Alendronate as Bioactive Coating on Titanium Surfaces: An Investigation of CaP–Alendronate Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Quantum Chemical Calculations
2.2. Materials, Chemicals and Solutions
2.3. Functionalisation of the Titanium Samples
2.4. Spontaneous Formation of Calcium Phosphates (CaP) on the Titanium Samples
2.5. Characterisation of the Titanium Samples
3. Results and Discussion
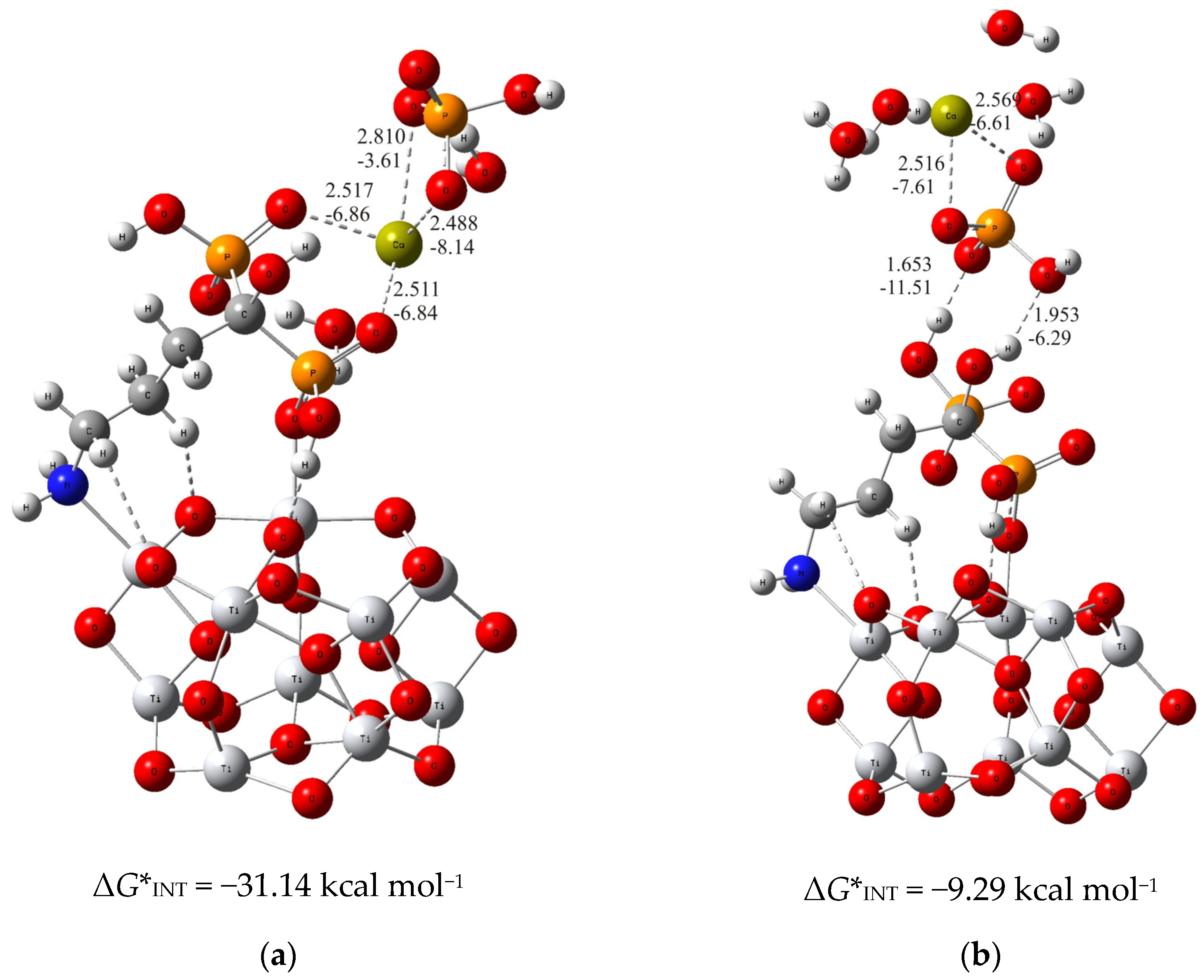
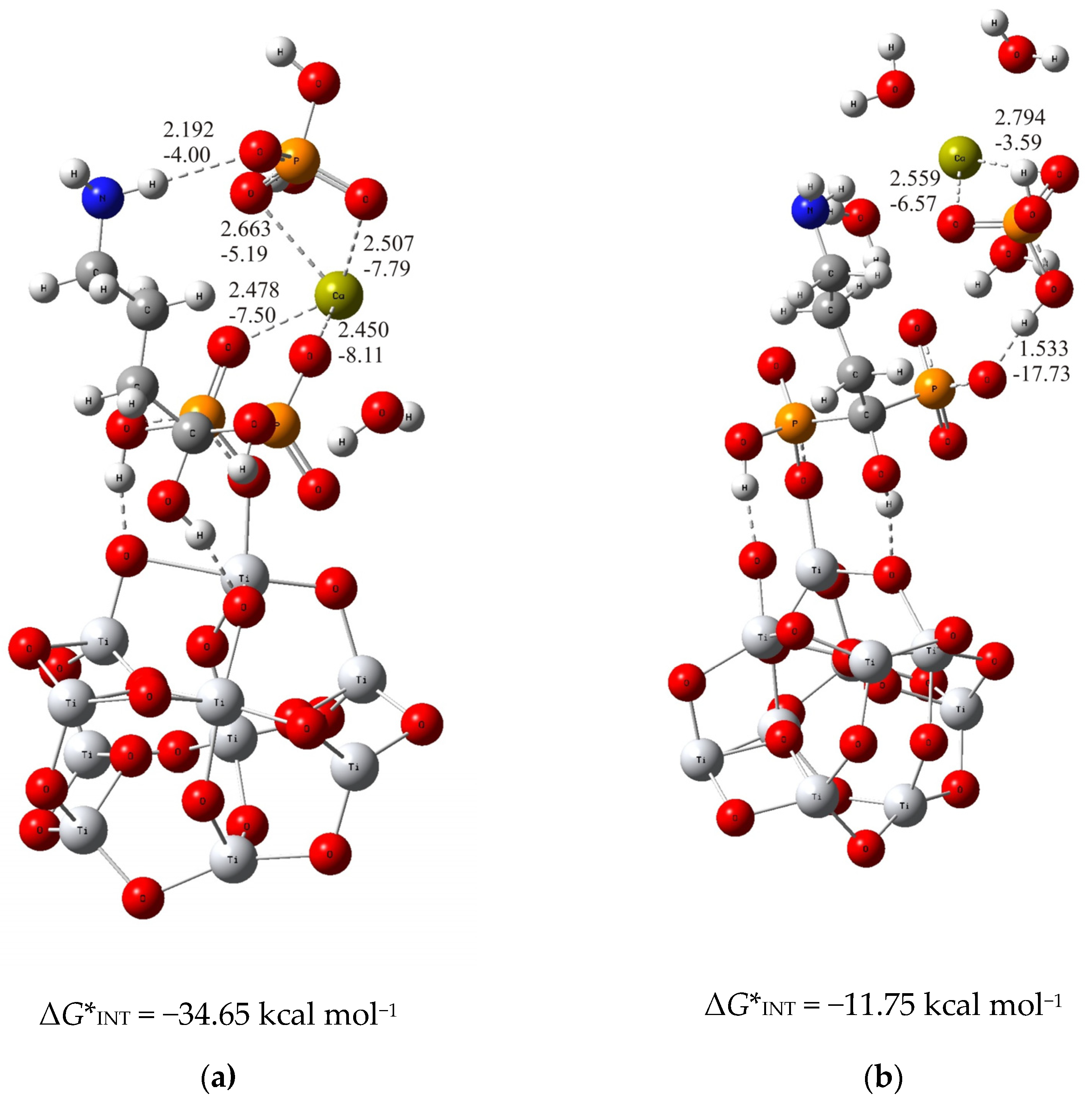
3.1. The Mechanism of Interaction of Calcium and Phosphate Ions with Ti/Oxide/Alendronate
3.2. Experimental Evidence for the Spontaneous Formation of Calcium Phosphates (CaP) on the Ti/oxide/Alendronate Surface
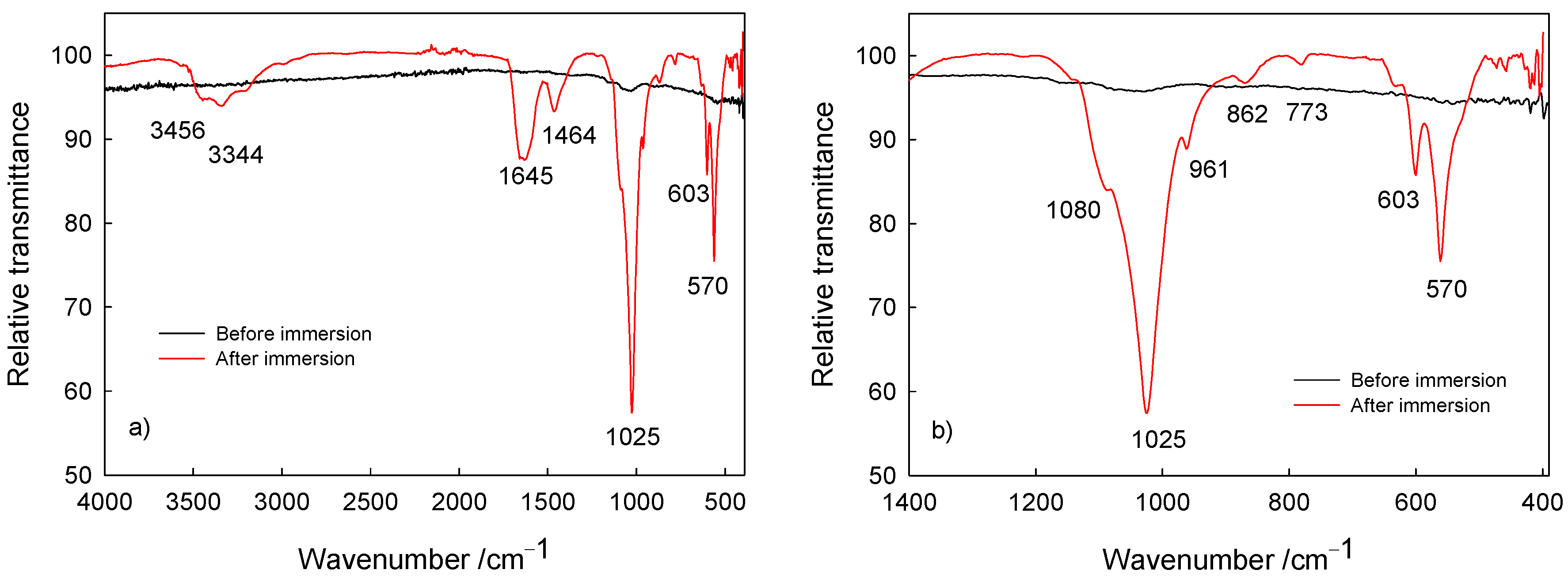
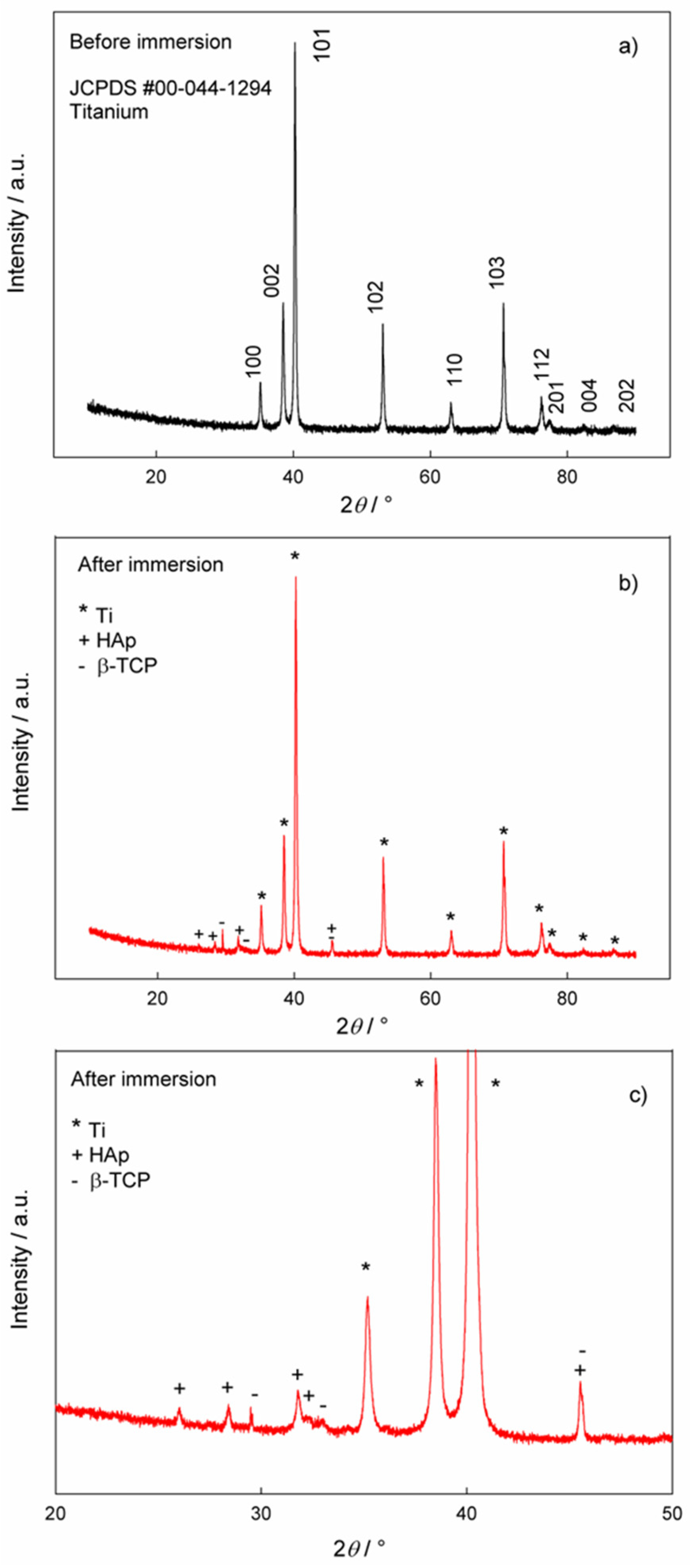
Morphology, Chemical and Phase Analysis of Ti/Oxide/Alendronate Samples after 100 Days Immersion in Artificial Saliva
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunete, D.M.; Tangvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine, 1st ed.; Springer: Berlin, Germany, 2001. [Google Scholar]
- Salernitano, E.; Migliaresi, C. Composite Materials for Biomedical Applications: A Review. J. Appl. Biomater. Biomech. 2003, 1, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Breme, J.; Biehl, V.; Hoffman, A. Tailor-Made Composites Based on Titanium for Medical Devices. Adv. Eng. Mater. 2000, 2, 270–275. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Saman, M.Z.M. A Comprehensive Review of Biomaterials and Its Characteristics for Bone Tissue Engineering Scaffold. J. Med. Device Technol. 2023, 2, 56–62. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 45, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Biocompatibility of titanium from the viewpoint of its surface. Sci. Technol. Adv. Mater. 2022, 23, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium-based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 603072. [Google Scholar] [CrossRef]
- Petrović, Ž.; Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Kralj, D.; Leskovac, M.; Petković, M. Influence of Biocompatible Coating on Titanium Surface Characteristics. ICMS 2020, 10, 37–46. [Google Scholar] [CrossRef]
- Vyas, V.; Kaur, T.; Kar, S.; Thirugnanam, A. Biofunctionalization of commercially pure titanium with chitosan/hydroxyapatite biocomposite via silanization: Evaluation of biological performances. J. Adhes. Sci. Technol. 2017, 31, 1768–1781. [Google Scholar] [CrossRef]
- Sharma, A.; Kokil, G.R.; He, Y.; Lowe, B.; Salam, A.; Altalhi, T.A.; Ye, Q.; Kumeria, T. Inorganic/organic combination: Inorganic particles/polymer composites for tissue engineering applications. Bioact. Mater. 2023, 24, 535–550. [Google Scholar] [CrossRef]
- Nayak, G.S.; Carradò, A.; Masson, P.; Pourroy, G.; Mouillard, F.; Migonney, V.; Falentin-Daudre, C.; Pereira, C.; Palkowski, H. Trends in Metal-Based Composite Biomaterials for Hard Tissue Applications. JOM 2022, 74, 102–125. [Google Scholar] [CrossRef]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Petković, M. A New Insight into Coating’s Mechanism Between TiO2 and Alendronate on Titanium Dental Implant. Materials 2020, 13, 3220. [Google Scholar] [CrossRef]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Ivanda, M.; Petković, M. Surface Functionalisation of Dental Implants with a Composite coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials 2022, 15, 5127. [Google Scholar] [CrossRef]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed]
- Meraw, S.J.; Reeve, C.M.; Wollan, P.C. Use of alendronate in peri-implant defect regeneration. J. Periodontol. 1999, 70, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Meraw, S.J.; Reeve, C.M. Qualitative analysis of peripheral peri-implant bone and influence of alendronate sodium on early bone regeneration. J. Periodontol. 1999, 70, 1228–1233. [Google Scholar] [CrossRef]
- Abtahi, J.; Tengvall, P.; Aspenberg, P. A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 2012, 50, 1148–1151. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwanf, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, J.; Mante, F.K.; Wunder, S.L.; Baran, G.R. The role of surface functional groups in calcium phosphate nucleation on titanium foil: A self-assembled monolayer technique. Biomaterials 2002, 23, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, W.; Zhang, Y.; Zhong, M. Biomimetic synthesis of enamel-like hydroxyapatite on self-assembled monolayers. Mater. Sci. Eng. C 2007, 27, 756–761. [Google Scholar] [CrossRef]
- Majewski, P.J.; Allidi, G. Synthesis of hydroxyapatite on titanium coated with organic self-assembled monolayers. Mater. Sci. Eng. A 2006, 420, 13–20. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Zeller, A.; Musyanovych, A.; Kappl, M.; Ethirajan, A.; Dass, M.; Markova, D.; Klapper, M.; Landfester, K. Nanostructured Coatings by Adhesion of Phosphonated Polystyrene Particles onto Titanium Surface for Implant Material Applications. ACS Appl. Mater. Interfaces 2010, 2, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, Y.; Zhang, Q.; Yu, L.; Hu, Z.; Liu, Y. Surface phosphonation treatment shows dose-dependent enhancement of the bioactivity of polyetheretherketone. RSC Adv. 2019, 9, 30076–30086. [Google Scholar] [CrossRef]
- Chang, R.; Liu, Y.-J.; Zhang, Y.-L.; Zhang, S.-Y.; Han, B.-B.; Chen, F.; Chen, Y.-X. Phosphorylated and Phosphonated Low-Complexity Protein Segments for Biomimetic Mineralization and Repair of Tooth Enamel. Adv. Sci. 2022, 9, 2103829. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Gao, Y.; Cheng, F.; Xiao, G.G.; Wang, J.; Jian, X. Apatite Formation on Poly(aryl ether sulfone ketone) Surfaces by Means of Polydopamine Layers Functionalized with Phosphonate Groups. Adv. Mater. Interfaces 2018, 5, 1800003. [Google Scholar] [CrossRef]
- Tan, G.; Ouyang, K.; Wang, H.; Zhou, L.; Wang, X.; Liu, Y.; Zhang, L.; Ning, C. Effect of Amino-, Methyl- and Epoxy-Silane Coupling as a Molecular Bridge for Formatting a Biomimetic Hydroxyapatite Coating on Titanium by Electrochemical Deposition. J. Mater. Sci. Technol. 2016, 32, 956–965. [Google Scholar] [CrossRef]
- Yakufu, M.; Wang, Z.; Wang, Y.; Jiao, Z.; Guo, M.; Liu, J.; Zhang, P. Covalently functionalized poly(etheretherketone) implants with osteogenic growth peptide (OGP) to improve osteogenesis activity. RSC Adv. 2020, 10, 9777–9785. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bodón, J.; Andrade Del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2021, 31, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Thrular, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density Functional Theory for Reaction Energies: Test of Meta and Hybrid Meta Functionals, Range-Separated Functionals, and Other High-Performance Functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Srinivasa Rao, J.; Dinadayalane, T.C.; Leszczynski, J.; Sastry, N. Comprehensive Study on the Solvation of Mono- and Divalent Metal Cations: Li+, Na+, K+, Be2+, Mg2+ and Ca2+. J. Phys. Chem. A 2008, 112, 12944–12953. [Google Scholar] [CrossRef]
- Pliego, J.R.; Riveros, J.M. The Cluster-Continuum Model for the Calculation of the Solvation Free Energy of Ionic Species. J. Phys. Chem. A 2001, 105, 7241–7247. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, NY, USA, 1994. [Google Scholar]
- Keith, T.A. AIMAll, Version 17.01.25; TK Gristmill Software; Overland Park: Johnson County, KS, USA, 2017; Available online: https://aim.tkgristmill.com/ (accessed on 18 January 2024).
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Qu, Z.; Kroes, G.-J. Theoretical Study of Stable, Defect-Free (TiO2)n Nanoparticles with n = 10−16. J. Phys. Chem. C 2007, 111, 16808–16817. [Google Scholar] [CrossRef]
- Katić, J.; Krivačić, S.; Petrović, Ž.; Mikić, D.; Marciuš, M. Titanium Implant Alloy Modified by Electrochemically Deposited Functional Bioactive Calcium Phosphate Coatings. Coatings 2023, 13, 640. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Wang, J.; Qu, S.; Weng, J.; Xin, R.; Leng, Y. Calcium phosphate crystal growth under controlled environment through urea hydrolysis. J. Cryst. Growth 2006, 297, 396–402. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ono, S.; Hirakura, S.; Oaki, Y.; Imai, H. Morphological variation of hydroxyapatite grown in aqueous solution based on simulated body fluid. CrystEngComm 2012, 14, 1143–1149. [Google Scholar] [CrossRef]
- Beaufils, S.; Rouillon, T.; Millet, P.; Le Bideau, J.; Weiss, P.; Chopart, J.P.; Daltin, A.L. Synthesis of calcium-deficient hydroxyapatite nanowires and nanotubes performed by template-assisted electrodeposition. Mater. Sci. Eng. C 2019, 98, 333–346. [Google Scholar] [CrossRef]
- Lin, K.; Zhou, Y.; Zhou, Y.; Qu, H.; Chen, F.; Zhu, Y.; Chang, J. Biomimetic hydroxyapatite porous microspheres with co-substituted essential trace elements: Surfactant-free hydrothermal synthesis, enhanced degradation and drug release. J. Mater Chem 2011, 21, 16558–16565. [Google Scholar] [CrossRef]
- Nespoli, A.; Passaretti, F.; Szentmiklósi, L.; Maróti, B.; Placidi, E.; Cassetta, M.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Biomedical NiTi and β-Ti Alloys: From Composition, Microstructure and Thermo-Mechanics to Application. Metals 2022, 12, 406. [Google Scholar] [CrossRef]
- Ochiuz, L.; Grigoras, C.; Popa, M.; Stoleriu, I.; Munteanu, C.; Timofte, D.; Profire, L.; Grigoras, A.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. [Google Scholar] [CrossRef] [PubMed]
- Albano, C.S.; Gomes, A.M.; da Silva Feltran, G.; da Costa Fernandes, C., Jr.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Biofunctionalization of titanium surfaces with alendronate and albumin modulates osteoblast performance. Heliyon 2020, 6, e04455. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 123–148. [Google Scholar]
- Chukanov, N.V.; Chervonnyi, A.D. IR Spectra of Minerals and Related Compounds, and Reference Samples’ Data. In Infrared Spectroscopy of Minerals and Related Compounds; Chukanov, N.V., Chervonnyi, A.D., Eds.; Springer: Cham, Switzerland, 2015; pp. 51–1047. [Google Scholar]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-K.; Im, J.-B.; Hwang, M.-J.; Kim, B.-J.; Kim, H.-Y.; Park, Y.-J. Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys. Metals 2015, 5, 850–862. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, Y.; Rho, H.T.; Kim, S. Microhardness and microstructural properties of a mixture of hydroxyapatite and b-tricalcium phosphate. J. Asian Ceram. Soc. 2023, 11, 11–17. [Google Scholar] [CrossRef]
- Vallet-Regı’, M.; Gonza’lez-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Allard, M.M.; Merlos, S.N.; Springer, B.N.; Cooper, J.; Zhang, G.; Boskovic, D.S.; Kwon, S.R.; Nick, K.E.; Perry, C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C 2018, 122, 29237–29248. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Borissova, A.O.; Antipin, M.Y.; Karapetyan, H.A.; Petrosyan, A.M.; Lyssenko, K.A. Cooperativity effects of H-bonding and charge transfer in an L-nitroarginine crystal with Z’ > 1. Mendeleev Commun. 2010, 20, 260–262. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Minaev, B.F.; Minaeva, V.A.; Nenajdenko, V.G. Single crystal architecture and absorption spectra of octathio[8]circulene and sym-tetraselenatetrathio[8]circulene: QTAIM and TD-DFT approach. J. Mol. Model. 2013, 19, 4511–4519. [Google Scholar] [CrossRef] [PubMed]
- Baryshnikov, G.V.; Minaev, B.F.; Korop, A.A.; Minaeva, V.A.; Gusev, A.N. Structure of zinc complexes with 3-(pyridin-2-yl)-5-(arylideneiminophenyl)-1HH-1,2,4-triazoles in different tautomeric forms: DFT and QTAIM study. Russ. J. Inorg. Chem. 2013, 58, 928–934. [Google Scholar] [CrossRef]
- Shahangi, F.; Chermahini, A.N.; Farrokhpour, H.; Teimouri, A. Selective complexation of alkaline earth metal ions with nanotubular cyclopeptides: DFT theoretical study. RSC Adv. 2014, 5, 2305–2317. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of EuIII-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 11095–11107. [Google Scholar] [CrossRef]

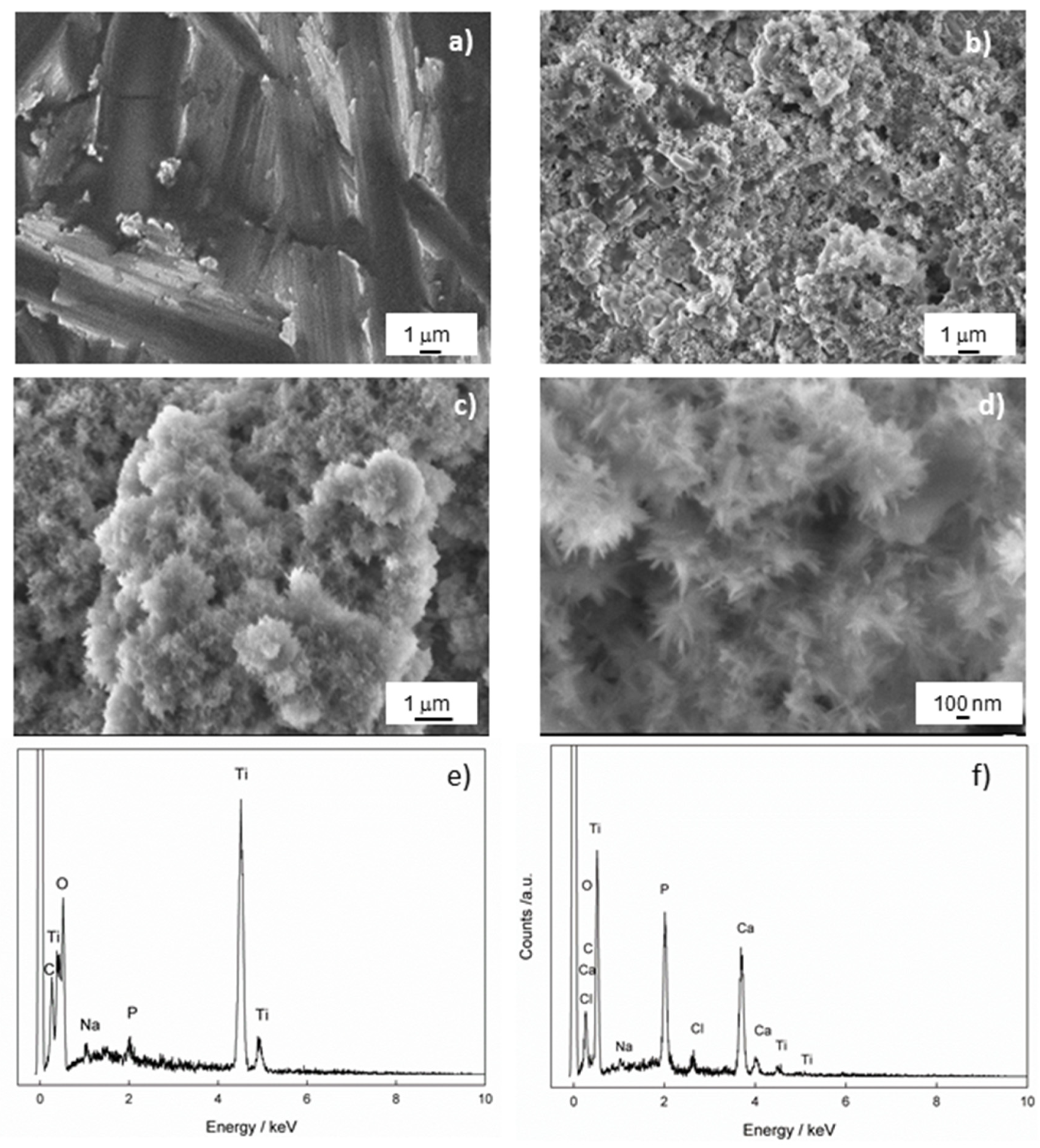
| Element, at. % | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Ti K | C K | O K | Na K | P K | Ca K | Cl K |
| Before immersion | 40.76 | 12.18 | 45.16 | 0.99 | 0.91 | / | / |
| After immersion | 1.41 | 16.68 | 54.22 | 0.49 | 10.41 | 15.74 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Despotović, I.; Petrović, Ž.; Katić, J.; Mikić, D. Alendronate as Bioactive Coating on Titanium Surfaces: An Investigation of CaP–Alendronate Interactions. Materials 2024, 17, 2703. https://doi.org/10.3390/ma17112703
Despotović I, Petrović Ž, Katić J, Mikić D. Alendronate as Bioactive Coating on Titanium Surfaces: An Investigation of CaP–Alendronate Interactions. Materials. 2024; 17(11):2703. https://doi.org/10.3390/ma17112703
Chicago/Turabian StyleDespotović, Ines, Željka Petrović, Jozefina Katić, and Dajana Mikić. 2024. "Alendronate as Bioactive Coating on Titanium Surfaces: An Investigation of CaP–Alendronate Interactions" Materials 17, no. 11: 2703. https://doi.org/10.3390/ma17112703
APA StyleDespotović, I., Petrović, Ž., Katić, J., & Mikić, D. (2024). Alendronate as Bioactive Coating on Titanium Surfaces: An Investigation of CaP–Alendronate Interactions. Materials, 17(11), 2703. https://doi.org/10.3390/ma17112703









