Phonon Properties and Lattice Dynamics of Two- and Tri-Layered Lead Iodide Perovskites Comprising Butylammonium and Methylammonium Cations—Temperature-Dependent Raman Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Raman Spectroscopy
3. Results and Discussion
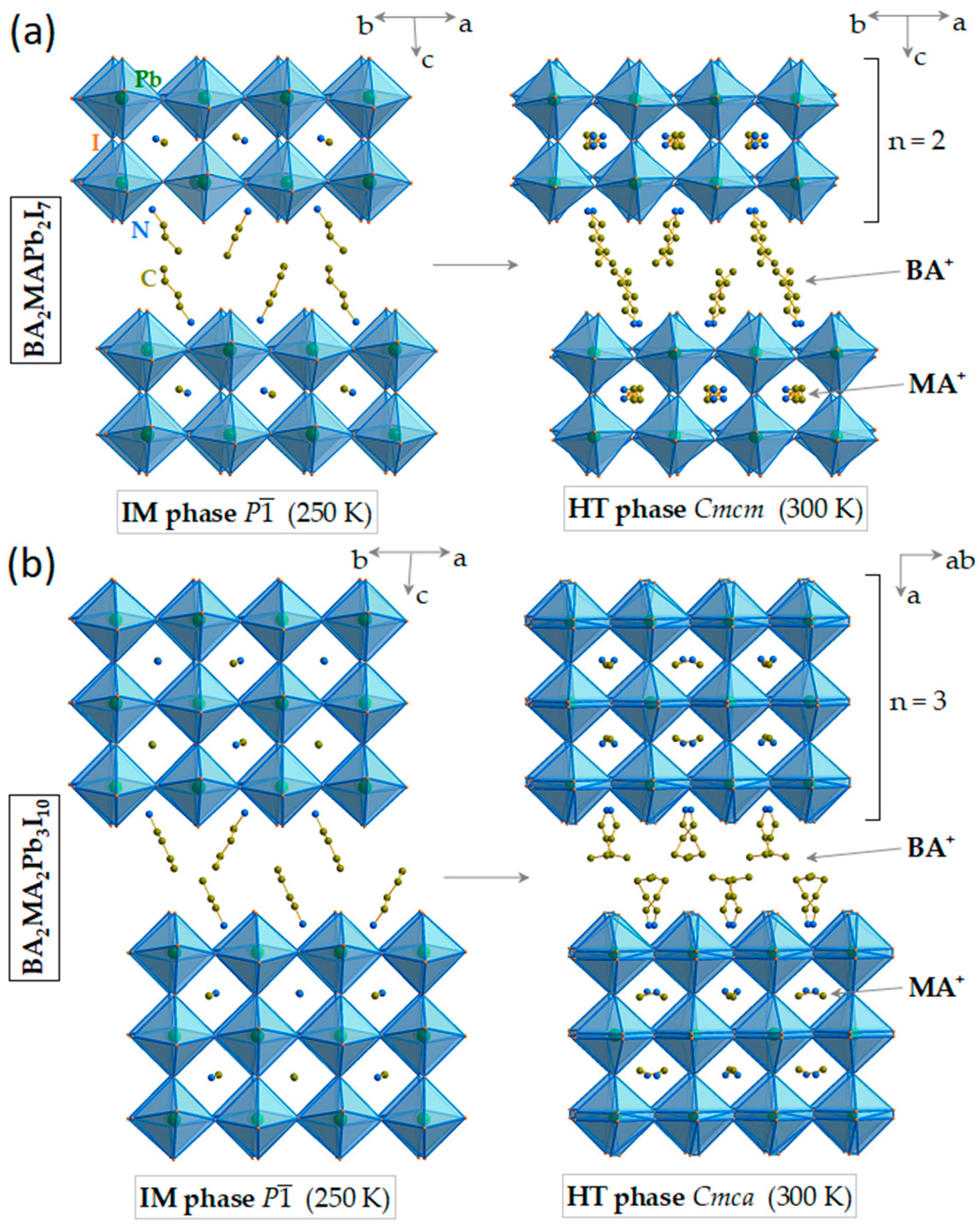
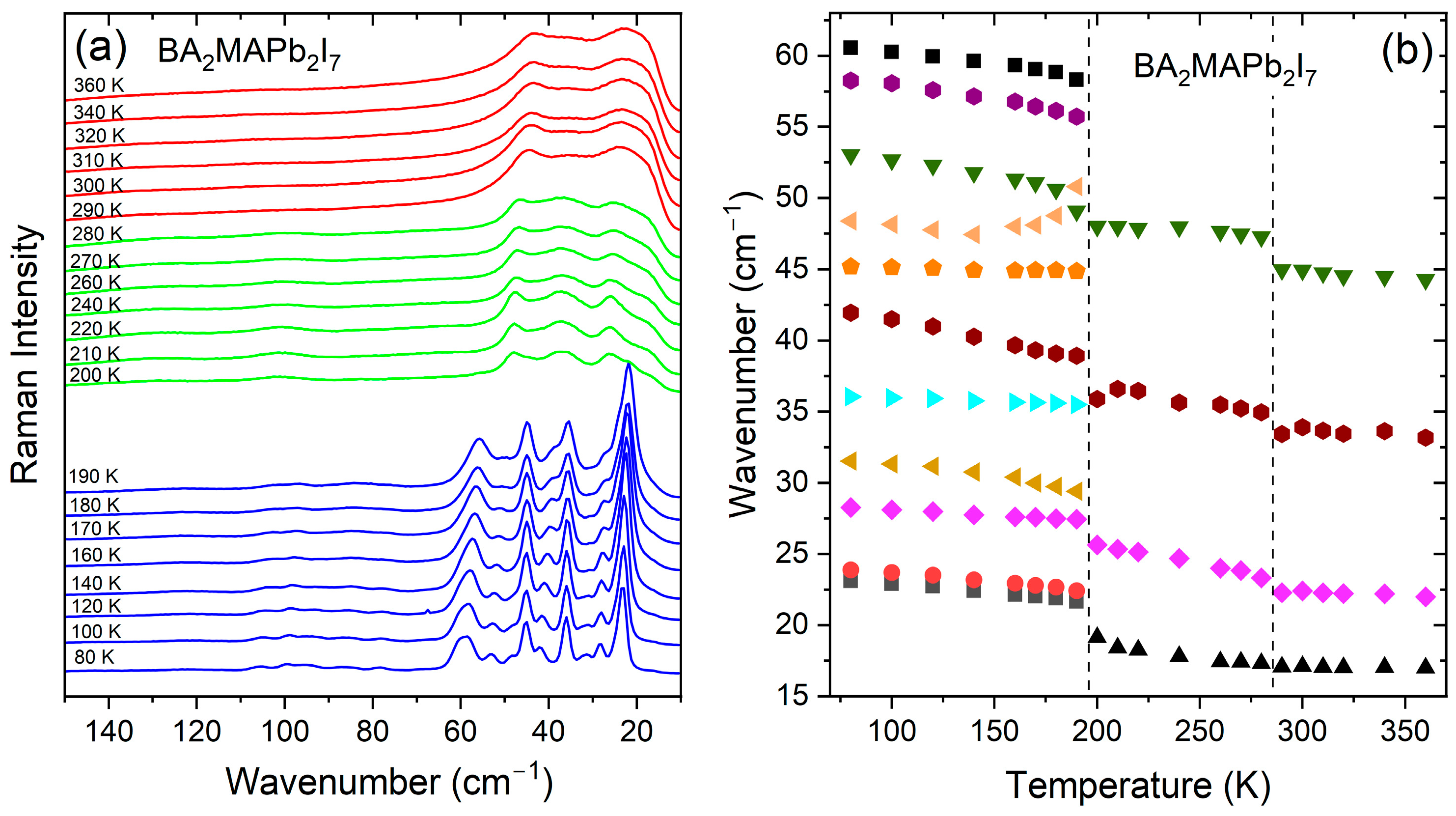
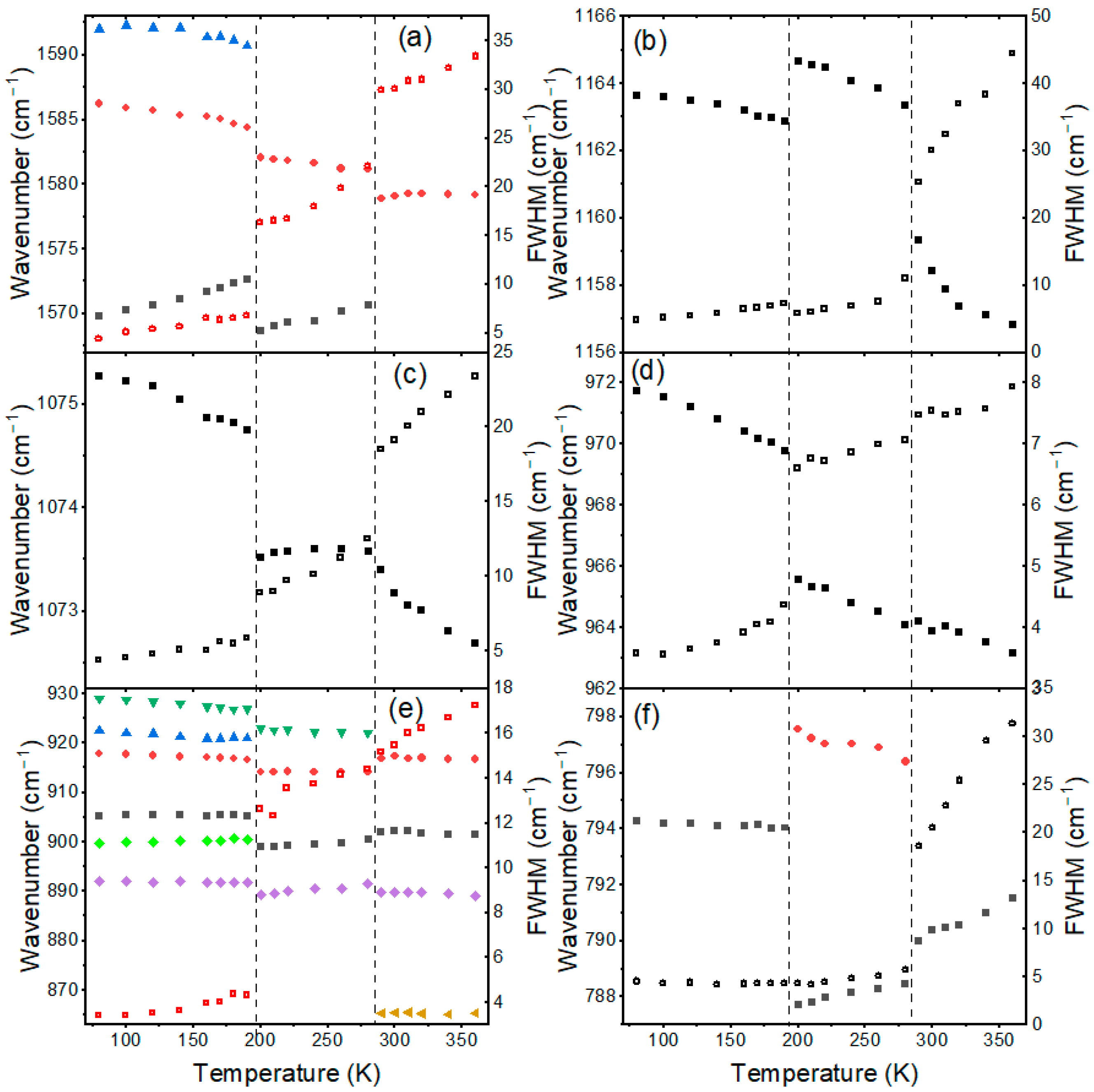
3.1. Temperature-Dependent Raman Study of BA2MAPb2I7
3.2. Temperature-Dependent Raman Study of BA2MA2Pb3I10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, Y.; Wu, J.; Xu, G.; Yang, X.; Cai, R.; Gong, Q.; Zhu, R.; Huang, W. Perovskite Solar Cells for Space Applications: Progress and Challenges. Adv. Mater. 2021, 33, 2006545. [Google Scholar] [CrossRef]
- Yang, T.; Gao, L.; Lu, J.; Ma, C.; Du, Y.; Wang, P.; Ding, Z.; Wang, S.; Xu, P.; Liu, D.; et al. One-stone-for-two-birds strategy to attain beyond 25% perovskite solar cells. Nat. Commun. 2023, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Oku, T. Crystal Structures of Perovskite Halide Compounds Used for Solar Cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zaręba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Maçzka, M.; Gagor, A.; Zareba, J.K.; Stefanska, D.; Drozd, M.; Balciunas, S.; Šimenas, M.; Banys, J.; Sieradzki, A.; Ma̧czka, M.; et al. Three-Dimensional Perovskite Methylhydrazinium Lead Chloride with Two Polar Phases and Unusual Second-Harmonic Generation Bistability above Room Temperature. Chem. Mater. 2020, 32, 4072–4082. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Petrosova, H.R.; Kucheriv, O.I.; Shova, S.; Gural’skiy, I.A. Aziridinium Cation Templating 3D Lead Halide Hybrid Perovskites. Chem. Commun. 2022, 58, 5745–5748. [Google Scholar] [CrossRef]
- Maczka, M.; Ptak, M.; Gagor, A.; Zareba, J.; Liang, X.; Balciunas, S.; Semenikhin, A.A.; Kucheriv, O.I.; Gural’skiy, I.A.; Shova, S.; et al. Phase Transitions, Dielectric Response, and Nonlinear Optical Properties of Aziridinium Lead Halide Perovskites. Chem. Mater. 2023, 35, 9725–9738. [Google Scholar] [CrossRef]
- Simenas, M.; Balciunas, S.; Wilson, J.N.; Svirskas, S.; Kinka, M.; Garbaras, A.; Kalendra, V.; Gagor, A.; Szewczyk, D.; Sieradzki, A.; et al. Suppression of Phase Transitions and Glass Phase Signatures in Mixed Cation Halide Perovskites. Nat. Commun. 2020, 11, 5103. [Google Scholar] [CrossRef]
- Simenas, M.; Gagor, A.; Banys, J.; Maczka, M. Phase Transitions and Dynamics in Mixed Three- and Low-Dimensional Lead Halide Perovskites. Chem. Rev. 2024, 124, 2281–2326. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cui, B.B. Low-dimensional metal halide perovskite materials: Structure strategies and luminescence applications. Adv. Sci. 2021, 8, 2004805. [Google Scholar] [CrossRef] [PubMed]
- Maczka, M.; Drozdowski, D.; Stefańska, D.; Gagor, A. Zero-dimensional mixed-cation hybrid lead halides with broadband emissions. Inorg. Chem. Front. 2023, 10, 7222–7230. [Google Scholar] [CrossRef]
- Li, X.; Hoffman, J.M.; Kanatzidis, M.G. The 2D Halide Perovskite Rulebook: How the Spacer Influences Everything from the Structure to Optoelectronic Device Efficiency. Chem. Rev. 2021, 121, 2230–2291. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Zarȩba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. [Methylhydrazinium]2PbBr4, a ferroelectric hybrid organic-inorganic perovskite with multiple nonlinear optical outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Y.; Chai, S.; Chen, S.; Xu, J. 2D organic-inorganic hybrid perovskite materials for nonlinear optics. Nanophotonics 2020, 9, 1787–1810. [Google Scholar] [CrossRef]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef]
- Chao, L.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Recent progress on low dimensional perovskite solar cells. J. Energy Chem. 2018, 27, 1091–1100. [Google Scholar] [CrossRef]
- Siwach, P.; Sikarwar, P.; Halpati, J.S.; Chandiran, K. Design of above-room-temperature ferroelectric two-dimensional layered halide perovskites. J. Mater. Chem. A 2022, 10, 8719–8738. [Google Scholar] [CrossRef]
- Niu, T.; Xue, Q.; Yip, H.Y. Advances in Dion-Jacobson phase two-dimensional metal halide perovskite solar cells. Nanophotonics 2021, 10, 2069–2102. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Lim, E.L.; Kong, T.; Zhang, Y.; Song, J.; Hagfeldt, A.; Bi, D. Stable Layered Perovskite Solar Cells with an Efficiency over 19% via Multifunctional Interfacial Engineering. J. Am. Chem. Soc. 2021, 143, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Hu, H.; Haselsberger, H.; Marcus, R.A.; Michel-Beyerle, M.E.; Lam, Y.M.; Zhu, J.X.; La-o-Vorakiat, C.; Beard, M.C.; Chia, E.E.M. Monitoring electron-phonon interactions in lead halide perovskites using time-resolved THz spectroscopy. ACS Nano 2019, 13, 8826–8835. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, M.J.; Robinson, P.J.; Abramovitch, D.J.; Tan, L.Z.; Rappe, A.M.; Reichman, D.R.; Egger, D.A. The Significance of Polarons and Dynamic Disorder in Halide Perovskites. ACS Energy Lett. 2021, 6, 2162–2173. [Google Scholar] [CrossRef]
- Herz, L.M. How Lattice Dynamics Moderate the Electronic Properties of Metal-Halide Perovskites. J. Phys. Chem. Lett. 2018, 9, 6853–6863. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, X.; Liu, Y.; Xu, Z.; Li, Y.; Hong, M.; Luo, J.; Sun, Z. High-Temperature Antiferroelectric of Lead Iodide Hybrid Perovskites. J. Am. Chem. Soc. 2019, 141, 12470–12474. [Google Scholar] [CrossRef] [PubMed]
- Paillard, C.; Bai, X.; Infante, I.C.; Guennou, M.; Geneste, G.; Alexe, M.; Kreisel, J.; Dkhil, B. Photovoltaics with Ferroelectrics: Current Status and Beyond. Adv. Mater. 2016, 28, 5153–5168. [Google Scholar] [CrossRef] [PubMed]
- Ptak, M.; Sieradzki, A.; Simenas, M.; Maczka, M. Molecular spectroscopy of hybrid organic-inorganic perovskites and related compounds. Coord. Chem. Rev. 2021, 448, 214180. [Google Scholar] [CrossRef]
- Nakada, K.; Matsumoto, Y.; Shimoi, Y.; Yamada, K.; Furukawa, Y. Temperature-Dependent Evolution of Raman Spectra of Methylammonium Lead Halide Perovskites, CH3NH3PbX3 (X=I, Br). Molecules 2019, 24, 626. [Google Scholar] [CrossRef]
- Ibaceta-Jaña, J.; Muydinov, R.; Rosado, P.; Mirhosseini, H.; Chugh, M.; Nazarenko, O.; Dirin, D.N.; Heinrich, D.; Wagner, M.R.; Kühne, T.D.; et al. Vibrational Dynamics in Lead Halide Hybrid Perovskites Investigated by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 5604–5614. [Google Scholar] [CrossRef]
- Ruan, S.; McMeekin, D.P.; Fan, R.; Webster, N.A.S.; Ebendorff-Heidepriem, H.; Cheng, Y.B.; Lu, J.; Ruan, Y.; McNeill, C.R. Raman Spectroscopy of Formamidinium-Based Lead Halide Perovskite Single Crystals. J. Phys. Chem. C 2020, 124, 2265–2272. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic Disorder, Phonon Lifetimes, and the Assignment of Modes to the Vibrational Spectra of Methylammonium Lead Halide Perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Ptak, M. Temperature-Dependent Raman Studies of FAPbBr3 and MAPbBr3 Perovskites: Effect of Phase Transitions on Molecular Dynamics and Lattice Distortion. Solids 2022, 3, 111–121. [Google Scholar] [CrossRef]
- Mączka, M.; Zienkiewicz, J.A.; Ptak, M. Comparative Studies of Phonon Properties of Three-Dimensional Hybrid Organic—Inorganic Perovskites Comprising Methylhydrazinium, Methylammonium, and Formamidinium Cations. J. Phys. Chem. C 2022, 126, 4048–4056. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M. Lattice Dynamics and Structural Phase Transitions in Two-Dimensional Ferroelectric Methylhydrazinium Lead Bromide Investigated Using Raman and IR spectroscopy. J. Phys. Chem. C 2022, 126, 7991–7998. [Google Scholar] [CrossRef]
- Spirito, D.; Asensio, Y.; Hueso, L.E.; Martin-Garcia, B. Raman spectroscopy in layered hybrid organic-inorganic metal halide perovskites. J. Phys. Mater. 2022, 5, 034004. [Google Scholar] [CrossRef]
- Fu, Y.; Hautzinger, M.P.; Luo, Z.; Wang, F.; Pan, D.; Aristov, M.M.; Guzei, I.A.; Pan, A.; Zhu, X.; Jin, S. Incorporating Large A Cations into Lead Iodide Perovskite Cages: Relaxed Goldschidt Tolerance Factor and Impact on Exciton-Phonon Interaction. ACS Cent. Sci. 2019, 5, 1377–1386. [Google Scholar] [CrossRef]
- Li, H.; Qin, Y.; Shan, B.; Shen, Y.; Ersan, F.; Soignard, E.; Ataca, C.; Tongay, S. Unusual Pressure-Driven Phase Transformation and Band Renormalization in 2D vdW Hybrid Lead Halide Perovskites. Adv. Mater. 2020, 32, 1907364. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, Y.; Bu, K.; Fu, Y.; Luo, H.; Chen, M.; Hautzinger, M.P.; Wang, Y.; Jin, S.; Yang, W.; et al. Pressure-Suppressed Carrier Trapping Leads to Enhanced Emission in Tow-Dimensional Perovskite (HA)2(GA)Pb2I7. Angew. Chem. Int. Ed. 2020, 59, 17533–17539. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Pedesseau, L.; Guo, P.; Cuthriell, S.; Hadar, I.; Even, J.; Katan, C.; Stoumpos, C.C.; Schaller, R.D.; et al. Negative Pressure Engineering with Large Cage Cations in 2D Halide Perovskites Causes Lattice Softening. J. Am. Chem. Soc. 2020, 142, 11486–11496. [Google Scholar] [CrossRef]
- Liang, M.; Lin, W.; Lan, Z.; Meng, J.; Zhao, Q.; Zou, X.; Castelli, I.E.; Pullerits, T.; Canton, S.E.; Zheng, K. Electronic Structure and Trap States of Two-Dimensional Ruddlesden–Popper Perovskites with the Relaxed Goldschmidt Tolerance Factor. ACS Appl. Electron. Mater. 2020, 2, 1402–1412. [Google Scholar] [CrossRef]
- Dahod, N.S.; France-Lanord, A.; Paritmongkol, W.; Grossman, J.C.; Tisdale, W.A. Low-frequency Raman spectrum of 2D layered perovskites: Local atomistic motion or superlattice modes? J. Chem. Phys. 2020, 153, 0044710. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yan, H.; Abdelwahab, I.; Lekina, Y.; Lü, X.; Yang, W.; Sun, H.; Leng, K.; Cai, Y.; Zhen, Z.X.; et al. Pressure driven rotational isomerism in 2D hybrid perovskites. Nat. Commun. 2023, 14, 411. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden-Popper Hybrid Lead Iodide perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Soe, C.M.M.; Tsai, H.; Nie, W.; Blancon, J.C.; Cao, D.H.; Liu, F.; Traore, B.; Katan, C.; Even, J.; et al. High Members of the 2D Ruddlesden-Popper Halide perovskites: Synthesis, Optical Properties, and Solar Cells of (CH3(CH2)3NH3)2(CH3NH3)4Pb5I16. Chem 2017, 2, 427–440. [Google Scholar] [CrossRef]
- Dang, Y.; Wei, J.; Liu, X.; Wang, X.; Xu, K.; Lei, M.; Hu, W.; Tao, X. Layered hybrid perovskite solar cells based on single-crystalline precursor solutions with superior reproducibility. Sustain. Energy Fuels 2018, 2, 2237–2243. [Google Scholar] [CrossRef]
- Saouma, F.O.; Stoumpos, C.C.; Wong, J.; Kanatzidis, M.G.; Jang, J.I. Selective enhancement of optical nonlinearity in two-dimensional organic-inorganic lead iodide perovskites. Nat. Commun. 2017, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Gelvez-Rueda, M.C.; Hutter, E.M.; Cao, D.H.; Renaud, N.; Stoumpos, C.C.; Hupp, J.T.; Savenije, T.J.; Kanatzidis, M.G.; Grozema, F.C. Interconversion between Free Charges and Bound Excitons in 2D Hybrid Lead Halide Perovskites. J. Phys. Chem. C 2017, 121, 26566–26574. [Google Scholar] [CrossRef] [PubMed]
- Cortecchia, D.; Neutzner, S.; Yin, J.; Salim, T.; Kandada, A.R.S.; Bruno, A.; Lam, Y.M.; Marti-Rujas, J.; Petrozza, A.; Soci, C. Structure-controlled optical thermoresponse in Ruddlesden-Popper layered perovskites. APL Mater. 2018, 6, 114207. [Google Scholar] [CrossRef]
- Paritmongkol, W.; Dahod, N.S.; Stollmann, A.; Mao, N.; Settens, C.; Zheng, S.L.; Tisdale, W.A. Synthetic Variation and Strcutural Trends in Layered Two-Dimensional Alkylammonium Lead Halide Perovskites. Chem. Mater. 2019, 31, 5592–5607. [Google Scholar] [CrossRef]
- Lyu, F.; Zheng, X.; Li, Z.; Chen, Z.; Shi, R.; Wang, Z.; Liu, H.; Lin, B.L. Spatiodynamics, Photodynamics, and Their Correlation in Hybrid Perovskites. Chem. Mater. 2021, 33, 3524–3533. [Google Scholar] [CrossRef]
- Koegel, A.A.; Oswald, I.W.H.; Rivera, C.; Miller, S.L.; Fallon, M.J.; Prisk, T.R.; Brown, C.M.; Neilson, J.R. Influence of Inorganic Layer Thickness on Methylammonium Dynamics in Hybrid Perovskite Derivatives. Chem. Mater. 2022, 34, 8316–8323. [Google Scholar] [CrossRef]
- Simenas, M.; Balciunas, S.; Gagor, A.; Pieniazek, A.; Tolborg, K.; Kinka, M.; Klimavicius, V.; Svirskas, S.; Kalendra, V.; Ptak, M.; et al. Mixology of MA1−xEAxPbI3 Hybrid Perovskites: Phase Transitions, Cation Dynamics, and Photoluminescence. Chem. Mater. 2022, 34, 10104–10112. [Google Scholar] [CrossRef] [PubMed]
- Maczka, M.; Ptak, M.; Fedoruk, K.; Stefanska, D.; Gagor, A.; Zareba, J.K.; Sieradzki, A. The lattice symmetrization worked, but with a plot twist: Effects of methylhydrazinium doping of MAPbI3 on phase transitions, cation dynamics and photoluminescence. J. Mater. Chem. C 2024, 12, 1396–1405. [Google Scholar] [CrossRef]
- Jeghnou, H.; Ouasri, A.; Rhandour, A.; Dhamelincourt, M.C.; Dhamelincourt, P.; Mazzah, A. Structural Phase Transition in (n-C4H9NH3)2SiF6: DSC and Raman Studies. J. Raman Spectrosc. 2003, 34, 126–130. [Google Scholar] [CrossRef]
- Jalbout, A.F.; Ouasri, A.; Jeghnou, H.; Rhandour, A. Experimental and Theoretical Studies of Monoalkylammonium Hexafluorosilicate [CH3(CH2)nNH3]2SiF6 (n = 2,3) and Ethylammonium Hexafluorosilicate [(C2H5)2NH2]2SiF6. Vib. Spectrosc. 2007, 44, 94–100. [Google Scholar] [CrossRef]
- Wright, A.; Verdi, C.; Milot, R.L.; Eperon, G.E.; Perez-Osorio, M.A.; Snaith, H.J.; Giustio, F.; Johnson, M.B.; Herz, L.M. Electron-Phonon Coupling in Hybrid Lead Halide Perovskites. Nat. Commun. 2016, 7, 11755. [Google Scholar] [CrossRef] [PubMed]
- Gelvez-Rueda, M.C.; Cao, D.H.; Patwardhan, S.; Renaud, N.; Stoumpos, C.C.; Schatz, G.C.; Hupp, J.T.; Farha, O.K.; Savenije, T.J.; Kanatzidis, M.G.; et al. Effect of Cation Rotation on Charge Dynamics in Hybrid Lead Halide Perovskites. J. Phys. Chem. C 2016, 120, 16577–16585. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, X.; Shen, Y.; Moehl, T.; Zakeeruddin, S.M.; Grätzel, M.; Wang, M. Photovoltaic behaviour of lead methylammonium triiodide perovskite solar cells down to 80 K. J. Mater. Chem. A 2015, 3, 11762–11767. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, M.; Smółka, S.; Ptak, M. Phonon Properties and Lattice Dynamics of Two- and Tri-Layered Lead Iodide Perovskites Comprising Butylammonium and Methylammonium Cations—Temperature-Dependent Raman Studies. Materials 2024, 17, 2503. https://doi.org/10.3390/ma17112503
Mączka M, Smółka S, Ptak M. Phonon Properties and Lattice Dynamics of Two- and Tri-Layered Lead Iodide Perovskites Comprising Butylammonium and Methylammonium Cations—Temperature-Dependent Raman Studies. Materials. 2024; 17(11):2503. https://doi.org/10.3390/ma17112503
Chicago/Turabian StyleMączka, Mirosław, Szymon Smółka, and Maciej Ptak. 2024. "Phonon Properties and Lattice Dynamics of Two- and Tri-Layered Lead Iodide Perovskites Comprising Butylammonium and Methylammonium Cations—Temperature-Dependent Raman Studies" Materials 17, no. 11: 2503. https://doi.org/10.3390/ma17112503
APA StyleMączka, M., Smółka, S., & Ptak, M. (2024). Phonon Properties and Lattice Dynamics of Two- and Tri-Layered Lead Iodide Perovskites Comprising Butylammonium and Methylammonium Cations—Temperature-Dependent Raman Studies. Materials, 17(11), 2503. https://doi.org/10.3390/ma17112503







