Economic and Accessible Portable Homemade Magnetic Hyperthermia System: Influence of the Shape, Characteristics and Type of Nanoparticles in Its Effectiveness
Abstract
1. Introduction
1.1. Magnetism and Heat Generation
1.2. SAR Calculation [35,36]
1.2.1. Non-Adiabatic Conditions
Box–Lucas Method
Corrected Initial Slope Method
2. Experimental Section
2.1. Equipment
2.2. Materials and Methods
2.2.1. Materials
2.2.2. Synthesis of Nanoparticles
Synthesis of Cubic-Shaped Magnetite Nanoparticles
Synthesis of Cubic-Shaped Magnetite Nanoparticles Coated with PEG
Synthesis of Magnetite Nanoparticles by Coprecipitation
Synthesis by Coprecipitation of Magnetite Nanoparticles Coated with PEG
Synthesis by Coprecipitation of Magnetite Nanoparticles Coated with Citric Acid
3. Results and Discussion
3.1. Nanoparticle Characterization
3.1.1. Phase Identification and Particle Morphology
3.1.2. SEM
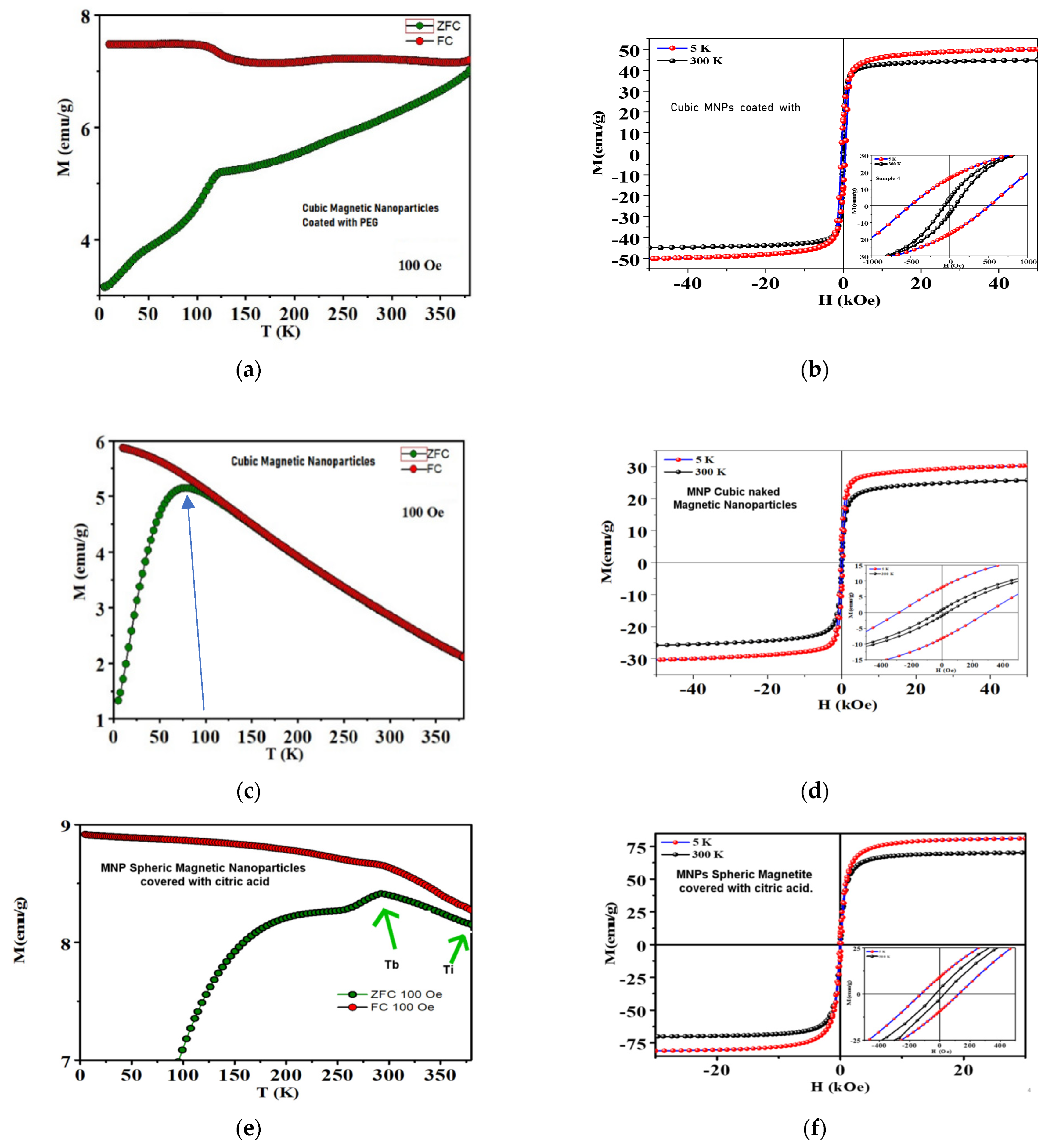
3.1.3. Magnetic Characterization
3.1.4. FTIR Analysis
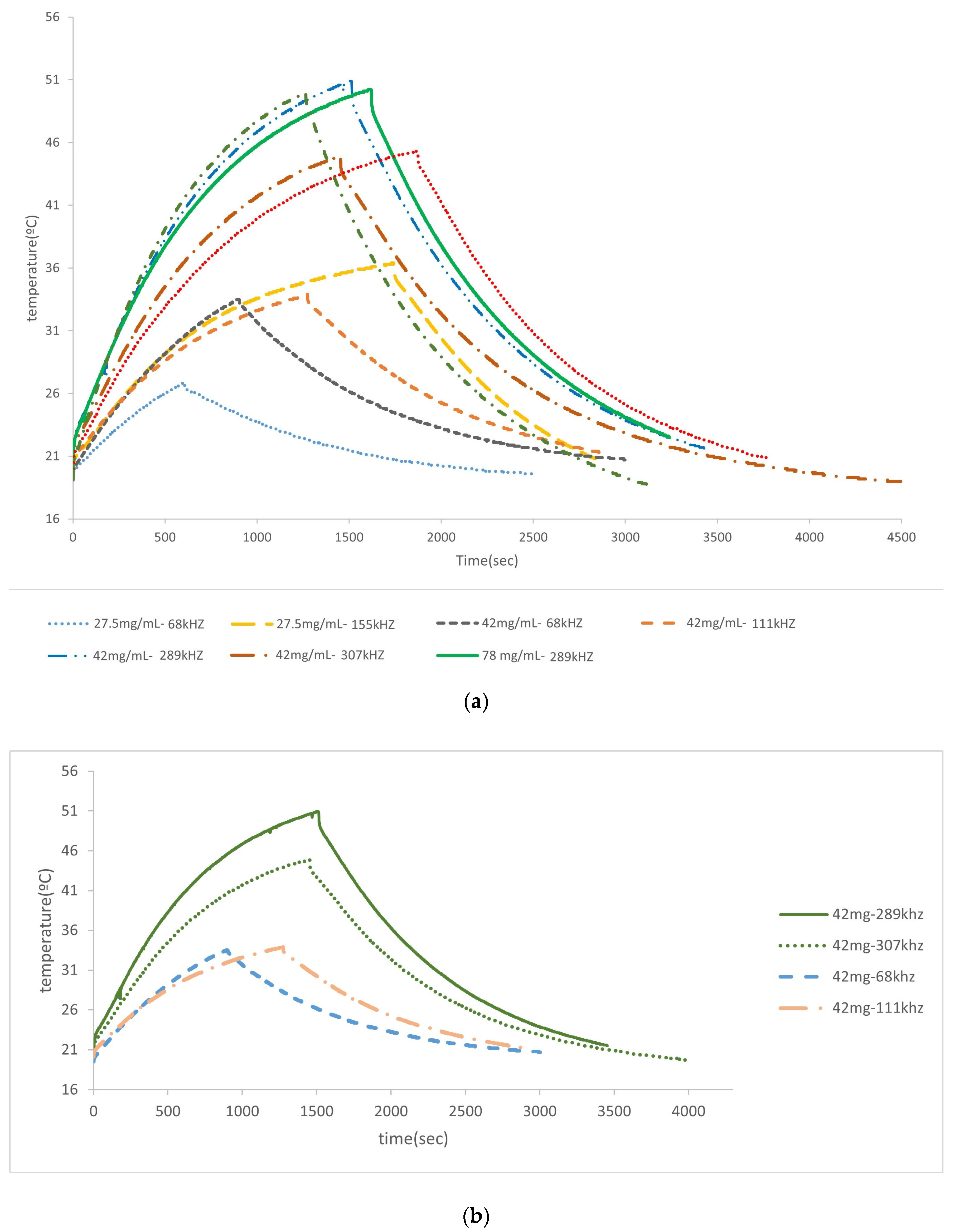
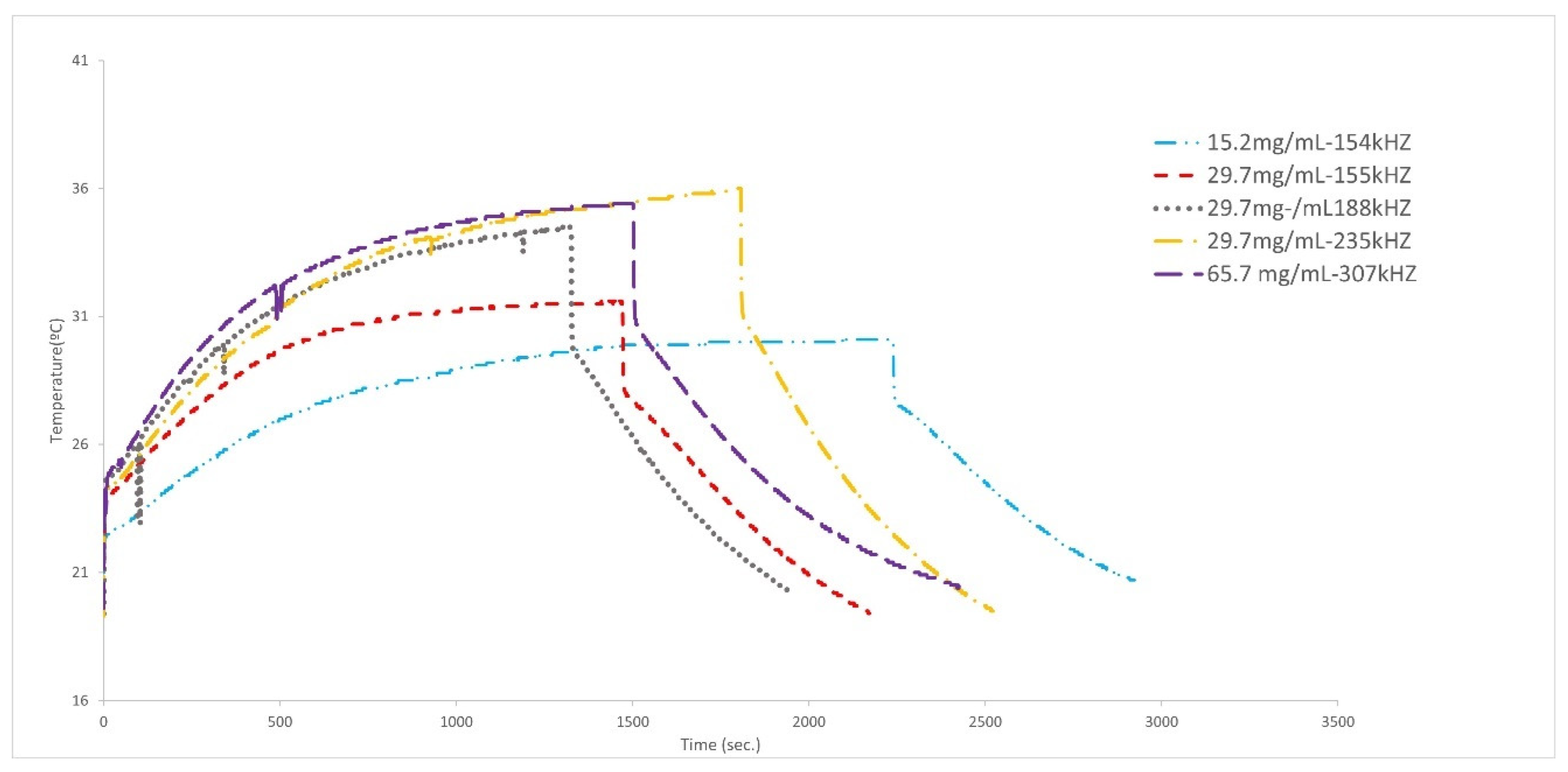
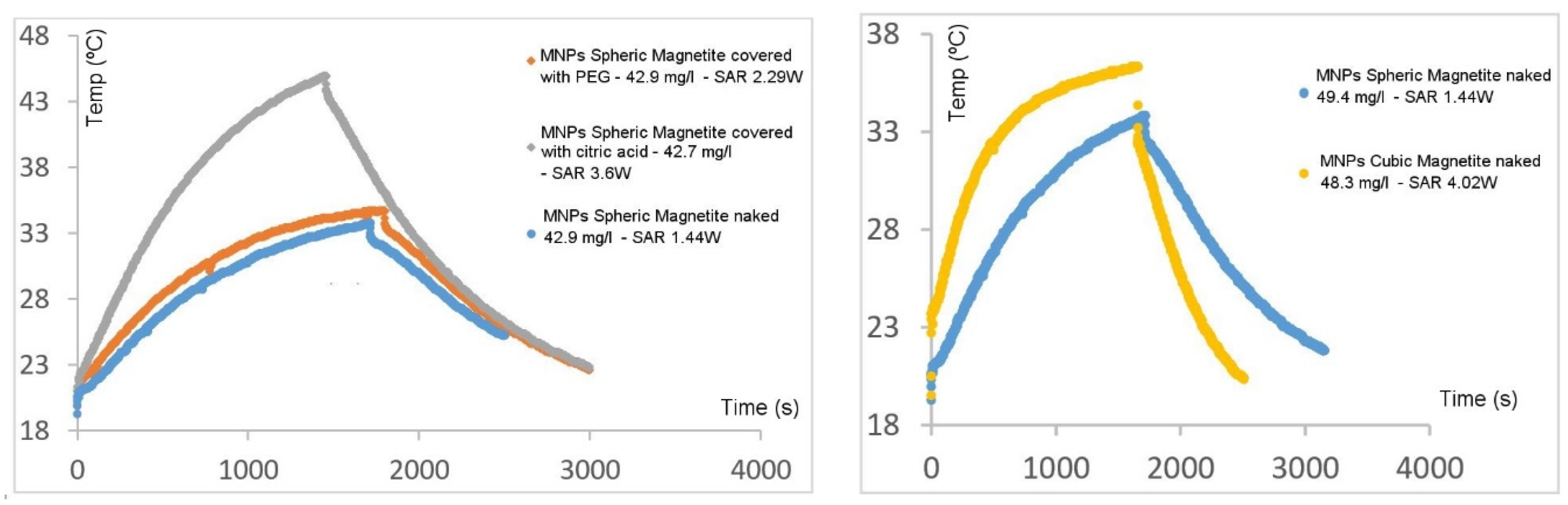
3.2. Hyperthermia Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corley, D.A.; Sedki, M.; Ritzwoller, D.P.; Greenlee, R.T.; Neslund-Dudas, C.; Rendle, K.A.; Honda, S.A.; Schottinger, J.E.; Udaltsova, N.; Vachani, A.; et al. Cancer Screening During the Coronavirus Disease-2019 Pandemic: A Perspective From the National Cancer Institute’s PROSPR Consortium. Gastroenterology 2021, 160, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.; González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Roussakow, S. The History of Hyperthermia Rise and Decline. Conf. Pap. Med. 2013, 2013, 428027. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Mesquita, M.S.; Garcia, L.M.; dos Santos, P.R.; Viveiros, C.C.d.M.d.; da Fonseca, R.D.; Xavier, M.A.; de Mendonça, G.W.; Rosa, S.S.; Silva, S.L.; et al. Radiofrequency driving antitumor effect of graphene oxide-based nanocomposites: A Hill model analysis. Nanomedicine 2024, 19, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Gillams, A.R. The use of radiofrequency in cancer. Br. J. Cancer 2005, 92, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrot, J.C.; Taylor, C.B. Selective Inductive Heating of Lymph Nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, J.; Yu, J.; Zheng, Z.; Liu, F.; Yang, A. Recent advances of high performance magnetic iron oxide nanoparticles: Controlled synthesis, properties tuning and cancer theranostics. Nano Sel. 2020, 2, 216–250. [Google Scholar] [CrossRef]
- Ma, Z.; Mohapatra, J.; Wei, K.; Liu, J.P.; Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 2021, 123, 3904–3943. [Google Scholar] [CrossRef]
- Zhu, K.; Ju, Y.; Xu, J.; Yang, Z.; Gao, S.; Hou, Y. Magnetic Nanomaterials: Chemical Design, Synthesis, and Potential Applications. Accounts Chem. Res. 2018, 51, 404–413. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Farhanian, D.; De Crescenzo, G.; Tavares, J.R. Large-Scale Encapsulation of Magnetic Iron Oxide Nanoparticles via Syngas Photo-Initiated Chemical Vapor Deposition. Sci. Rep. 2018, 8, 12223. [Google Scholar] [CrossRef] [PubMed]
- Mona, L.P.; Songca, S.P.; Ajibade, P.A. Synthesis and encapsulation of iron oxide nanorods for application in magnetic hyperthermia and photothermal therapy. Nanotechnol. Rev. 2021, 11, 176–190. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Arafa, K.; Fytory, M. 1—Physical properties, classification, synthesis, and functionalization of magnetic nanomaterials. In Magnetic Nanomaterials in Analytical Chemistry; Ahmadi, M., Afkhami, A., Madrakian, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Sun, N.; Deng, C. Facile synthesis of thiol-polyethylene glycol functionalized magnetic titania nanomaterials for highly efficient enrichment of N-linked glycopeptides. J. Chromatogr. A 2017, 1512, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Libutti, S.K.; Pingpank, J.F.; Bartlett, D.L.; Beresnev, T.H.; Mavroukakis, S.M.; Steinberg, S.M.; Liewehr, D.J.; Kleiner, D.E.; Alexander, H.R. Analysis of Factors Associated With Outcome in Patients With Malignant Peritoneal Mesothelioma Undergoing Surgical Debulking and Intraperitoneal Chemotherapy. J. Clin. Oncol. 2003, 21, 4560–4567. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Kouzoudis, D.; Samourgkanidis, G.; Kolokithas-Ntoukas, A.; Zoppellaro, G.; Spiliotopoulos, K. Magnetic Hyperthermia in the 400–1100 kHz Frequency Range Using MIONs of Condensed Colloidal Nanocrystal Clusters. Front. Mater. 2021, 8. [Google Scholar] [CrossRef]
- Augusto, P.A.; Castelo-Grande, T.; Vargas, D.; Hernández, L.; Merchán, L.; Estevez, A.M.; Gómez, J.; Compaña, J.M.; Barbosa, D. Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters. Materials 2020, 13, 2219. [Google Scholar] [CrossRef] [PubMed]
- Augusto, P.A.; Castelo-Grande, T.; Estévez, A.M.; Barbosa, D.; Costa, P.M. Method to evaluate and prove-the-concept of magnetic separation and/or classification of particles. J. Magn. Magn. Mater. 2017, 426, 405–414. [Google Scholar] [CrossRef]
- Pacheco, A.R.F.; Cardoso, B.D.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Carvalho, V.M.; Rodrigues, R.O.; Coutinho, P.J.G.; Castelo-Grande, T.; Augusto, P.A.; et al. Development of pH-Sensitive Magnetoliposomes Containing Shape Anisotropic Nanoparticles for Potential Application in Combined Cancer Therapy. Nanomaterials 2023, 13, 1051. [Google Scholar] [CrossRef] [PubMed]
- Augusto, P.A.; Castelo-Grande, T.; Vargas, D.; Pascual, A.; Hernández, L.; Estevez, A.M.; Barbosa, D. Upscale Design, Process Development, and Economic Analysis of Industrial Plants for Nanomagnetic Particle Production for Environmental and Biomedical Use. Materials 2020, 13, 2477. [Google Scholar] [CrossRef] [PubMed]
- Bentarhlia, N.; Elansary, M.; Belaiche, M.; Mouhib, Y.; Lemine, O.; Zaher, H.; Oubihi, A.; Kartah, B.; Monfalouti, H. Evaluating of novel Mn–Mg–Co ferrite nanoparticles for biomedical applications: From synthesis to biological activities. Ceram. Int. 2023, 49, 40421–40434. [Google Scholar] [CrossRef]
- Gaona-Esquivel, A.; Hernandez-M, D.S.; Hernández-Rodríguez, Y.; Cigarroa-Mayorga, O. The role of Nd as a dopant in Mn3O4NPs on the heat induction of artificial breast tissue due to the irradiation of microwaves. Mater. Chem. Phys. 2022, 292, 126822. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.; Ipus, J.; Law, J.; Moreno-Ramírez, L.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Korolev, V.V.; Arefyev, I.M.; Ramazanova, A.G. The magnetocaloric effect of superfine magnets. J. Therm. Anal. Calorim. 2008, 92, 691–695. [Google Scholar] [CrossRef]
- Korolev, V.V.; Aref’ev, I.M.; Ramazanova, A.G. The magnetocaloric effect and heat capacity of ferrimagnetic nanosystems: High-dispersity magnetite. Russ. J. Phys. Chem. A 2007, 81, 949–951. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Anandhi, J.S.; Arun, T.; Joseyphus, R.J. Role of magnetic anisotropy on the heating mechanism of Co-doped Fe3O4 nanoparticles. Phys. B Condens. Matter 2020, 598, 412429. [Google Scholar] [CrossRef]
- Nemala, H.; Thakur, J.S.; Naik, V.M.; Vaishnava, P.P.; Lawes, G.; Naik, R. Investigation of magnetic properties of Fe3O4 nanoparticles using temperature dependent magnetic hyperthermia in ferrofluids. J. Appl. Phys. 2014, 116, 034309. [Google Scholar] [CrossRef]
- Mazon, E.E.; Villa-Martínez, E.; Hernández-Sámano, A.; Córdova-Fraga, T.; Ibarra-Sánchez, J.J.; Calleja, H.A.; Cruz, J.A.L.; Barrera, A.; Estrada, J.C.; Paz, J.A.; et al. A high-resolution frequency variable experimental setup for studying ferrofluids used in magnetic hyperthermia. Rev. Sci. Instrum. 2017, 88, 084705. [Google Scholar] [CrossRef] [PubMed]
- Andreu, I.; Natividad, E. Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.; Efthimiadou, E.K.; Pissas, M.; Fuentes, D.; Boukos, N.; Psycharis, V.; Kordas, G.; Loukopoulos, V.C.; Kagadis, G.C. Magnetic fluid hyperthermia simulations in evaluation of SAR calculation methods. Phys. Medica 2020, 71, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef]
- Ring, H.L.; Sharma, A.; Ivkov, R.; Bischof, J.C. The impact of data selection and fitting on SAR estimation for magnetic nanoparticle heating. Int. J. Hyperth. 2020, 37, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
- Castelo-Grande, T.; Augusto, P.A.; Gomes, L.; Calvo, E.; Barbosa, D. An Economic and accessible portable homemade magnetic hyperthermia system. 2024. submitted. [Google Scholar]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Dossett, J.; Totten, G. Nanoparticle heating using induction in hyperthermia. In ASM Handbook; ASM International: Materials Park, OH, USA, 2014; Volume 4, pp. 4–5. [Google Scholar]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Yu, J.; Gao, S. Chapter Nanomagnetism: Principles, Nanostructures, and Biomedical Applications. In Synthesis and Biomedical Applications of Magnetic Nanomaterials; EDP Sciences: Les Ulis, France, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Huo, Y.; Yu, J.; Gao, S. Magnetic-mediated hyperthermia for cancer treatment: Research progress and clinical trials. In Synthesis and Biomedical Applications of Magnetic Nanomaterials; EDP Sciences: Les Ulis, France, 2022; pp. 228–260. [Google Scholar] [CrossRef]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.-H.; Kandala, S.K.; Yang, C.-T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell–mediated tumor suppression in models of breast cancer. Sci. Adv. 2020, 6, eaay1601. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.; Inman, A.; Young, S.-H.; Muhammed, M.; Burks, T.; Uheida, A.; Tkach, A.; Waltz, M.; Castranova, V.; et al. Oxidative Stress and Dermal Toxicity of Iron Oxide Nanoparticles In Vitro. Cell Biochem. Biophys. 2012, 67, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Daldrup-Link, H.E. Clinical Tracking of Cell Transfer and Cell Transplantation: Trials and Tribulations. Radiology 2018, 289, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Sánchez, O.S.; Castelo-Grande, T.; Augusto, P.A.; Compaña, J.M.; Barbosa, D. Cubic Nanoparticles for Magnetic Hyperthermia: Process Optimization and Potential Industrial Implementation. Nanomaterials 2021, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Goldberg, S.N. Basic Science Research in Thermal Ablation. Surg. Oncol. Clin. N. Am. 2011, 20, 237–258. [Google Scholar] [CrossRef]
- Mihai, C.T.; Luca, A.; Danciu, M.; Alexa-Stratulat, T.; Ciobanu, R.; Tamba, B.I.; Stanciu, G.D.; Ciortescu, I.; Mihai, C.; Stefanescu, G.; et al. In vitro evaluation of novel functionalized nanoparticles for terahertz imaging. Med.-Surg. J. 2019, 123, 139–146. [Google Scholar]
- Mansurov, Z.; Smagulova, G.; Kaidar, B.; Imash, A.; Lesbayev, A. PAN—Composite Electrospun-Fibers Decorated with Magnetite Nanoparticles. Magnetochemistry 2022, 8, 160. [Google Scholar] [CrossRef]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Saragi, T.; Permana, B.; Therigan, A.; Sinaga, H.D.; Maulana, T.; Risdiana, R. Study of Magnetic Properties and Relaxation Time of Nanoparticle Fe3O4-SiO2. Materials 2022, 15, 1573. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y.S. Size Effects on Properties of NiO Nanoparticles Grown in Alkalisalts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]
- Ramana, P.; Rao, K.S.; Kumar, K.R.; Kapusetti, G.; Choppadandi, M.; Kiran, J. A study of uncoated and coated nickel-zinc ferrite nanoparticles for magnetic hyperthermia. Mater. Chem. Phys. 2021, 266, 124546. [Google Scholar] [CrossRef]
- Andhare, D.D.; Patade, S.R.; Khedkar, M.V.; Nawpute, A.A.; Jadhav, K.M. Intensive analysis of uncoated and surface modified Co-Zn nanoferrite as a heat generator in magnetic fluid hyperthermia applications. Appl. Phys. A 2022, 128, 502. [Google Scholar] [CrossRef]
- Cheraghipour, E.; Javadpour, S.; Mehdizadeh, A.R. Citrate capped superparamagnetic iron oxide nanoparticles used for hyperthermia therapy. J. Biomed. Sci. Eng. 2012, 05, 715–719. [Google Scholar] [CrossRef]
- Sadat, E.; Bud’ko, S.L.; Ewing, R.C.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Effect of Dipole Interactions on Blocking Temperature and Relaxation Dynamics of Superparamagnetic Iron-Oxide (Fe3O4) Nanoparticle Systems. Materials 2023, 16, 496. [Google Scholar] [CrossRef]
- Banerjee, A.; Blasiak, B.; Pasquier, E.; Tomanek, B.; Trudel, S. Synthesis, characterization, and evaluation of PEGylated first-row transition metal ferrite nanoparticles as T 2 contrast agents for high-field MRI. RSC Adv. 2017, 7, 38125. [Google Scholar] [CrossRef]
- Malega, F.; Indrayana, I.P.T.; Suharyadi, E. Synthesis and characterization of the microstructure and functional group bond of Fe3o4 nanoparticles from natural iron sand in Tobelo North Halmahera. J. Ilm. Pendidik. Fis. Al-Biruni 2018, 7, 13–22. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.E.; Airinei, A. Citric-acid-coated magnetite nanoparticles for biological applications. Eur. Phys. J. E 2006, 21, 117–121. [Google Scholar] [CrossRef]
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Abu Noqta, O.; Khaniabadi, P.M.; Mehrdel, B. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Sci. Rep. 2020, 10, 10793. [Google Scholar] [CrossRef]
- Arefi, M.; Miraki, M.K.; Mostafalu, R.; Satari, M.; Heydari, A. Citric acid stabilized on the surface of magnetic nanoparticles as an efficient and recyclable catalyst for transamidation of carboxamides, phthalimide, urea and thiourea with amines under neat conditions. J. Iran. Chem. Soc. 2018, 16, 393–400. [Google Scholar] [CrossRef]
- Singh, D.; Gautam, R.K.; Kumar, R.; Shukla, B.K.; Shankar, V.; Krishna, V. Citric acid coated magnetic nanoparticles: Synthesis, characterization and application in removal of Cd(II) ions from aqueous solution. J. Water Process. Eng. 2014, 4, 233–241. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.; Sampaio, L.; Macêdo, M.; Nakamura, M.; Toma, H. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.-T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224001. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.-P. Magnetic hyperthermia performance of magnetite nanoparticle assemblies under different driving fields. AIP Adv. 2017, 7, 056327. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of magnetic field parameters and iron oxide nanoparticle properties on heat generation for use in magnetic hyperthermia. J. Magn. Magn. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef]
- Usov, N.A.; Nesmeyanov, M.S.; Gubanova, E.M.; Epshtein, N.B. Heating ability of magnetic nanoparticles with cubic and combined anisotropy. Beilstein J. Nanotechnol. 2019, 10, 305–314. [Google Scholar] [CrossRef] [PubMed]



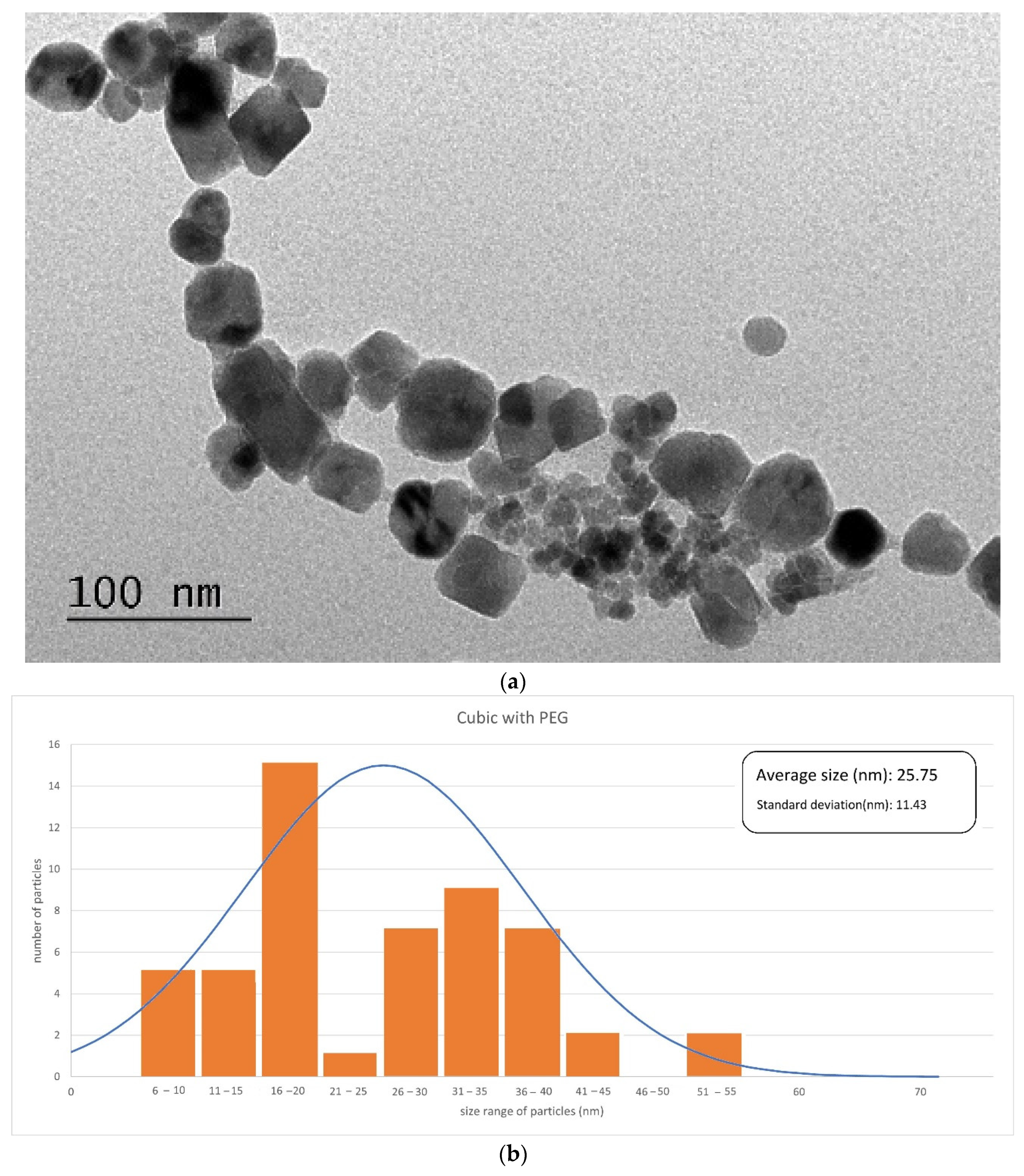



| Type of Particles | Size of Crystals (nm) |
|---|---|
| Cubic | 7.70 ± 0.05 |
| Cubic/PEG | 20.40 ± 0.06 |
| Spherical/CA | 15.00 ± 0.08 |
| Type of MNP | Avg. Size (nm) | MS emu/g (5 K/300 K) | MR emu/g (5 K/300 K) | MR/MS | HC kOe (5 K/300 K) | χ0 (emu/g·Oe) |
|---|---|---|---|---|---|---|
| Cubic MNPs | 35.39 | 3.00 | 8.00 | 0.27 | −337.85 | 2.37 × 10−2 |
| Cubic (100 K) | 35.39 | 25.9 | −1.47 | −0.01 | 31.08 | 4.72 × 10−2 |
| Cubic w/PEG | 25.75 | 50.0 | 16.48 | 0.32 | −667.01 | 2.75 × 10−2 |
| MNPs w/CA | 12.37 | 70.0 | 8.40 | 0.12 | 534.91 | 1.57 × 10−2 |
| Particles | Susceptibility (m3/kg) |
|---|---|
| MNPs covered with CA | 2.34 × 10−1 |
| Cubic MNPs | 2.34 × 10−2 |
| Cubic MNPs covered with PEG | 1.40 × 10−2 |
| Particles | Frequency (kHz) | Concentration (mg/mL) | Ti/Tfin./ΔT (K) | SAR (Increment) (W/g) | SAR (BLM) (W/g) |
|---|---|---|---|---|---|
| Cubic MNPs coated with PEG | 235 | 29.7 | 19.3/35.9/16.6 | 4.0 ± 0.7 | 4.4 |
| 189 | 29.7 | 19.4/34.5/15.1 | 5.0 ± 0.9 | 5.8 | |
| 155 | 29.7 | 20.4/31.6/11.2 | 4.0 ± 0.6 | 5.0 | |
| 155 | 15.2 | 19.8/30.1/10.3 | 4.2 ± 0.7 | 5.4 | |
| 307 | 65.7 | 19.3/35.4 | 2.8+ 0.4 | 3.8 | |
| MNPs coated with citric acid | 289 | 42.7 | 20.2/50.1/29.9 | 4.1 ± 0.4 | 4.5 |
| 289 | 78.2 | 19.7/50.2/30.5 | 2.3 ± 0.2 | 2.5 | |
| 307 | 42.7 | 20.2/44.9/24.7 | 3.1 ± 0.3 | 3.6 | |
| 307 | 78.2 | 19.5/45.3/25.8 | 1.6 ± 0.1 | 1.8 | |
| 155 | 27.5 | 19.5/36.4 | 3.5 ± 0.4 | 4.1 | |
| Cubic MNPs (naked) | 155 | 122.0 | 19.9/37.1 | 1.1 ± 0.1 | 1.1 |
| 307 | 73.2 | 18.9/38.0 | 1.6 ± 0.2 | 1.6 | |
| 307 | 48.6 | 19.5/36.3 | 3.3 ± 0.3 | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelo-Grande, T.; Augusto, P.A.; Gomes, L.; Lopes, A.R.C.; Araújo, J.P.; Barbosa, D. Economic and Accessible Portable Homemade Magnetic Hyperthermia System: Influence of the Shape, Characteristics and Type of Nanoparticles in Its Effectiveness. Materials 2024, 17, 2279. https://doi.org/10.3390/ma17102279
Castelo-Grande T, Augusto PA, Gomes L, Lopes ARC, Araújo JP, Barbosa D. Economic and Accessible Portable Homemade Magnetic Hyperthermia System: Influence of the Shape, Characteristics and Type of Nanoparticles in Its Effectiveness. Materials. 2024; 17(10):2279. https://doi.org/10.3390/ma17102279
Chicago/Turabian StyleCastelo-Grande, Teresa, Paulo A. Augusto, Lobinho Gomes, Ana Rita Castro Lopes, João Pedro Araújo, and Domingos Barbosa. 2024. "Economic and Accessible Portable Homemade Magnetic Hyperthermia System: Influence of the Shape, Characteristics and Type of Nanoparticles in Its Effectiveness" Materials 17, no. 10: 2279. https://doi.org/10.3390/ma17102279
APA StyleCastelo-Grande, T., Augusto, P. A., Gomes, L., Lopes, A. R. C., Araújo, J. P., & Barbosa, D. (2024). Economic and Accessible Portable Homemade Magnetic Hyperthermia System: Influence of the Shape, Characteristics and Type of Nanoparticles in Its Effectiveness. Materials, 17(10), 2279. https://doi.org/10.3390/ma17102279







