Static Compaction on Coupled Precursors and Optimizing Molarity for Enhanced Strength and Durability of Geopolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Testing
2.3.1. Physico-Mechanical Performance
2.3.2. Durability Performance
2.4. Sustainability Quantification
3. Results and Discussions
3.1. Optimizing Compressive Strength
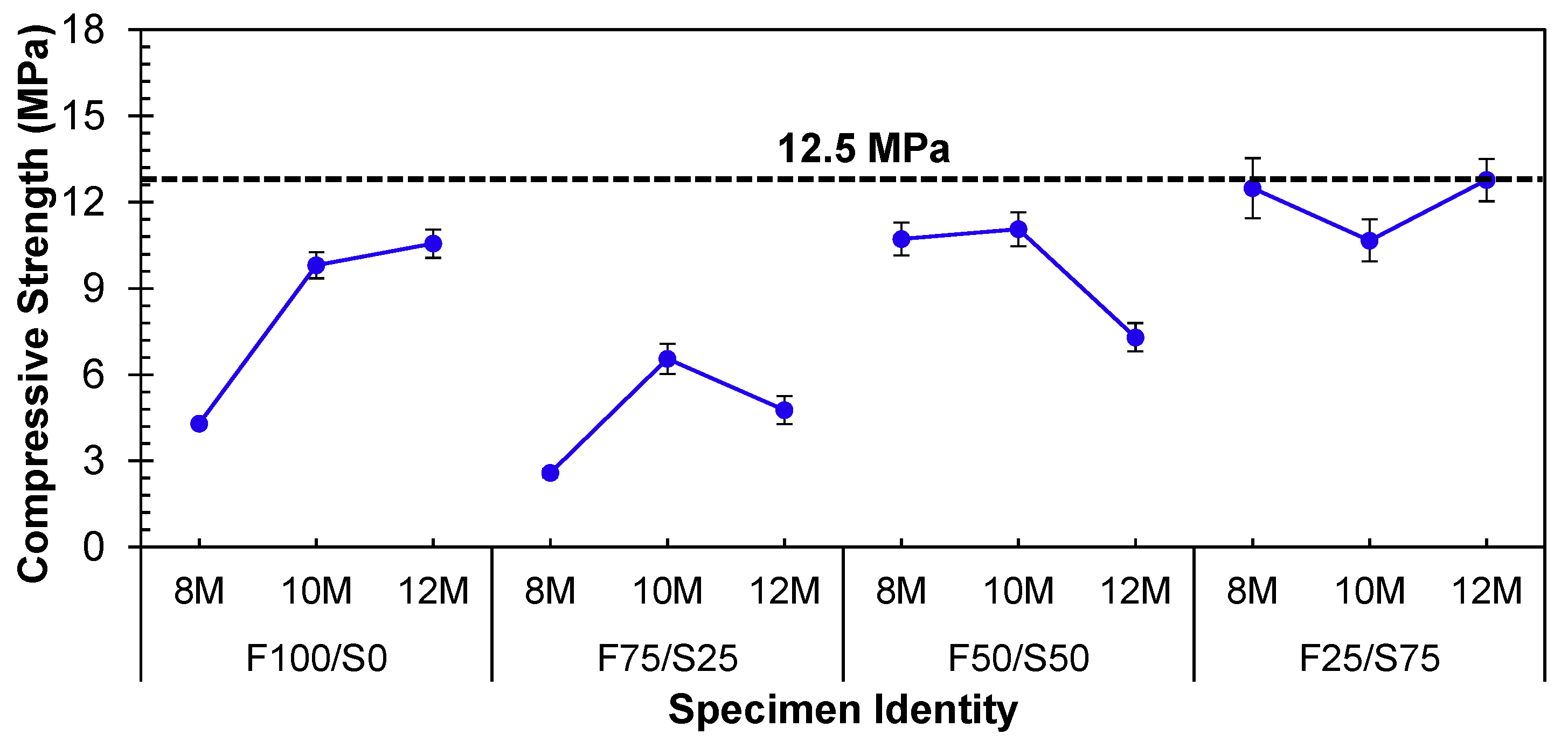
3.1.1. Impact of Molarity under Ambient Curing Conditions
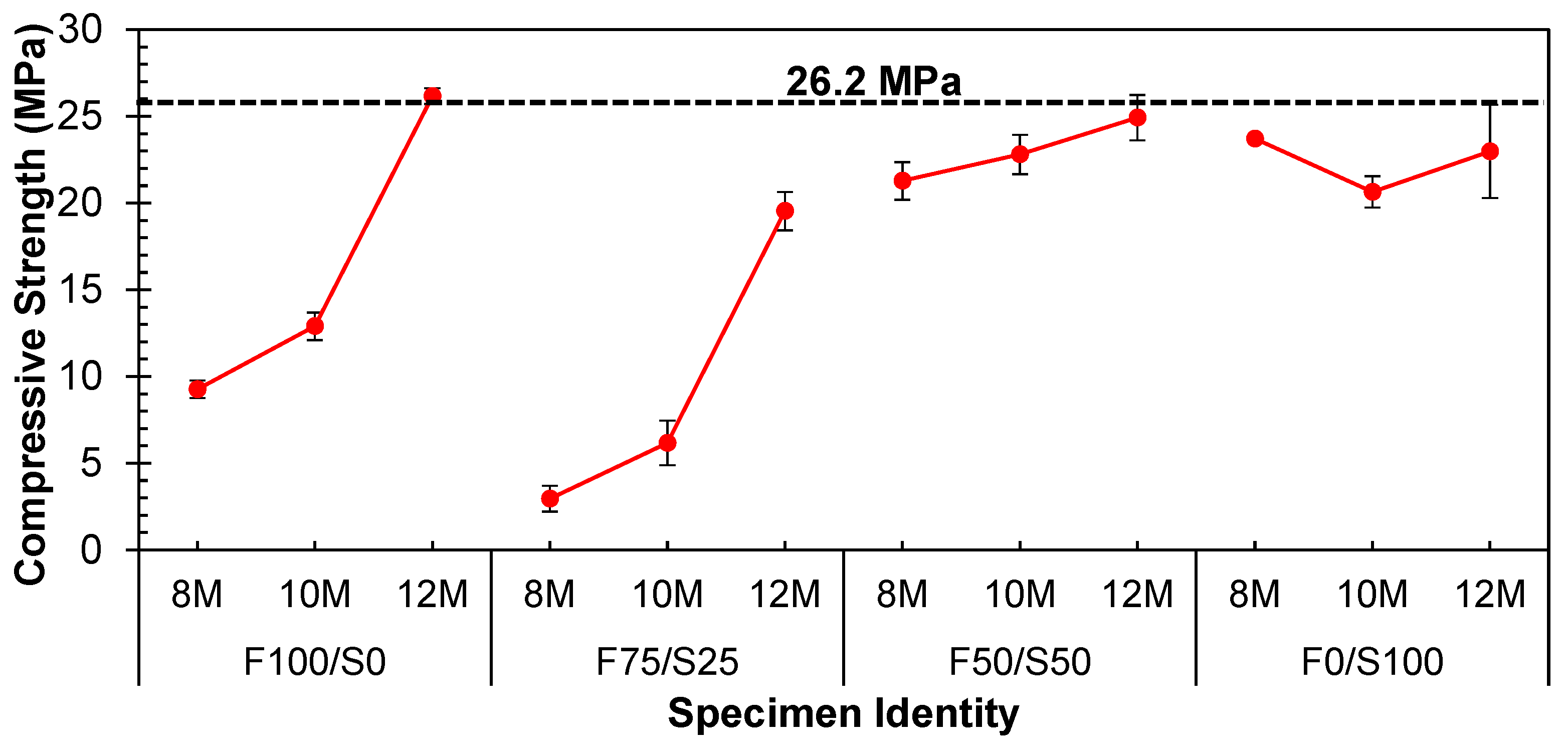
3.1.2. Impact of Molarity under Ambient Curing Conditions
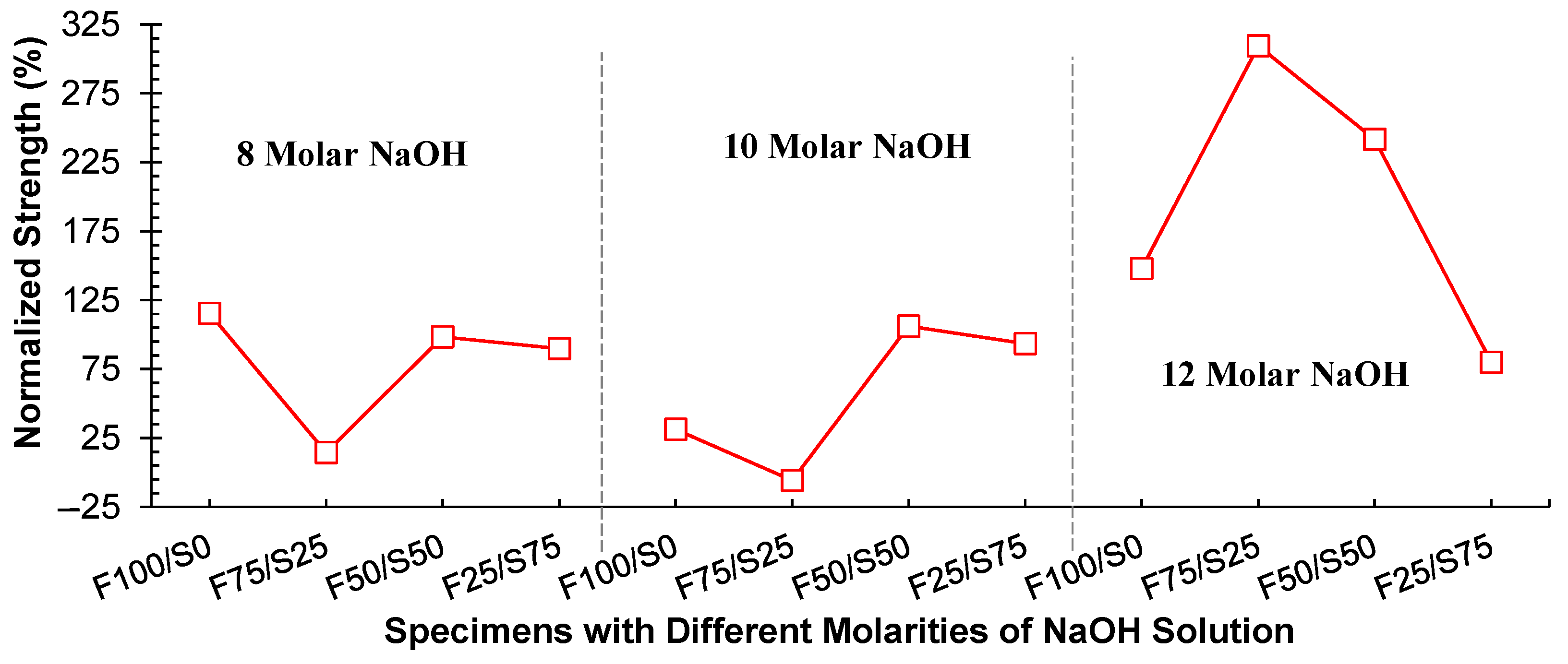
3.1.3. Comparison between Curing Conditions
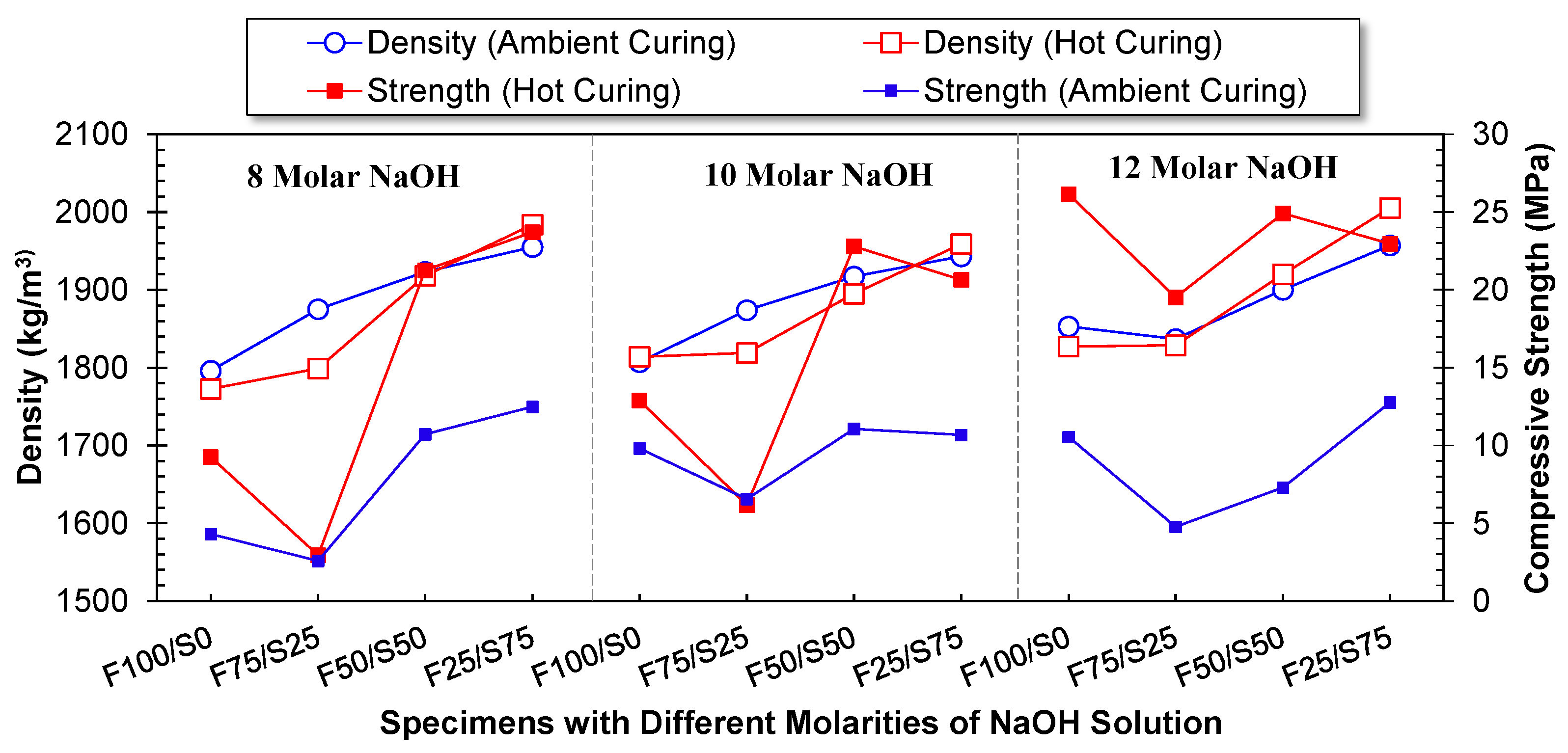
3.2. Bulk Density
3.3. Optimizing Specimen Based on Physico-Mechanical Performance
3.4. Durability of Structural Block
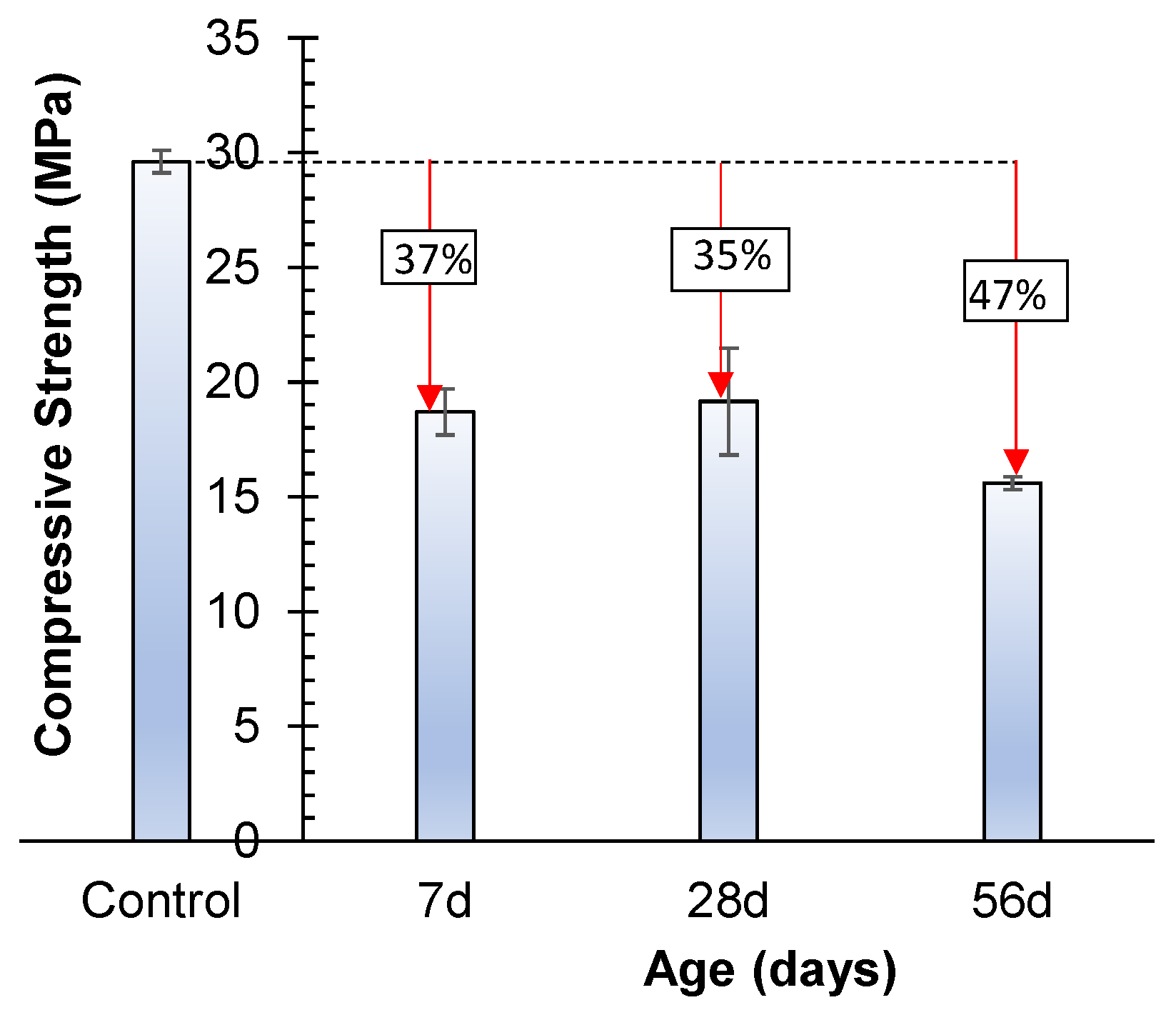
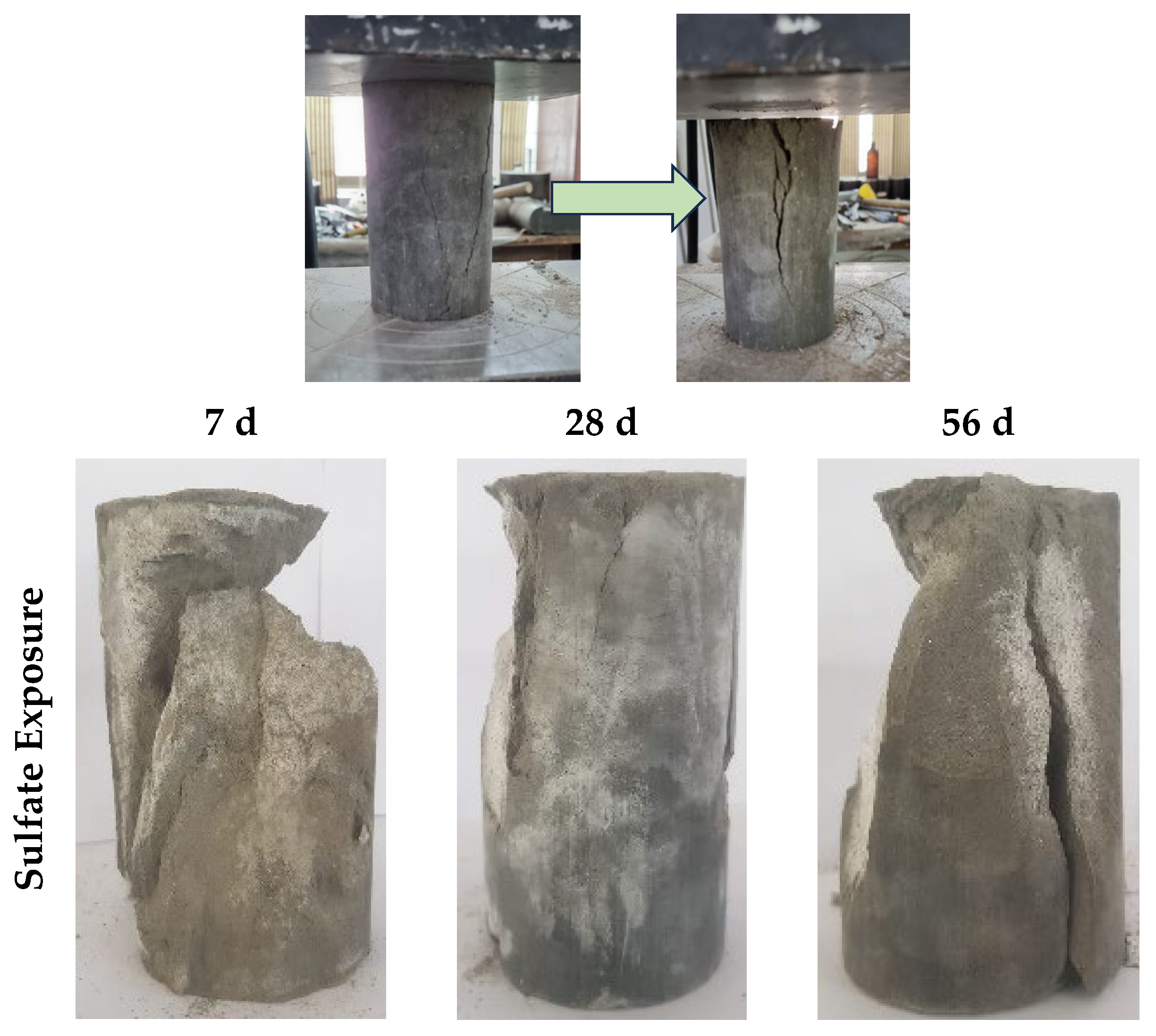
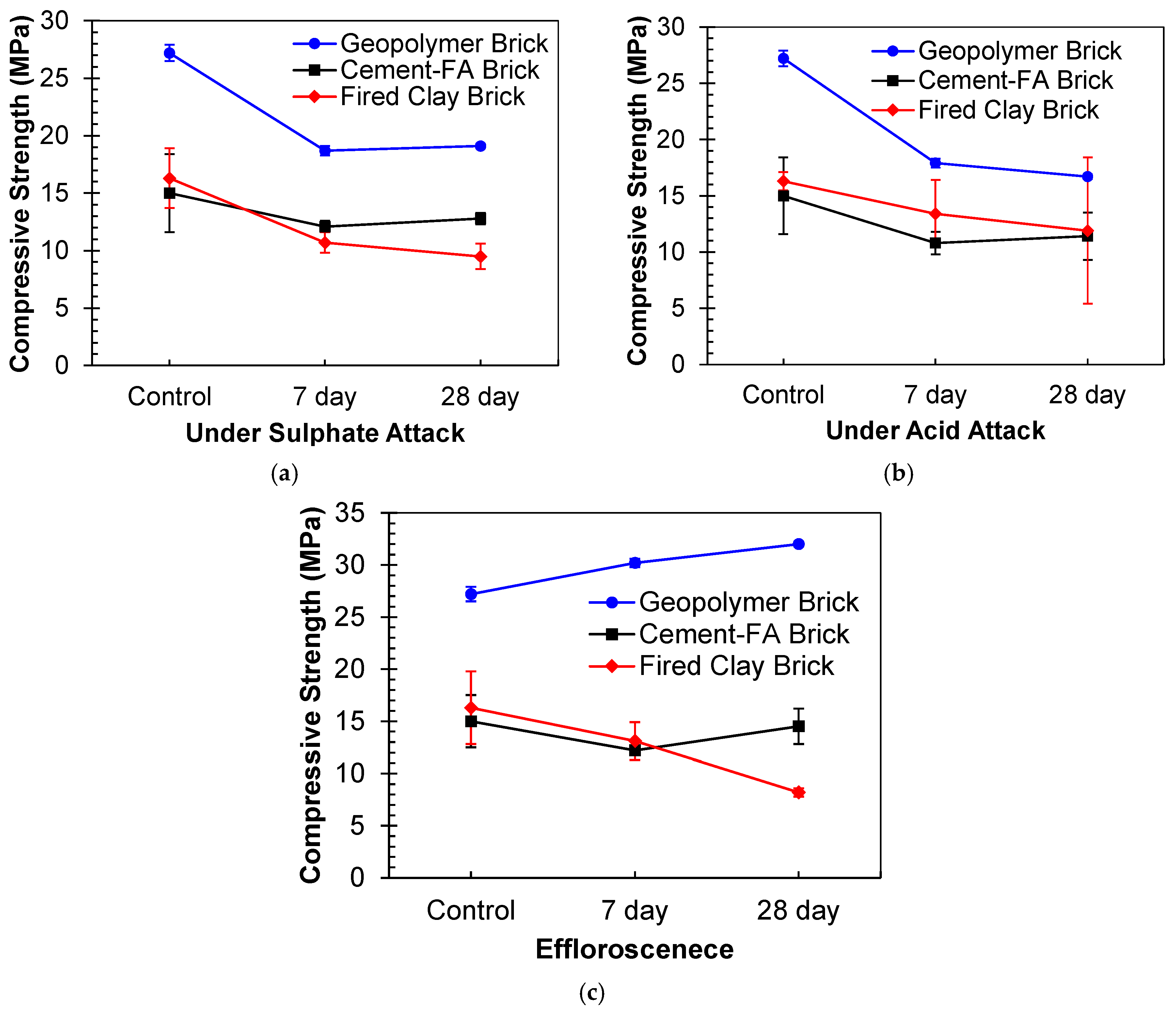
3.4.1. Sulfate Attack
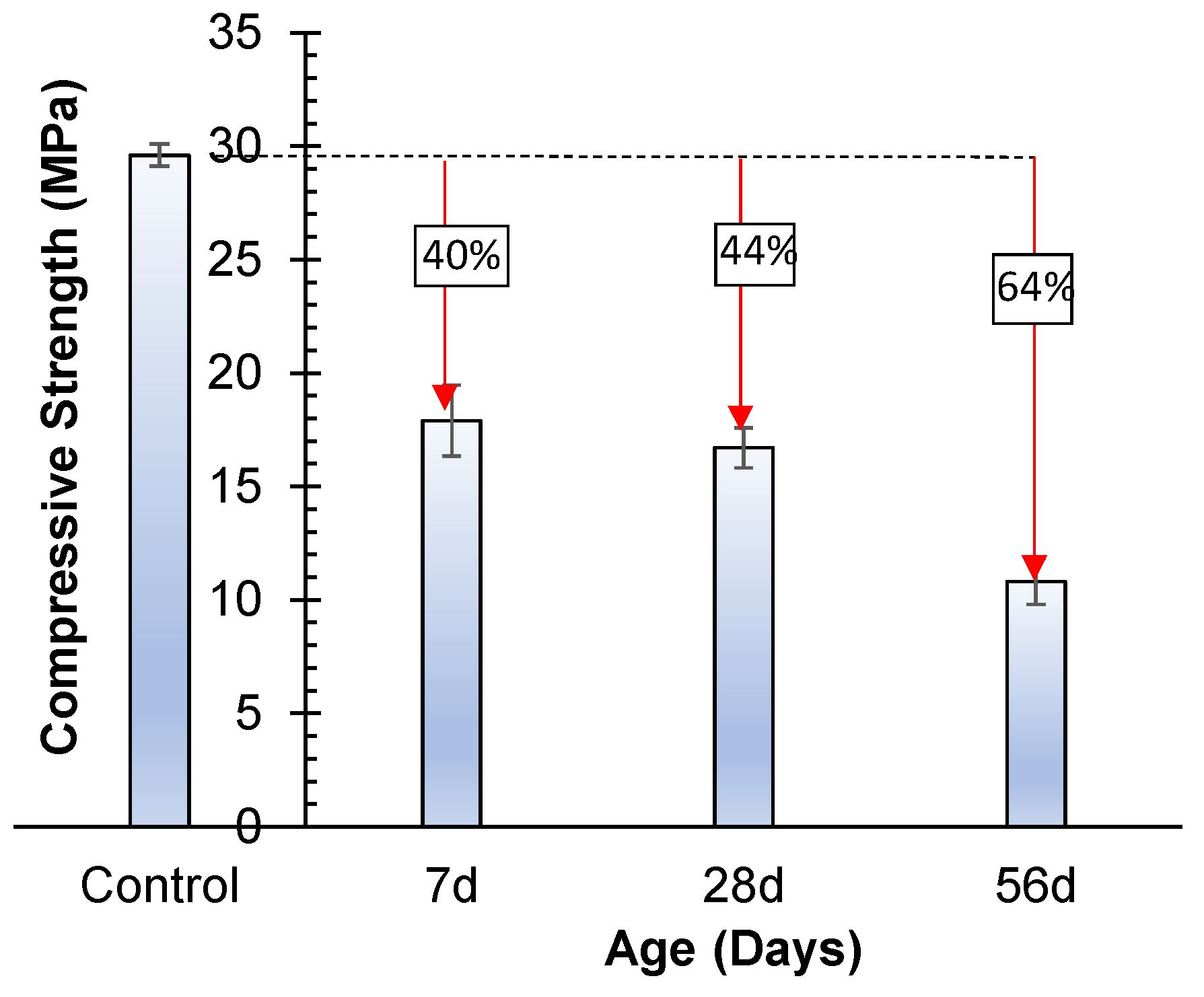
3.4.2. Acid Attack
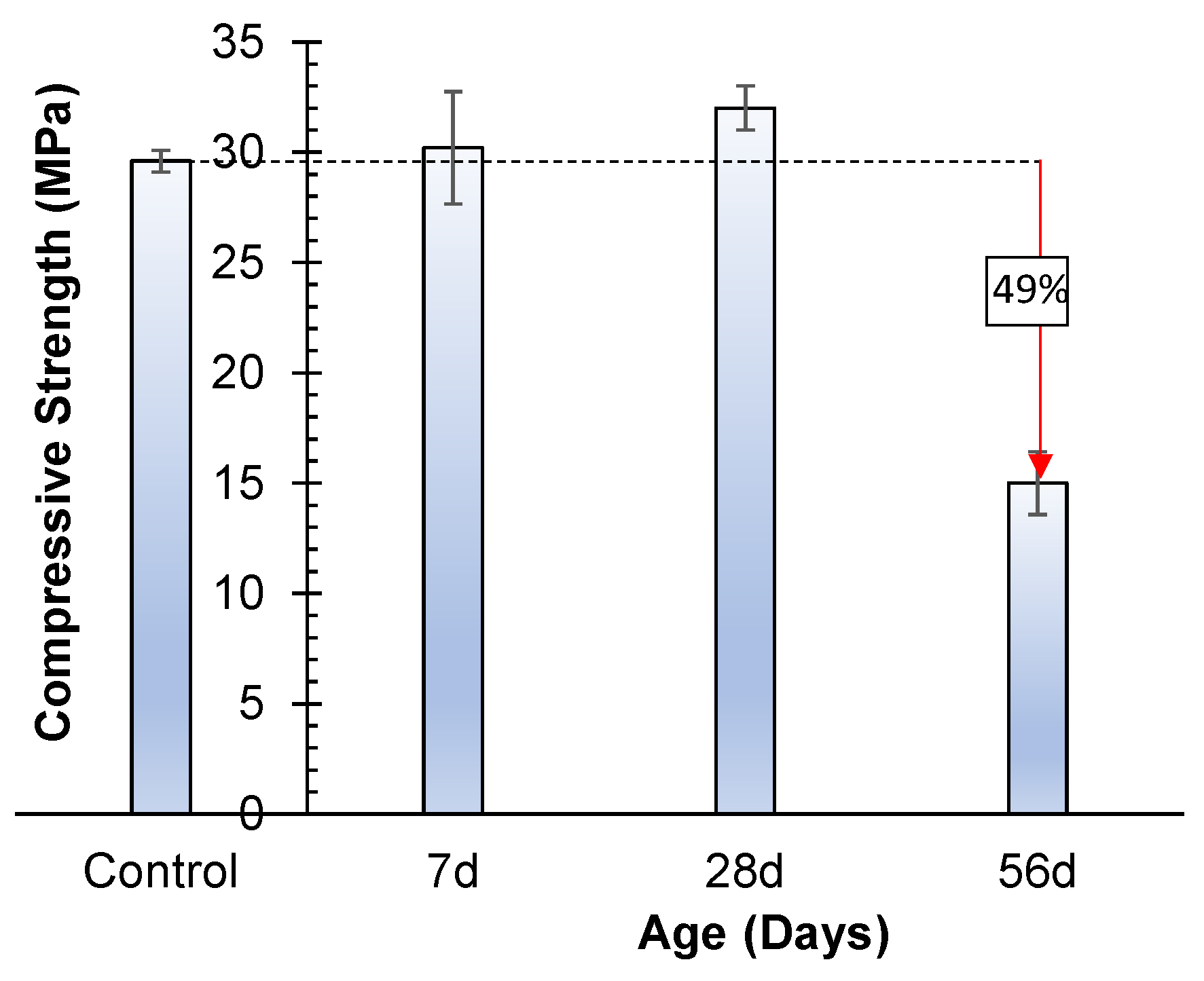
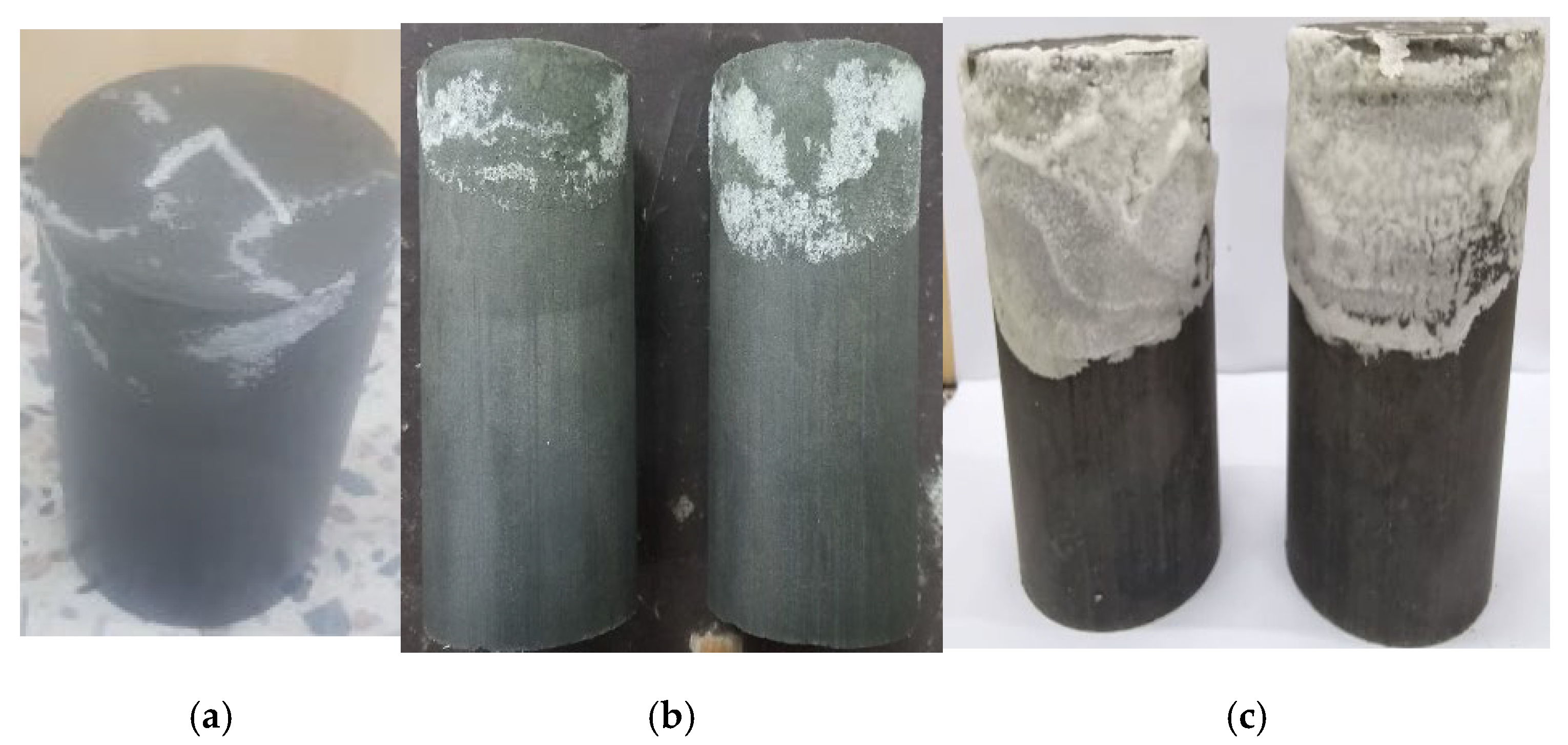
3.4.3. Efflorescence
3.4.4. FTIR Curves
3.4.5. Failure Modes
3.5. Comparative Analysis with Commercial Bricks
3.6. Sustainability and Feasibility Assessment of Developed Structural Block
4. Conclusions
- Higher molarity yielded an increase in the strength of the FA-based geopolymer, but when coupled with GGBS, higher molarity is detrimental to the strength. This is due to the leaching of Al and Si at different concentrations from low- (FA) and high- (GGBS) calcium precursors;
- Compressive strength was optimized using 100% FA as a precursor and a 12-molar concentration of NaOH solution to develop a geopolymer structural block by employing a pressure catalysis approach and curing at hot conditions. The value was 26.2 MPa. However, the strength was increased three times when shifted from ambient curing to hot curing conditions with the 75% FA and 25% GGBS specimen;
- The density of the FA-based specimen has the least bulk density (1773 kg/m3), and by increasing the proportion of GGBS, the bulk density increases linearly and reaches up to 2000 kg/m3. However, the influence of the molarity of NaOH solution and curing condition has a marginal effect on the bulk density;
- Both sulfate and acidic exposure have a detrimental influence on the strength of geopolymer, and more than 40% reduction in strength was observed when exposed for 56 days. The acidic exposure is very damaging, and the reduction in strength is up to 64%;
- The strength was increased up to 28 days by partially dipping in water, and no efflorescence was observed, whereas at 56 days, significantly salt crystalline formations appeared, and strength was also decreased by 49%;
- The failure mode was catastrophic, and layer splitting was observed after failure. This was aligned with the significant loss in the reduction in the strength of the specimens;
- The durability performance was also compared with conventional commercially available fired clay brick and cement–FA-based brick. The developed brick had better performance up to 28 days of investigation. However, its cost is higher, and it may have a lower economic index and seem more feasible than conventional materials. It can be considered an alternative to traditional masonry blocks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Horpibulsuk, S. A review of studies on bricks using alternative materials and approaches. Constr. Build. Mater. 2018, 188, 1101–1118. [Google Scholar] [CrossRef]
- Iftikhar, S.; Rashid, K.; Haq, E.U.; Zafar, I.; Alqahtani, F.K.; Khan, M.I. Synthesis and characterization of sustainable geopolymer green clay bricks: An alternative to burnt clay brick. Constr. Build. Mater. 2020, 259, 119659. [Google Scholar] [CrossRef]
- Wang, Y.; Zafar, I.; Rashid, K.; Ltifi, M.; Ju, M. Multicriteria analysis for quantifying sustainability of developed load bearing lightweight geopolymer. J. Clean. Prod. 2024, 434, 140266. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S. Introduction to geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–11. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z.; Wang, H.; Liu, Y. A review on mixture design methods for geopolymer concrete. Compos. Part B Eng. 2019, 178, 107490. [Google Scholar] [CrossRef]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different admixtures and additives on the properties of alkali-activated fly ash. Mater. Des. 2014, 53, 1005–1025. [Google Scholar] [CrossRef]
- Takeda, H.; Hashimoto, S.; Matsui, H.; Honda, S.; Iwamoto, Y. Rapid fabrication of highly dense geopolymers using a warm press method and their ability to absorb neutron irradiation. Constr. Build. Mater. 2014, 50, 82–86. [Google Scholar] [CrossRef]
- Ahmad, M.; Rashid, K.; Hameed, R.; Haq, E.U.; Farooq, H.; Ju, M. Physico-mechanical performance of fly ash based geopolymer brick: Influence of pressure-temperature-time. J. Build. Eng. 2022, 50, 104161. [Google Scholar] [CrossRef]
- Zafar, I.; Rashid, K.; Ahmad, M.; Ltifi, M. Thermo-chemico-mechanical activation of bagasse ash to develop geopolymer based cold-pressed block. Constr. Build. Mater. 2022, 352, 129053. [Google Scholar] [CrossRef]
- Ahmad, M.; Rashid, K. Novel approach to synthesize clay-based geopolymer brick: Optimizing molding pressure and precursors’ proportioning. Constr. Build. Mater. 2022, 322, 126472. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Ou, Z. Durability of alkali-activated materials in aggressive environments: A review on recent studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Acid resistance of inorganic polymer binders. 1. Corrosion rate. Mater. Struct. 2011, 45, 1–14. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, X.; Long, F. Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sturm, P.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H.; Kühne, H.C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Lee, Y.H.; Lee, J.; Ahn, N. Acid Resistance and Curing Properties for Green Fly Ash-geopolymer Concrete. J. Asian Archit. Build. Eng. 2018, 12, 317–322. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and subflorescence induced microstructural and mechanical evolution in fly ash-based geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Temuujin, J.; Ruescher, C.; Minjigmaa, A.; Darkhijav, B.; Davaabal, B.; Battsetseg, B.E. Characterization of Effloresences of Ambient and Elevated Temperature Cured Fly Ash Based Geopolymer Type Concretes. Adv. Mater. Res. 2016, 1139, 25–29. [Google Scholar] [CrossRef]
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Effect of hydrophobic surface-modified fine aggregates on efflorescence control in geopolymer. Cem. Concr. Compos. 2022, 126, 104337. [Google Scholar] [CrossRef]
- Mierzwiński, D.; Korniejenko, K.; Łach, M.; Mikuła, J.; Krzywda, J. Effect of Coffee Grounds Addition on Efflorescence in Fly Ash-based Geopolymer. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012035. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of slag substitution on physical and mechanical properties of fly ash-based alkali activated binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Kani, E.N.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- ASTM C 1437-07; Standard Test Method for FLow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM C 39; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM C 1012; Standard Test Method for Length Change of Hydraulic Cement Mortars Exposed to a Sulfate Solution. ASTM International: West Conshohocken, PA, USA, 2015.
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Almakayeel, N.; Khan, T.Y. Development and optimization of an eco-friendly geopolymer brick production process for sustainable masonry construction. Case Stud. Constr. Mater. 2023, 18, e02133. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Anand, N.; Raj, R.S.; Lubloy, E. Techno-socio-economic aspects of Portland cement, Geopolymer, and Limestone Calcined Clay Cement (LC3) composite systems: A-State-of-Art-Review. Constr. Build. Mater. 2023, 398, 132484. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Khadar, S.D.A. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Kuenzel, C.; Ranjbar, N. Dissolution mechanism of fly ash to quantify the reactive aluminosilicates in geopolymerisation, Resources. Conserv. Recycl. 2019, 150, 104421. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41. [Google Scholar] [CrossRef]
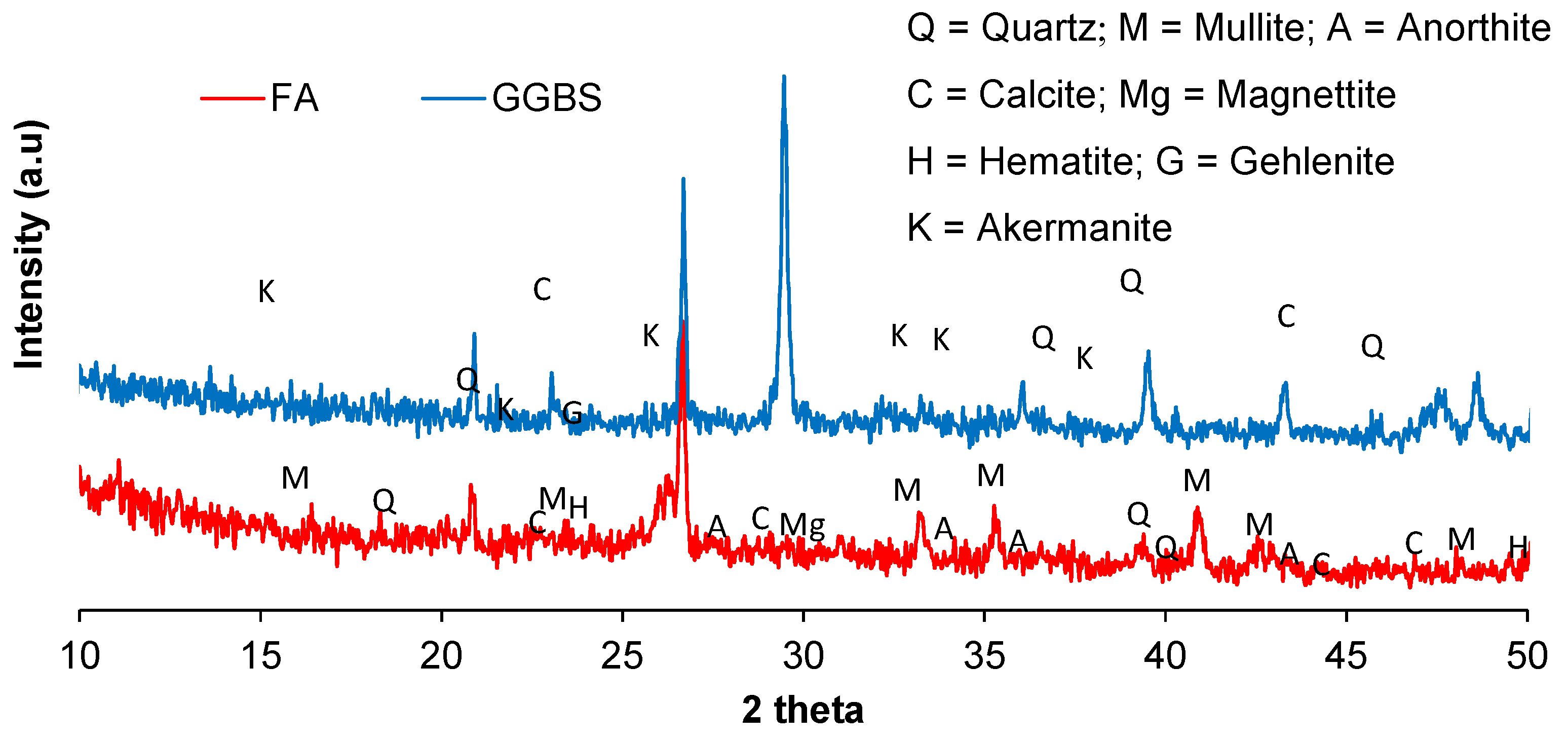






| Material | Oxides (%) | LOI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | Na2O | SO3 | (%) | |
| FA | 56.3 | 23.1 | 9.0 | 6.4 | 1.7 | 0.6 | 0.3 | --- | <3.0 |
| GGBS | 37.4 | 13.3 | 40.9 | 1.3 | 1.6 | --- | 0.4 | 0.6 | |
| Sr. No | FA (%) | GGBS (%) | Sand (%) | NaOH Molarity | Acronyms |
|---|---|---|---|---|---|
| 1 | 50 | 0 | 50 | 8 | F100/S0 |
| 2 | 50 | 0 | 50 | 10 | F100/S0 |
| 3 | 50 | 0 | 50 | 12 | F100/S0 |
| 4 | 37.5 | 12.5 | 50 | 8 | F75/S25 |
| 5 | 37.5 | 12.5 | 50 | 10 | F75/S25 |
| 6 | 37.5 | 12.5 | 50 | 12 | F75/S25 |
| 7 | 25 | 25 | 50 | 8 | F50/S50 |
| 8 | 25 | 25 | 50 | 10 | F50/S50 |
| 9 | 25 | 25 | 50 | 12 | F50/S50 |
| 10 | 12.5 | 37.5 | 50 | 8 | F25/S75 |
| 11 | 12.5 | 37.5 | 50 | 10 | F25/S75 |
| 12 | 12.5 | 37.5 | 50 | 12 | F25/S75 |
| Sr. No | Material | Cost (USD Per Ton) | Quantity for 1 m3 | CO2 Emission Per Ton | Ref. ** |
|---|---|---|---|---|---|
| 1 | Fly ash | 8.8 | 817.7 | 19.6 | [34] |
| 2 | Sand | 2.5 | 817.7 | 1.3 | [34] |
| 3 | Na2SiO3 | 122.8 | 146 | 237 | [35] |
| 4 | NaOH | 350.9 | 58.4 * | 1120 | [34] |
| Description | Fired Clay Brick | Cement–FA Brick | Geopolymer Brick |
|---|---|---|---|
| Control strength | 16.3 | 15 | 29.6 |
| MgSO4—7 d | 10.7 | 12.1 | 18.7 |
| MgSO4—28 d | 9.5 | 12.8 | 19.1 |
| H2SO4—7 d | 13.4 | 10.8 | 17.9 |
| H2SO4—28 d | 11.9 | 11.4 | 16.7 |
| Cost (USD per 1k) | 52.6 | 56.1 | 67.4 |
| CO2 emissions | 0.549 | 0.3 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, K.; Ltifi, M.; Zafar, I.; Rafiqi, M.H.; Raoof, M.N. Static Compaction on Coupled Precursors and Optimizing Molarity for Enhanced Strength and Durability of Geopolymer. Materials 2024, 17, 2509. https://doi.org/10.3390/ma17112509
Rashid K, Ltifi M, Zafar I, Rafiqi MH, Raoof MN. Static Compaction on Coupled Precursors and Optimizing Molarity for Enhanced Strength and Durability of Geopolymer. Materials. 2024; 17(11):2509. https://doi.org/10.3390/ma17112509
Chicago/Turabian StyleRashid, Khuram, Mounir Ltifi, Idrees Zafar, Muhammad Hashim Rafiqi, and Muhammad Naeem Raoof. 2024. "Static Compaction on Coupled Precursors and Optimizing Molarity for Enhanced Strength and Durability of Geopolymer" Materials 17, no. 11: 2509. https://doi.org/10.3390/ma17112509
APA StyleRashid, K., Ltifi, M., Zafar, I., Rafiqi, M. H., & Raoof, M. N. (2024). Static Compaction on Coupled Precursors and Optimizing Molarity for Enhanced Strength and Durability of Geopolymer. Materials, 17(11), 2509. https://doi.org/10.3390/ma17112509






