Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2022; Plastics Europe: Brussells, Belgium, 2023. [Google Scholar]
- Tran, M.H.; Lee, E.Y. Production of Polyols and Polyurethane from Biomass: A Review. Environ. Chem. Lett. 2023, 21, 2199–2223. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. Synthesis and Characterization of Biopolyols through Biomass Liquefaction of Wood Shavings and Their Application in the Preparation of Polyurethane Wood Composites. Eur. J. Wood Wood Prod. 2022, 80, 57–74. [Google Scholar] [CrossRef]
- Abdel Hakim, A.A.; Nassar, M.; Emam, A.; Sultan, M. Preparation and Characterization of Rigid Polyurethane Foam Prepared from Sugar-Cane Bagasse Polyol. Mater. Chem. Phys. 2011, 129, 301–307. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane Foams from Vegetable Oil-Based Polyols: A Review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Alagi, P.; Hong, S.C. Vegetable Oil-Based Polyols for Sustainable Polyurethanes. Macromol. Res. 2015, 23, 1079–1086. [Google Scholar] [CrossRef]
- Cifarelli, A.; Boggioni, L.; Vignali, A.; Tritto, I.; Bertini, F.; Losio, S. Flexible Polyurethane Foams from Epoxidized Vegetable Oils and a Bio-based Diisocyanate. Polymers 2021, 13, 612. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of Transesterified Palm Olein-Based Polyol and Rigid Polyurethanes from This Polyol. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural Oil Based Highly Functional Polyols as Feedstock for Rigid Polyurethane Foam Thermal Insulation. Ind. Crops Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Stirna, U.; Fridrihsone, A.; Lazdiņa, B.; Misāne, M.; Vilsone, D. Biobased Polyurethanes from Rapeseed Oil Polyols: Structure, Mechanical and Thermal Properties. J. Polym. Environ. 2013, 21, 952–962. [Google Scholar] [CrossRef]
- Asare, M.A.; Kote, P.; Chaudhary, S.; de Souza, F.M.; Gupta, R.K. Sunflower Oil as a Renewable Resource for Polyurethane Foams: Effects of Flame-Retardants. Polymers 2022, 14, 5282. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Fang, Z.; Wan, Z.D.; Chen, H.C.; He, W.; Li, X.L.; Guo, K. Rigid Polyurethane Foam Based on Modified Soybean Oil. Adv. Mater. Res. 2013, 724–725, 1681–1684. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of Edible Oil vs. Non-Edible Oil vs. Waste Edible Oil as Biodiesel Feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel Production from Waste Cooking Oil: 1. Process Design and Technological Assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marson, A.; Masiero, M.; Modesti, M.; Scipioni, A.; Manzardo, A. Life Cycle Assessment of Polyurethane Foams from Polyols Obtained through Chemical Recycling. ACS Omega 2021, 6, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M. New Poly(Lactide-Urethane-Isocyanurate) Foams Based on Bio-PolylactideWaste. Polymers 2019, 11, 481. [Google Scholar] [CrossRef]
- Dîrloman, F.M.; Toader, G.; Rotariu, T.; Țigănescu, T.V.; Ginghina, R.E.; Petre, R.; Alexe, F.; Ungureanu, M.I.; Rusen, E.; Diacon, A.; et al. Novel Polyurethanes Based on Recycled Polyethylene Terephthalate: Synthesis, Characterization, and Formulation of Binders for Environmentally Responsible Rocket Propellants. Polymers 2021, 13, 3828. [Google Scholar] [CrossRef]
- Tran, T.K.N.; Pilard, J.F.; Pasetto, P. Recycling Waste Tires: Generation of Functional Oligomers and Description of Their Use in the Synthesis of Polyurethane Foams. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products – A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef]
- Nowshehri, J.A.; Bhat, Z.A.; Shah, M.Y. Blessings in Disguise: Bio-Functional Benefits of Grape Seed Extracts. Food Res. Int. 2015, 77, 333–348. [Google Scholar] [CrossRef]
- Jithender, B.; Rathod, P.J. Nutritional and Anti-Nutritional Factors Present in Oil Seeds: An Overview Phytochemical Characterization of Algal Species from Coastal Belt of Okha Region View Project. Int. J. Chem. Stud. 2019, 7, 1159–1165. [Google Scholar]
- De Haro, J.C.; Rodríguez, J.F.; Carmona, M.; Pérez, Á.; Ángel, P. Revalorization of Grape Seed Oil for Innovative Non-Food Applications. In Grapes and Wines—Advances in Production, Processing, Analysis and Valorization; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef][Green Version]
- Vijayan, J.G.; Chandrashekar, A.; AG, J.; Prabhu, T.N.; Kalappa, P. Polyurethane and Its Composites Derived from Bio-Sources: Synthesis, Characterization and Adsorption Studies. Polym. Polym. Compos. 2022, 30, 09673911221110347. [Google Scholar] [CrossRef]
- Clark, A.J.; Ross, A.H.; Bon, S.A.F. Synthesis and Properties of Polyesters from Waste Grapeseed Oil: Comparison with Soybean and Rapeseed Oils. J. Polym. Environ. 2017, 25, 1–10. [Google Scholar] [CrossRef]
- Malewska, E.; Polaczek, K.; Kurańska, M. Impact of Various Catalysts on Transesterification of Used Cooking Oil and Foaming Processes of Polyurethane Systems. Materials 2022, 15, 7807. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, U.; Sercheli, R.; Matheus, R. Transesterification of Vegetable Oils: A Review General Aspects of Transesterification Transesterification of Vegetable Oils Acid-Catalyzed Processes Base-Catalyzed Processes. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar]
- Yakushin, V.; Stirna, U.; Bikovens, O.; Misane, M.; Sevastyanova, I.; Vilsone, D. Synthesis and Characterization of Novel Polyurethanes Based on Tall Oil. Medziagotyra 2013, 19, 390–396. [Google Scholar] [CrossRef]
- Kurańska, M.; Leszczyńska, M.; Malewska, E.; Prociak, A.; Ryszkowska, J. Implementation of Circular Economy Principles in the Synthesis of Polyurethane Foams. Polymers 2020, 12, 2068. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of Bio-Polyols with Different Chemical Structures on Foaming of Polyurethane Systems and Foam Properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]
- Zemła, M.; Prociak, A.; Michałowski, S.; Cabulis, U.; Kirpluks, M.; Simakovs, K. Thermal Insulating Rigid Polyurethane Foams with Bio-Polyol from Rapeseed Oil Modified by Phosphorus Additive and Reactive Flame Retardants. Int. J. Mol. Sci. 2022, 23, 12386. [Google Scholar] [CrossRef]
- Noureddine, B.; Zitouni, S.; Achraf, B.; Houssém, C.; Jannick, D.; Jean-François, G.; Noureddine, B.; Zitouni, S.; Achraf, B.; Houssém, C.; et al. Development and Characterization of Tailored Polyurethane Foams for Shock Absorption to Cite This Version: HAL Id: Hal-04143390 Applied Sciences Development and Characterization of Tailored Polyurethane Foams for Shock Absorption. Appl. Sci. 2022, 12, 2206. [Google Scholar] [CrossRef]
- Stefano, F.; Alice, L.; Georgios, K.; Dmytro, L.; Gianluca, S.; Europe, P. Closed and Open-Cell Spray Polyurethane Foam. Energy Procedia 2013, 441–446. [Google Scholar]
- Prociak, A.; Ryszkowska, J.; Rokicki, G. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014; Volume 1, ISBN 9788301187842. [Google Scholar]
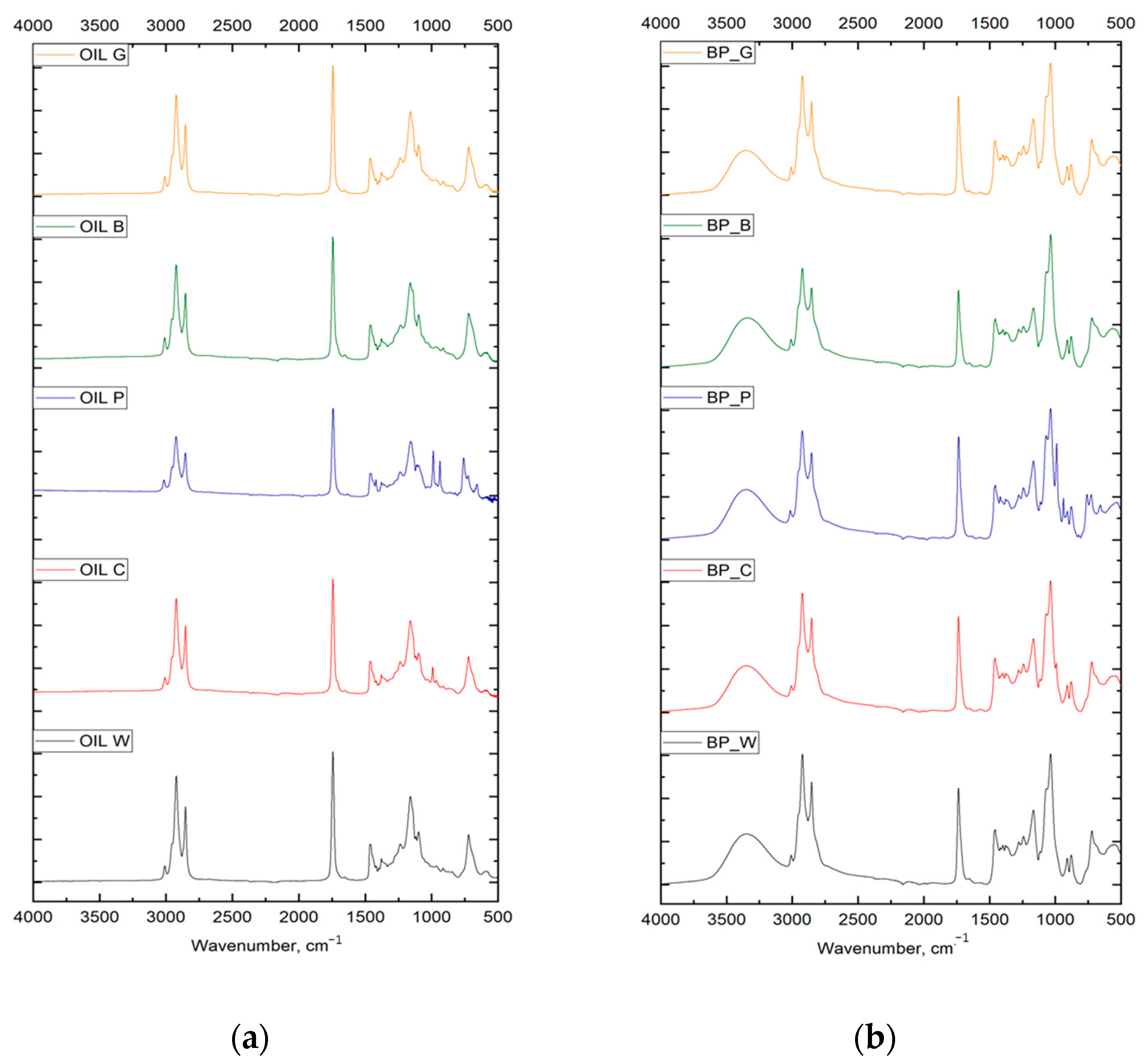
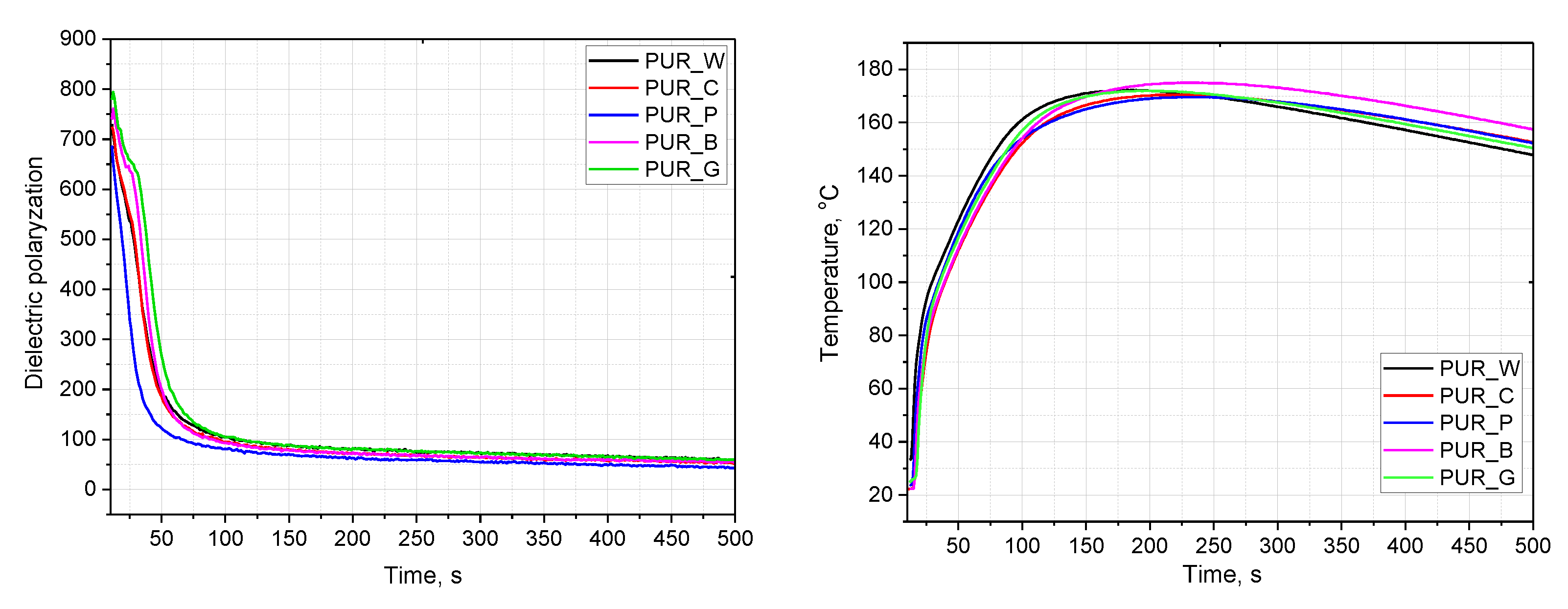

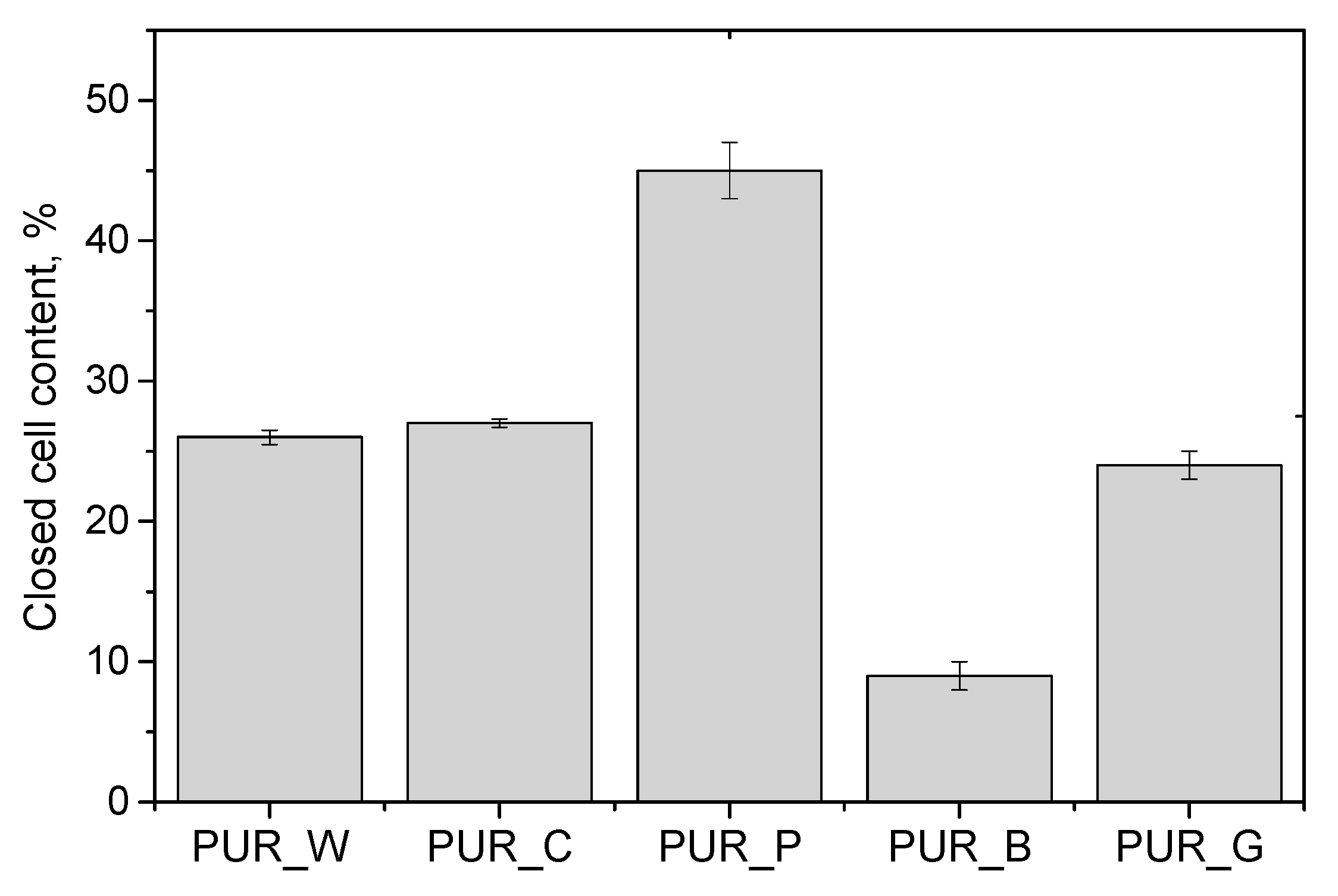
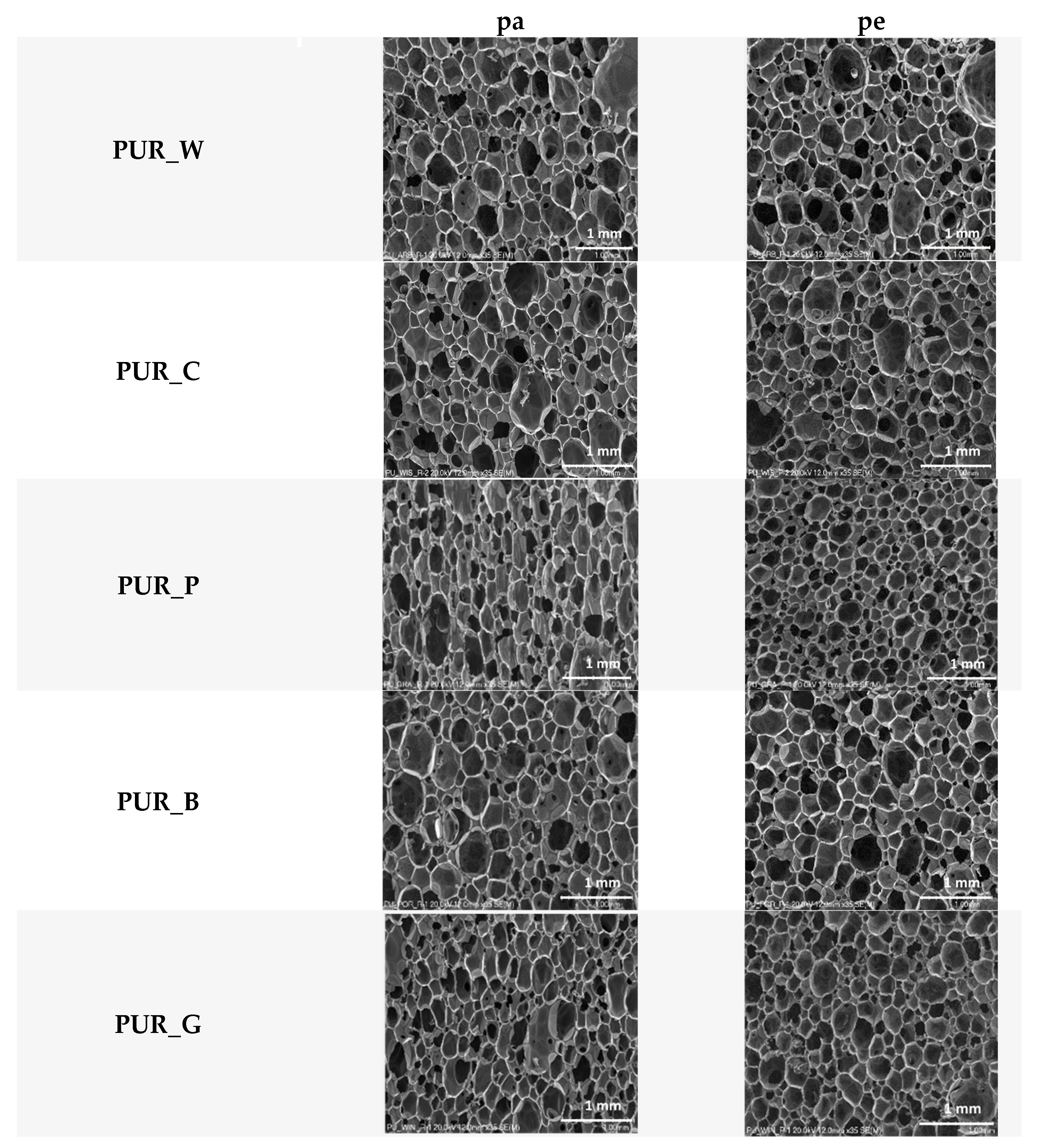
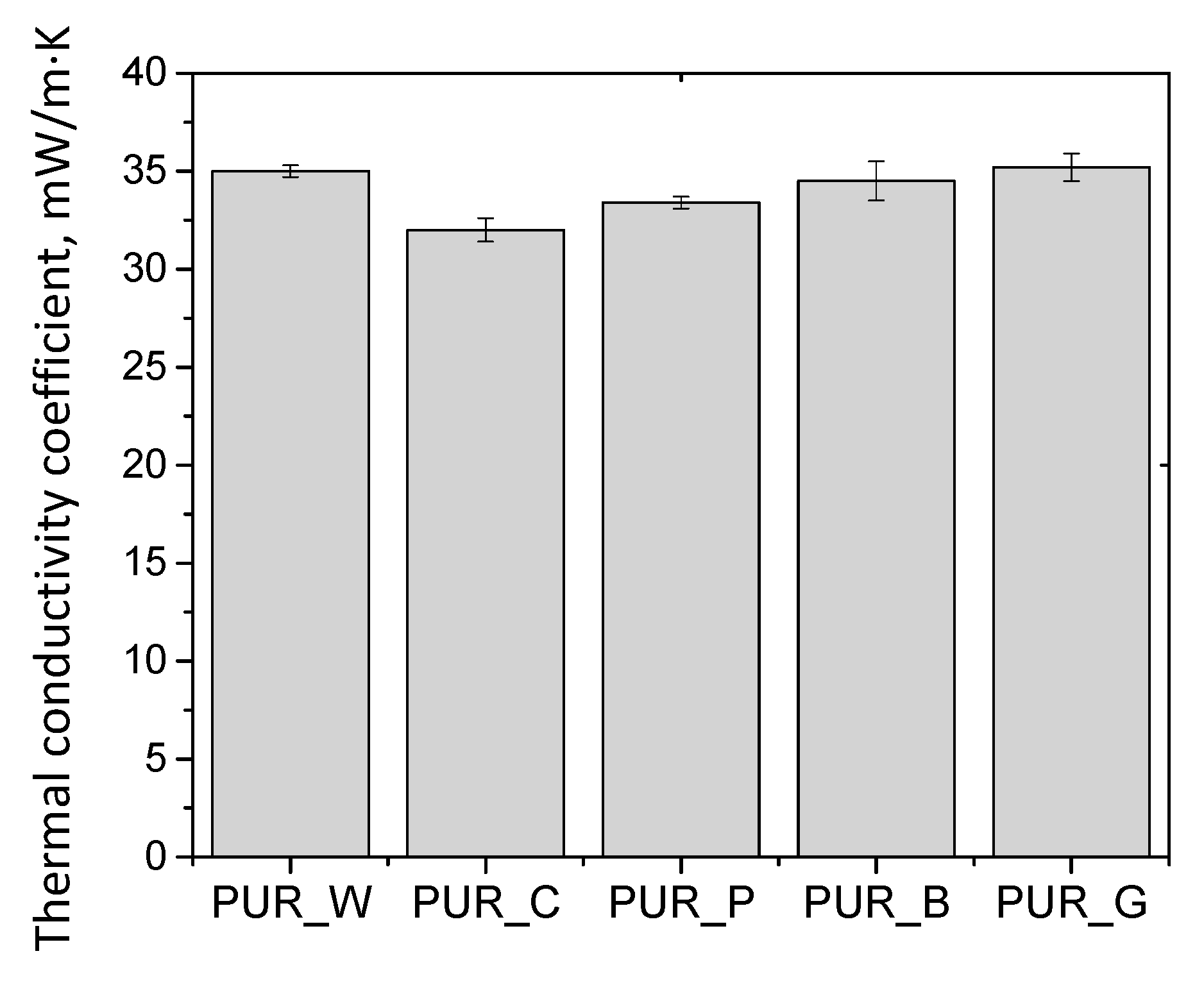
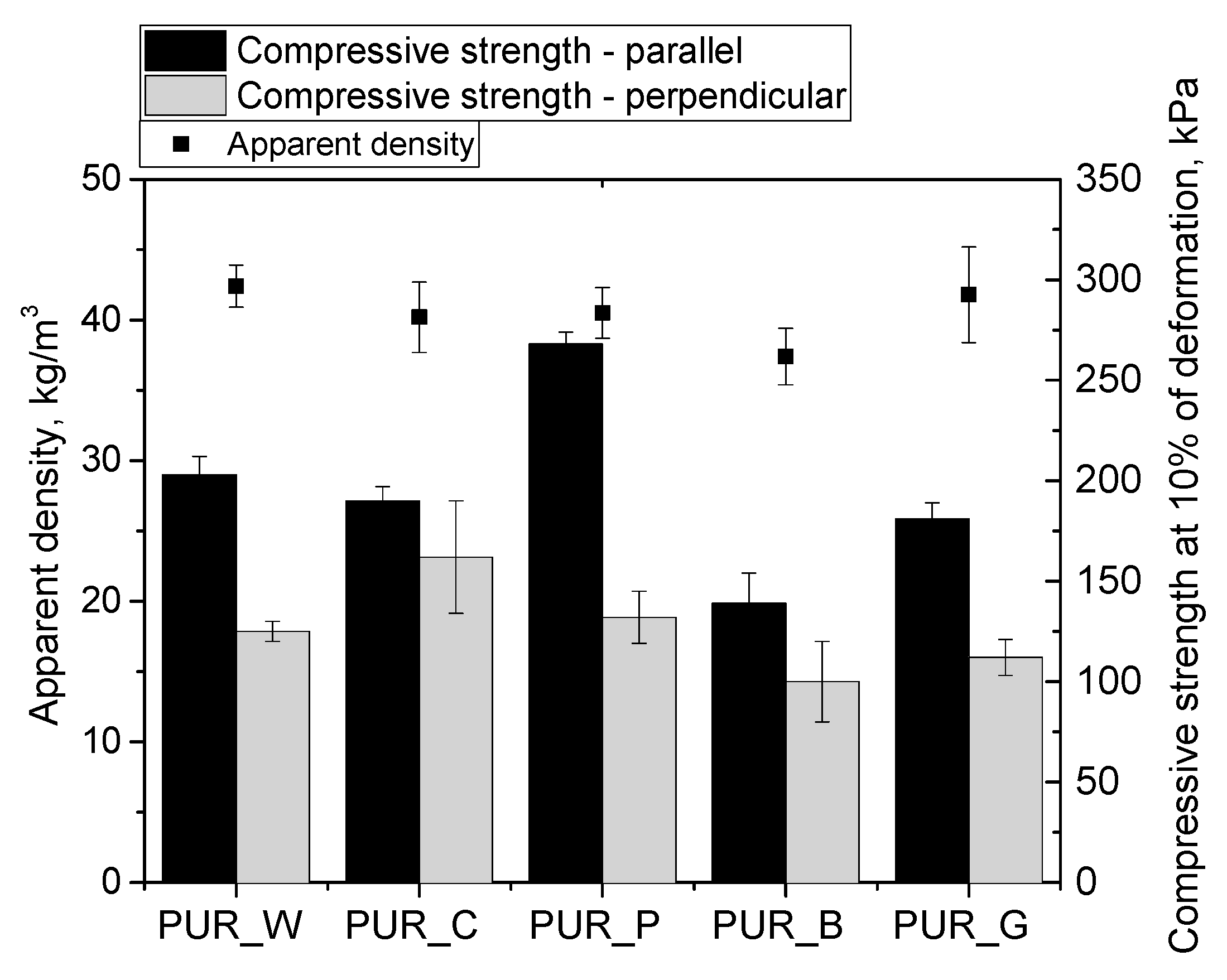
| Biopolyol | Biofoam | ||||
|---|---|---|---|---|---|
| PUR_W | PUR_C | PUR_P | PUR_B | PUR_G | |
| Mass of the Component, g | |||||
| BP_W | 75 | 0 | 0 | 0 | 0 |
| BP_C | 0 | 75 | 0 | 0 | 0 |
| BP_P | 0 | 0 | 75 | 0 | 0 |
| BP_B | 0 | 0 | 0 | 75 | 0 |
| BP_G | 0 | 0 | 0 | 0 | 75 |
| Surfactant | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Water | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Isocyanate | 148.5 | 148.1 | 148.5 | 151.9 | 147.3 |
| Oil | Source of Oil | Ival, gI2/100 g | Mn, g/mol | Mw, g/mol | Ƞ, mPa·s |
|---|---|---|---|---|---|
| OIL_W | watermelon | 118 | 865 | 871 | 53 |
| OIL_C | cherry | 137 | 859 | 865 | 55 |
| OIL_P | pomegranate | 101 | 881 | 886 | 200 |
| OIL_B | blackcurrant | 139 | 822 | 830 | 72 |
| OIL_G | grape | 133 | 859 | 865 | 46 |
| Biopolyol | Source of Oil | OHval, mgKOH/g | Mn, g/mol | Mw, g/mol | Ƞ, mPa·s |
|---|---|---|---|---|---|
| BP_W | watermelon | 361 | 279 | 471 | 165 |
| BP_C | cherry | 359 | 289 | 497 | 169 |
| BP_P | pomegranate | 361 | 335 | 558 | 413 |
| BP_B | blackcurrant | 381 | 276 | 473 | 160 |
| BP_G | grape | 355 | 281 | 484 | 157 |
| PUR_W | PUR_C | PUR_P | PUR_B | PUR_G | |
|---|---|---|---|---|---|
| ts, s | <11 | <11 | <11 | <11 | <11 |
| tg, s | 21 | 22 | 20 | 20 | 25 |
| td, s | 80 | 80 | 79 | 70 | 81 |
| Tmax, °C | 172 | 170 | 170 | 175 | 172 |
| t Tmax, s | 182 | 205 | 222 | 224 | 196 |
| Foam | Brittleness, % | Water Absorption, % |
|---|---|---|
| PUR_W | 5.3 ± 0.42 | 1.4 ± 0.31 |
| PUR_C | 7.2 ± 0.76 | 1.6 ± 0.35 |
| PUR_P | 5.5 ± 1.71 | 1.0 ± 0.15 |
| PUR_B | 10.2 ± 0.42 | 1.9 ± 0.27 |
| PUR_G | 4.1 ± 0.40 | 1.4 ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malewska, E.; Kurańska, M.; Tenczyńska, M.; Prociak, A. Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials. Materials 2024, 17, 158. https://doi.org/10.3390/ma17010158
Malewska E, Kurańska M, Tenczyńska M, Prociak A. Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials. Materials. 2024; 17(1):158. https://doi.org/10.3390/ma17010158
Chicago/Turabian StyleMalewska, Elżbieta, Maria Kurańska, Maria Tenczyńska, and Aleksander Prociak. 2024. "Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials" Materials 17, no. 1: 158. https://doi.org/10.3390/ma17010158
APA StyleMalewska, E., Kurańska, M., Tenczyńska, M., & Prociak, A. (2024). Application of Modified Seed Oils of Selected Fruits in the Synthesis of Polyurethane Thermal Insulating Materials. Materials, 17(1), 158. https://doi.org/10.3390/ma17010158







