3.1. Fungal Strain Selection
With rising interest in sustainable biomaterials development, the need to screen existing fungal biodiversity, considering substrate and growing conditions, to find the most suitable species according to the desired material application is a priority [
9]. To date, fungi, mainly from the phylum Basidiomycota, have been successfully used for biomaterials production [
19,
29]. Species from the genera Trametes, Ganoderma and Pleurotus are useful in the production of bio-composites and leather-like materials [
29,
30,
31,
32], whereas recent studies have demonstrated that species of the genus Fomitopsis can be used for growing mycelium skin [
30].
The first stage of this study consisted of evaluating the mycelium growth rate of
G. carnosum,
T. versicolor,
P. ostreatus,
F. pinicola and
P. eryngii using three different nutrient-rich agar media (PDA, MEA and MMN) at 22 °C, 25 °C and 28 °C. The temperatures and media tested were chosen by considering typical reported conditions for growing and maintaining the selected fungal species [
21,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48], and that the general optimum for hyphal growth of wood decay fungi is 25 °C [
46]. MMN agar was included as it is often used for fungal species isolation and maintenance in the laboratory. The main objective was to select the fastest growing organisms and conditions to obtain a dense mycelium mat, as well as to establish the optimal temperature for lignocellulosic substrate selection.
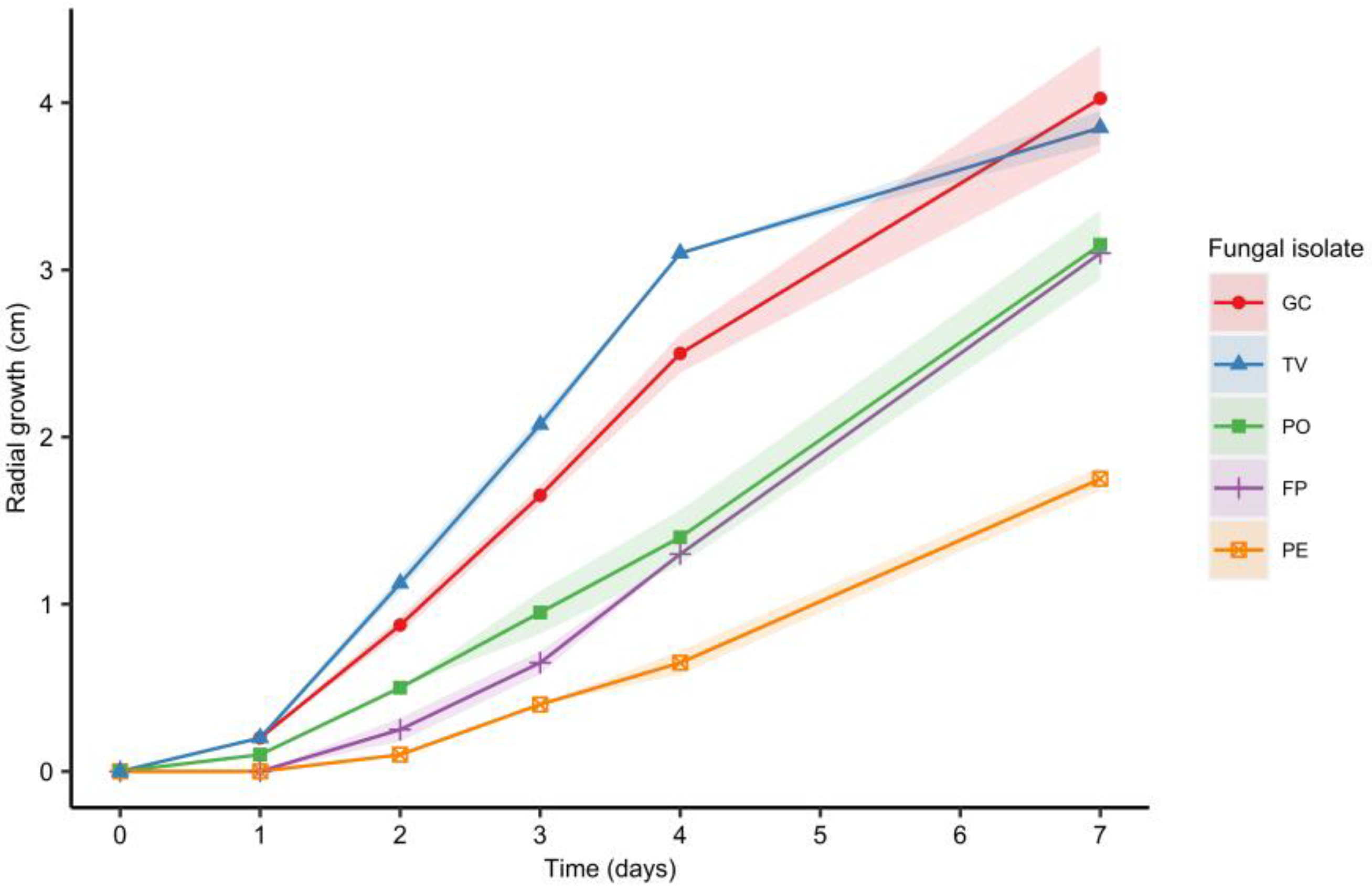
As shown in
Figure 1, the results obtained from the multivariate statistical analysis demonstrate that temperatures above 22 °C were preferable for growing the mycelium, but there is no significant difference between the growth rate obtained when using 25 °C or 28 °C. In addition, the mycelium growth on PDA was significantly faster than that on MEA or MMN. Based on these results, a fungal growth temperature of 25 °C and the PDA medium were chosen for the subsequent experiments.
Pictures of the mycelium of the five fungal isolates, in Petri dishes with the three different media, after 7 days of incubation at 25 °C, are shown in
Figure 2. Mycelium of all the isolates was more well developed and had faster growth, in terms of ability to cover the medium, when grown on PDA.
The faster-growing fungal isolates were
G. carnosum and
T. versicolor 3086, compared to
P. ostreatus FPRL 40C and
F. pinicola, as shown in
Figure 3 (
p < 0.05). These results are comparable to those reported by [
26] referring to different
Pleurotus species,
G. lucidum and
T. versicolor mycelium growth on MEA measured for 7 days. The authors reported
T. versicolor to have the highest radial growth of the strains mentioned, followed by the
Ganoderma species and, lastly, by the
Pleurotus ones. In the present study
P. eryngii showed the slowest growth rate, therefore this strain was excluded from subsequent experiments. In addition, it has been reported that
F. pinicola strains grow optimally at 30 °C [
29]. Considering room temperature as the most convenient condition for a future scale up of the process, the following experiments were carried out only with
G. carnosum,
T. versicolor and
P. ostreatus at 25 °C.
3.2. Grain Spawn Selection
Spawn is a nutritious substrate (e.g., grains) previously colonized by the mycelium that is used to inoculate the substrate for the bio-composite production [
49]. This is a practice inherited from the cultivation of edible, medicinal and therapeutic mushrooms [
50,
51]. The grain provides many nutrient-rich inoculation sites, facilitates the homogenization of the substrate–inoculum mixture, and contributes to the rapid growth of the mycelium [
48]. Common substrates used in spawn production include grains such as rye, rice, wheat, sorghum, millet, and corn, as well as cotton waste [
49,
50,
51,
52,
53,
54,
55,
56], and the selection depends mainly on the material’s availability and the fungal strain grown on it.
The second stage of this study included the evaluation of three different grain spawn media (millet, wheat and a 1:1 mix of the two) for mycelium growth of the three selected fungal strains (
G. carnosum,
T. versicolor and
P. ostreatus). Wheat and millet grains have been used to provide nitrogen to accelerate fungal growth during the first stage of the biomaterial’s production [
57], indicating that they are not only readily available, but also a great option regarding nutritional content for spawn media. The effect of 1%
w/
w CaCO
3 supplementation was also analyzed, as it is often used as a pH regulator [
52].
As shown in
Figure 4, results from the statistical analysis indicate that, for all fungal isolates, growth rate is significantly faster when using the mixed grains instead of millet or wheat separately. This difference may be due to the grain’s complementing mineral composition and carbohydrate availability, as both wheat and millet have similar protein (around 10 to 15%) and starch (between 60 to 70%) contents [
58,
59]. The use of 1%
w/
w CaCO
3 generated an average delay of 0.05 cm/day on the velocity of mycelium colonization of the grains, as shown in
Table 3. As the buffering action of CaCO
3 was proven to not have a positive impact on mycelium growth enhancement at this stage of the process, it was not added for subsequent spawn production. These results may also be linked with reduced moisture availability in the grains supplemented with CaCO
3, as the supplement may act as a desiccant and prevent the mycelium from developing properly.
In
Figure 5, the growth tendency of the three previously selected fungal isolates, using the mixed grains spawn media at 25 °C, is presented. The fastest strain was
T. versicolor, the mycelium of which took 11 days to fully colonize the grain, followed by
G. carnosum and
P. ostreatus, which took 13 and 15 days, respectively. This behavior is analogous to that observed with the growth of the strains on PDA. Taking this into consideration, one can also state that, even if many parameters can influence fungal growth [
58], using a standard approach with typical media in Petri dishes to evaluate the growth rate of different mycelia is a feasible and easy option for comparison between various fungal isolates.
3.3. Lignocellulosic Substrate Evaluation
Mycelium growth density and the mycelium–substrate degree of interfacial bonding varies significantly by species and agricultural or wood by-product employed [
19]. Different samples were developed, using the previously selected fungal strains and the mixed grains as inoculum, to determine the compatibility of the fungi with the selected substrates (
Figure 6).
Table 4 shows the results for the specimens obtained for each fungus–substrate combination evaluated. In general, pine sawdust performed poorly regardless of the isolate used, resulting in brittle materials that crumbled easily. This can be attributed to the physical state of this substrate, as it mainly consists of fine particles (<1 mm) that the fungal hyphae could not properly bind together. However, it was observed that this issue could be overcome by permitting the fungal skin to develop on the surface of the composite, which helps to keep the small particles together and to maintain the material’s shape. Similarly, it has been reported that, when the mycelium skin is not present, the composite tends to be less dense [
55].
The tree of heaven wood chips were of particular interest for this study as it is categorized as a toxic and invasive species in Europe that is linked with negative impacts, including infrastructure damage, invasive insect proliferation and human health risks, such as allergic responses [
49,
50]. A similar situation to the one observed with the sawdust samples was obtained with the tree of heaven wood chips and
G. carnosum composites. The wood chip dimensions (∼4 mm), and their lack of fibrous components, hindered the capacity of fungal hyphae to completely unify the substrate, resulting in brittle materials that also crumbled easily, as reported in
Table 2. The
T. versicolor and
P. ostreatus isolates achieved better results in terms of the final material’s integrity, yet they took longer times to colonize the same quantity of substrate than
G. carnosum. Fungal skin growth time differences between fungal isolates have also been reported by other authors, such as Bruscato et al. (2019) [
52], who noticed that
Pleurotus albidus requires longer growing periods to produce a mycelial ‘envelope’ when compared to
Pycnoporus sanguineus and
Lentinus velutinus.
Even though oak wood and its by-products have been used by several authors for growing the same fungal species tested in this study [
47,
48,
49], the oak shavings substrate generally performed poorly, except with
P. ostreatus, which produced a homogeneous final product with a reasonable growth time. In addition, this specific substrate was difficult to hand press to achieve the desired final form, so, overall, it was not one of the best options to work with. On the other hand, shredded beech wood composites resulted in compact and resistant composites regardless of the fungal strain. These results are consistent with the reported building components, boards and blocks produced using beech wood as a substrate and different fungal species [
19,
48].
Wheat straw was only evaluated using
T. versicolor because of a lack of material availability. Results using this substrate were promising, as the final composite was light and homogeneous, and the fungal colonization of the substrate was quick. Other authors have reported mycelium composites produced using wheat straw to be good thermal insulating materials [
19,
45]. The properties of the composites will be discussed further in the next section.
Overall, the bulk densities obtained in this study range between the expected values for as-grown mycelium composites containing forestry by-product substrates (87–300 kg/m
3) [
19]. With the production method used in this study this characteristic depends highly on the pressure applied during the mold-filling process, which is why the obtained values varied considerably, even when using the same substrate and isolate.
The differences found between the three fungal isolates and the substrates can be attributed to the substrates’ composition, pH, nutrient availability [
19,
21], mycelium anatomy and structure, overall growth preferences and natural habitat of the fungal species [
56].
Radiographies of the composites produced using
T. versicolor and the five different evaluated substrates, organized from left to right according to the free space present in the final product, are shown in
Figure 7. The color, from black to white, represents a value of apparent density and the distribution between particles and voids.
Shredded beech wood gave the most compact material whereas the wheat straw composite appeared as the lightest one, which is consistent with the bulk density data obtained and discussed earlier. These observations contribute to the information discussed in
Section 3.4 regarding the material’s properties.
The best samples (in terms of material integrity) were selected for thermal conductivity and moisture absorption tests, as the main goal of the experiment was to find suitable fungal isolate–substrate combinations for the production of insulation panels.
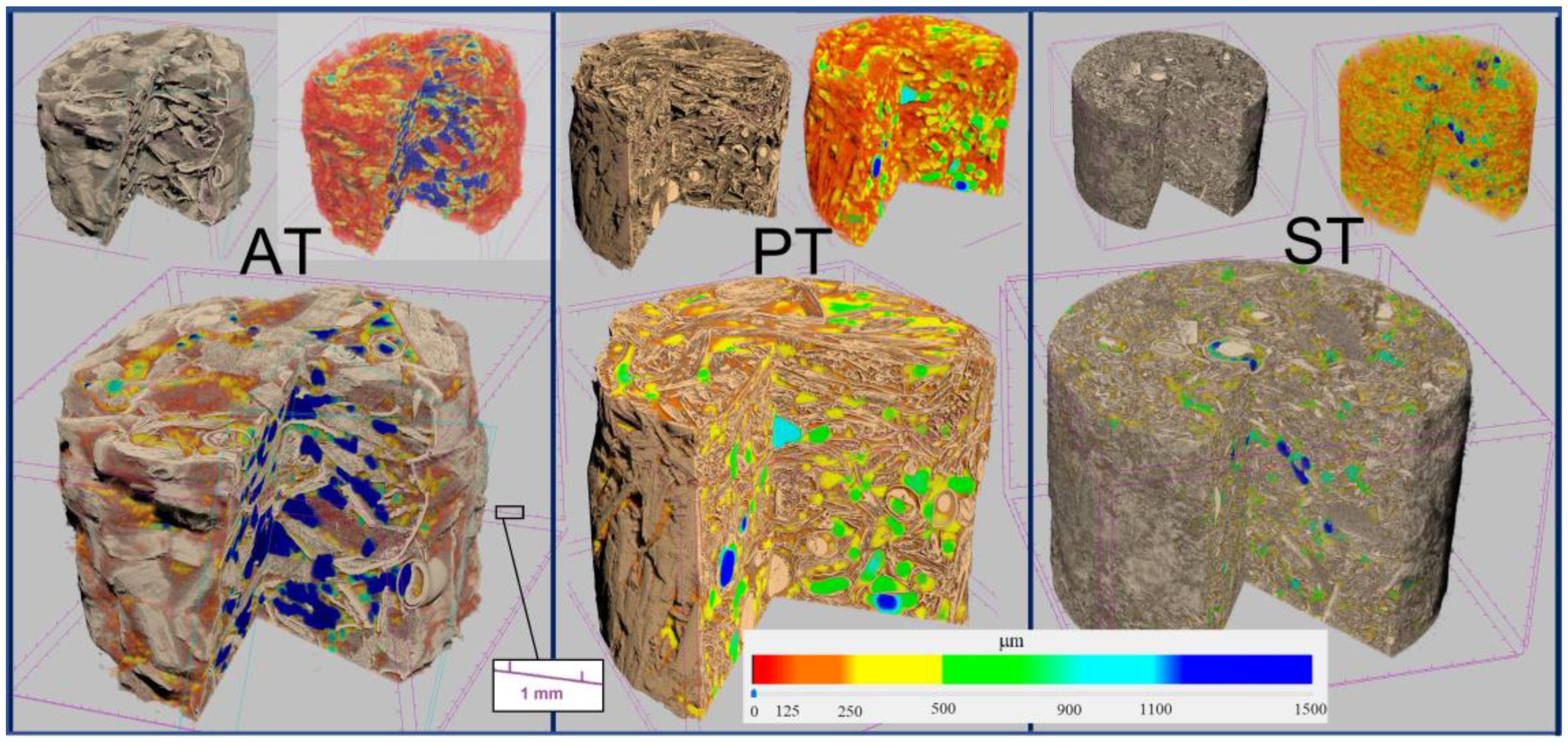
3.4. Internal Structure Characterization by X-ray Micro-CT and 3D Image Analysis
An example of 3D images of the biomaterial specimens reconstructed by the X-ray micro-CT, showing three different substrates inoculated with
Trametes versicolor, is reported in
Figure 8.
The structure of the specimens was characterized, as a whole, by determining the overall morphometric parameters reported in
Table 5 and defined in
Table 2. Total porosity, mean pore size, specimen specific surface and solid-phase mean thickness are shown in the graphs in
Figure 9. It can be noted that the total porosity (P) of the specimens is independent of both substrate and fungal mycelium and that it ranges between 63% and 78%, whereas the average pore diameter (P size) is significantly lower (about 120 μm on average) for specimens of pine sawdust (see two-factor ANOVA results in
Supplementary Materials) compared to tree of heaven wood chips and wheat straw (about 420 μm on average). The average thickness of solid substrate (SP thick) is not significantly different between the specimens (about 70 μm on average), whereas specimens with pine sawdust substrate showed significantly higher specific surface values (SP surf/S vol) of about 310 cm
−1, on average, compared to those based on tree of heaven wood chips and wheat straw (about 190 cm
−1 and 150 cm
−1, respectively). The fractal dimension (FD) of the specimen structure was also significantly larger for specimens with pine sawdust substrate.
With regard to the structure characterization of the biomaterial, the binarized images of the solid and porous phases were processed using mathematical morphology algorithms allowing the measurement of the internal size distributions. The size classes were about 15 microns wide for both the thickness of the solid substrate and the distance between the walls within the structure of the specimens. The graphs in
Figure 9 show the results expressed as both quantity distribution and cumulative curves. The marked similarity of the shape of the distributions within the same substrate clearly indicates that the internal geometry of the biomaterial specimens was basically defined by the type of lignocellulosic substrate used, and much less influenced by the fungal isolate used for the inoculum.
With regard to the study of the solid phase, the biomaterials consisting of tree of heaven wood chips showed thicknesses mainly below 300 μm with two well-expressed modal values, one around 25 μm and the other at 40 μm, along with a third nested around 75 μm. For the biomaterials made of wheat straw, the thicknesses were mainly less than 170 μm, with two well-expressed modes, one around 30 μm and the other at 70 μm, as well as one nested around 120 μm. The biomaterials consisting of pine sawdust, on the other hand, showed thicknesses mainly less than 120 μm, with a distinctly leptocurtic distribution with a modal value around 35 μm and a nested one, barely detectable around 70 μm.
The study of the pore space inside the structure of all the specimens showed, first of all, a range of dimensional variability, much greater than that of the corresponding solid-phase thicknesses, and a higher degree of multimodality, not limited only to the smaller pore sizes (
Figure 9). The most frequent pore sizes (modes) found in the size range below 150 μm were 20 μm, 45 μm and 75 μm for all tree of heaven wood chips specimens, and 30 μm, 70 μm and 120 μm for both wheat straw and pine sawdust specimens, but in a much greater amount in the latter substrate. The observation of the cumulative distributions in
Figure 10, moreover, allows us to highlight that the maximum size of the pores found exceeds 1.5 mm in the case of tree of heaven wood chips specimens, but is less than 1.3 mm in the case of wheat straw and less than 0.7 mm in the case of pine sawdust.
Finally, the differences between the size distributions found in the different fungal isolates show solid-phase and pore volumes of the most frequent dimensional ranges that are slightly larger for specimens inoculated with Trametes versicolor than for those obtained with the other two fungi.
3.5. Thermal Conductivity Assessment and Moisture Absorption Determination
As mentioned in the
Section 3.3, some of the samples produced tended to crumble easily. For this reason, the more consistent specimens (38 in total) were selected for thermal conductivity and moisture absorption determinations.
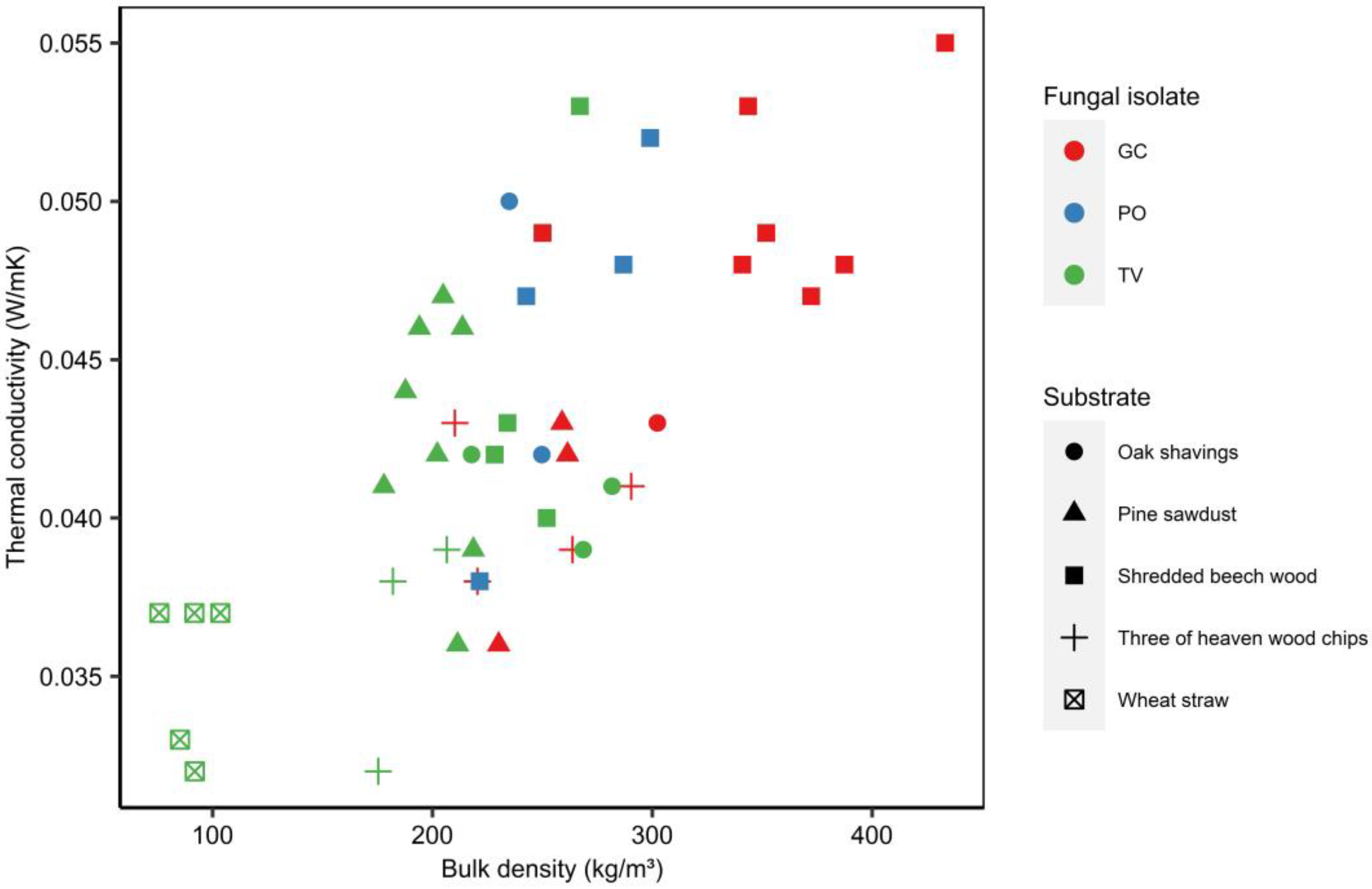
Figure 11 shows thermal conductivity in relation to bulk density of the different composites produced.
As expected, at higher densities, higher values of thermal conductivity were obtained. The association between the properties of these two materials is due to the presence of air-filled empty space in the matrix and the low thermal conductivity of air (26.2 × 10
−3 W/mK at 0.1 MPa, 300 K) [
19]. Shredded beech wood, oak shavings and pine sawdust tended to behave as a paste when moistened, leaving few voids to be filled by air. On the other hand, wheat straw and tree of heaven wood chip materials’ physical states permitted more empty spaces to remain occupied by air, attributing lower values of thermal conductivity and densities in relation to the other bio-composites.
When compared to other commonly used insulating materials, such as glass wool (57 kg/m
3, 0.04 W/mK), sheep’s wool (18 kg/m
3, 0.05 W/mK) and kenaf (105 kg/m
3, 0.04 W/mK) [
19,
54], wheat straw, tree of heaven wood chips and pine sawdust performed well in terms of thermal conductivity. As thermal insulators are usually low-density and highly porous materials [
60,
61,
62,
63], the wheat straw composites are the most promising option from the substrates tested in this study.
When analyzing specifically the differences between the fungal isolates used for the materials’ production and focusing on shredded beechwood composites, it is evident that
G. carnosum bonded materials tend to be denser in comparison with the
P. ostreatus and
T. versicolor ones. This might be explained by the differences in mycelial density due to hyphae thickness and branching. It has been reported that the hyphae of
Ganoderma species tend to be thicker (13 µm) than those of
Trametes (1.3 µm), for example [
57]. Moreover, density differences in film applications have shown that
Ganoderma hyphae are more likely to branch and generate a more compact structure with fewer pores than those of
Pleurotus, whose growth tends to be more lengthwise [
21].
On the other hand, moisture absorption is also inherent and of special interest for composite materials, as it can influence other characteristics, such as mechanical and thermal properties, and its application possibilities [
21,
46]. A material’s thermal conductivity coefficient tends to increase with increasing moisture content due to the presence of water in the previously air-occupied pores, and it can also lead to faster material decay [
46]. As shown in
Table 6, moisture absorption did not highlight particular differences between substrates and fungal isolates. All biomaterials reached a moisture content between 9% to 11% after one month at 75 ± 5% RH, with wheat straw and tree of heaven wood chips being at the lower end of that interval. The results obtained in the present study are in line with the results reported by Tacer-Caba et al. (2020) [
21], who obtained a 10% moisture absorption in composites made from oat husk and rapeseed cake using
Trichoderma asperellum and
Agaricus bisporus strains when evaluating a 75% RH scenario.
Volumetric swelling (
VS) was calculated using the following formula:
where
Vf represents the final volume after conditioning in the chamber and
Wd the dry volume.
A commercial cork panel was used as the reference for moisture absorption (6.51 ± 0.07%), the value obtained is lower than that of the biocomposites considered here. This is a known issue that must be addressed to properly introduce this type of biomaterial into existing markets, yet it was not studied further during this research. On the other hand, the fact that the samples in this study were produced by manual pressing of the colonized substrate into the desired shape must be taken in consideration, as the human factor in the production process increases variability of the specimens obtained. Nevertheless, despite all the positive results obtained regarding the use of these biocomposites, it is important to remark that people appreciate the advantages of this type of material but could be reluctant to use these materials in their own homes [
15].