Ball Milling Innovations Advance Mg-Based Hydrogen Storage Materials Towards Practical Applications
Abstract
1. Introduction
2. Synthesis of Mg-Based Hydrides via Ball Milling
2.1. Mg-Based Binary Hydrides
2.2. Mg-Based Ternary Hydrides
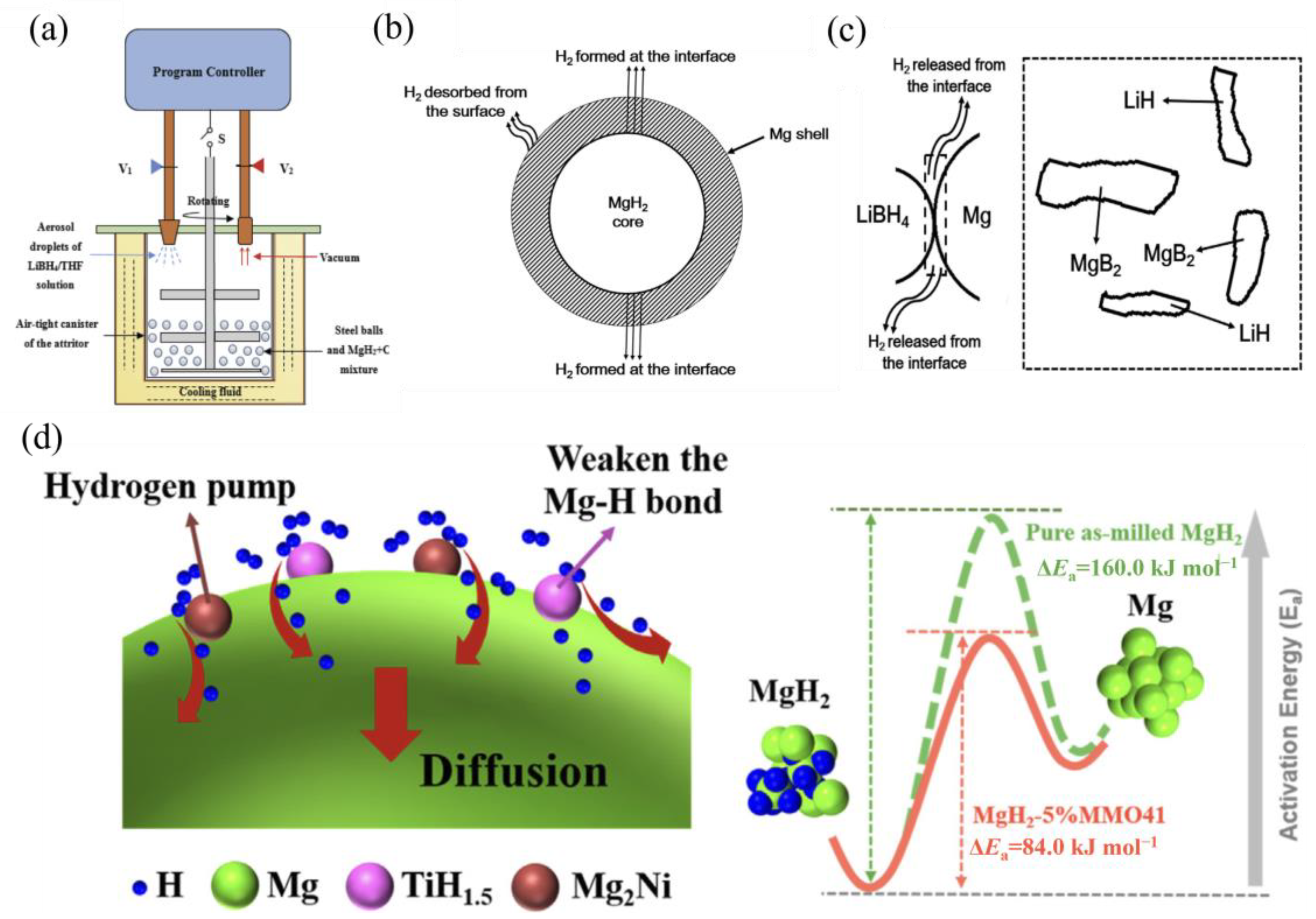
2.3. Mechanisms of Mg-Based Hydride Formation during Ball Milling
3. Nanostructuring of Mg-Based Hydrides via Ball Milling
3.1. Nanocrystalline Mg-Based Hydrides
3.2. Amorphous Mg-Based Hydrides
3.3. Mechanisms of Nanostructure/Amorphous Formation during Ball Milling

4. Catalytic Modification of Mg-Based Hydrides via Ball Milling
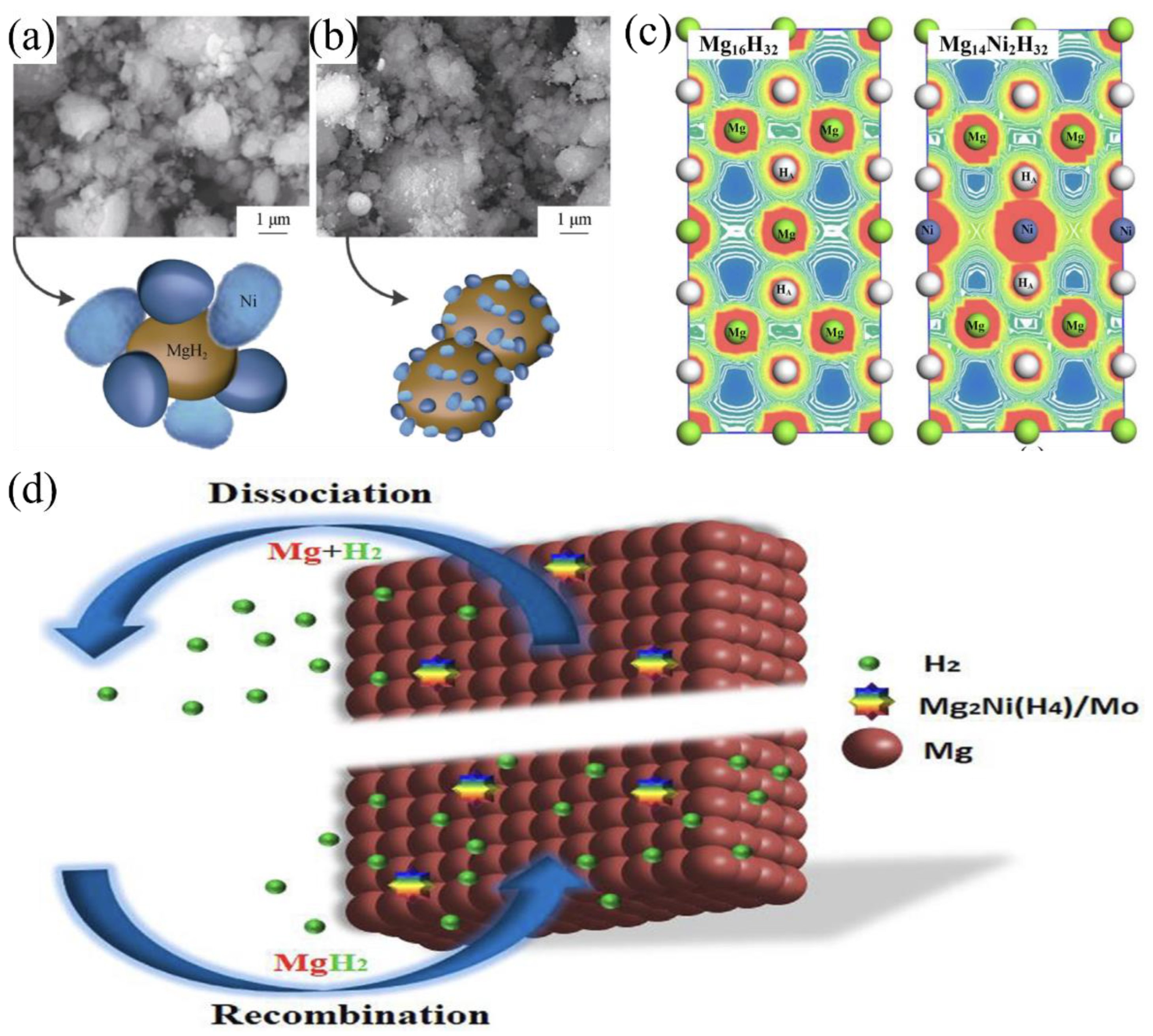
4.1. Transition Metal Catalysts
4.2. Metal Oxide Catalysts
4.3. Mechanisms of Catalytic Effects during Ball Milling
5. Nanocomposite Mg-Based Hydrides via Ball Milling
5.1. Carbon-Containing Nanocomposites
5.2. Metal Hydride-Containing Nanocomposites
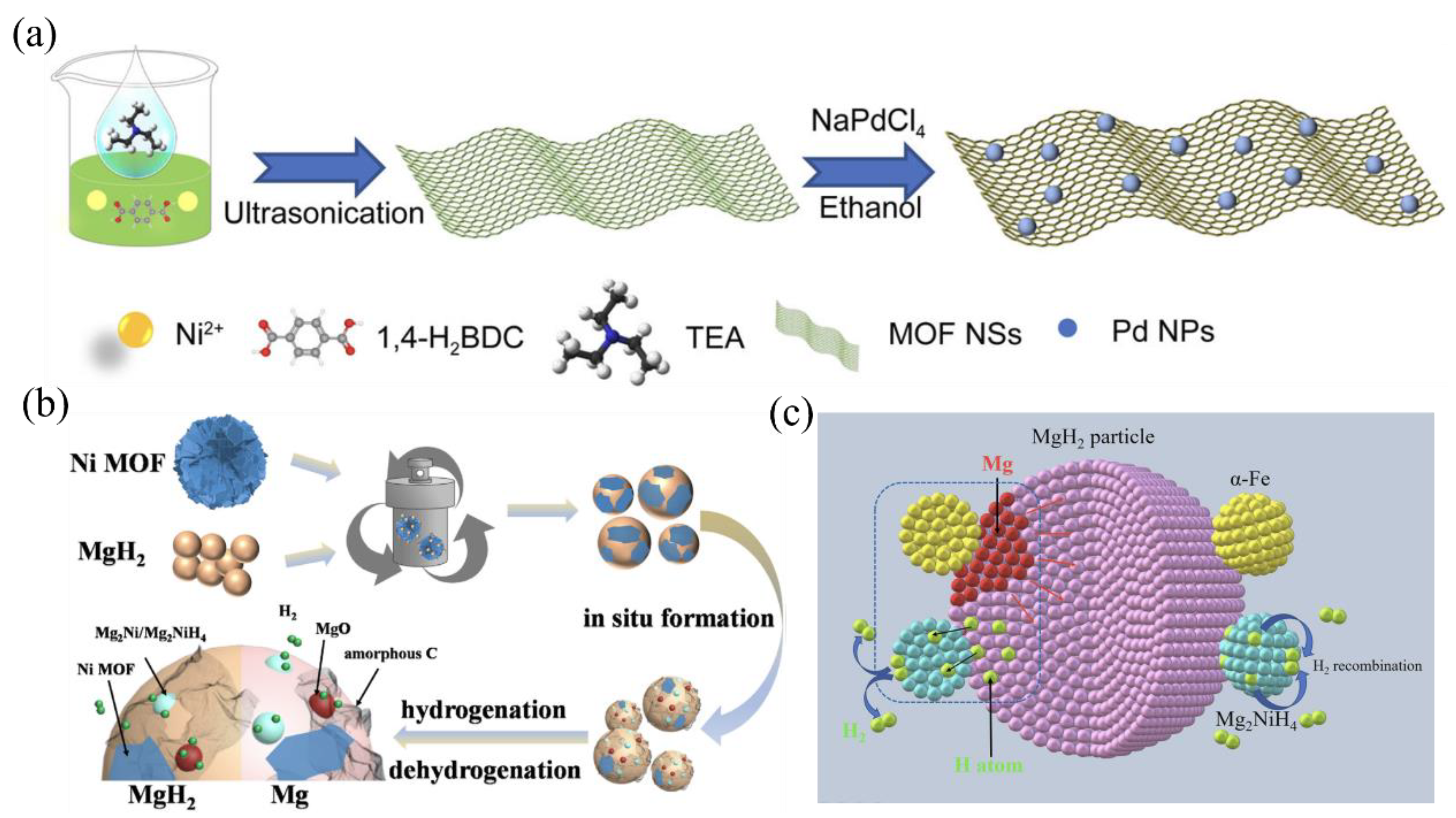
5.3. Metal–Organic Framework-Containing Nanocomposites
5.4. Mechanisms of Nanocomposite Formation and Synergistic Effects
6. Conclusions and Perspectives
- (1)
- The hydrogen storage capacity of Mg-based hydrides is still lower than the theoretical value due to the presence of impurities, oxides, and by-products introduced during the ball milling process and the hydrogen absorption/desorption cycles. The development of high-purity starting materials, optimized ball milling conditions, and effective purification methods is necessary to maximize the hydrogen storage capacity of Mg-based hydrides.
- (2)
- The hydrogen absorption/desorption kinetics of Mg-based hydrides at low temperatures (<100 °C) is still not satisfactory for practical applications, especially for on-board hydrogen storage in fuel cell vehicles. The development of novel catalysts, nanostructures, and nanocomposites with enhanced low-temperature kinetics is crucial to meet the requirements of practical hydrogen storage systems.
- (3)
- The cyclic stability and reversibility of Mg-based hydrides are still limited by the sintering, coarsening, and degradation of the nanostructure during extended cycling. The development of advanced nanoconfinement and nanoencapsulation strategies, as well as the introduction of anti-sintering additives and coatings, is important to improve the long-term stability and reversibility of Mg-based hydrides.
- (4)
- The safety and compatibility of Mg-based hydrides with the container materials and the fuel cell components are still not well understood and may pose risks for practical applications. The development of advanced characterization techniques and testing protocols, as well as the investigation of the interactions between Mg-based hydrides and other materials, is necessary to ensure the safe and reliable operation of Mg-based hydrogen storage systems.
- (5)
- The cost and scalability of ball milling processes for the production of Mg-based hydrides are still not competitive with other hydrogen storage methods such as compression and liquefaction. The development of low-cost and high-efficiency ball milling techniques, as well as the optimization of the process parameters and the energy consumption, is important to reduce the cost and increase the throughput of Mg-based hydrides.
- (1)
- The development of novel Mg-based alloys and composites with a high hydrogen storage capacity, fast kinetics, and good reversibility. The use of machine learning and high-throughput screening methods, combined with experimental validation and optimization, can accelerate the discovery and design of new Mg-based hydride materials.
- (2)
- The development of advanced ball milling techniques and equipment for the synthesis and modification of Mg-based hydrides. The use of high-energy and high-frequency ball milling methods, such as planetary ball milling and attritor ball milling, as well as the in situ monitoring and control of the ball milling process, can improve the efficiency and reproducibility of the ball milling process.
- (3)
- The development of multi-scale characterization and modeling tools for the understanding of the structure–property relationships and the hydrogen storage mechanisms of ball-milled Mg-based hydrides. The combination of experimental techniques such as in situ XRD, TEM, and neutron scattering with theoretical methods such as DFT, MD, and phase-field modeling can provide a comprehensive and predictive understanding of the hydrogen storage behavior of Mg-based hydrides.
- (4)
- The development of advanced nanoconfinement and catalysis strategies for the enhancement of the hydrogen storage properties of Mg-based hydrides. The use of novel nanoporous materials, such as MOFs, covalent organic frameworks (COFs), and porous organic polymers (POPs), as well as the functionalization and doping of the catalyst nanoparticles, can create new opportunities for the design and optimization of high-performance Mg-based hydrides.
- (5)
- The development of prototype Mg-based hydrogen storage systems and their integration with fuel cells and other hydrogen utilization technologies. The demonstration and testing of Mg-based hydrogen storage systems under realistic operating conditions, as well as the assessment of their performance, durability, and safety, can provide valuable feedback and guidance for the further improvement and scale-up of Mg-based hydrides.
Author Contributions
Funding
Conflicts of Interest
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Ding, Z. Research Progress and Application Prospects of Solid-State Hydrogen Storage Technology. Molecules 2024, 29, 1767. [Google Scholar] [CrossRef]
- Georgeson, L.; Maslin, M.; Poessinouw, M. Clean up energy innovation. Nature 2016, 538, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M. The matter of a clean energy future. Science 2022, 376, 1361. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies and Future Outlook for Clean Energy Applications. Molecules 2024, in press. [Google Scholar]
- Hou, Q.; Zhang, J.; Zheng, Z.; Yang, X.; Ding, Z. Ni3Fe/BC nanocatalysts based on biomass charcoal self-reduction achieves excellent hydrogen storage performance of MgH2. Dalton Trans. 2022, 51, 14960–14969. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Ravnsbæk, D.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T. Mechanochemical synthesis of hydrogen storage materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Recent Advances in the Preparation Methods of Magnesium-Based Hydrogen Storage Materials. Molecules 2024, in press. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Bassetti, A.; Bonetti, E.; Pasquini, L.; Montone, A.; Grbovic, J.; Antisari, M.V. Hydrogen desorption from ball milled MgH2 catalyzed with Fe. Eur. Phys. J. B 2005, 43, 19–27. [Google Scholar] [CrossRef]
- Recham, N.; Bhat, V.; Kandavel, M.; Aymard, L.; Tarascon, J.-M.; Rougier, A. Reduction of hydrogen desorption temperature of ball-milled MgH2 by NbF5 addition. J. Alloys Compd. 2008, 464, 377–382. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Duan, C.; Tian, Y.; Wang, X.; Wu, J.; Liu, B.; Fu, D.; Zhang, Y.; Lv, W.; Hu, L.; Wang, F.; et al. Anchoring Mo single atoms on N-CNTs synchronizes hydrogenation/dehydrogenation property of Mg/MgH2. Nano Energy 2023, 113. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmidt, J.; Kahnt, A.; Osvet, A.; Romeis, S.; Zolnhofer, E.M.; Marthala, V.R.; Guldi, D.M.; Peukert, W.; et al. Noble-Metal-Free Photocatalytic Hydrogen Evolution Activity: The Impact of Ball Milling Anatase Nanopowders with TiH2. Adv Mater. 2017, 29, 1604747. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Y.; Li, Z.; Lin, X.; Lu, C.; Ding, W.; Zou, J. Boosting Hydrogen Storage Performance of MgH2 by Oxygen Vacancy-Rich H-V2O5 Nanosheet as an Excited H-Pump. Nano-Micro Lett. 2024, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, S.; Chen, X.; Liu, L.; Deng, Y.; Zhou, L.; Cai, X. Mechanism of hydrogenation and dehydrogenation in Mg/Cu9Al4 @Mg and MgH2/Cu9Al4 @MgH2: A DFT and experimental investigation. J. Alloys Compd. 2024, 978, 173542. [Google Scholar] [CrossRef]
- Jia, Y.; Zou, J.; Yao, X. Catalytically enhanced dehydrogenation of MgH2 by activated carbon supported Pd-VOx (x = 2.38) nanocatalyst. Int. J. Hydrogen Energy 2012, 37, 13393–13399. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Li, Y.-T.; Li, S.-Y.; Wu, P.-K.; Hou, Q.-H.; Zheng, Y.; Gao, B.; Huo, K.-F.; Du, W.-J.; et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023, 42, 2906–2927. [Google Scholar] [CrossRef]
- Lu, W.-C.; Ou, S.-F.; Lin, M.-H.; Wong, M.-F. Hydrogen absorption/desorption performance of Mg Al alloys synthesized by reactive mechanical milling and hydrogen pulverization. J. Alloys Compd. 2016, 682, 318–325. [Google Scholar] [CrossRef]
- Danaie, M.; Tao, S.; Kalisvaart, P.; Mitlin, D. Analysis of deformation twins and the partially dehydrogenated microstructure in nanocrystalline magnesium hydride (MgH2) powder. Acta Mater. 2010, 58, 3162–3172. [Google Scholar] [CrossRef]
- Varin, R.; Li, S.; Wronski, Z.; Morozova, O.; Khomenko, T. The effect of sequential and continuous high-energy impact mode on the mechano-chemical synthesis of nanostructured complex hydride Mg2FeH6. J. Alloys Compd. 2005, 390, 282–296. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.; Ström-Olsen, J.; Schulz, R. Catalytic effect of Pd on hydrogen absorption in mechanically alloyed Mg2Ni, LaNi5 and FeTi. J. Alloys Compd. 1995, 217, 295–300. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J. Synergy of hydrogen sorption in ball-milled hydrides of Mg and Mg2Ni. J. Alloys Compd. 1999, 289, 197–206. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, J.; Wu, Y. Catalytic effects of Mg(BH4)2 on the desorption properties of 2LiNH2-MgH2 mixture. Int. J. Hydrogen Energy 2019, 44, 19294–19301. [Google Scholar]
- Hu, M.; Sun, X.; Li, B.; Li, P.; Xiong, M.; Tan, J.; Ye, Z.; Eckert, J.; Liang, C.; Pan, H. Interaction of metallic magnesium with ammonia: Mechanochemical synthesis of Mg(NH2)2 for hydrogen storage. J. Alloys Compounds 2022, 907, 164397. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.; Jensen, T.R.; Filinchuk, Y.; Dovgaliuk, I.; Chernyshov, D.; He, L.; Li, Y.; Li, H.-W. Magnesium borohydride Mg(BH4)2 for energy applications: A review. J. Mater. Sci. Technol. 2023, 161, 170–179. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.A.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Czujko, T.; Oleszek, E.E.; Szot, M. New Aspects of MgH2 Morphological and Structural Changes during High-Energy Ball Milling. Materials 2020, 13, 4550. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Zhang, Y.; Isobe, S.; Kubo, M.; Nakamura, T. Characterization of Mg-Ni alloy hydrides prepared by reactive mechanical grinding. J. Alloys Compd. 2007, 446, 170–175. [Google Scholar]
- Le Ruyet, R.; Fleutot, B.; Berthelot, R.; Benabed, Y.; Hautier, G.; Filinchuk, Y.; Janot, R. Mg3(BH4)4(NH2)2 as Inorganic Solid Electrolyte with High Mg2+ Ionic Conductivity. ACS Appl. Energy Mater. 2020, 3, 6093–6097. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Xia, G.; He, H.; Zhang, X.; Zou, W.; Yu, X. First-principles study of decomposition mechanisms of Mg(BH4)2·2NH3 and LiMg(BH4)3·2NH3. RSC Adv. 2017, 7, 31027–31032. [Google Scholar] [CrossRef]
- de Castro, J.; Yavari, A.; LeMoulec, A.; Ishikawa, T.T.; Botta F, W.J. Improving H-sorption in MgH2 powders by addition of nanoparticles of transition metal fluoride catalysts and mechanical alloying. J. Alloys Compd. 2005, 389, 270–274. [Google Scholar] [CrossRef]
- Huot, J.; Akiba, E.; Iba, H. Preparation of a Mg-Ni alloy for hydrogen storage by ball milling in a hydrogen gas atmosphere. J. Alloys Compd. 1995, 231, L5–L8. [Google Scholar]
- Varin, R.A.; Czujko, T.; Wronski, Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling. Nanotechnology 2006, 17, 3856–3865. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Particle size, grain size and lattice parameter of nanocrystalline Mg-Ni alloys synthesized by controlled mechanical milling. J. Alloys Compd. 2006, 416, 356–364. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Nicolaisen, T.; Fernandez, J.A.; Klassen, T.; Bormann, R. Effect of nanosized oxides on MgH2 (de)hydriding kinetics. J. Alloys Compd. 2007, 434, 738–742. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.; Tan, J.; Ouyang, Y.; Du, Y.; Sun, L. First-principles study on the dehydrogenation thermodynamics and kinetics of Ti, Zr, V and Nb doped MgH2. J. Energy Storage 2024, 83, 110612. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Bu, W.; Cai, Y.; Qi, Y.; Guo, S. Improved hydrogen storage dynamics of amorphous and nanocrystalline Ce-Mg-Ni-based CeMg12-type alloys synthesized by ball milling. Renew. Energy 2019, 132, 167–175. [Google Scholar] [CrossRef]
- Liang, H.; Li, J.; Shen, X.; Cao, B.; Zhu, J.; Geng, B.; Zhu, S.; Li, W. The study of amorphous La@Mg catalyst for high efficiency hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 18404–18411. [Google Scholar] [CrossRef]
- Fecht, H.-J. Nanostructure formation by mechanical attrition. Nanostruct. Mater. 1995, 6, 33–42. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, X.; Li, T.; Zhai, T.; Lin, Y.; Feng, D.; Zhang, Y. Improved hydrogen storage performances of nanocrystalline RE5Mg41-type alloy synthesized by ball milling. J. Energy Storage 2022, 46, 103702. [Google Scholar] [CrossRef]
- Révész, M. Gajdics, Improved H-Storage Performance of Novel Mg-Based Nanocomposites Prepared by High-Energy Ball Milling: A Review. Energies 2021, 14, 6400. [Google Scholar] [CrossRef]
- Fecht, H.J.; Hellstern, E.; Fu, Z.; Johnson, W.L. Nanocrystalline metals prepared by high-energy ball milling. Met. Trans. A 1990, 21, 2333–2337. [Google Scholar] [CrossRef]
- Koch, C.; Whittenberger, J. Mechanical milling/alloying of intermetallics. Intermetallics 1996, 4, 339–355. [Google Scholar] [CrossRef]
- Takacs, L.; McHenry, J.S. Temperature of the milling balls in shaker and planetary mills. J. Mater. Sci. 2006, 41, 5246–5249. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Cai, X.; Zhou, L. Hydrogen storage behaviour of Cr- and Mn-doped Mg2Ni alloys fabricated via high-energy ball milling. Int. J. Hydrogen Energy 2023, 48, 17202–17215. [Google Scholar] [CrossRef]
- Cuevas, F.; Korablov, D.; Latroche, M. Synthesis, structural and hydrogenation properties of Mg-rich MgH2-TiH2 nanocomposites prepared by reactive ball milling under hydrogen gas. Phys. Chem. Chem. Phys. 2012, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; He, L.; Zhang, P.; Zhang, Z.; Pan, S.; Li, W. Tailoring hydrogen storage properties of amorphous Mg 65 Cu 25 Y 10 alloy via minor alloying addition of Ag. Intermetallics 2018, 97, 22–26. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, L.; Yan, N.; Zheng, J.; Bian, T.; Yang, Z.; Su, S. Realizing Hydrogen De/Absorption Under Low Temperature for MgH2 by Doping Mn-Based Catalysts. Nanomaterials 2020, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, X.; Ji, L.; Yan, N.; Sun, Z.; Zhu, X. Catalytic Effect of Facile Synthesized TiH(1.971) Nanoparticles on the Hydrogen Storage Properties of MgH2. Nanomaterials 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, X.; Liu, Y.; Li, Y.; Zhang, Z.; Chen, K.; Han, S. Synergetic catalysis of Ni@C@CeO2 for driving ab/desorption of MgH2 at moderate temperature. Fuel 2024, 357, 129726. [Google Scholar] [CrossRef]
- Bahou, S.; Labrim, H.; Ez-Zahraouy, H. Improvement of decomposition temperature and gravimetric density of MgH2 by transition metals and vacancies: A comparison study. Solid State Commun. 2023, 371, 115170. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, B.; Zhang, G.; Wang, Y.; Li, X. Catalytic effect of double transition metal sulfide NiCo2S4 on hydro-gen storage properties of MgH2. Appl. Surf. Sci. 2024, 645, 158801. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Cai, W.; Ouyang, L.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems–A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Hussain, T.; Maark, T.A.; Chakraborty, S.; Ahuja, R. Improvement in Hydrogen Desorption from β- and γ-MgH2 upon Transition-Metal Doping. ChemPhysChem 2015, 16, 2481. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2-Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Nyahuma, F.M.; Zhang, L.; Song, M.; Lu, X.; Xiao, B.; Zheng, J.; Wu, F. Significantly improved hydrogen storage behaviors in MgH2 with Nb nanocatalyst, International Journal of Minerals. Metall. Mater. 2022, 29, 1788–1797. [Google Scholar]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Rönnebro, E. Hydrogen Storage Properties of Nanosized MgH2-0.1TiH2 Prepared by Ultrahigh-Energy-High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 1, 5603–5611. [Google Scholar] [CrossRef]
- Jin, S.; Shim, J.; Ahn, J.; Cho, Y.; Yi, K. Improvement in hydrogen sorption kinetics of MgH2 with Nb hydride catalyst. Acta Mater. 2007, 55, 5073–5079. [Google Scholar] [CrossRef]
- Gattia, D.M.; Jangir, M.; Jain, I. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Tian, G.; Wu, F.; Zhang, H.; Wei, J.; Zhao, H.; Zhang, L. Boosting the hydrogen storage performance of MgH2 by Vanadium based complex oxides. J. Phys. Chem. Solids 2023, 174, 111187. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J. Alloys Compd. 2004, 364, 242–246. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Plocinski, T. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles. Int. J. Hydrogen Energy 2008, 33, 1859–1867. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Zhu, Y.; Zhao, Y.; Lin, H.; Zhang, Y.; Li, H.; Zhang, J.; Liu, Y.; Gao, W.; et al. Crystal-facet-dependent catalysis of anatase TiO2 on hydrogen storage of MgH2. J. Alloys Compd. 2020, 822, 153553. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Sun, P.; Zhou, C.; Guo, X.; Fang, Z.Z. The role of oxide in hydrogen absorption and desorption kinetics of MgH2-based material. J. Alloys Compd. 2023, 934. [Google Scholar] [CrossRef]
- Larsson, P.; Araújo, C.M.; Larsson, J.A.; Jena, P.; Ahuja, R. Role of catalysts in dehydrogenation of MgH2 nanoclusters. Proc. Natl. Acad. Sci. USA 2008, 105, 8227–8231. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yang, X.; Zhou, S.; Ran, L.; Lu, Y.; Chen, Y.; Wang, J.; Pan, F. Enhancing hydrogen storage properties of MgH2 using FeCoNiCrMn high entropy alloy catalysts. J. Mater. Sci. Technol. 2023, 149, 88–98. [Google Scholar] [CrossRef]
- Lan, Z.; Liang, H.; Wen, X.; Hu, J.; Ning, H.; Zeng, L.; Liu, H.; Tan, J.; Eckert, J.; Guo, J. Experimental and theoretical studies on two-dimensional vanadium carbide hybrid nanomaterials derived from V4AlC3 as excellent catalyst for MgH2. J. Magnes. Alloy. 2023, 11, 3790–3799. [Google Scholar] [CrossRef]
- Bolarin, J.A.; Zhang, Z.; Cao, H.; Li, Z.; He, T.; Chen, P. NaH doped TiO2 as a high-performance catalyst for Mg/MgH2 cycling stability and room temperature absorption. J. Magnes. Alloy. 2023, 11, 2740–2749. [Google Scholar] [CrossRef]
- Huang, H.; Xu, T.; Chen, J.; Yuan, J.; Yang, W.; Liu, B.; Zhang, B.; Wu, Y. Enhanced catalysis of Pd single atoms on Sc2O3 nanoparticles for hydrogen storage of MgH2. Chem. Eng. J. 2024, 483. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Lu, J.; Zhang, X. Thermodynamic and kinetic destabilization of magnesium hydride using Mg–In solid solution alloys. J. Am. Chem. Soc. 2013, 135, 10982–10985. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Liu, B.; Lu, X.; Zhang, T.; Gu, Q. The cycling stability of the in situ formed Mg-based nanocomposite catalyzed by YH2. J. Mater. Chem. A 2017, 5, 17532–17543. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Q.; Zhu, Y.; Liu, Y.; Zhang, J.; Ba, Z.; Hu, X.; Li, L. Assisting Ni catalysis by CeO2 with oxygen vacancy to optimize the hydrogen storage properties of MgH2. J. Mater. Sci. Technol. 2023, 159, 62–71. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Si, T.-Z.; Zhang, X.-Y.; Feng, J.-J.; Ding, X.-L.; Li, Y.-T. Enhancing hydrogen sorption in MgH2 by controlling particle size and contact of Ni catalysts. Rare Met. 2018, 40, 995–1002. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.; Yu, L.; Lu, X.; Zhou, D. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2. Renew. Energy 2020, 154, 1229–1239. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, Q.; Liu, Y.; Yang, X. A fancy hydrangea shape bimetallic Ni-Mo oxide of remarkable catalytic effect for hydrogen storage of MgH2. J. Ind. Eng. Chem. 2023, 118, 393–406. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and Reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Hong, F.; Shi, W.; Zhao, R.; Li, R.; Fan, Y.; Liu, H.; Zhou, W.; Ning, H.; Guo, J. Effect of MOF-derived carbon–nitrogen nanosheets co-doped with nickel and titanium dioxide nanoparticles on hydrogen storage performance of MgH2. Chem. Eng. J. 2023, 468, 143692. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.; Ding, C.; Niu, H.; Zhang, T.; Wang, N.; Zhang, Q.; Liu, D.; Han, S.; Yu, H. Effectiveness of crystallitic carbon from coal as milling aid and for hydrogen storage during milling with magnesium. Fuel 2013, 109, 68–75. [Google Scholar] [CrossRef]
- Pal, P.; Agarwal, S.; Tiwari, A.; Ichikawa, T.; Jain, A.; Dixit, A. Improved hydrogen desorption properties of exfoliated graphite and graphene nanoballs modified MgH2. Int. J. Hydrogen Energy 2022, 47, 41891–41897. [Google Scholar] [CrossRef]
- Rather, S.U.; Taimoor, A.A.; Muhammad, A.; Alhamed, Y.A.; Zaman, S.F.; Ali, A.M. Kinetics of hydrogen adsorption on MgH2/CNT composite. Mater. Res. Bull. 2016, 77, 23–28. [Google Scholar] [CrossRef]
- Peng, D.; Ding, Z.; Zhang, L.; Fu, Y.; Wang, J.; Li, Y.; Han, S. Remarkable hydrogen storage properties and mechanisms of the shell–core MgH2@carbon aerogel microspheres. Int. J. Hydrogen Energy 2018, 43, 3731–3740. [Google Scholar] [CrossRef]
- Zhong, Y.; Wan, X.; Ding, Z.; Shaw, L.L. New dehydrogenation pathway of LiBH4 + MgH2 mixtures enabled by nanoscale LiBH4. Int. J. Hydrogen Energy 2016, 41, 22104–22117. [Google Scholar] [CrossRef]
- Ding, Z.; Shaw, L. Enhancement of Hydrogen Desorption from Nanocomposite Prepared by Ball Milling MgH2 with In Situ Aerosol Spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New insights into the solid-state hydrogen storage of nanostructured LiBH4-MgH2 system. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, P.; Shaw, L. Solid-state hydrogen desorption of 2 MgH2 + LiBH4 nano-mixture: A kinetics mechanism study. J. Alloys Compd. 2019, 806, 350–360. [Google Scholar] [CrossRef]
- Kwak, Y.-J.; Song, M.-Y.; Lee, K.-T. Improvement in the Hydrogen Storage Properties of MgH2 by Adding NaAlH4. Metals 2024, 14, 227. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M.; Al-Ajmi, F.; Banyan, M. Performance and fuel cell applications of reacted ball-milled MgH2/5.3 wt% TiH2 nanocomposite powders. RSC Adv. 2018, 8, 38175–38185. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lu, Y.; Liu, X.; Tang, W.; Li, X.; Wang, F.; Shui, J.; Yu, R. Layered double hydroxide-derived Mg2Ni/TiH1.5 composite catalysts for enhancing hydrogen storage performance of MgH2. J. Magnes. Alloy. 2023. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z.; Ding, H.; Jiang, H.; Yang, W.; Du, Y.; Zheng, K.; Huo, L.L. Shaw, MOFs-Based Materials for Sol-id-State Hydrogen Storage: Strategies and Perspectives. Chem. Eng. J. 2024, 485, 149665. [Google Scholar] [CrossRef]
- Shao, H.; Huang, Y.; Guo, H.; Liu, Y.; Guo, Y.; Wang, Y. Thermally stable Ni MOF catalyzed MgH2 for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 37977–37985. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Sun, L.-X.; Xu, F.; Luo, Y.-M.; Xia, Y.-P.; Wei, S.; Zhang, C.-C.; Cheng, R.-G.; Ye, C.-F.; Liu, M.-Y.; et al. Modulated noble metal/2D MOF heterostructures for improved hydrogen storage of MgH2. Rare Met. 2023, 43, 1672–1685. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Shao, Y.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalysis derived from flower-like Ni MOF towards the hydrogen storage performance of magnesium hydride. Int. J. Hydrogen Energy 2022, 47, 9346–9356. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Zhu, W.; Khan, D.; Hu, C.; Huang, T.; Ding, W.; Zou, J. Nano Fe and Mg2Ni derived from TMA-TM (TM = Fe, Ni) MOFs as synergetic catalysts for hydrogen storage in MgH2. Sustain. Energy Fuels 2020, 4, 2192–2200. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L.; Huot, J.; Schulz, R. Effect of ball milling on the corrosion resistance of magnesium in aqueous media. Electrochim. Acta 2004, 49, 2461–2470. [Google Scholar] [CrossRef]
- Park, H.; Jung, C.; Yi, S.; Choi, P.-P. Elucidating the ball-milling-induced crystallization mechanism of amorphous NbCo1.1Sn via atomic-scale compositional analysis. J. Alloys Compd. 2023, 968, 172014. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Ritter, J.A. Kinetic behavior of Ti-doped NaAlH4 when co-doped with carbon nanostructures. J. Phys. Chem. B 2006, 110, 17353–17358. [Google Scholar] [CrossRef] [PubMed]
- Wagemans, R.W.; van Lenthe, J.H.; de Jongh, P.E.; van Dillen, A.J.; de Jong, K.P. Hydrogen Storage in Magnesium Clusters: Quantum Chemical Study. J. Am. Chem. Soc. 2005, 127, 16675–16680. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Lee, E.Y.; Lee, K.S. Catalytic effects of metal oxide on hydrogen absorption of magnesium metal hydride. J. Alloys Compd. 2006, 421, 179–184. [Google Scholar] [CrossRef]
- Pan, Y.B.; Wu, Y.F.; Li, Q. Modeling and analyzing the hydriding kinetics of Mg-LaNi5 composites by Chou model. Int. J. Hydrogen Energy 2011, 36, 12892–12901. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Calka, A.; Wexler, D.; Liu, H. Effects of carbon black, graphite and carbon nanotube additives on hydrogen storage properties of magnesium. J. Alloys Compd. 2007, 427, 94–100. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Utke, R. Ternary LiBH4–MgH2–NaAlH4 hydride confined into nanoporous carbon host for reversible hydrogen storage. J. Phys. Chem. Solids 2016, 90, 80–86. [Google Scholar] [CrossRef]
- Lu, Z.; He, J.; Song, M.; Zhang, Y.; Wu, F.; Zheng, J.; Zhang, L.; Chen, L. Bullet-like vanadium-based MOFs as a highly active catalyst for promoting the hydrogen storage property in MgH2. Int. J. Miner. Met. Mater. 2022, 30, 44–53. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Khan, D.; Zhu, W.; Hu, C.; Zeng, X.; Ding, W. Preparation and hydrogen storage properties of MgH2-trimesic acid-TM MOF (TM = Co, Fe) composites. J. Mater. Sci. Technol. 2019, 35, 2132–2143. [Google Scholar] [CrossRef]
| Hydride | Preparation Method | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Hydrogen Pressure (MPa) | Dehydrogenation Temperature (°C) | Hydrogen Capacity (wt.%) | Desorption Activation Energy (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| Mg2CoH5 | Ball milling of MgH2 and Co | 2 | 400 | 30:1 | 3.0 | 265 | 4.2 | 121 |
| Mg2NiH4 | Ball milling of MgH2 and Mg2Ni | 3 | \ | 30:1 | 1.0 | 240 | 5 | \ |
| Mg(NH2)2 | Ball milling of MgH2 and LiNH2 | 3 | 300 | 20:1 | - | 150 | 7.2 | 76 |
| Mg(BH4)2 | Ball milling of MgB2 and LiBH4 | 1 | 200 | 10:1 | 0.1 | 260 | 14.9 | 118 |
| Li2Mg(NH)2 | Decomposition of Mg(NH2)2 | - | - | - | - | 150 | 5.6 | 54 |
| LiMg(BH4)3 | Ball milling of Mg(BH4)2 and LiBH4 | 2 | 250 | 15:1 | 0.1 | 180 | 11.5 | 92 |
| Hydride | Composition | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder ratio | Hydrogen Pressure (MPa) | Grain Size (nm) | Dehydrogenation Temperature (°C) | Activation Energy (kJ/mol) | Hydrogen Capacity (wt.%) | Reversible Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| MgH2 | MgH2 | 20 | 400 | 10:1 | 1.0 | 5–10 | 200 | 76 | 7.2 | 6.8 |
| Mg2NiH4 | Mg2Ni + MgH2 | 25 | 300 | 20:1 | 3.0 | 3–5 | 200 | 87 | 3.0 | 2.8 |
| MgH2 | MgH2 + 10 wt.% LiH | 1 | 200 | 10:1 | 1.0 | 7 | 150 | 65 | 6.0 | 5.8 |
| MgH2 | MgH2 + 5 wt.% Nb2O5 | 20 | 400 | 30:1 | 1.0 | 5–10 | 200 | 61 | 6.5 | 6.2 |
| Mg2NiH4 | Mg2Ni + 10 wt.% TiH2 | 30 | 250 | 40:1 | 3.0 | 10–20 | 220 | 85 | 3.5 | 3.2 |
| Hydride | Composition | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Hydrogen Pressure (MPa) | Dehydrogenation Temperature (°C) | Dehydrogenation Activation Energy (kJ/mol) | Hydrogen Capacity (wt.%) | Reversible Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| MgH2 | MgH2 + 10 wt.% Ni | 20 | 400 | 30:1 | 1.0 | 150 | 72 | 6.8 | 6.5 |
| Mg2NiH4 | Mg2Ni + MgH2 | 100 | 200 | 50:1 | 3.0 | 200 | 98 | 3.0 | 2.8 |
| Mg-Ni-Y | Mg65Ni30Y5 | 20 | 300 | 40:1 | 3.0 | 200 | 105 | 3.5 | 3.2 |
| Mg2NiH4 | Mg2Ni + 5 wt.% TiF3 | 50 | 250 | 60:1 | 3.0 | 180 | 84 | 3.2 | 3.0 |
| MgH2 | MgH2 + 10 wt.% VTiCr | 10 | 500 | 20:1 | 1.0 | 120 | 63 | 6.0 | 5.5 |
| Hydride | Catalyst (mol%) | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Dehydrogenation Temperature (°C) | Activation Energy (kJ/mol) | Hydrogen Capacity (wt.%) | Reversible Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|
| MgH2 | 1% Ti, V, Mn, Fe, Ni | 1 | 400 | 10:1 | 250 (Ti, V), 300 (Mn, Fe, Ni) | 61 (Ti, V), 92 (Mn, Fe, Ni) | 6.5 (Ti, V), 6.0 (Mn, Fe, Ni) | 6.2 (Ti, V), 5.8 (Mn, Fe, Ni) |
| MgH2 | 1% Nb | 2 | 500 | 20:1 | 200 | 61 | 6.8 | 6.5 |
| MgH2 | 10% Ni | 0.5–5 | 300 | 30:1 | 250 (5 h) | 67 (5 h) | 6.2 (5 h) | 6.0 (5 h) |
| MgH2 | Ti-Fe-Nb (1:1:1) | 2 | 400 | 40:1 | 150 | 53 | 6.5 | 6.2 |
| MgH2 | 5% VTiCr | 10 | 500 | 20:1 | 180 | 59 | 6.0 | 5.8 |
| Mg2NiH4 | 10% TiH2 | 30 | 250 | 60:1 | 220 | 85 | 3.5 | 3.2 |
| Hydride | Catalyst (mol%) | Milling Atmosphere | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Dehydrogenation Temperature (°C) | Activation Energy (kJ/mol) | Hydrogen Capacity (wt.%) | Reversible Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| Mgh2 | 0.5% Nb2O5 | Ar | 20 | 400 | 30:1 | 250 | 85 | 6.5 | 6.2 |
| MgH2 | 1% TiO2 nanoparticles | Ar | 10 | 500 | 20:1 | 275 | 96 | 6.2 | 6.0 |
| MgH2 | 1% Cr2O3 | H2 | 5 | 400 | 40:1 | 225 | 75 | 6.8 | 6.5 |
| MgH2 | TiO2 nanotubes (5%) | Ar | 20 | 300 | 50:1 | 250 | 81 | 6.5 | 6.2 |
| MgH2 | 2% Nb2O5 | H2 | 10 | 500 | 20:1 | 225 | 68 | 7.0 | 6.8 |
| MgH2 | 5% V2O5 | Ar | 30 | 200 | 60:1 | 240 | 78 | 6.0 | 5.8 |
| Hydride | Carbon Additive (wt.%) | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Dehydrogenation Temperature (°C) | Activation Energy (kJ/mol) | Hydrogen Capacity (wt.%) | Reversible Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|
| MgH2 | 5% graphite | 10 | 400 | 30:1 | 300 | 108 | 6.5 | 6.2 |
| MgH2 | 10% CNTs | 2 | 500 | 20:1 | 275 | 102 | 6.0 | 5.8 |
| MgH2 | 5% graphene | 5 | 400 | 40:1 | 250 | 91 | 6.5 | 6.3 |
| MgH2 | 2% C60 | 10 | 300 | 50:1 | 265 | 97 | 6.8 | 6.5 |
| Mg2NiH4 | 10% CNTs | 25 | 200 | 60:1 | 220 | 83 | 3.2 | 3.0 |
| Hydride | Metal Hydride Additive | Composition (mol%) | Milling Time (h) | Milling Speed (rpm) | Ball-to-Powder Ratio | Dehydrogenation Temperature (°C) | Activation Energy (kJ/mol) | Reversible Hydrogen Capacity (wt.%) |
|---|---|---|---|---|---|---|---|---|
| MgH2 | LiBH4 | 5% LiBH4 | 1 | 400 | 20:1 | 225 | 98 | 8.0 |
| MgH2 | NaAlH4 | 30% NaAlH4 | 2 | 500 | 30:1 | 250 | 102 | 5.5 |
| MgH2 | TiH2 | 10% TiH2 | 5 | 300 | 40:1 | 275 | 115 | 6.0 |
| MgH2 | CaH2 | 5% CaH2 | 10 | 200 | 50:1 | 280 | 121 | 5.8 |
| Mg2NiH4 | LaNi5 | 10% LaNi5 | 20 | 250 | 60:1 | 240 | 95 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, Y.; Hou, Q.; Hao, Y.; Ding, Z. Ball Milling Innovations Advance Mg-Based Hydrogen Storage Materials Towards Practical Applications. Materials 2024, 17, 2510. https://doi.org/10.3390/ma17112510
Xu Y, Li Y, Hou Q, Hao Y, Ding Z. Ball Milling Innovations Advance Mg-Based Hydrogen Storage Materials Towards Practical Applications. Materials. 2024; 17(11):2510. https://doi.org/10.3390/ma17112510
Chicago/Turabian StyleXu, Yaohui, Yuting Li, Quanhui Hou, Yechen Hao, and Zhao Ding. 2024. "Ball Milling Innovations Advance Mg-Based Hydrogen Storage Materials Towards Practical Applications" Materials 17, no. 11: 2510. https://doi.org/10.3390/ma17112510
APA StyleXu, Y., Li, Y., Hou, Q., Hao, Y., & Ding, Z. (2024). Ball Milling Innovations Advance Mg-Based Hydrogen Storage Materials Towards Practical Applications. Materials, 17(11), 2510. https://doi.org/10.3390/ma17112510








