]
- Tielens, F.; Gierada, M.; Handzlik, J.; Calatayud, M. Characterization of amorphous silica based catalysts using DFT computational methods. Catal. Today 2020, 354, 3–18. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Q.; Yan, K.; Cheng, F.; Lou, H. Novel process for alumina extraction via the coupling treatment of coal gangue and bauxite red mud. Ind. Eng. Chem. Res. 2014, 53, 4518–4521. [Google Scholar] [CrossRef]
- Liu, F.; Xie, M.; Yu, G.; Ke, C.; Zhao, H. Study on calcination catalysis and the desilication mechanism for coal gangue. ACS Sustain. Chem. Eng. 2021, 9, 10318–10325. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, R.; Chang, L. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Wang, P.; Qi, T.; Li, X.; Wang, Y.; Shen, L.; Liu, G.; Peng, Z.; Zhou, Q. Leaching mechanism of SiO2 in activated kaolinite clinker with low modulus sodium silicate solution. Int. J. Coal Prep. Util. 2024, 4, 1–15. [Google Scholar]
- Xing, L.; Li, X.; Cao, P.; Luo, J.; Jiang, H. Stepwise extraction and utilization of silica and alumina from coal fly ash by mild hydrothermal process. Process Saf. Environ. 2024, 182, 918–929. [Google Scholar] [CrossRef]
- Cao, S.; Chang, L.; Bi, X.; Luo, S.; Liu, J. Alkaline hydrothermal treatment and leaching kinetics of silicon from laterite nickel ore. Min. Metall. Explor. 2022, 39, 129–138. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Bai, C.; Yang, K.; Zheng, T.; Lu, S.; Li, H.; Qiao, Y.; Colomob, P. Porous alkali-activated material from hypergolic coal gangue by microwave foaming for methylene blue removal. J. Am. Ceram. Soc. 2023, 106, 1473–1489. [Google Scholar] [CrossRef]
- Hanumananaik, M.; Subramaniam, K.V.L. Shrinkage in low-calcium fly ash geopolymers for precast applications: Reaction product content and pore structure under drying conditions. J. Build. Eng. 2023, 78, 107583–107595. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Jiang, R.; Song, S.; Feng, S.; Lian, M. Extraction of aluminum and iron ions from coal gangue by acid leaching and kinetic analyses. Minerals 2022, 12, 215. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R. The thermal behavior of kaolinite intercalation complexes, a review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef]
- Gao, P.; Shi, H.; Ma, T.; Liang, S.; Xia, Y.; Xu, Z.; Xu, Z.; Wang, S.; Min, C.; Liu, L. MXene/TiO2 heterostructure-decorated hard carbon with stable Ti-O-C bonding for enhanced sodium-ion storage. ACS Appl. Mater. Interfaces 2021, 13, 51028–51038. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Hao, Z. The thermal activation process of coal gangue selected from Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhou, Q.; Zhang, Y.; Sun, J. Thermal kinetics analysis of coal-gangue selected from Inner Mongolia in China. J. Therm. Anal. Calorim. 2018, 131, 1835–1843. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar] [CrossRef]
- Wang, A.; Ye, T.; Liu, Y.; Song, M.; Lou, N.; Wu, G.; Niu, Y.; Zheng, T. Facile in-situ synthesis of carbon black@poly (ionic liquid) composites with a smooth U-link chain macrostructure within surfactant-free ionic liquid microemulsions. Compos. Part A Appl. Sci. Manuf. 2024, 176, 107859–107867. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Jha, R.; Kumar, S.A.; Sharma, D. Sol-gel-mediated synthesis of TiO2 nanocrystals: Structural, optical, and electrochemical properties. Int. J. Appl. Ceram. Technol. 2020, 17, 1400–1409. [Google Scholar]
- Gui, Q.; Khan, M.I.; Wang, S.; Zhang, L. The ultrasound leaching kinetics of gold in the thiosulfate leaching process catalysed by cobalt ammonia. Hydrometallurgy 2020, 196, 105426–105433. [Google Scholar] [CrossRef]
- Valeev, D.; Pankratov, D.; Shoppert, A.; Sokolov, A.; Kasikov, A.; Mikhailova, A.; Salazar-Concha, C.; Rodionov, I. Mechanism and kinetics of iron extraction from high silica boehmite-kaolinite bauxite by hydrochloric acid leaching. Trans. Nonferrous Met. Soc. China 2021, 31, 3128–3149. [Google Scholar] [CrossRef]
- Moghadam, M.; Ajalloeian, R.; Hajiania, A. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
- Nie, H.; Sun, C.; Liu, G.; Du, W.; He, Z. Dissolution pore types of the Wufeng formation and the Longmaxi formation in the Sichuan Basin, south China: Implications for shale gas enrichment. Mar. Pet. Geol. 2019, 101, 243–251. [Google Scholar] [CrossRef]
- Wang, R.C.; Zhai, Y.C.; Ning, Z. Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution. Trans. Nonferrous Met. Soc. China 2014, 24, 1928–1936. [Google Scholar] [CrossRef]
- Du, H.; Wu, Y.; Gao, Y.; Ma, L.; Liu, X.; Zhao, B.; Zhang, J. CO2 uptake behavior on an MCM-41 structure mesoporous SiO2 material from coal gangue. J. Chin. Inst. Eng. 2020, 43, 856–865. [Google Scholar] [CrossRef]
- Xia, H.; Platt, J.P. Quartz grainsize evolution during dynamic recrystallization across a natural shear zone boundary. J. Struct. Geol. 2018, 109, 120–126. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Wang, C.; Gao, L.; Liu, M.; Xia, S.; Han, Y. Self-crystallization behavior of paraffin and the mechanism study of SiO2 nanoparticles affecting paraffin crystallization. Chem. Eng. J. 2023, 452, 139287. [Google Scholar] [CrossRef]
- Bezerra, B.P.; Morelli, M.R.; Luz, A.P. Effect of reactive silica sources on the properties of Na-metakaolin-based geopolymer binder. Constr. Build. Mater. 2023, 364, 129989–129998. [Google Scholar] [CrossRef]


| Instrument | Type | Production Location | Working Conditions | Usage |
|---|---|---|---|---|
| X-ray fluorescence spectrometer (XRF) | Supermini 200 | Rigaku Corporation, Tokyo, Japan | Power: 4 kW; maximum voltage; and current: 60 kV, 160 mA. | Component analysis |
| X-ray polycrystalline diffractometer (XRD) | 6100 | Shimadzu Corporation, Kyoto, Japan | Cu Kα1 (λ is Kα = 1.54059 Å); 2θ = 5° (min)–65° (max); step: 0.02°; voltage: 40 kV; current: 35 mA | Phase analysis |
| Scanning electron microscope (SEM) | EVO18 | Analytisches Instrument, Jena, Germany | The surface of the sample is sprayed with carbon and amplified 1000–2000 times | Morphology analyses |
| Energy-dispersive X-ray spectroscopy (EDS) | Extreme | Oxford Instrument Corporation, Oxford, UK | Map scanning analysis | Energy spectrum analyses |
| Fourier transform infrared spectrometer (FT-IR) | 7600 | Tianjin Gangdong Science and Technology Corporation, Tianjin, China | Background calibration with KBr at a ratio of m(sample): m(KBr) = 1:500, and the spectral analysis range was from 4000 cm−1 to 400 cm−1 | Infrared spectral analysis |
| SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|
| 78 | 2.84 | 1.28 | 0.02 | 15.5 | 0.78 | 0.02 | 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Gao, Y.; Song, S.; Jiang, R. Kinetics and Mechanism of SiO2 Extraction from Acid-Leached Coal Gangue Residue by Alkaline Hydrothermal Treatment. Materials 2024, 17, 4168. https://doi.org/10.3390/ma17174168
Kong D, Gao Y, Song S, Jiang R. Kinetics and Mechanism of SiO2 Extraction from Acid-Leached Coal Gangue Residue by Alkaline Hydrothermal Treatment. Materials. 2024; 17(17):4168. https://doi.org/10.3390/ma17174168
Chicago/Turabian StyleKong, Deshun, Yuan Gao, Shuojiang Song, and Rongli Jiang. 2024. "Kinetics and Mechanism of SiO2 Extraction from Acid-Leached Coal Gangue Residue by Alkaline Hydrothermal Treatment" Materials 17, no. 17: 4168. https://doi.org/10.3390/ma17174168
APA StyleKong, D., Gao, Y., Song, S., & Jiang, R. (2024). Kinetics and Mechanism of SiO2 Extraction from Acid-Leached Coal Gangue Residue by Alkaline Hydrothermal Treatment. Materials, 17(17), 4168. https://doi.org/10.3390/ma17174168





