A Review on Concrete Superplasticizers and Their Potential Applications for Enhancing the Performance of Thermally Activated Recycled Cement
Abstract
1. Introduction
2. Classification and Mechanisms of Superplasticizers
3. Comparison of Physical and Chemical Properties between RC and OPC
4. Potential Superplasticizers for Thermally Activated RC
4.1. Polyacrylate-Type Superplasticizers
4.2. Polymer Polycarboxylate Superplasticizers
4.3. Sulfate-Based Superplasticizers
4.4. Inorganic Superplasticizers
5. Conclusions
- The differences between OPC and RC include:
- (1)
- Chemical composition: RC contains more CaO than OPC and lacks C3S.
- (2)
- Specific surface area: The specific surface area of RC is larger than that of OPC.
- (3)
- Hydration rate: RC releases a lot of heat in the early stage of hydration, and the hydration rate of RC is faster than that of OPC.
- The different mechanisms of superplasticizers when applied to ordinary cement and RC include:
- (1)
- Surface property disparity: More rougher surfaces and impurities may be contained in RC particles, which necessitate the use of superplasticizers with stronger dispersive capabilities to enhance their workability.
- (2)
- Chemical reactivity: The potential presence of any residual chemical additives or aging products in RC necessitates that the superplasticizer possesses chemical reactivity that is compatible with these components to prevent performance degradation caused by chemical reactions.
- (3)
- Adsorption behavior: The adsorption behavior of the superplasticizer can be altered by the different mineral components in RC, which affects its water-reducing effectiveness and stability.
- Characteristics of superplasticizers that may be suitable for RC include:
- (1)
- With high-efficiency dispersing capabilities, dispersion effects at the molecular level can be optimized through the design of long-chain structures and side chains; this method is suitable for addressing the irregular and diverse particle size distribution in RC and can effectively cope with any potential impurities.
- (2)
- The ability to adapt to complex cementitious matrices can be enhanced by altering the molecular structure, such as the introduction of functional groups and the adjustment of molecular chain lengths.
- (3)
- The performance of polyacrylate superplasticizers in thermally activated RC could be significantly impacted by their molecular structure. By designing and adjusting the molecular structure, the performance of thermally activated RC-based materials can be improved by altering the surface charge properties.
- (4)
- Temperature sensitivity of superplasticizers is another important factor. The initial hydration heat release of RC is large and can easily have an impact on the high temperature sensitivity of the superplasticizers, such as polycarboxylate superplasticizers. They can be considered when supplemented together with plasticizers based on lignosulfonates.
- Future research and development directions:
- (1)
- The impact of different components in RC from different sources on the adsorption of superplasticizers should be investigated. This includes assessing the surface charge characteristics of various mineral phases and how they are affected by the adsorption and dispersing performance of superplasticizers.
- (2)
- An in-depth study of the adsorption and self-assembly behavior of polyacrylate-based superplasticizers within the RC system should be conducted to better understand their mechanisms of action in the dispersion of RC particles. This involves exploring the structure and function of the superplasticizer molecular layer on the surface of RC particles.
- (3)
- Compounding technology can be utilized to blend two or more high-efficiency superplasticizers in specific proportions, thereby altering some of their individual properties while internally coordinating to produce a synergistic effect for RC.
- (4)
- Furthermore, further systematical research is needed to investigate the adsorption and lubrication behaviors of different types of superplasticizers and their possible participation in RC rehydration in RC pastes, in order to gain a deeper understanding of their mechanisms of action in RC paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- China Cement Association. Cement Industry Analysis Report 2023. Available online: http://lwzb.stats.gov.cn/pub/lwzb/fbjd/202405/W020240527578179123377.pdf (accessed on 24 May 2024).
- Ali, N.; Abbas, J.; Anwer, M.; Alwi, S.K.K.; Anjum, M.N.; Author, C.; Jaffar, A. The greenhouse gas emissions produced by cement production and its impact on environment: A review of global cement processing. Int. J. Res. Sci. 2015, 2, 488–500. [Google Scholar]
- CBS News. Cement Industry Accounts for about 8% of CO2 Emissions. One Startup Seeks to Change That. Available online: https://www.cbsnews.com/news/cement-industry-co2-emissions-climate-change-brimstone/ (accessed on 4 March 2024).
- Wang, J.J.; Wang, Y.L.; Yu, J.; Xu, L.; Li, M.L.; Cheng, J.H.; Li, Z. Effects of sodium sulfate and potassium sulfate on the properties of calcium sulfoaluminate (CSA) cement based grouting materials. Constr. Build. Mater. 2022, 353, 129045. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, L.; Lyu, K.; Li, T.Y.; Zhao, P.Z.; Liu, R.D.; Zuo, J.Q.; Fu, F.; Shah, S.P. Enhanced early hydration and mechanical properties of cement-based materials with recycled concrete powder modified by nano-silica. J. Build. Eng. 2022, 50, 104175. [Google Scholar] [CrossRef]
- El-Hawary, M.; Al-Sulily, A. Internal curing of recycled aggregates concrete. J. Clean. Prod. 2020, 275, 122911. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Li, K.; Lin, S.; Li, M.; Hao, T.; Ling, Z.; Xiang, D.; Wang, T. A systematic review of factors affecting properties of thermal-activated recycled cement. Resour. Conserv. Recycl. 2022, 185, 106432. [Google Scholar] [CrossRef]
- Crook, D.N.; Murray, M.J. Regain of strength after firing of concrete. Mag. Concr. Res. 1970, 22, 149–154. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high temperature environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Pereira, M.F.C. Mechanical characterization of thermal activated low-carbon recycled cement mortars. J. Clean. Prod. 2019, 218, 377–389. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A. Comparison of energy consumption and carbon emissions from clinker and recycled cement production. J. Clean. Prod. 2021, 306, 127277. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Ma, Z.M.; Sui, T.B.; Akbarnezhad, A.; Duan, Z.H. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Xi, X.Y.; Liu, H.; Du, C.W.; Lu, H.B. A review: Enhanced performance of recycled cement and CO2 emission reduction effects through thermal activation and nanosilica incorporation. Constr. Build. Mater. 2024, 422, 135763. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Chen, W.; Yu, R.; Zhang, R. Cementitious characteristics of hydrated cement paste subjected to various dehydration temperatures. Constr. Build. Mater. 2009, 23, 531–537. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.J.; Li, K.F.; Li, M.L.; Lin, S.Y.; Hao, T.Y.; Wang, T.Y.; Guo, Y.P.; Ling, Z. Investigations on the rehydration of recycled blended SCMs cement. Cem. Concr. Res. 2023, 163, 107036. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.J.; Li, K.F.; Hao, T.Y.; Li, Z.; Li, L.; Ran, B.; Du, H. New insights on dehydration at elevated temperature and rehydration of GGBS blended cement. Cem. Concr. Compos. 2023, 139, 105068. [Google Scholar] [CrossRef]
- Xu, L.; Hu, X.C.; Yang, Q.R.; Ran, B.; Li, K.F.; Wang, J.J.; Zhang, X. Insight into multi-ionic adsorption behavior of recycled cement paste exposed to chloride solutions. Constr. Build. Mater. 2024, 426, 136142. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.J.; Hu, X.C.; Ran, B.; Huang, R.; Tang, H.Y.; Li, Z.; Li, B.W.; Wu, S.H. Modification of recycled cement with phosphogypsum and ground granulated blast furnace slag. Constr. Build. Mater. 2024, 426, 136241. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, L.; Li, M.L.; Wang, Y.L.; He, H.; Xiang, D.; Li, K.F.; Hao, T.Y. Investigations on factors influencing physical properties of recycled cement and the related carbon emissions and energy consumptions. J. Clean. Prod. 2023, 414, 137715. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Li, M.L.; He, H.; Wang, Y.; Xiang, D.; Lin, S.Y.; Zhong, Y.; Zhao, H.Y. Effect of pre-carbonation on the properties of cement paste subjected to high temperatures. J. Build. Eng. 2022, 51, 104337. [Google Scholar] [CrossRef]
- Carriço, A.; Real, S.; Bogas, J.A. Durability performance of thermoactivated recycled cement concrete. Cem. Concr. Compos. 2021, 124, 104270. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, J.J.; Li, M.L.; Xu, L.; Xiang, D.; Wang, Y.L.; He, H.; Zhu, Y.; Zhao, J.X. Estimation of chloride diffusion coefficient from water permeability test of cementitious materials. Constr. Build. Mater. 2022, 340, 127816. [Google Scholar] [CrossRef]
- Real, S.; Bogas, J.A.; Carriço, A.; Hu, S. Mechanical Characterisation and Shrinkage of Thermoactivated Recycled Cement Concrete. Appl. Sci. 2021, 11, 2454. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Shu, X.; Ran, Q.; Yang, Y. Recent advance of chemical admixtures in concrete. Cem. Concr. Res. 2019, 124, 105834. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, W.; Xiang, S.; Yang, X.; Yuan, M.; Zhou, H.; Yu, H.; Zheng, T.; Zhang, J.; Jiang, Z.; et al. Synthesis and Modification of Polycarboxylate Superplasticizers—A Review. Materials 2024, 17, 1092. [Google Scholar] [CrossRef]
- Zhou, B.W.; Ha, C.Y.; Mo, J.Q.; Deng, L.L.; Shen, M.M. Process of Researches and Applications on Lignosulfonate Surfactants. Polym. Bull. 2013, 5, 76–82. [Google Scholar] [CrossRef]
- Jiang, Y.M. Synthetic Naphthalene Superplasticizer and Performance Study. Master’s Thesis, Chongqing Normal University, Chongqing, China, 2017. Available online: https://kns.cnki.net/kcms2/article/abstract?v=sxrP1m9hSI9aIt1D1R2HaQdbTAIziEFPGNDR0xn2eHOPc_07CowB8EDchZgR8Hgu7rG6naA7x_XFuhPY-saflx6vqMhJ_tl0vrYygkvcP7z8zL851-ucIfRU8frgBjcKsFWDEbOreoTWwo3nKcquTFFjvimVixa274L1A9u9ENc7HHhr4IvyHxs-lUHDcwhrxFN31jNPSw=&uniplatform=NZKPT&language=CHS (accessed on 1 May 2017).
- Li, X.G.; Wang, L.Y. Research Progress of Polycarboxylate Superplasticizer. Res. Appl. Build. Mater. 2021, 3, 24–26+12. [Google Scholar] [CrossRef]
- Wu, H.X.; Gao, J.M.; Liu, C.; Luo, X.; Chen, G.F. Combine use of 100% thermoactivated recycled cement and recycled aggregate for fully recycled mortar: Properties evaluation and modification. J. Clean. Prod. 2024, 450, 141841. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, E. The relationship between steady-state chloride diffusion and migration coefficients in cementitious materials. Mag. Concr. Res. 2020, 72, 1016–1026. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, M.Z.; Li, Q.Z. Synthesis and behavior analysis of amino-sulfonic acid-based high performance water-reducer. Concrete 2001, 11, 25–28. [Google Scholar]
- Zhu, B.S.; Qiao, M.; Wu, J.Z.; Ran, Q.P. The factors affecting the properties of the aminosulfonate superplasticizer and the exploration of structural modification. New Build. Mater. 2019, 46, 5–8+18. [Google Scholar] [CrossRef]
- Cao, S.Y.; Dong, T.N.; Cao, Y.C.; Lei, J.B.; Zhang, S.Y.; Hang, Z.S.; Huang, Y. Monodisperse sulfonated melamine formaldehyde resin microspheres: Synthesis by dispersion polymerization and light-diffusing performance characterization. AIP Adv. 2024, 14, 035325. [Google Scholar] [CrossRef]
- Pei, M.S.; Wang, D.J.; Hu, X.B.; Xu, D.F. Synthesis of sodium sulfanilate-phenol-formaldehyde condensate and its application as a superplasticizer in concrete. Cem. Concr. Res. 2000, 30, 1841–1845. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Glasser, F.P. Influence of sulphonated melamine formaldehyde superplasticizer on cement hydration and microstructure. Adv. Cem. Res. 1989, 2, 111–119. [Google Scholar] [CrossRef]
- Yan, R.F.; Yin, S.H.; Zhang, H.S.; Wang, L.M.; Chen, D.P. Effect of superplasticizer on the setting behaviors and mechanical properties of tailings-waste rock cemented paste backfills. Case Stud. Constr. Mater. 2022, 18, e01714. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Liu, J.T.; Li, R. Research Progress of Naphthalene Series Highly Efficient Water Reducing Agent. Chem. World 2017, 58, 124–128. [Google Scholar] [CrossRef]
- Han, Y.L. Comparative analysis of batch and continuous preparation processes for synthetic aliphatic water reducers. Process Manag. 2015, 03, 202–214. [Google Scholar]
- Zhou, M.S.; Qiu, X.Q.; Yang, D.J.; Wang, W.X. Synthesis and evaluation of sulphonated acetone–formaldehyde resin applied as dispersant of coal–water slurry. Energy Convers. Manag. 2007, 48, 204–209. [Google Scholar] [CrossRef]
- Wang, X. Study on Selection and Dosage of Polycarboxylate and Aliphatic Series Superplasticizer. J. Taiyuan Univ. 2019, 37, 1–5. [Google Scholar] [CrossRef]
- Wei, A.M.; Wang, L.J.; Niu, L.Q. Mechanism of action and research progress of polyacrylic acid high efficient water reducing agents. Creat. Living 2008, 36–38. [Google Scholar]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78 Part A, 81–99. [Google Scholar] [CrossRef]
- Yuan, J.T.; Liu, B.; Lu, C.F. Research Review of Water Reducing Agents for Concrete. Sichuan Build. Mater. 2015, 41, 9–10+12. [Google Scholar]
- He, T.S.; Li, P.; Xu, Y.L.; Qian, Q.; Dan, W. Effect of Compound Use of Micro Inorganic Salt and Different Efficient Water-reducing Agents on the Performance of Concrete. Bull. Chin. Ceram. Soc. 2016, 35, 753–757. [Google Scholar] [CrossRef]
- Feng, H.; Feng, Z.J.; Wang, W.S.; Deng, Z.L.; Zheng, B.C. Impact of polycarboxylate superplasticizers (PCEs) with novel molecular structures on fluidity, rheological behavior and adsorption properties of cement mortar. Constr. Build. Mater. 2021, 292, 123285. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.B.; Li, H.; Bai, Y. Progress in the polycarboxylate superplasticizer and their structure-activity relationship—A review. Mater. Today Commun. 2023, 35, 105838. [Google Scholar] [CrossRef]
- Xiong, G.Y.; Guo, X.L. Effects and mechanism of superplasticizers and precursor proportions on the fresh properties of fly ash-slag powder based geopolymers. Constr. Build. Mater. 2022, 350, 128734. [Google Scholar] [CrossRef]
- Ruckstuhl, S.; Suter, M.J.F.; Giger, W. Sorption and mass fluxes of sulfonated naphthalene formaldehyde condensates in aquifers. J. Contam. Hydrol. 2003, 67, 1–12. [Google Scholar] [CrossRef]
- Huynh, L.; Feiler, A.; Jenkins, P. The effect of adsorbing naphthalene sulfonate formaldehyde condensates upon the interactions between metal oxides. Colloids Surf. A 2001, 181, 79–89. [Google Scholar] [CrossRef]
- Fan, Z.R.; Kong, L.J.; Lu, J.T.; Wang, X.B. Mechanism study of effect of superplasticizers on the fluidity of alkali-activated materials. Mater. Struct. 2023, 56, 29. [Google Scholar] [CrossRef]
- Ji, X.P.; Pan, T.H.; Zhao, W.H.; Liu, J.Z.; Sha, J.F.; Han, F.Y. Interaction of superplasticizers with C3A: Understanding the superplasticizer compatibility with cement. J. Mater. Civ. Eng. 2023, 35, 04023276. [Google Scholar] [CrossRef]
- Du, K.L. Synthesis and application of aliphatic superplasticizer by two-stage acetone process. Earth Environ. Sci. 2020, 571, 012159. [Google Scholar] [CrossRef]
- Martin, K.; Joerg, S.; Alfred, T.; Andreas, K. Drying and curing behaviour of melamine formaldehyde resin impregnated papers. J. Appl. Polym. Sci. 2014, 131, 39860. [Google Scholar] [CrossRef]
- Mangane, M.B.C.; Argane, R.; Trauchessec, R.; Lecomte, A.; Benzaazoua, M. Influence of superplasticizers on mechanical properties and workability of cemented paste backfill. Miner. Eng. 2018, 116, 3–14. [Google Scholar] [CrossRef]
- Zhang, R.G.; Li, Q.; Liu, Y.; Guo, H.L.; Lei, X.J. The synthesis of polyacrylate type superplasticizer and its application. Chem. Technol. Mark. 2006, 09, 42–43+58. [Google Scholar]
- Yamada, K.; Takahashi, T. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Ferrari, G.; Cerulli, T.; Clemente, P.; Dragoni, M.; Gamba, M.; Surico, F. Influence of carboxylic acid carboxylic ester ratio of carboxylic acid ester superplasticizer on characteristics of cement mixtures. Spec. Publ. 2000, 195, 505–520. [Google Scholar]
- Winnefeld, F.; Becker, S.; Gtz, J.P.T. Effects of the molecular architecture of comb-shaped superplasticizers on their performance in cementitious systems. Cem. Concr. Compos. 2007, 29, 251–262. [Google Scholar] [CrossRef]
- Puertas, F.; Santos, H.; Palacios, M.; Martnez-Ramrez, S. Polycarboxylate superplasticiser admixtures: Effect on hydration, microstructure and rheological behaviour in cement pastes. Adv. Cem. Res. 2005, 17, 77–89. [Google Scholar] [CrossRef]
- Borget, P.; Galmiche, L.; Meins, J.F.L.; Lafuma, F. Microstructural characterisation and behaviour in different salt solutions of sodium polymethacrylate-g-PEO comb copolymers. Colloids Surf. A-Physicochem. Eng. Asp. 2005, 260, 173–182. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.R.; Gao, Y.F. Synthesis of a water-soluble comb polymer containing mPEG side chains and the restricted crystallization behavior of their side chains. Acta Polym. Sin. 2006, 1, 26–31. [Google Scholar] [CrossRef]
- Wang, S.H.; Yan, H.X.; Ma, X.Y.; Huang, Y.; Zhang, Q.L. Synthesis, structure and properties of comb-like copolymer P(MMA-co-MAh)-g-PEGME. Acta Polym. Sin. 2008, 9, 880–886. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhang, J.L.; Sun, S.M.; Zhong, K.H.; Shao, Q.; Xu, H.J.; Huang, H.L.; Wei, J.X. Dispersion. fluidity retention and retardation effect of polyacrylate-based ether superplasticizer nanomicelles in Portland cement. Constr. Build. Mater. 2021, 290, 123149. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yu, J.; Wang, J.J.; Xiang, D.; Gu, H.; Cheng, J.H. Effects of sodium aluminate and quicklime on the properties of CSA grouting materials. J. Build. Eng. 2022, 58, 105060. [Google Scholar] [CrossRef]
- Zhang, L.L.; Ji, Y.S.; Huang, G.D.; Li, J.; Hu, Y.J. Modification and enhancement of mechanical properties of dehydrated cement paste using ground granulated blast-furnace slag. Constr. Build. Mater. 2018, 164, 525–534. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.J.; Li, K.F.; Li, Z.; Li, L. The role of C12A7, α′H-C2S and dehydrated amorphous nesosilicate in rehydration of recycled cement. In Proceedings of the 16th International Congress on the Chemistry of Cement, Bangkok, Thailand, 18–22 September 2023. [Google Scholar]
- Kwon, E.; Ahn, J.; Cho, B.; Park, D. A study on development of recycled cement made from waste cementitious powder. Constr. Build. Mater. 2015, 83, 174–180. [Google Scholar] [CrossRef]
- GB175-2023; General Portland Cement, China. China National Standardization Administration: Beijing, China, 2023.
- Wang, J.J.; Mu, M.L.; Liu, Y.L. Recycled cement. Constr. Build. Mater. 2018, 190, 1124–1132. [Google Scholar] [CrossRef]
- Rui, Y.; Shui, Z. Effect of dehydrated cement paste (DCP) adidition on cementitious of cement in early age. In Proceedings of the International Conference on Waste Engineering and Management, Shanghai, China, 3–15 October 2010. [Google Scholar]
- Wu, A.X.; Li, H.; Cheng, H.Y.; Wang, Y.M.; Li, C.P.; Ruan, Z.E. Status and prospects of researches on rheology of paste backfill using unclassified-tailings (Part 1): Concepts, characteristics and models. Chin. J. Eng. 2020, 42, 803–813. [Google Scholar] [CrossRef]
- EN 196-6; Methods of Testing Cement—Part 6: Determination of Fineness. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- Bogas, J.A.; Carriço, A.; Tenza-Abril, A.J. Microstructure of thermoactivated recycled cement pastes. Cem. Concr. Res. 2020, 138, 106226. [Google Scholar] [CrossRef]
- Guo, H.; Gao, R.; Liu, S.H.; Feng, C.H.; Qin, M.J.; Sun, G.L. Effect of ultra-low dosage graphene oxide on the properties of recycled cement-based materials. J. Build. Eng. 2024, 91, 109637. [Google Scholar] [CrossRef]
- EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- Bogas, J.A.; Real, S.; Cruz, R.; Azevedo, B. Mechanical performance and shrinkage of compressed earth blocks stabilised with thermoactivated recycled cement. J. Build. Eng. 2023, 79, 107892. [Google Scholar] [CrossRef]
- Bogas, J.A.; Real, S.; Carriço, A.; Abrantes, J.C.C.; Guedes, M. Hydration and phase development of recycled cement. Cem. Concr. Compos. 2022, 127, 104405. [Google Scholar] [CrossRef]
- Yu, R.; Shui, Z.H. Influence of agglomeration of a recycled cement additive on the hydration and microstructure development of cement based materials. Constr. Build. Mater. 2013, 49, 841–851. [Google Scholar] [CrossRef]
- Serpell, R.; Lopez, M. Properties of mortars produced with reactivated cementitious materials. Cem. Concr. Compos. 2015, 64, 16–26. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, X.Z.; Ong, C.K.; Tan, B.T.G.; Yang, J. Dielectric and electrical properties of ordinary Portland cement and slag cement in the early hydration period. J. Mater. Sci. 1996, 31, 1345–1352. [Google Scholar] [CrossRef]
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- Abile, R.; Russo, A.; Limone, C.; Montagnaro, F. Impact of the charge density on the behaviour of polycarboxylate ethers as cement dispersants. Constr. Build. Mater. 2018, 180, 477–490. [Google Scholar] [CrossRef]
- Wen, X.D.; Feng, L.; Hu, D.Y.; Wang, K.; Zhang, Z.Y. Effect of side-chain length in polycarboxylic superplasticizer on the early-age performance of cement-based materials. Constr. Build. Mater. 2019, 211, 26–32. [Google Scholar] [CrossRef]
- Xue, H.; Tian, Y.; Wang, Z.D. Research on Compatibility of Low-heat Cement and Water Reducing Agent. China Concr. 2024, 5, 32–35. [Google Scholar]
- Gay, C.; Raphael, E. Comb-like polymers inside nanoscale pores. Adv. Colloid Interface Sci. 2001, 94, 229–236. [Google Scholar] [CrossRef]
- Flatt, R.J.; Houst, Y.F. A simplified view on chemical effects perturbing the action of superplasticizers. Cem. Concr. Res. 2001, 31, 1169–1176. [Google Scholar] [CrossRef]
- Plank, J.; Dai, Z.; Andres, P.R. Preparation and characterization of new Ca–Al–polycarboxylate layered double hydroxides. Mater. Lett. 2006, 60, 3614–3617. [Google Scholar] [CrossRef]
- Giraudeau, C.; Lacaillerie, J.B.E.; Souguir, Z.; Nonat, A.; Flatt, R.J. Surface and intercalation chemistry of polycarboxylate copolymers in cementitious systems. J. Am. Ceram. Soc. 2009, 92, 2471–2488. [Google Scholar] [CrossRef]
- Plank, J.; Dai, Z.M.; Keller, H.; Hossle, F.; Seidl, W. Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement. Cem. Concr. Res. 2010, 40, 45–57. [Google Scholar] [CrossRef]
- Yoshioka, K.; Tazawa, E.; Kawai, K.; Enohat, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L.; Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; et al. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kawa, H.U.; Hanehara, S.; Rasaka, T.K.S.; Sawaki, D. Effect of admixture on hydration of cement, adsorptive behavior of admixture and fluidity and setting of fresh cement paste. Cem. Concr. Res. 1992, 22, 1115–1129. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Towards reliable X-ray photoelectron spectroscopy: Sputter-damage effects in transition metal borides, carbides, nitrides, and oxides. Appl. Surf. Sci. 2021, 542, 148599. [Google Scholar] [CrossRef]
- Kauppi, A.; Andersson, K.M.; Bergstriim, L. Probing the effect of superplasticizer adsorption on the surface forces using the colloidal probe AFM technique. Cem. Concr. Res. 2005, 35, 133–140. [Google Scholar] [CrossRef]
- Houst, Y.F.; Bowen, P.; Perche, F.; Kauppi, A.; Borget, P.; Galmiche, L.; Meins, J.F.L.; Lafuma, F.; Flatt, R.J.; Schober, I.; et al. Design and function of novel superplasticizers for more durable high-performance concrete (superplast project). Cem. Concr. Res. 2008, 38, 1197–1209. [Google Scholar] [CrossRef]
- Hamada, H.M.; Abed, F.; Katman, H.Y.B.; Humada, A.M.; Jawahery, M.S.A.; Majdi, A.; Yousif, S.T.; Thomas, B.S. Effect of silica fume on the properties of sustainable cement concrete. J. Mater. Res. Technol. 2023, 24, 8887–8908. [Google Scholar] [CrossRef]
- Sun, T.; Shui, Z.H.; Huo, T. Rehydration performance of binary binders made with dehydrated cement paste and phosphogypsum. Key Eng. Mater. 2011, 474–476, 1238–1242. [Google Scholar] [CrossRef]
- Zhang, L.L.; Ji, Y.S.; Li, J.; Gao, F.R.; Huang, G.D. Effect of retarders on the early hydration and mechanical properties of reactivated cementitious material. Constr. Build. Mater. 2019, 212, 192–201. [Google Scholar] [CrossRef]
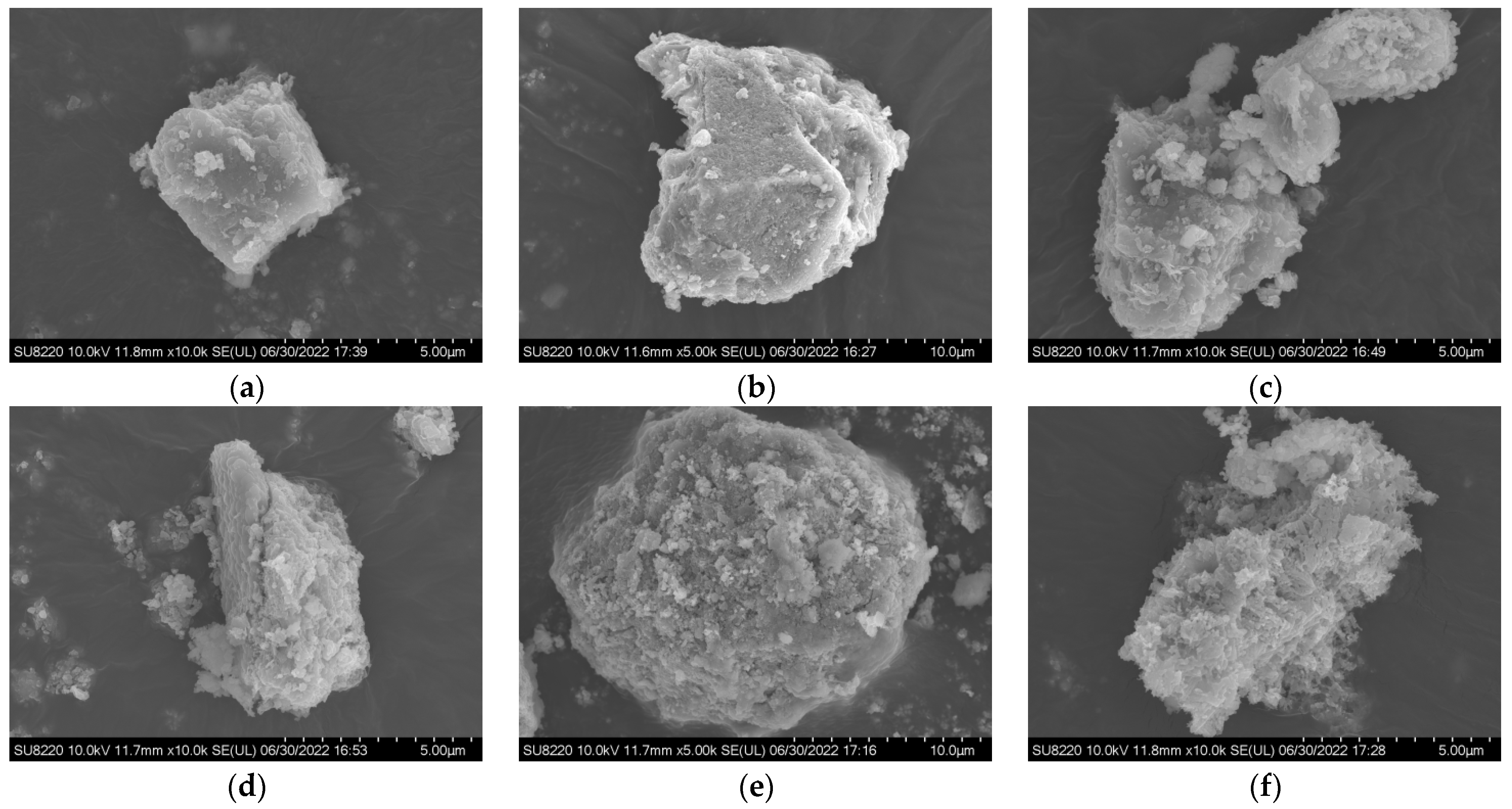
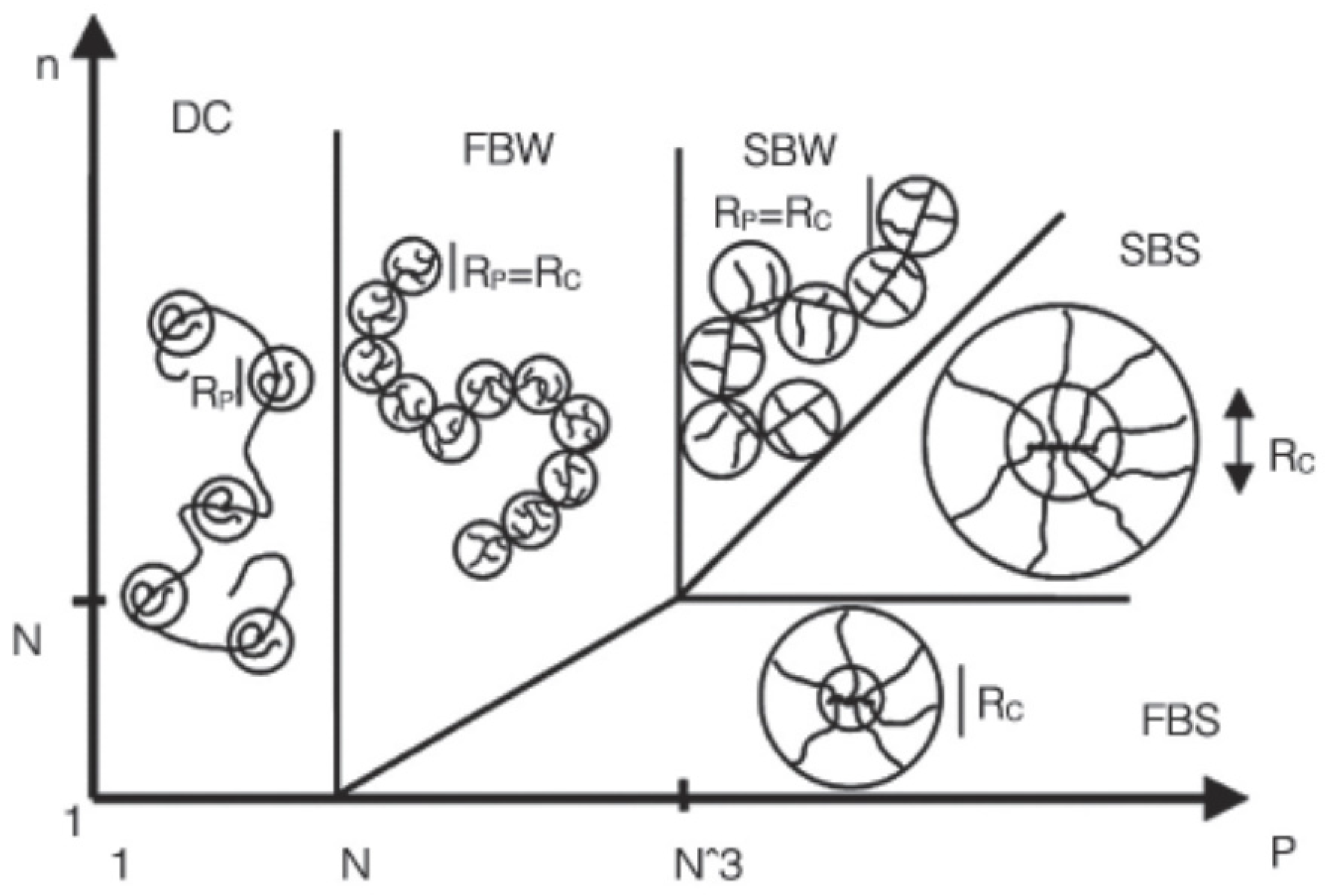
| Type | Characteristic | Mechanisms | Dosage | Application | Reference |
|---|---|---|---|---|---|
| Polycarboxylate superplasticizers (PCEs) | This type of superplasticizer is currently the most advanced in technology and has the best application prospects. It has characteristics such as low dosage, high water-reducing efficiency, low slump loss, significant enhancing effect, and is a green and environmentally friendly high-efficiency superplasticizer. | PCEs are adsorbed onto the surface of cement particles through their negatively charged anchoring groups (such as carboxyl groups) to form a thicker adsorption layer, generating electrostatic repulsion and steric hindrance, thereby enhancing the flowability of the cement slurry. | The amount added is low, with a high water-reduction efficiency, typically at 0.5–2.0% of the total weight of the cementitious material. | Suitable for preparing high-durability, high-fluidity, high-slump-retention, and high-strength concrete. | [25,45,46,47] |
| Naphthalene sulfonate formaldehyde condensates | A higher water-reducing efficiency (15–25%) can be achieved, no air-entrainment is induced, there is minimal impact on setting time, and it exhibits good compatibility with cement, as well as various other admixtures. Furthermore, it is relatively cost-effective. | By forming complexes with calcium ions on the surface of cement particles through its sulfonate groups, the flowability of the cement slurry is increased. | The amount added is typically 0.2–2.0% of the total weight of the cementitious material, commonly used at 0.2–0.5%. | Applicable for prestressed concrete engineering; can enhance the early strength and later strength of concrete. | [48,49] |
| Amine sulfonate superplasticizers | The molecule has a complex structure, containing a large number of hydrophilic functional groups such as sulfonate, amino, hydroxyl, etc., and has a very good water-reducing effect and improves the durability of concrete. | Because its amino and sulfonate groups interact with cement particles, the dispersion and flowability of the cement slurry are improved, while the cement dosage and water requirement are reduced. | The amount added is relatively high, typically at 1.0–3.0% of the total weight of the cementitious material. | Suitable for improving the durability of concrete. | [50,51] |
| Aliphatic superplasticizers | The strengthening effect on concrete is obvious, with minimal slump loss. | Having longer carbon chains, it can form a protective film on the surface of cement particles, reducing inter-particle friction, thus improving flowability and reducing water requirements. | The amount added typically ranged from 1.5 to 2.0%. | Applicable for situations where the reinforcement effect on concrete is significant, and the slump loss is minimal. | [52] |
| Melamine formaldehyde superplasticizers | The appearance is a white powder, soluble in water, with good dispersibility for powdery materials, a high water-reduction efficiency, and good fluidity and self-healing properties. | By its melamine resin structure interacting with the surface of cement particles, the flowability of the cement slurry is improved, and the cement dosage is reduced. | The amount added varies depending on the product, but is typically 0.5–1.5% of the total weight of the cementitious material. | Applicable for improving poor workability of concrete caused by poor aggregate quality. | [53,54] |
| Polyacrylate-type superplasticizers | This type is not only highly efficient in reducing water and improving the concrete structure but can also control the slump loss, is compatible with a variety of cement types, works at a low dosage, and still maintains high mobility. | Increasing the dispersion of cement particles is mainly due to improving the spatial exclusion between the particles and the polyacrylate-type superplasticizers’ air-entraining isolation “ball” effect. | The water decrease rate is as high as 21.3% at a dosage of 0.35% of the total weight of the cementitious material. | Can be applied to many kinds of cement-based concrete. | [55,56,57,58,59,60,61,62] |
| Standard | OPC | RC | Reference | |
|---|---|---|---|---|
| Particle size (μm) | GB175-2023 [69] | <45 | <75–150 | [69,70,71,72] |
| Blaine specific surface (m2/kg) | EN 196-6 [73] | 300–450 | 800–4400 | [20,69,74,75] |
| Chemical composition | C3S, C2S, C4AF, C3A, gypsum, limestone | Contains more f-CaO and polycrystalline C2S, amorphous AFm phase, but lacks C3S. | [20,69] | |
| w/b (water-cement ratio) | EN 196-3 [76] | 0.25–0.35 | 0.5–0.75 | [21,66,69,77,78,79,80,81] |
| 28-day compressive strength (MPa) | EN 196-1 [82] GB175-2023 [69] | 32.5–62.5 | 3–32 | [7,10,14,69,70,77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Xu, L.; Xu, Z.; Zhang, Q.; Wang, J. A Review on Concrete Superplasticizers and Their Potential Applications for Enhancing the Performance of Thermally Activated Recycled Cement. Materials 2024, 17, 4170. https://doi.org/10.3390/ma17174170
Huang R, Xu L, Xu Z, Zhang Q, Wang J. A Review on Concrete Superplasticizers and Their Potential Applications for Enhancing the Performance of Thermally Activated Recycled Cement. Materials. 2024; 17(17):4170. https://doi.org/10.3390/ma17174170
Chicago/Turabian StyleHuang, Rong, Lei Xu, Zihang Xu, Qihang Zhang, and Junjie Wang. 2024. "A Review on Concrete Superplasticizers and Their Potential Applications for Enhancing the Performance of Thermally Activated Recycled Cement" Materials 17, no. 17: 4170. https://doi.org/10.3390/ma17174170
APA StyleHuang, R., Xu, L., Xu, Z., Zhang, Q., & Wang, J. (2024). A Review on Concrete Superplasticizers and Their Potential Applications for Enhancing the Performance of Thermally Activated Recycled Cement. Materials, 17(17), 4170. https://doi.org/10.3390/ma17174170







