RETRACTED: Cr–Diamond/Cu Composites with High Thermal Conductivity Fabricated by Vacuum Hot Pressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Cr–Diamond/Cu Composites
2.2. Material Characterization
3. Results and Discussion
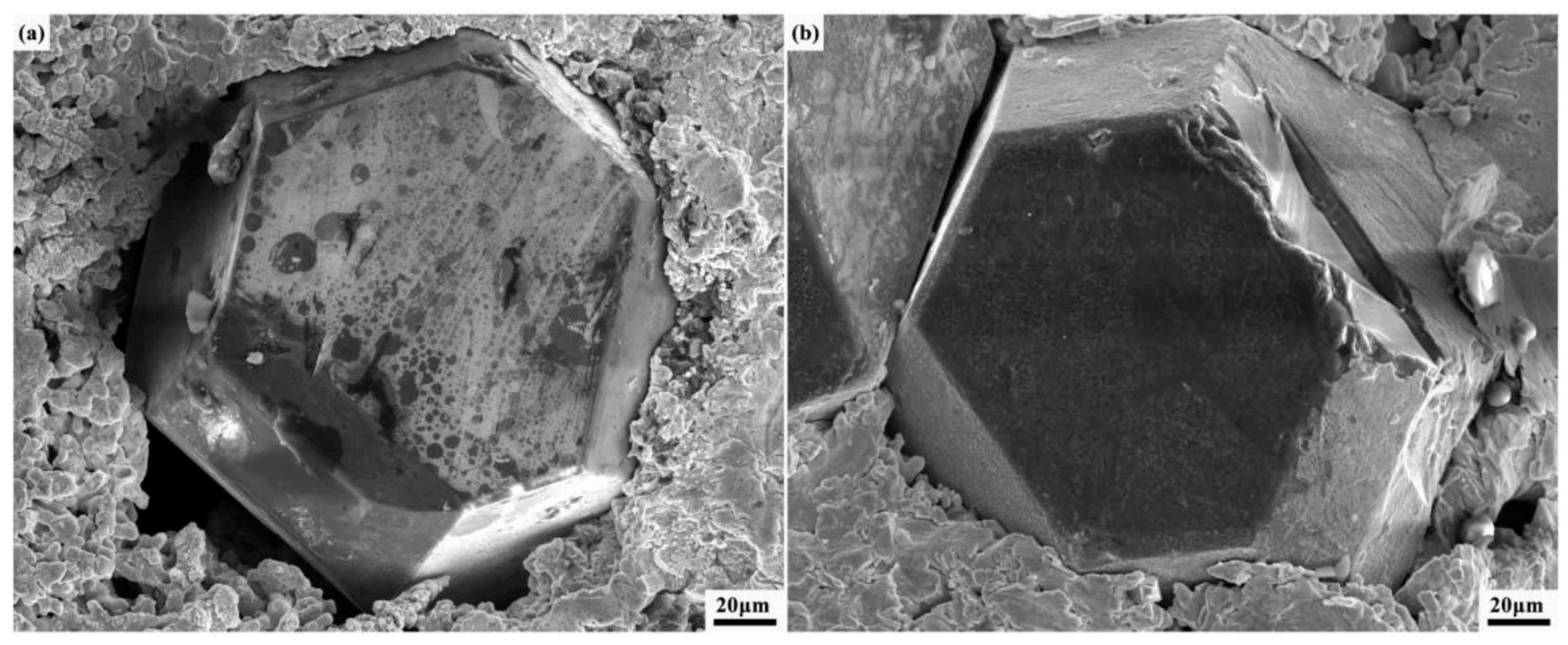
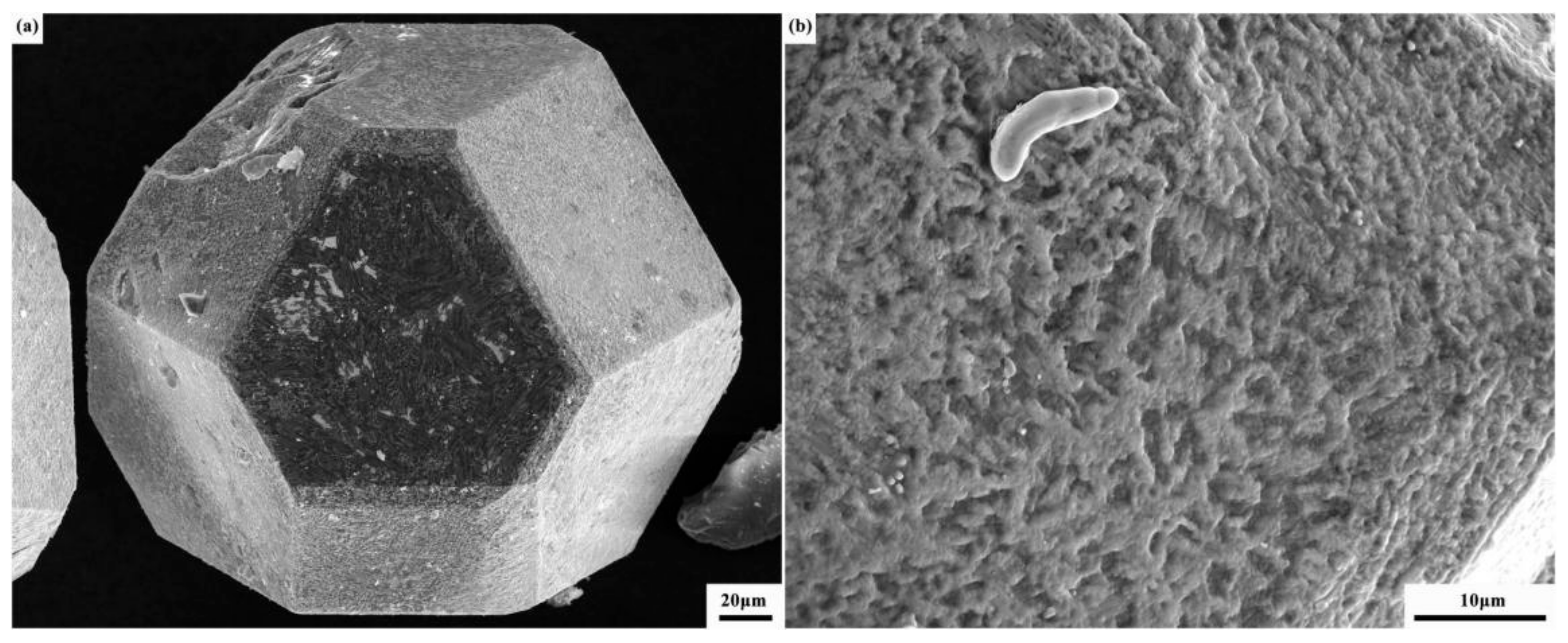
3.1. Properties of Composites Prepared with Different Chromium Layer Thicknesses
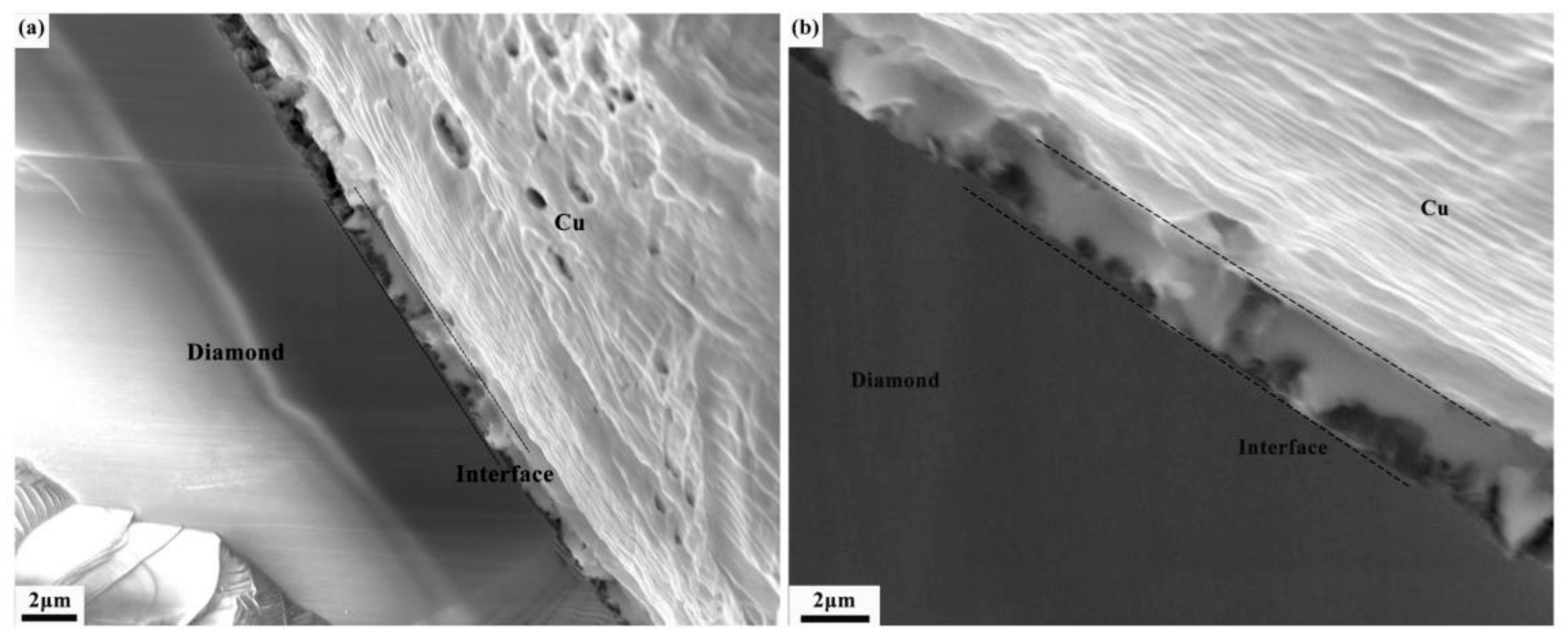
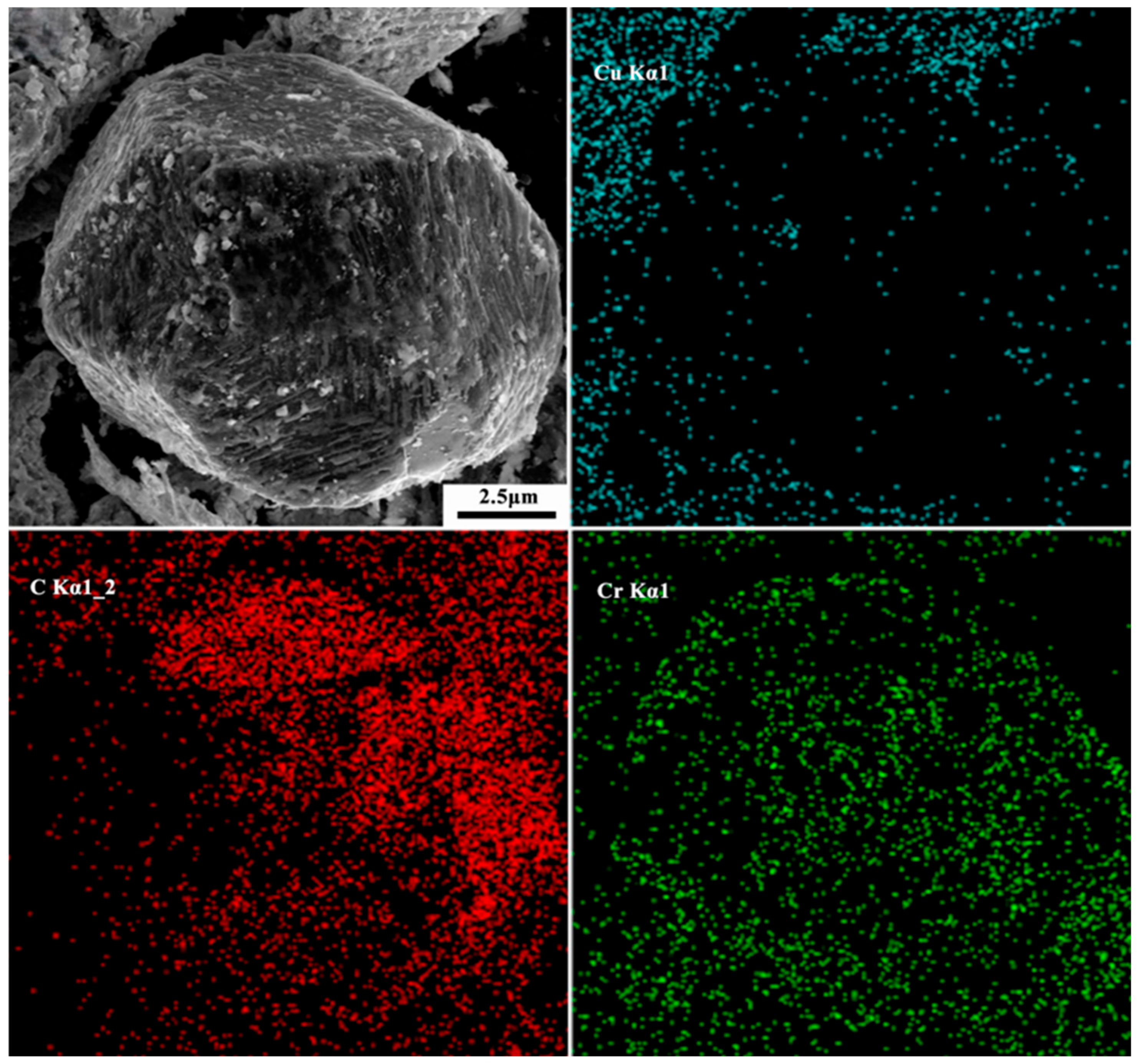
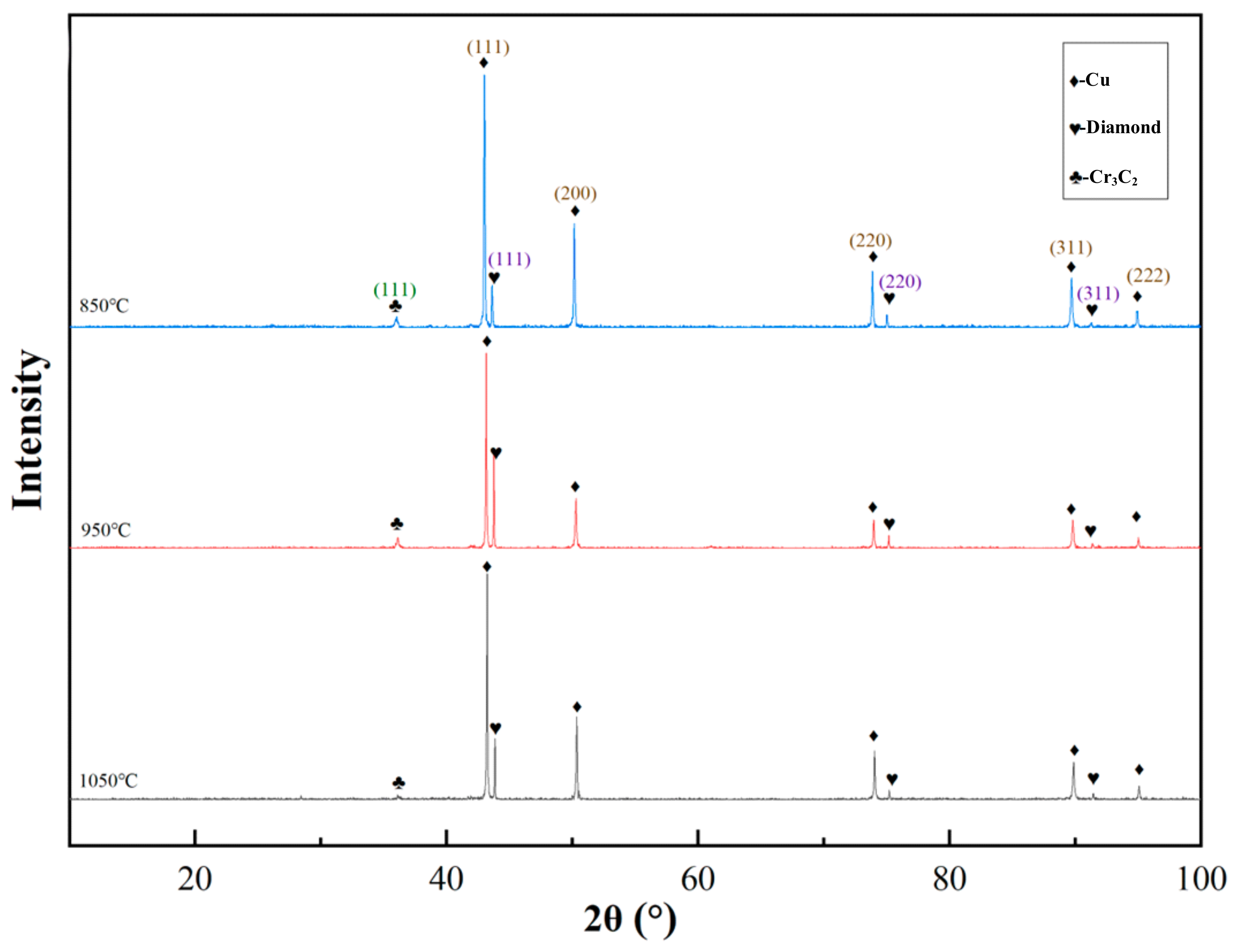
3.2. Interface Structure of Cr–Diamond/Copper Composite Material
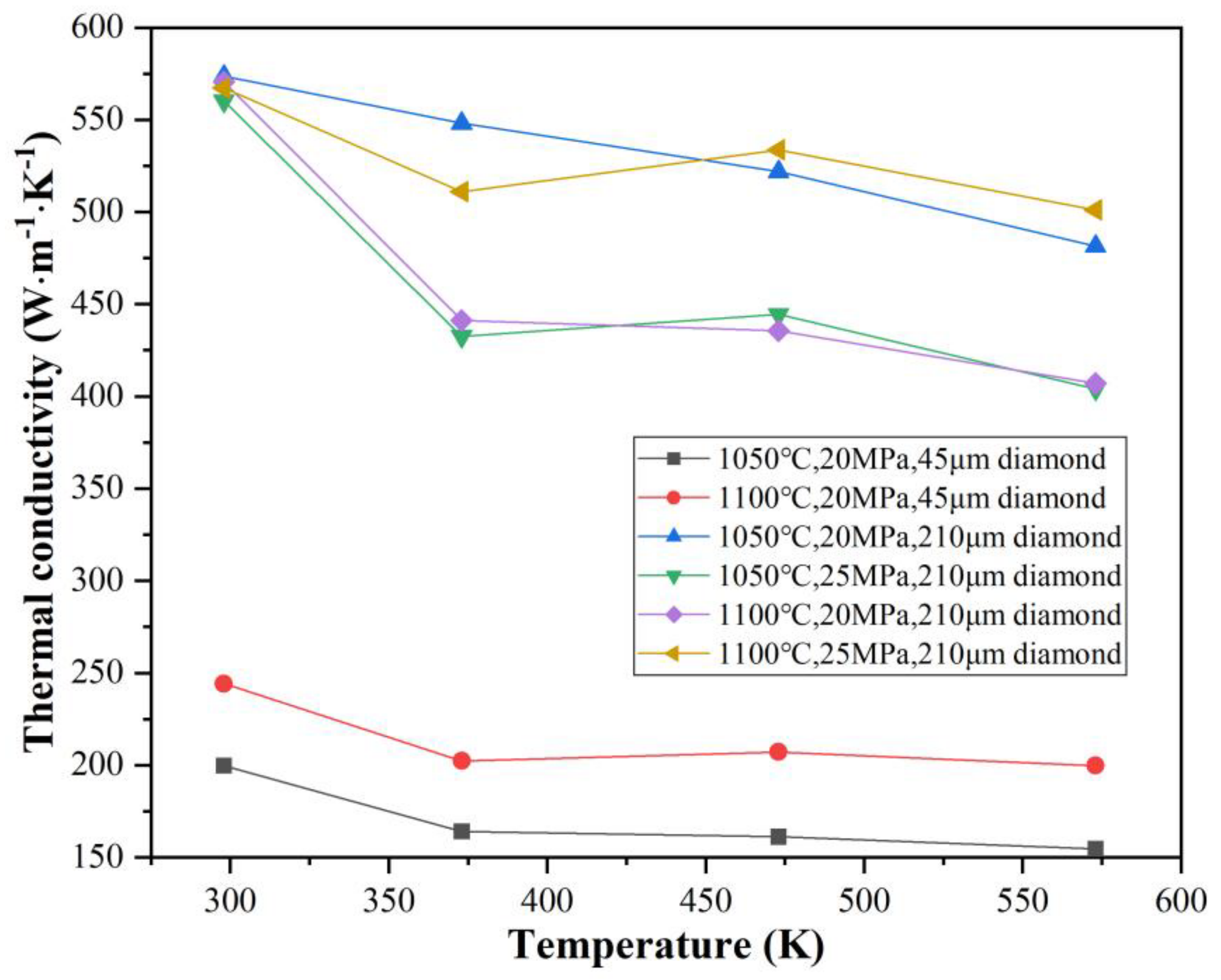
3.3. Thermal Conductivity and Its Influencing Mechanism in Cr–Diamond/Copper Composite Materials
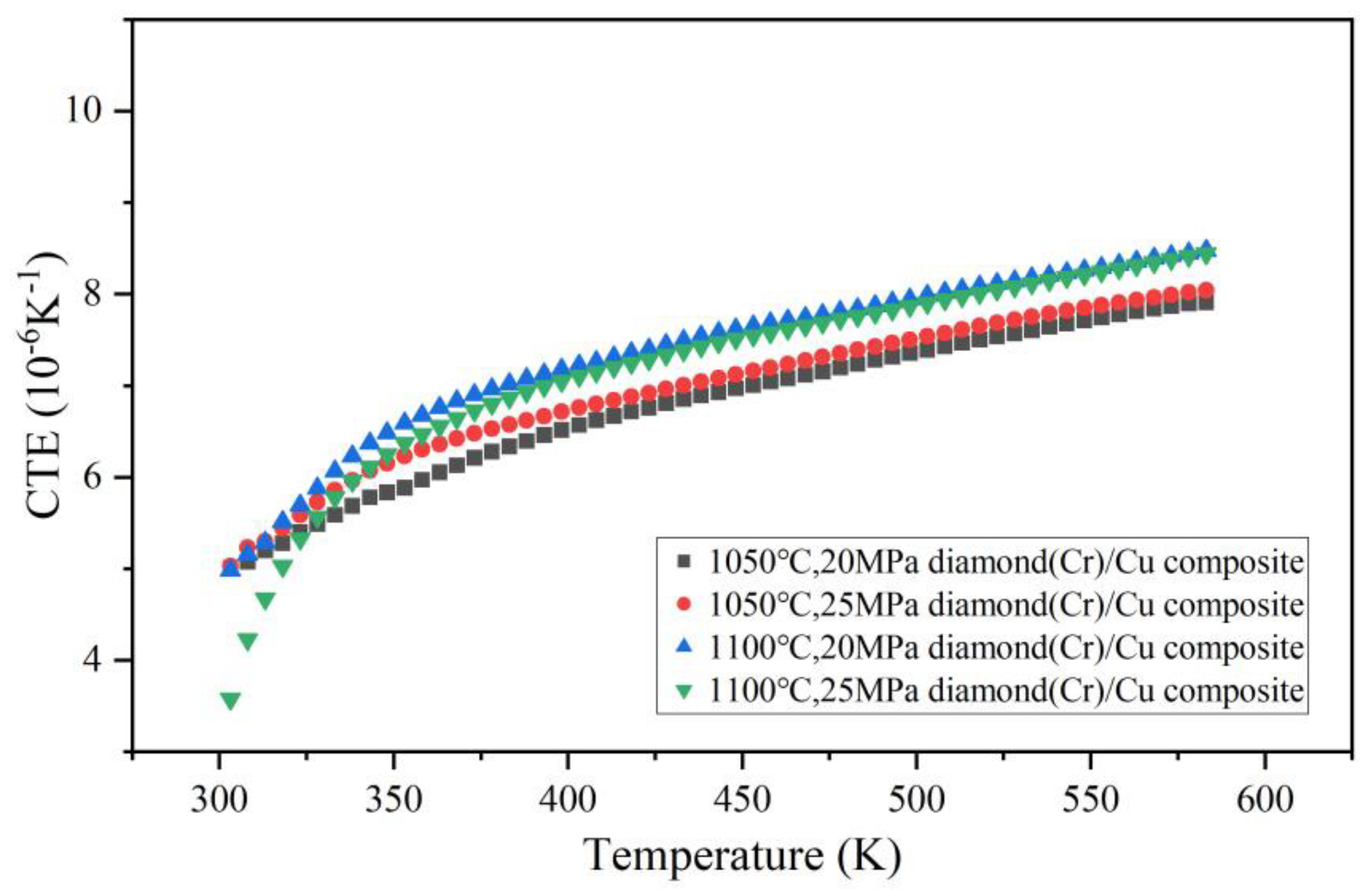
3.4. Thermal Expansion Coefficient of Cr–Diamond/Copper Composite Materials
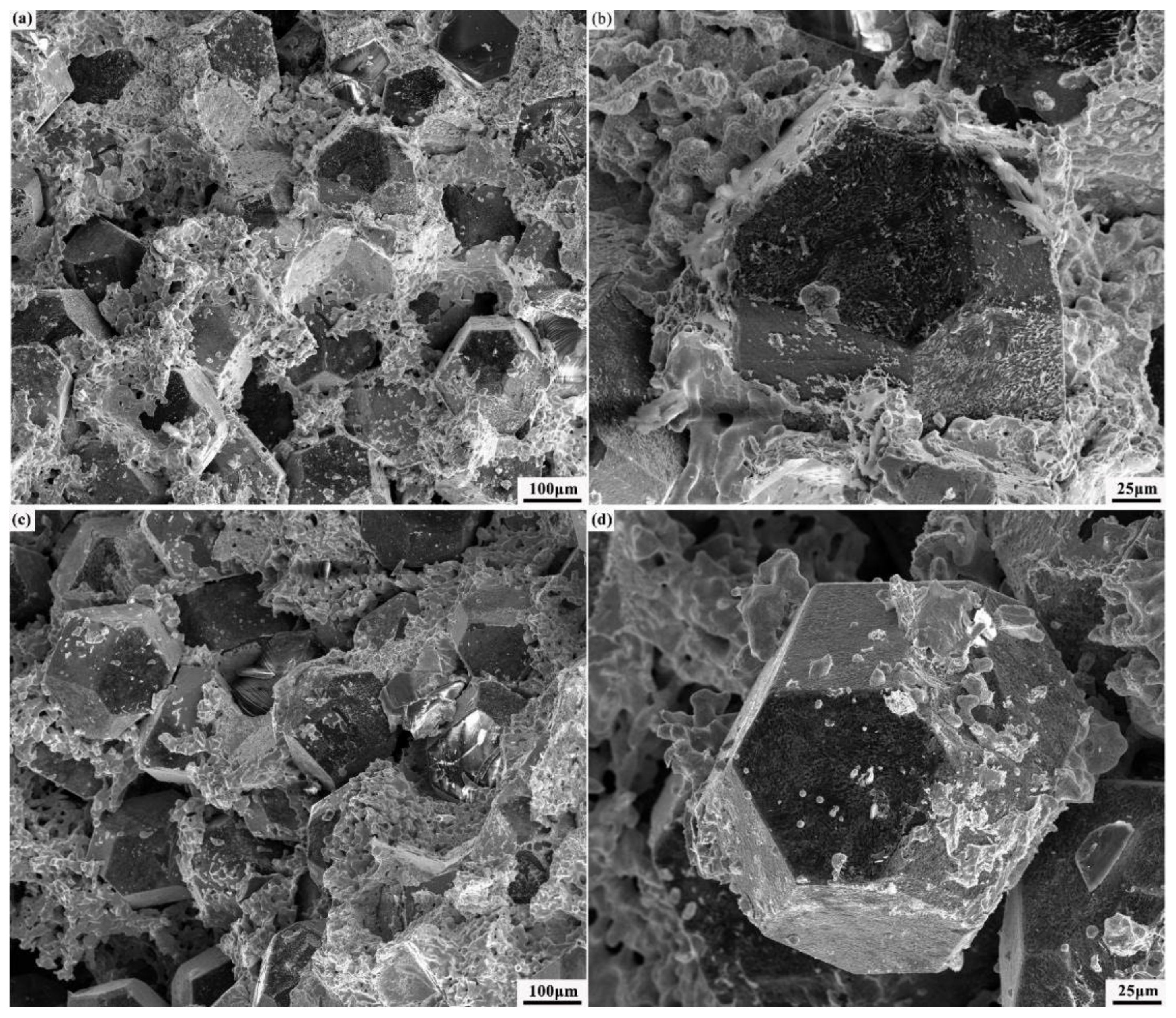
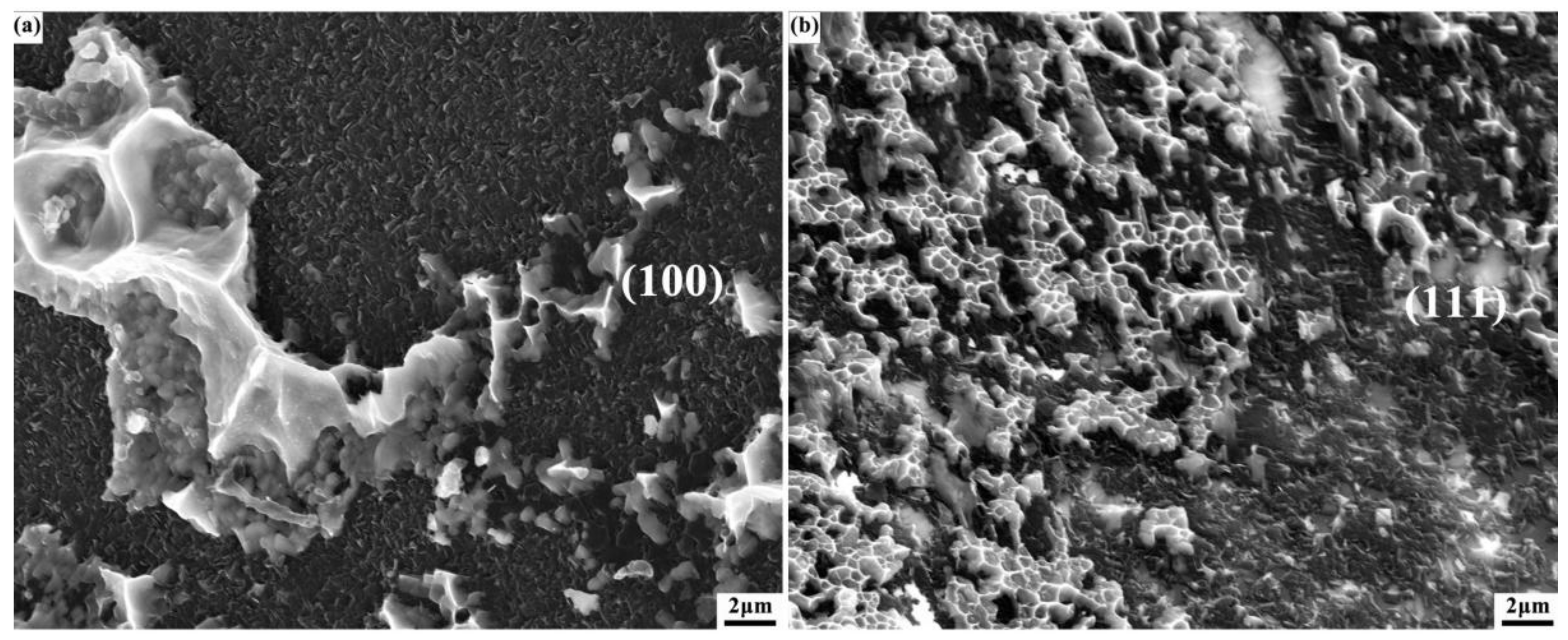
3.5. Fracture Mechanism of Cr–Diamond/Copper Composite Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arai, S.; Ueda, M. Fabrication of high thermal conductivity copper/diamond composites by electrodeposition under potentiostatic conditions. J. Appl. Electrochem. 2020, 50, 631–638. [Google Scholar] [CrossRef]
- Azina, C.; Cornu, I.; Silvain, J.-F.; Lu, Y.; Battaglia, J.-L. Effect of titanium and zirconium carbide interphases on the thermal conductivity and interfacial heat transfers in copper/diamond composite materials. AIP Adv. 2019, 9, 055315. [Google Scholar] [CrossRef]
- Wort, C.J.; Balmer, R.S. Diamond as an electronic material. Mater. Today 2008, 11, 22–28. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Bai, G.; Li, N.; Wang, X.; Zhang, H.; Wang, J. Interfacial structure evolution and thermal conductivity of Cu-Zr/diamond composites prepared by gas pressure infiltration. J. Alloys Compd. 2019, 781, 800–809. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Wang, J.; Chang, J. Effect of tungsten based coating characteristics on microstructure and thermal conductivity of diamond/Cu composites prepared by pressueless infiltration. Ceram. Int. 2019, 45, 10810–10818. [Google Scholar] [CrossRef]
- Guillemet, T.; Heintz, J.M.; Mortaigne, B.; Lu, Y.; Silvain, J.F. Formation of Cu nanodots on diamond surface to improve heat transfer in Cu/D composites. Adv. Eng. Mater. 2017, 20, 1700894. [Google Scholar] [CrossRef]
- Yunlong, W.; Kaiyue, D.; Kaikun, W.; Zhigang, D.; Zhihong, X. Structure and thermal properties of layered Ti-clad diamond/Cu composites prepared by SPS and HP. Rare Met. Mater. Eng. 2018, 47, 2011–2016. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhou, K.; Jiao, Z.; Yao, Y.; Wu, J.; Ma, L.; Wei, Q. Thermal conductivity of tungsten-modified diamond/copper-boron composites by vacuum vapor deposition. Chin. J. Nonferrous Met. 2024, 1–15. [Google Scholar]
- Wu, D.; Chen, W.; Hu, Q.; Zhang, L.; Liu, Q.; Liu, J.; Huang, G.; Jia, J.; Zou, J. Effects of Diamond Content on the Thermal Conductivity of Copper Matrix Composite Materials Prepared by Cold Spraying. J. Phys. Conf. Ser. 2024, 2694, 012029. [Google Scholar] [CrossRef]
- Ziang, Z.; Sun, F.; Feng, Y. Analysis of the effect of interfacial thermal conductivity on the thermal conductivity of copper/diamond composites. J. Eng. Thermophys. 2023, 44, 2514–2520. [Google Scholar]
- Ma, J.N.; Bolzoni, L.; Yang, F. Interface manipulation and its effects on the resultant thermal conductivity of hot-forged copper/Ti-coated diamond composites. J. Alloys Compd. 2021, 868, 159182. [Google Scholar] [CrossRef]
- Lei, L.; Bolzoni, L.; Yang, F. High thermal conductivity and strong interface bonding of a hot-forged Cu/Ti-coated-diamond composite. Carbon 2020, 168, 553–563. [Google Scholar] [CrossRef]
- Schubert, T.; Trindade, B.; Weißgärber, T.; Kieback, B. Interfacial design of Cu-based composites prepared by powder metallurgy for heat sink applications. Mater. Sci. Eng. A 2008, 475, 39–44. [Google Scholar] [CrossRef]
- Hagio, T.; Park, J.H.; Naruse, Y.; Goto, Y.; Kamimoto, Y.; Ichino, R.; Bessho, T. Electrodeposition of nano-diamond/copper composite platings: Improved interfacial adhesion between diamond and copper via formation of silicon carbide on diamond surface. Surf. Coat. Technol. 2020, 403, 126322. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Yu, G.; Du, R.; He, P. A novel ultrasonic consolidation method for rapid preparation of diamond/Cu composites. Mater. Lett. 2022, 323, 132498. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, S.; Gong, P.; Chen, Q.; Geng, H.; Sun, M.; Sun, Q.; Hao, L. Fabrication of titanium and copper-coated diamond/copper composites via selective laser melting. Micromachines 2022, 13, 724. [Google Scholar] [CrossRef]
- Constantin, L.; Fan, L.; Pontoreau, M.; Wang, F.; Cui, B.; Battaglia, J.L.; Silvain, J.F.; Lu, Y.F. Additive manufacturing of copper/diamond composites for thermal management applications. Manuf. Lett. 2020, 24, 61–66. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, J.; Jiang, Y.; Xu, D.; Guan, Y.; Huang, N.; Liu, L.; Jiang, X.; Yang, B. Tailoring thermal conductivity of diamond/InSnBi composite by surface treatment of diamonds. Diam. Relat. Mater. 2024, 146, 111231. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Wu, C.; Yin, Z.; Liu, C.; Huang, Y.; Liu, J.; Shi, Z. The interface and fabrication process of diamond/Cu composites with nanocoated diamond for heat sink applications. Metals 2021, 11, 196. [Google Scholar] [CrossRef]
- Wang, L.; Bai, G.; Li, N.; Gao, L.; Li, J.; Xu, K.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Unveiling interfacial structure and improving thermal conductivity of Cu/diamond composites reinforced with Zr-coated diamond particles. Vacuum 2022, 202, 111133. [Google Scholar] [CrossRef]
- AlHazaa, A.; Haneklaus, N.; Almutairi, Z. Impulse Pressure-Assisted Diffusion Bonding (IPADB): Review and Outlook. Metals 2021, 11, 323. [Google Scholar] [CrossRef]
- Liu, X.; Sun, F.; Wang, L.; Wu, Z.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. The role of Cr interlayer in determining interfacial thermal conductance between Cu and diamond. Appl. Surf. Sci. 2020, 515, 146046. [Google Scholar] [CrossRef]
- Chu, K.; Liu, Z.; Jia, C.; Chen, H.; Liang, X.; Gao, W.; Tian, W.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloys Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Cui, W.; Qu, X. Preparation of copper-diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Bai, Q.; Zhang, Y.; Wang, Y.; He, X. Study on the effect of microcrack defects on the damage failure of CVD diamond coated microtools. Surf. Technol. 2021, 50, 355–362. [Google Scholar]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Ukhina, A.V.; Dudina, D.V.; Esikov, M.A.; Samoshkin, D.A.; Stankus, S.V. The Influence of the Carbide-Forming Metallic Additives (W, Mo, Cr, Ti) on the Microstructure and Thermal Conductivity of Copper–Diamond Composites. J. Compos. Sci. 2023, 7, 219. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, Y.; Zhang, F.; Fan, T.; Zhang, D. Unveiling the interfacial configuration in diamond/Cu composites by using statistical analysis of metallized diamond surface. Scr. Mater. 2018, 152, 84–88. [Google Scholar] [CrossRef]
- Chang, G.; Sun, F.; Wang, L.; Zhang, Y.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Mo-interlayer-mediated thermal conductance at Cu/diamond interface measured by time-domain thermoreflectance. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105921. [Google Scholar] [CrossRef]
- Sang, J.; Zhou, L.; Yang, W.; Zhu, J.; Fu, L.; Li, D. Enhanced thermal conductivity of copper/diamond composites by fine-regulating microstructure of interfacial tungsten buffer layer. J. Alloys Compd. 2021, 856, 157440. [Google Scholar] [CrossRef]
- Ren, S.; Shen, X.; Guo, C.; Liu, N.; Zang, J.; He, X.; Qu, X. Effect of coating on the microstructure and thermal conductivities of diamond-Cu composites prepared by powder metallurgy. Compos. Sci. Technol. 2011, 71, 1550–1555. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Che, Z.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Combining Cr pre-coating and Cr alloying to improve the thermal conductivity of diamond particles reinforced Cu matrix composites. J. Alloys Compd. 2018, 749, 1098–1105. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, W.; Xu, M.; Cao, S.; Feng, Y.; Zhou, H. Improvement of diamond/copper composite interface by adding rare earth Nd. Surf. Technol. 2018, 47, 27–32. [Google Scholar]
- Zhou, L.; Liu, J.; Ding, R.; Cao, J.; Zhan, K.; Zhao, B. A review of diamond interfacial modification and its effect on the properties of diamond/Cu matrix composites. Surf. Interfaces 2023, 40, 103143. [Google Scholar] [CrossRef]
- Jia, X.; Wei, J.; Huang, Y.; Shao, S.; Kong, Y.; Liu, J.; Chen, L.; Li, C.; Ye, H. Progress of diamond thermal dissipation substrates in GaN-based power devices. Surf. Technol. 2020, 49, 111–123. [Google Scholar]



| No. | Chromium Layer Thickness/(nm) | Density /(g∙cm3) | Compactness /(%) | Thermal Diffusivity /(mm2∙s−1) | Specific Heat Capacity/(J∙g−1∙K−1) | TC /(W∙m−1∙K−1) |
|---|---|---|---|---|---|---|
| 1 | 0 | 5.18 | 90.99 | 20.76 | 0.44 | 47.29 |
| 2 | 150 | 5.51 | 95.57 | 78.66 | 0.40 | 173.28 |
| 3 | 200 | 5.56 | 96.04 | 72.79 | 0.40 | 161.77 |
| No. | Temperature /°C | Pressure /MPa | Density /(g∙cm−3) | Compactness /% | Thermal Diffusivity /(mm2∙s−1) | Specific Heat Capacity /(J∙g−1∙K−1) | TP /(W∙m−1∙K−1) |
|---|---|---|---|---|---|---|---|
| 1 (without Cr) | 850 | 20 | 5.27 | 92.5 | 63.40 | 0.38 | 127.14 |
| 2 | 850 | 20 | 5.40 | 94.6 | 199.87 | 0.39 | 417.52 |
| 3 | 1050 | 20 | 5.43 | 95.2 | 235.29 | 0.45 | 593.67 |
| 4 | 1050 | 25 | 5.58 | 97.8 | 251.55 | 0.40 | 590.14 |
| 5 | 1050 | 30 | 5.36 | 93.9 | 227.18 | 0.43 | 578.08 |
| 6 | 1100 | 20 | 5.40 | 94.6 | 265.68 | 0.41 | 580.54 |
| 7 | 1100 | 25 | 5.43 | 95.2 | 251.27 | 0.42 | 587.15 |
| Sample | Thermal Diffusion Coefficient/(mm2∙s−1) | |||
|---|---|---|---|---|
| 298 K | 373 K | 473 K | 573 K | |
| 1050 °C, 20 MPa, 45 μm diamond | ||||
| 1100 °C, 20 MPa, 45 μm diamond | ||||
| 1050 °C, 20 MPa, 210 μm diamond | ||||
| 1050 °C, 25 MPa, 210 μm diamond | ||||
| 1100 °C, 20 MPa, 210 μm diamond | ||||
| 1100 °C, 25 MPa, 210 μm diamond | ||||
| Sample | Specific Heat Capacity/(J∙g−1∙s−1) | |||
|---|---|---|---|---|
| 298 K | 373 K | 473 K | 573 K | |
| 1050 °C, 20 MPa, 45 μm diamond | ||||
| 1100 °C, 20 MPa, 45μm diamond | ||||
| 1050 °C, 20 MPa, 210μm diamond | ||||
| 1050 °C, 25 MPa, 210μm diamond | ||||
| 1100 °C, 20 MPa, 210μm diamond | ||||
| 1100 °C, 25 MPa, 210μm diamond | ||||
| No. | Temperature /°C | Pressure /MPa | Thermal Expansion Coefficient /(×10−6K−1) | ||
|---|---|---|---|---|---|
| 25~100 °C | 25~200 °C | 25~300 °C | |||
| 1 | 1050 | 20 | 6.37 | 7.25 | 7.92 |
| 2 | 1050 | 25 | 6.48 | 7.31 | 7.99 |
| 3 | 1100 | 20 | 6.90 | 7.78 | 8.42 |
| 4 | 1100 | 25 | 6.73 | 7.69 | 8.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Cao, X.; Liu, Y.; Xu, Y.; Wu, J. RETRACTED: Cr–Diamond/Cu Composites with High Thermal Conductivity Fabricated by Vacuum Hot Pressing. Materials 2024, 17, 3711. https://doi.org/10.3390/ma17153711
Xu Q, Cao X, Liu Y, Xu Y, Wu J. RETRACTED: Cr–Diamond/Cu Composites with High Thermal Conductivity Fabricated by Vacuum Hot Pressing. Materials. 2024; 17(15):3711. https://doi.org/10.3390/ma17153711
Chicago/Turabian StyleXu, Qiang, Xiaodie Cao, Yibo Liu, Yanjun Xu, and Jiajun Wu. 2024. "RETRACTED: Cr–Diamond/Cu Composites with High Thermal Conductivity Fabricated by Vacuum Hot Pressing" Materials 17, no. 15: 3711. https://doi.org/10.3390/ma17153711
APA StyleXu, Q., Cao, X., Liu, Y., Xu, Y., & Wu, J. (2024). RETRACTED: Cr–Diamond/Cu Composites with High Thermal Conductivity Fabricated by Vacuum Hot Pressing. Materials, 17(15), 3711. https://doi.org/10.3390/ma17153711






