Copper-Based Diamond-like Thermoelectric Compounds: Looking Back and Stepping Forward
Abstract
1. Introduction
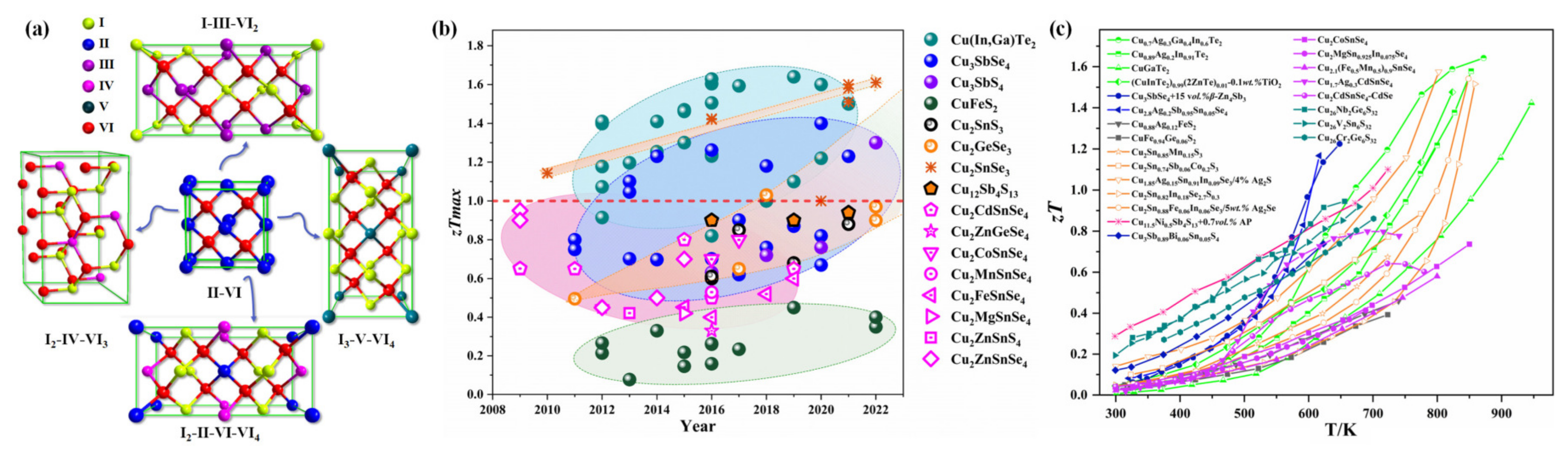
2. Copper-Based Diamond-like Thermoelectric Compounds
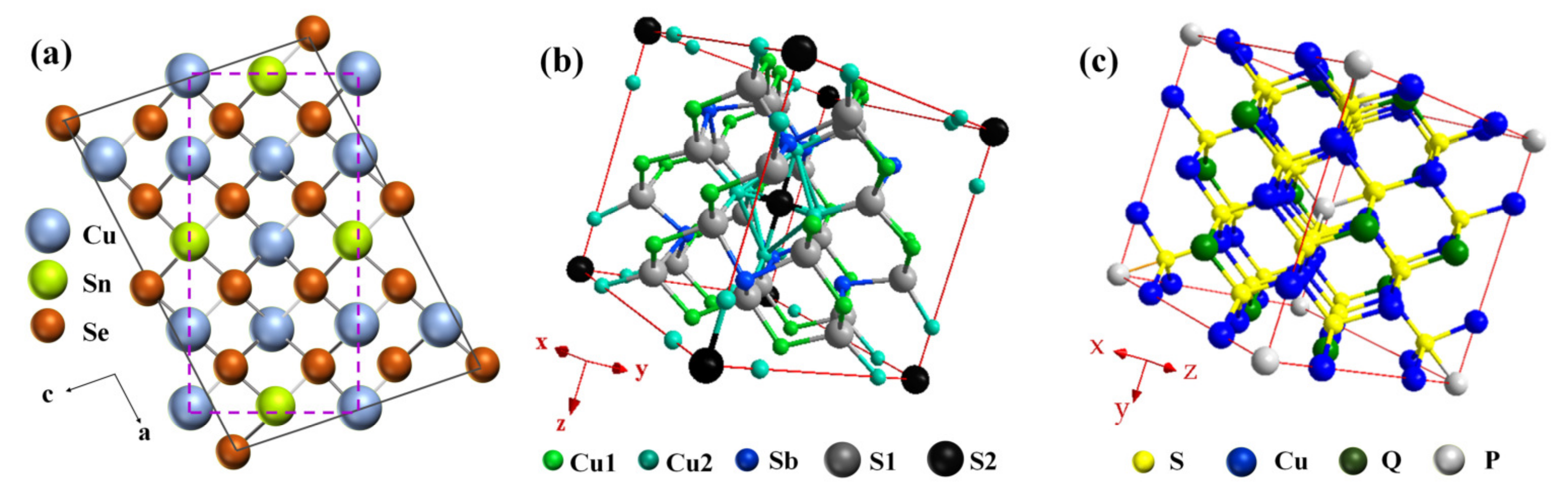
| Composition | zTmax | zTave | α2σ (μW·cm−1·K−2) | κL (W·m−1·K−1) | n@RT (1019cm−3) | Synthesis Method * | Ref. |
|---|---|---|---|---|---|---|---|
| Cu0.75Ag0.2InTe2 | 1.24@850 K | 0.47 | 7.26 | 0.3 | 1.11 | M + HP | [23] |
| Polycrystalline CuGaTe2 | 1.40@950 K | 0.43 | 8.9 | 0.45 | 0.11 | M | [29] |
| Cu0.7Ag0.3Ga0.4In0.6Te2 | 1.64@873 K | 0.73 | 5.22 | 0.24 | 0.007 | M + HP | [26] |
| (CuInTe2)0.99(2ZnTe)0.01–0.1 wt% TiO2 | 1.47@823 K | 0.50 | 12.93 | 0.45 | 6.01 | M | [36] |
| Cu0.89Ag0.2In0.91Te2 | 1.60@850 K | 0.49 | 8.81 | 0.36 | 0.07 | M + SPS | [38] |
| Cu7.9In8.1Ga0.3Te16 | 1.22@850 K | 0.51 | 11.92 | 0.55 | 7.03 | M + SPS | [39] |
| Cu0.7Ag0.3GaTe2 | 1.00@750 K | 0.57 | 12.26 | 0.68 | 4.8 | M + HP | [40] |
| Cu0.8Ag0.2In0.2Ga0.8Te2 | 1.50@ 850 K | 0.78 | 14 | 0.49 | 0.043 | M + SPS | [41] |
| Cu3SbSe4+15 vol% β–Zn4Sb3 | 1.23@648 K | 0.43 | 12.7 | 0.14 | 5.5 | ST | [45] |
| Cu3Sb0.96Sn0.04Se4–5 wt% AgSb0.98Ge0.02Se2 | 1.23@675 K | 0.50 | 13.8 | 0.54 | 8.72 | M + SPS | [47] |
| Cu2.8Ag0.2Sb0.95Sn0.05Se4 | 1.18@623 K | 0.36 | 9.54 | 0.27 | 12.0 | MAH + SPS | [48] |
| Cu2.85Ag0.15SbSe4 | 0.90@623 K | 0.52 | 10.98 | 0.66 | 0.57 | M + SPS | [49] |
| Cu3SbSe4–4 wt% CuAlSe2 | 1.40@723 K | 0.72 | 16 | 0.35 | 10 | M + HP | [27] |
| Cu0.92Zn0.08FeS2 | 0.26@623 K | 0.14 | 5.4 | 2.24 | 39.6 | M | [52] |
| Cu0.92In0.08FeS2 | 0.35@723 K | 0.19 | 4.7 | 0.79 | 41.2 | M + SPS | [53] |
| Cu0.88Ag0.12FeS2 | 0.45@723 K | 0.22 | 7.6 | 1.15 | 3.6 | M + PAS | [54] |
| CuFe0.94Ge0.06S2 | 0.40@723 K | 0.17 | 6 | 1.04 | 4.7 | M + HP | [55] |
| Cu2Sn0.9In0.1S3 | 0.60@773 K | 0.32 | 6.23 | 1.01 | 126 | MA + SPS | [56] |
| Cu2Sn0.85Mn0.15S3 | 0.68@723 K | 0.28 | 9.2 | 0.4 | 462 | M + SPS | [57] |
| Cu2Sn0.74Sb0.06Co0.2S3 | 0.88@773 K | 0.43 | 10.4 | 0.41 | 237 | M + SPS | [58] |
| Cu1.85Ag0.15(Sn0.88Ga0.1Na0.02)Se3 | 1.60@823 K | 0.50 | 12.75 | 0.28 | 91.8 | M | [62] |
| Cu1.85Ag0.15Sn 0.9In0.1Se3 | 1.42@823 K | 0.38 | 9.70 | - | 73.4 | SHS | [63] |
| Cu1.85Ag0.15Sn0.91In0.09Se3/4% Ag2S | 1.58@800 K | 0.59 | 12.6 | 0.12 | 133.4 | SHS + PAS | [64] |
| Cu2Sn0.82In0.18Se2.7S0.3 | 1.51@858 K | 0.33 | 9.3 | 0.35 | 151 | M | [65] |
| Cu2Sn0.88Fe0.06In0.06Se3–5 wt% Ag2Se | 1.61@848 K | 0.40 | 7.6 | 0.2 | 163 | M + MA + HP | [66] |
| Cu1.9Ag0.1Ge0.997Ga0.003Se3 | 1.03@768 K | 0.58 | 7.3 | 0.46 | 3.5 | M + SPS | [60] |
| Cu1.8Ag0.2Ge0.95In0.05Se3 | 0.97@723 K | 0.44 | 6.4 | 0.38 | 4.6 | M + HP | [61] |
| Cu11.7Gd0.3Sb4S13 | 0.94@749 K | 0.46 | 16 | - | 60.3 | M + HP | [68] |
| Cu11.25Cd0.75Sb4S13 | 0.90@623 K | 0.72 | 12.1 | 0.33 | 42 | M + HP | [98] |
| Cu11.5Ni0.5Sb4S13+0.7 vol% AP | 1.15@723 K | 0.66 | 12.8 | 0.17 | - | MA + SPS + | [103] |
| Cu3SbS4–9 wt% CuAlS2–1.5 wt% AgAlS2 | 1.30@773 K | 0.77 | 16.1 | 0.72 | 42.2 | M + HP | [28] |
| Cu3Sb0.95Sn0.05S4 | 0.72@623 K | 0.37 | 11.3 | 0.85 | 41.4 | MA + SPS | [50] |
| Cu3Sb0.89Bi0.06Sn0.05S4 | 0.76@623 K | 0.38 | 13.98 | 0.78 | 74 | MA + SPS | [51] |
| Cu2.10Cd0.90SnSe4 | 0.65@700 K | 0.27 | 5.1 | 0.23 | - | M + SPS | [70] |
| Cu2CoSnSe4 | 0.70@850 K | 0.31 | 6.83 | 0.45 | 19 | M + SPS | [76] |
| Cu2MgSn0.925In0.075Se4 | 0.42@700 K | 0.17 | 5.8 | - | 14 | M + SPS | [79] |
| Cu2.1(Fe0.5Mn0.5)0.9SnSe4 | 0.60@800 K | 0.22 | 6.1 | - | 30 | M + SPS | [77] |
| Cu2.1Fe0.9SnSe4 | 0.52@800 K | 0.23 | 5.9 | 0.60 | 23 | M + SPS | [78] |
| Cu2CdSnSe4 | 0.50@760 K | 0.17 | 3.1 | 0.42 | 1.15 | CS + M + SPS | [71] |
| Cu2.1Cd0.8SnSe3.4 | 0.65@723 K | 0.27 | 6.96 | 0.42 | - | ST + HP | [72] |
| Cu1.7Ag0.3CdSnSe4 | 0.80@688 K | 0.43 | 6.5 | 0.37 | - | MAH + SPS | [73] |
| Cu2CdSnSe4–CdSe | 0.65@725 K | 0.34 | 5.1 | 0.56 | 60 | M + HP | [74] |
| Cu26Nb2Ge6.0S32 | 1.00@670 K | 0.50 | 8 | 0.51 | - | M + HP | [104] |
| Cu26V2Sn6S32 | 0.93@675 K | 0.55 | 7.73 | 0.4 | 380 | MA + SPS | [106] |
| Cu26Cr2Ge6S32 | 1.00@700 K | 0.48 | 19.4 | 0.48 | - | MA + SPS | [108] |
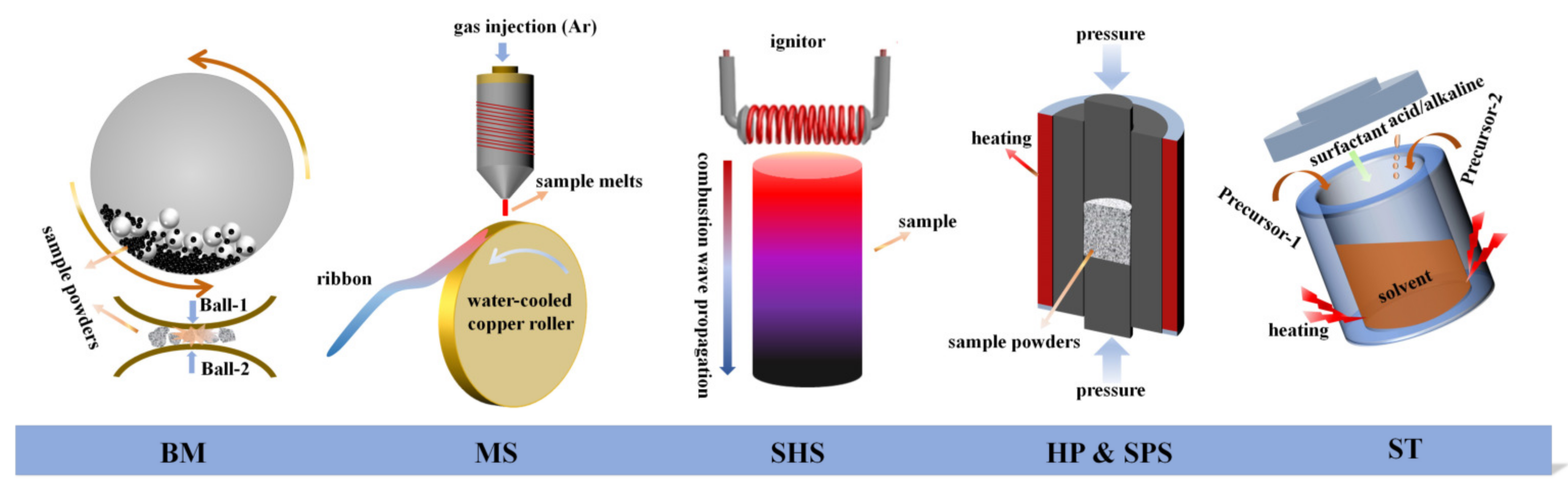
3. Material Synthesis Recipes
4. Strategies for Optimizing the TE Performances of CBDL Compounds
4.1. Several Representative Strategies for Enhancing Power Factor
4.1.1. Optimization in Carrier Concentration
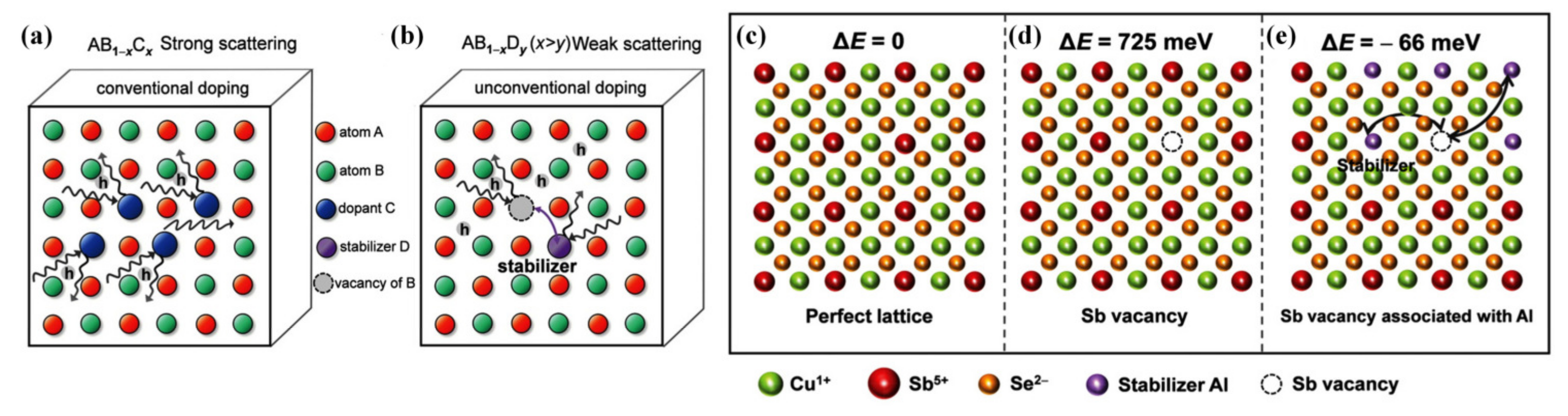
4.1.2. Modulation Doping
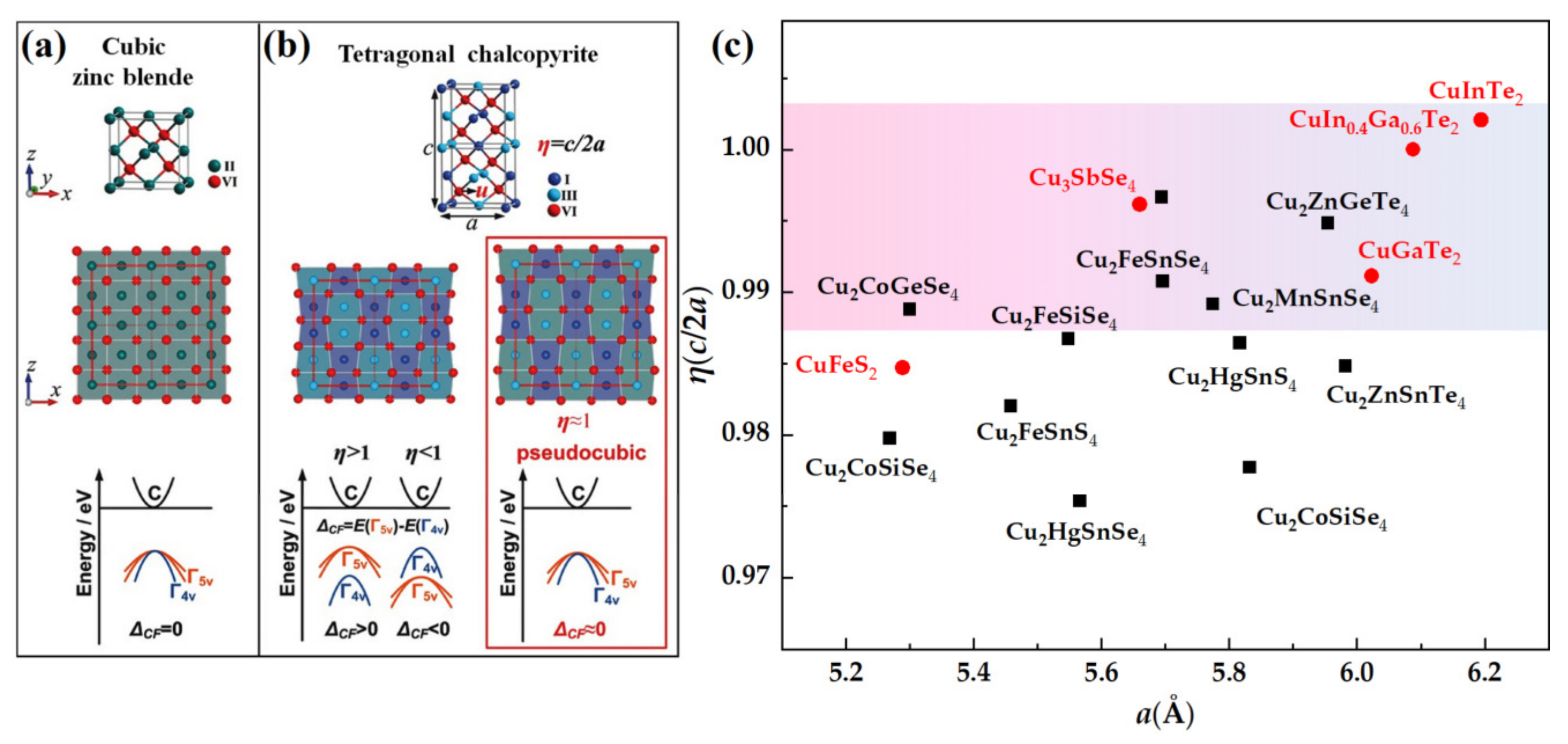
4.1.3. Pseudocubic Structure
4.1.4. Softening p-d Hybridization
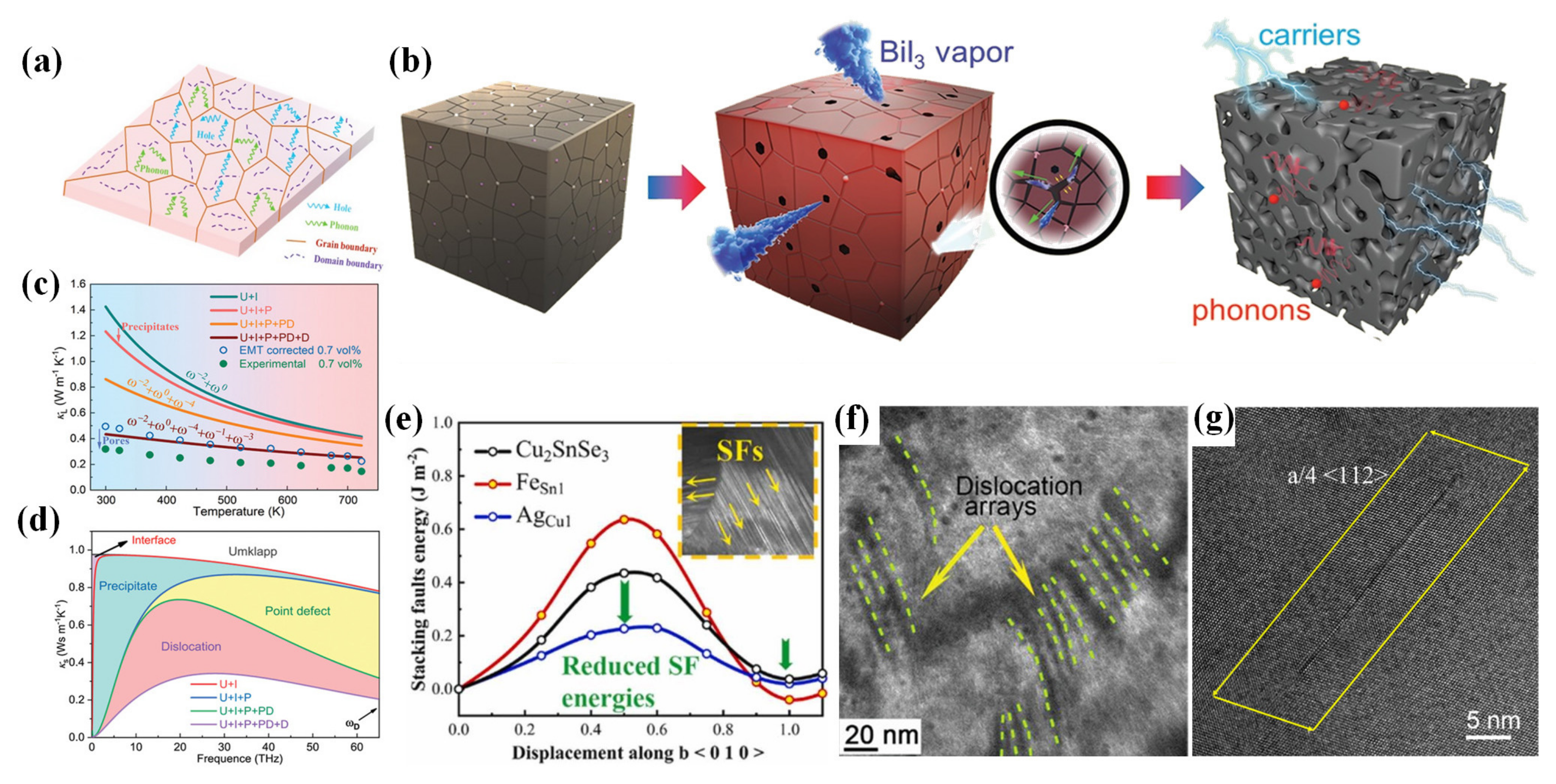
4.2. Strategies for Reducing Lattice Thermal Conductivity
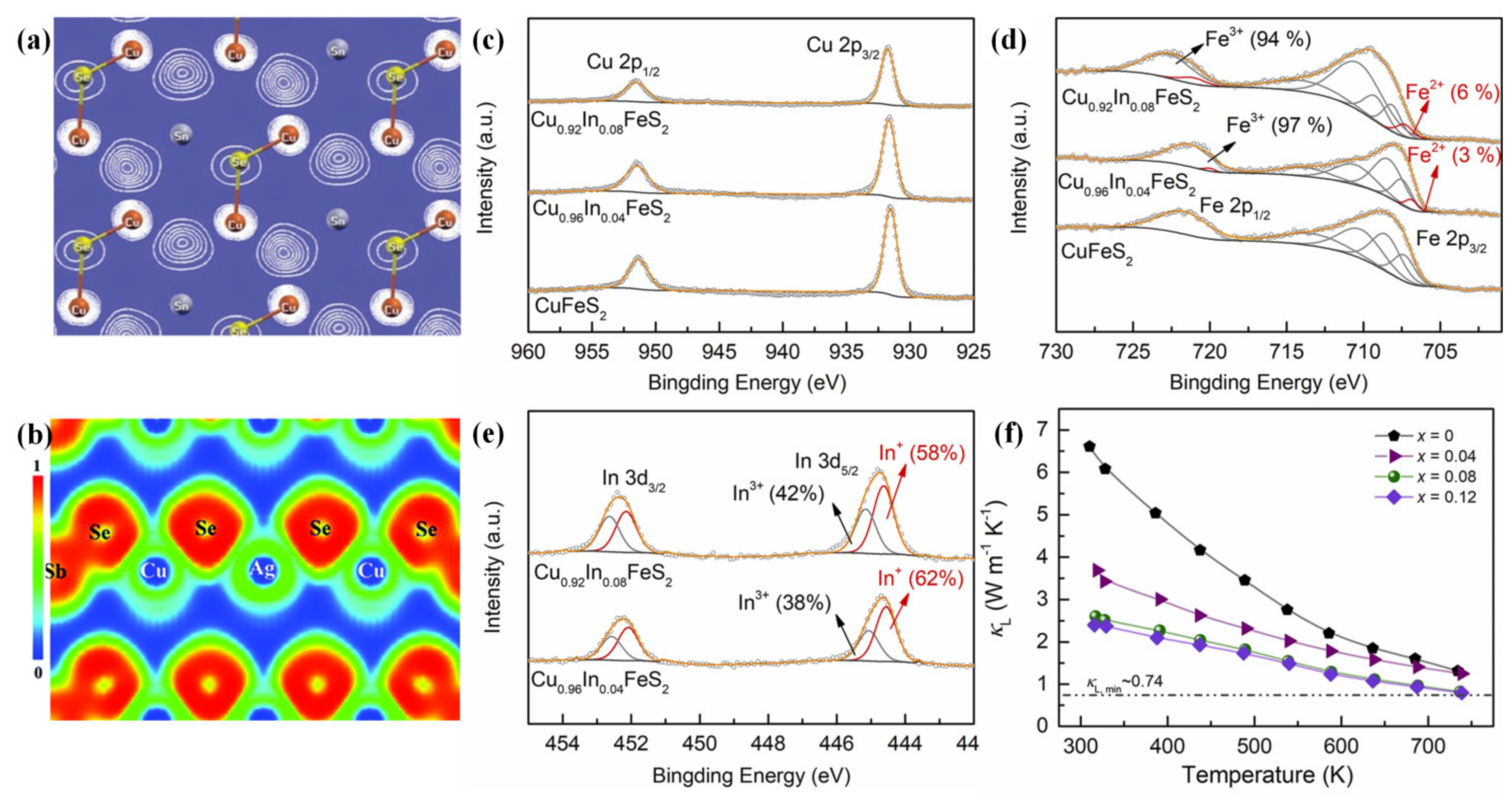
4.2.1. Point-Defect Scattering
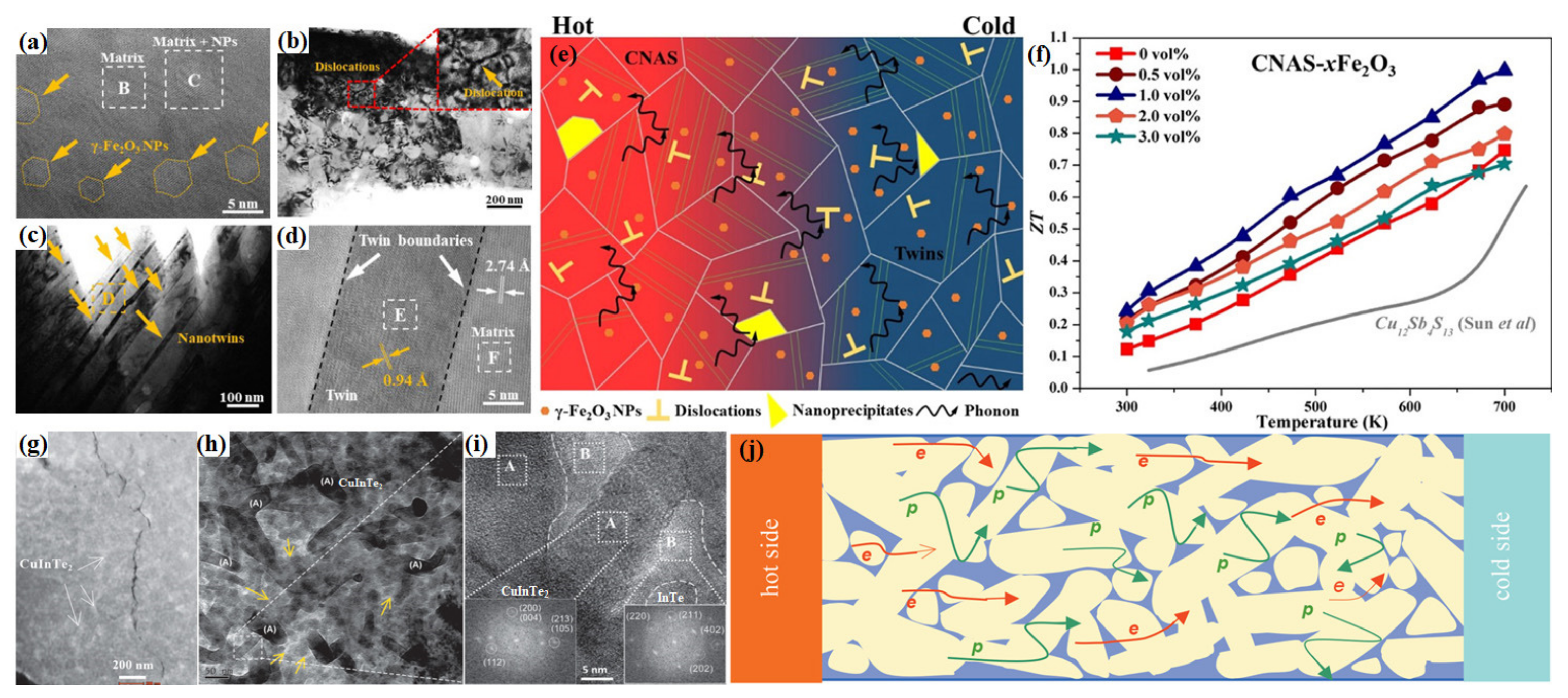
4.2.2. Nanostructure Engineering
4.2.3. Nanocomposite
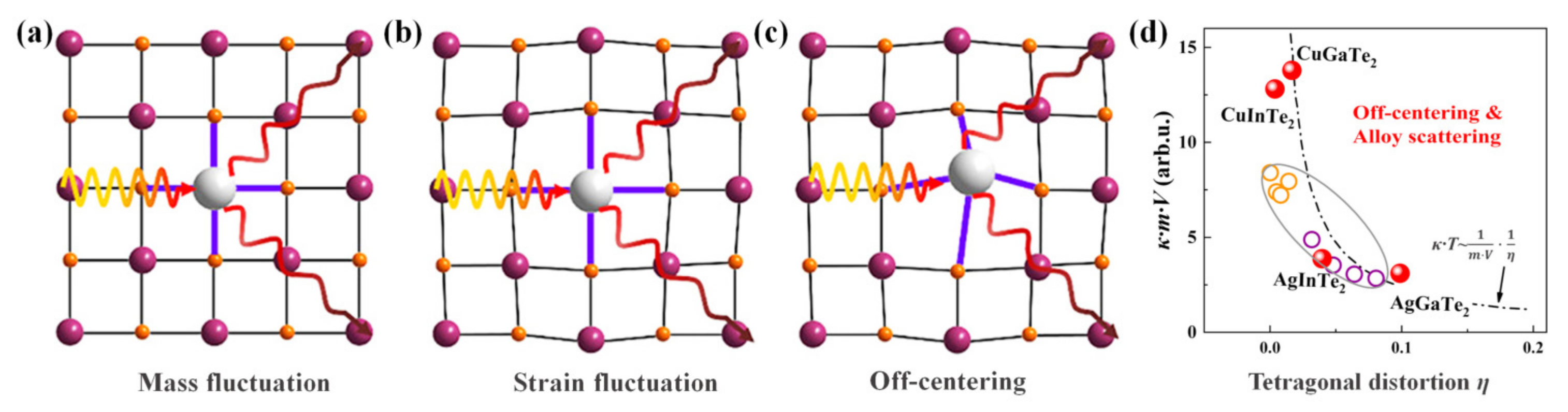
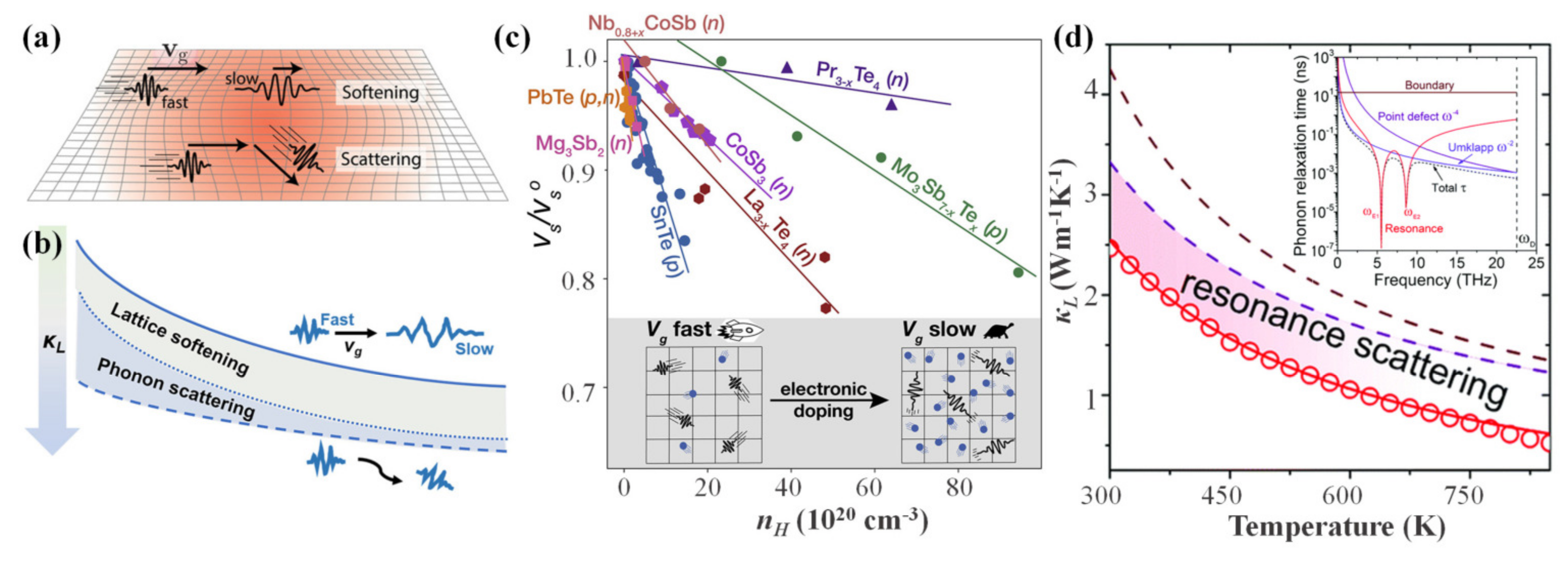
4.2.4. Lattice Softening Effects
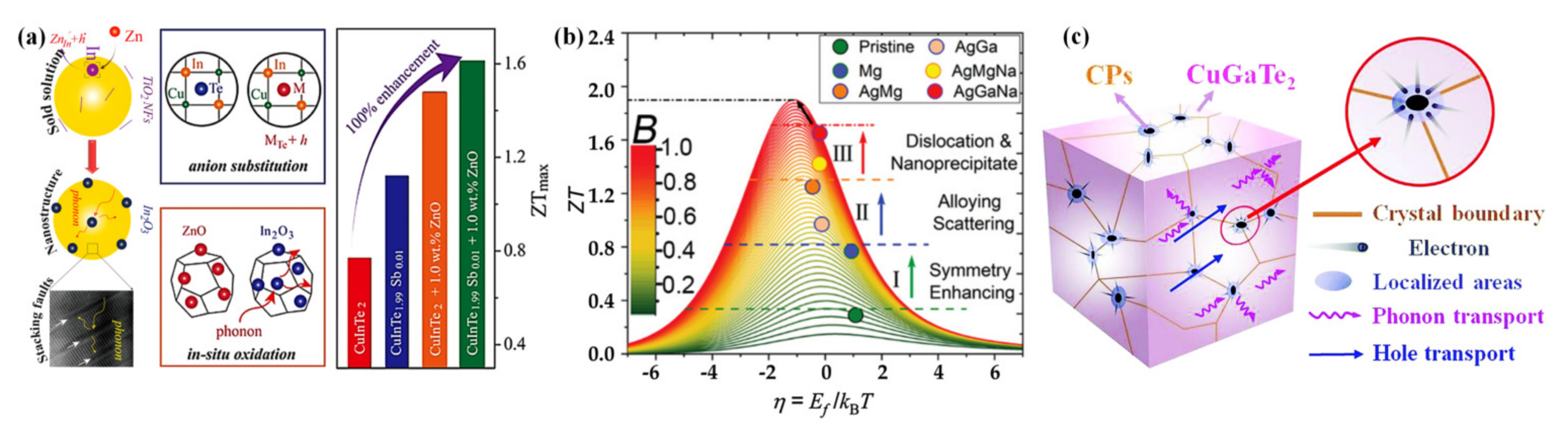
4.3. Synergistic Regulation
4.3.1. Entropy Engineering
4.3.2. Progressive Regulation Strategy
5. CBDL-Based TE Devices
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Zou, J.; Chen, Z.G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef] [PubMed]
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhao, L.D.; Kanatzidis, M.G. Rationally Designing High-Performance Bulk Thermoelectric Materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef]
- Mukherjee, M.; Srivastava, A.; Singh, A.K. Recent Advances in Designing Thermoelectric Materials. J. Mater. Chem. C 2022, 10, 12524–12555. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in Thermoelectric Materials Research: Looking Back and Moving Forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, S.; Liu, Z.; Mu, E.; Hu, Z. Thermoelectric Converter: Strategies from Materials to Device Application. Nano Energy 2022, 91, 106692. [Google Scholar] [CrossRef]
- Pei, Y.Z.; Shi, X.Y.; LaLonde, A.; Wang, H.; Chen, L.D.; Snyder, G.J. Convergence of Electronic Bands for High Performance Bulk Thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef]
- Liu, W.; Yin, K.; Zhang, Q.J.; Uher, C.; Tang, X.F. Eco-Friendly High-Performance Silicide Thermoelectric Materials. Nat. Sci. Rev. 2017, 4, 611–626. [Google Scholar] [CrossRef]
- Liu, W.S.; Jie, Q.; Kim, H.S.; Ren, Z.F. Current Progress and Future Challenges in Thermoelectric Power Generation: From Materials to Devices. Acta Mater. 2015, 87, 357–376. [Google Scholar] [CrossRef]
- Li, J.F.; Pan, Y.; Wu, C.H.; Sun, F.H.; Wei, T.R. Processing of Advanced Thermoelectric Materials. Sci. China Technol. Sci. 2017, 60, 1347–1364. [Google Scholar] [CrossRef]
- He, Y.; Day, T.; Zhang, T.; Liu, H.L.; Shi, X.; Chen, L.D.; Snyder, G.J. High Thermoelectric Performance in Non-Toxic Earth-Abundant Copper Sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef]
- Cao, Y.; Sheng, Y.; Li, X.; Xi, L.L.; Yang, J. Application of Materials Genome Methods in Thermoelectrics. Fron. Mater. 2022, 9, 861817. [Google Scholar] [CrossRef]
- Liu, R.H.; Chen, H.Y.; Zhao, K.P.; Qin, Y.T.; Jiang, B.B.; Zhang, T.S.; Sha, G.; Shi, X.; Uher, C.; Zhang, W.Q.; et al. Entropy as a Gene-Like Performance Indicator Promoting Thermoelectric Materials. Adv. Mater. 2017, 29, 1702712. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ponsford, D.; Lay, C.L.; Wang, X.; Wang, X.; Neo, D.C.J.; Wu, T.; Thitsartarn, W.; Yeo, J.C.C.; Guan, G.; et al. Thermoelectric Silver-Based Chalcogenides. Adv. Sci. 2022, 9, 2204624. [Google Scholar] [CrossRef]
- Slack, G.A. New Materials and Performance Limits for Thermoelectric Cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Qiu, P.F.; Shi, X.; Chen, L.D. Cu-Based Thermoelectric Materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Skoug, E.J.; Cain, J.D.; Morelli, D.T. High Thermoelectric Figure of Merit in the Cu3SbSe4–Cu3SbS4 solid Solution. Appl. Phys. Lett. 2011, 98, 261911. [Google Scholar] [CrossRef]
- Liu, R.H.; Xi, L.L.; Liu, H.L.; Shi, X.; Zhang, W.Q.; Chen, L.D. Ternary Compound CuInTe2: A Promising Thermoelectric Material with Diamond-Like Structure. Chem. Commun. 2012, 48, 3818–3820. [Google Scholar] [CrossRef]
- Suekuni, K.; Takabatake, T. Research Update: Cu-S Based Synthetic Minerals as Efficient Thermoelectric Materials at Medium Temperatures. APL Mater. 2016, 4, 104503. [Google Scholar] [CrossRef]
- Fan, J.; Carrillo-Cabrera, W.; Akselrud, L.; Antonyshyn, I.; Chen, L.D.; Grin, Y.R. New Monoclinic Phase at the Composition Cu2sSnSe3 and Its Thermoelectric Properties. Inorg. Chem. 2013, 52, 11067–11074. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Qin, Y.T.; Cheng, N.; Zhang, J.W.; Shi, X.; Grin, Y.R.; Chen, L.D. Thermoelectric Performance of Cu1−X−δAgXInTe2 Diamond-Like Materials with a Pseudocubic Crystal Structure. Inorg. Chem. Front. 2016, 3, 1167–1177. [Google Scholar] [CrossRef]
- Skoug, E.J.; Morelli, D.T. Role of Lone-Pair Electrons in Producing Minimum Thermal Conductivity in Nitrogen-Group Chalcogenide Compounds. Phys. Rev. Lett. 2011, 107, 235901. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xi, L.L.; Fan, J.; Zhang, W.Q.; Chen, L.D. Cu-Se Bond Network and Thermoelectric Compounds with Complex Diamondlike Structure. Chem. Mater. 2010, 22, 6029–6031. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.L.; Zhu, C.; Zhou, C.J.; Jabar, B.; Li, J.; Zhu, X.G.; Wang, L.; Song, C.J.; Xin, H.X.; et al. Design of Domain Structure and Realization of Ultralow Thermal Conductivity for Record-High Thermoelectric Performance in Chalcopyrite. Adv. Mater. 2019, 31, e1905210. [Google Scholar] [CrossRef]
- Huang, Y.L.; Zhang, B.; Li, J.W.; Zhou, Z.Z.; Zheng, S.K.; Li, N.H.; Wang, G.W.; Zhang, D.; Zhang, D.L.; Han, G.; et al. Unconventional Doping Effect Leads to Ultrahigh Average Thermoelectric Power Factor in Cu3SbSe4-Based Composites. Adv. Mater. 2022, 34, 2109952. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.C.; Wu, H.; Huang, Y.L.; Zheng, S.K.; Zhang, B.; Fu, H.X.; Cheng, Z.E.; Jiang, P.F.; Han, G.; et al. High Thermoelectric Performance in Earth-Abundant Cu3SbS4 by Promoting Doping Efficiency via Rational Vacancy Design. Adv. Funct. Mater. 2023, 33, 2214163. [Google Scholar] [CrossRef]
- Plirdpring, T.; Kurosaki, K.; Kosuga, A.; Day, T.; Firdosy, S.; Ravi, V.; Snyder, G.J.; Harnwunggmoung, A.; Sugahara, T.; Ohishi, Y.; et al. Chalcopyrite CuGaTe2: A High-Efficiency Bulk Thermoelectric Material. Adv. Mater. 2012, 24, 3622–3626. [Google Scholar] [CrossRef]
- Luo, Y.B.; Yang, J.Y.; Jiang, Q.H.; Li, W.X.; Zhang, D.; Zhou, Z.W.; Cheng, Y.D.; Ren, Y.Y.; He, X. Progressive Regulation of Electrical and Thermal Transport Properties to High-Performance CuInTe2 thermoelectric Materials. Adv. Energy Mater. 2016, 6, 160007. [Google Scholar] [CrossRef]
- Zhang, J.W.; Liu, R.H.; Cheng, N.; Zhang, Y.B.; Yang, J.H.; Uher, C.; Shi, X.; Chen, L.D.; Zhang, W. High-Performance Pseudocubic Thermoelectric Materials from Non-Cubic Chalcopyrite Compounds. Adv. Mater. 2014, 26, 3848–3853. [Google Scholar] [CrossRef]
- Wei, T.R.; Qin, Y.T.; Deng, T.T.; Song, Q.F.; Jiang, B.B.; Liu, R.H.; Qiu, P.F.; Shi, X.; Chen, L.D. Copper Chalcogenide Thermoelectric Materials. Sci. China Mater. 2018, 62, 8–24. [Google Scholar] [CrossRef]
- Zhang, D.; Bai, H.C.; Li, Z.L.; Wang, J.L.; Fu, G.S.; Wang, S.F. Multinary Diamond-Like Chalcogenides for Promising Thermoelectric Application. Chin. Phys. B 2018, 27, 047206. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Gao, Y.; Wu, Y.; Wang, B.Y.; Sun, W.L.; Yu, L.; Wei, S.T.; Zheng, S.Q. P-Type Doping of Transition Metal Elements to Optimize the Thermoelectric Properties of CuGaTe2. Chem. Eng. J. 2022, 427, 131807. [Google Scholar] [CrossRef]
- Qin, Y.T.; Qiu, P.F.; Liu, R.H.; Li, Y.L.; Hao, F.; Zhang, T.S.; Ren, D.D.; Shi, X.; Chen, L.D. Optimized Thermoelectric Properties in Pseudocubic Diamond-Like CuGaTe2 Compounds. J. Mater. Chem. A 2016, 4, 1277–1289. [Google Scholar] [CrossRef]
- Luo, Y.B.; Yang, J.Y.; Jiang, Q.H.; Li, W.X.; Xiao, Y.; Fu, L.W.; Zhang, D.; Zhou, Z.W.; Cheng, Y.D. Large Enhancement of Thermoelectric Performance of CuInTe2 via a Synergistic Strategy of Point Defects and Microstructure Engineering. Nano Energy 2015, 18, 37–46. [Google Scholar] [CrossRef]
- Yan, Y.C.; Lu, X.; Wang, G.Y.; Zhou, X.Y. Zt = 1.1 in CuInTe2 Solid Solutions Enabled by Rational Defect Engineering. ACS Appl. Energy Mater. 2019, 3, 2039–2048. [Google Scholar] [CrossRef]
- Xie, H.Y.; Hao, S.Q.; Cai, S.T.; Bailey, T.P.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow Thermal Conductivity in Diamondoid Lattices: High Thermoelectric Performance in Chalcopyrite Cu0.8+YAg0.2In1−YTe2. Energy Environ. Sci. 2020, 13, 3693–3705. [Google Scholar] [CrossRef]
- Li, M.; Luo, Y.; Hu, X.J.; Cai, G.M.; Han, Z.K.; Du, Z.L.; Cui, G.L. Synergistic Regulation of Phonon and Electronic Properties to Improve the Thermoelectric Performance of Chalcogenide CuIn1−xGaxTe2:yInTe (x = 0–0.3) with In Situ Formed Nanoscale Phase InTe. Adv. Electron. Mater. 2020, 6, 190114. [Google Scholar] [CrossRef]
- Shen, J.W.; Zhang, X.Y.; Chen, Z.W.; Lin, S.Q.; Li, J.; Li, W.; Li, S.S.; Chen, Y.; Pei, Y.Z. Substitutional Defects Enhancing Thermoelectric CuGaTe2. J. Mater. Chem. A 2017, 5, 5314–5320. [Google Scholar] [CrossRef]
- Xie, H.Y.; Hao, S.Q.; Bailey, T.P.; Cai, S.T.; Zhang, Y.Y.; Slade, T.J.; Snyder, G.J.; Dravid, V.P.; Uher, C.; Wolverton, C.; et al. Ultralow Thermal Conductivity in Diamondoid Structures and High Thermoelectric Performance in (Cu1−xAgx)(In1–yGay)Te2. J. Am. Chem. Soc. 2021, 143, 5978–5989. [Google Scholar] [CrossRef]
- Wang, B.Y.; Zheng, S.Q.; Chen, Y.X.; Wu, Y.; Li, J.; Ji, Z.; Mu, Y.N.; Wei, Z.B.; Liang, Q.; Liang, J.X. Band Engineering for Realizing Large Effective Mass in Cu3SbSe4 by Sn/La Codoping. J. Phys. Chem. C 2020, 124, 10336–10343. [Google Scholar] [CrossRef]
- Wang, B.Y.; Zheng, S.Q.; Wang, Q.; Li, Z.L.; Li, J.; Zhang, Z.P.; Wu, Y.; Zhu, B.S.; Wang, S.Y.; Chen, Y.X.; et al. Synergistic Modulation of Power Factor and Thermal Conductivity in Cu3SbSe4 Towards High Thermoelectric Performance. Nano Energy 2020, 71, 104658. [Google Scholar] [CrossRef]
- Kumar, A.; Dhama, P.; Saini, D.S.; Banerji, P. Effect of Zn Substitution at a Cu Site on The Transport Behavior and Thermoelectric Properties in Cu3SbSe4. RSC Adv. 2016, 6, 5528–5534. [Google Scholar] [CrossRef]
- Zou, T.H.; Qin, X.Y.; Li, D.; Li, L.L.; Sun, G.L.; Wang, Q.Q.; Zhang, J.; Xin, H.X.; Liu, Y.F.; Song, C.J. Enhanced Thermoelectric Performance of β-Zn4Sb3 Based Composites Incorporated with Large Proportion of Nanophase Cu3SbSe4. J. Alloys Compd. 2014, 588, 568–572. [Google Scholar] [CrossRef]
- Li, J.M.; Li, D.; Song, C.J.; Wang, L.; Xin, H.X.; Zhang, J.; Qin, X.Y. Realized High Power Factor and Thermoelectric Performance in Cu3SbSe4. Intermetallics 2019, 109, 68–73. [Google Scholar] [CrossRef]
- Li, J.M.; Ming, H.W.; Zhang, B.L.; Song, C.J.; Wang, L.; Xin, H.X.; Zhang, J.; Qin, X.Y.; Li, D. Ultra-Low Thermal Conductivity and High Thermoelectric Performance Realized in a Cu3SbSe4 Based System. Mater. Chem. Front. 2021, 5, 324–332. [Google Scholar] [CrossRef]
- Xie, D.D.; Zhang, B.; Zhang, A.; Chen, Y.; Yan, Y.; Yang, H.; Wang, G.; Wang, G.; Han, X.; Han, G.; et al. High Thermoelectric Performance of Cu3SbSe4 Nanocrystals with Cu2−xSe in Situ Inclusions Synthesized by a Microwave-Assisted Solvothermal Method. Nanoscale 2018, 10, 14546–14553. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, J.Y.; Bai, H.C.; Luo, Y.B.; Wang, B.; Hou, S.H.; Li, Z.L.; Wang, S. Significant Average Zt Enhancement in Cu3SbSe4-Based Thermoelectric Material via Softening P-D Hybridization. J. Mater. Chem. A 2019, 7, 17648–17654. [Google Scholar] [CrossRef]
- Chen, K.; Di Paola, C.; Du, B.; Zhang, R.Z.; Laricchia, S.; Bonini, N.; Weber, C.; Abrahams, I.; Yan, H.; Reece, M. Enhanced Thermoelectric Performance of Sn-Doped Cu3SbS4. J. Mater. Chem. C 2018, 6, 8546–8552. [Google Scholar] [CrossRef]
- Shen, M.J.; Lu, S.Y.; Zhang, Z.F.; Liu, H.Y.; Shen, W.X.; Fang, C.; Wang, Q.Q.; Chen, L.C.; Zhang, Y.W.; Jia, X.P. Bi and Sn Co-Doping Enhanced Thermoelectric Properties of Cu3SbS4 Materials with Excellent Thermal Stability. ACS Appl. Mater. Interfaces 2020, 12, 8271–8279. [Google Scholar] [CrossRef]
- Xie, H.Y.; Su, X.L.; Zheng, G.; Zhu, T.; Yin, K.; Yan, Y.G.; Uher, C.; Kanatzidis, M.G.; Tang, X.F. The Role of Zn in Chalcopyrite CuFeS2: Enhanced Thermoelectric Properties of Cu1−xZnxFeS2 with in Situ Nanoprecipitates. Adv. Energy Mater. 2016, 7, 601299. [Google Scholar]
- Ge, B.Z.; Lee, H.; Zhou, C.J.; Lu, W.Q.; Hu, J.B.; Yang, J.; Cho, S.P.; Qiao, G.J.; Shi, Z.Q.; Chung, I. Exceptionally Low Thermal Conductivity Realized in the Chalcopyrite CuFeS2 via Atomic-Level Lattice Engineering. Nano Energy 2022, 94, 106941. [Google Scholar] [CrossRef]
- Ge, B.Z.; Shi, Z.Q.; Zhou, C.J.; Hu, J.B.; Liu, G.W.; Xia, H.Y.; Xu, J.T.; Qiao, G.J. Enhanced Thermoelectric Performance of N-Type Eco-Friendly Material Cu1−xAgxFeS2 (X=0–0.14) via Bandgap Tuning. J. Alloys Compd. 2019, 809, 151717. [Google Scholar] [CrossRef]
- Tippireddy, S.; Azough, F.; Vikram; Bhui, A.; Chater, P.; Kepaptsoglou, D.; Ramasse, Q.; Freer, R.; Grau-Crespo, R.; Biswas, K.; et al. Local Structural Distortions and Reduced Thermal Conductivity in Ge-Substituted Chalcopyrite. J. Mater. Chem. A 2022, 10, 23874–23885. [Google Scholar] [CrossRef]
- Tan, Q.; Sun, W.; Li, Z.L.; Li, J.F. Enhanced Thermoelectric Properties of Earth-Abundant Cu2SnS3 via in Doping Effect. J. Alloys Compd. 2016, 672, 558–563. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.W.; Wang, Y.F.; Hu, X.H.; Lyu, Y.; Cheng, C.C.; Pan, L.; Lu, C.H. Role of Crystal Transformation on the Enhanced Thermoelectric Performance in Mn-Doped Cu2SnS3. J. Alloys Compd. 2019, 780, 618–625. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Gu, Y.; Zhang, P.; Hu, X.H.; Wang, Y.F.; Zong, P.; Pan, L.; Lyu, Y.; Koumoto, K. Enhanced Thermoelectric Performance in Polymorphic Heavily Co-Doped Cu2SnS3 through Carrier Compensation by Sb Substitution. Sci Technol. Adv. Mater. 2021, 22, 363–372. [Google Scholar] [CrossRef]
- Huang, T.Y.; Yan, Y.C.; Peng, K.L.; Tang, X.D.; Guo, L.J.; Wang, R.F.; Lu, X.; Zhou, X.Y.; Wang, G.Y. Enhanced Thermoelectric Performance in Copper-Deficient Cu2GeSe3. J. Alloys Compd. 2017, 723, 708–713. [Google Scholar] [CrossRef]
- Wang, R.F.; Li, A.; Huang, T.; Zhang, B.; Peng, K.L.; Yang, H.Q.; Lu, X.; Zhou, X.Y.; Han, X.D.; Wang, G.Y. Enhanced Thermoelectric Performance in Cu2GeSe3 via (Ag,Ga)-Co-Doping on Cation Sites. J. Alloys Compd. 2018, 769, 218–225. [Google Scholar] [CrossRef]
- Yang, J.; Lu, B.B.; Song, R.F.; Hou, H.G.; Zhao, L.J.; Zhang, X.Z.; Liu, G.W.; Qiao, G.J. Realizing Enhanced Thermoelectric Properties in Cu2GeSe3 via a Synergistic Effect of in and Ag Dual-Doping. J. Eur. Ceram. Soc. 2022, 42, 169–174. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.B.; Fang, Y.W.; Qin, F.Y.; Cao, X.; Xie, H.Y.; Liu, J.W.; Dong, J.F.; Sanson, A.; Giarola, M.; et al. High Thermoelectric Performance through Crystal Symmetry Enhancement in Triply Doped Diamondoid Compound Cu2SnSe3. Adv. Energy Mater. 2021, 11, 2100661. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, G.H.; Cao, T.F.; Liu, L.M.; Li, J.T.; Chen, K.X.; Li, L.F.; Han, Y.M.; Zhou, M. Enhanced Thermoelectric Properties of Cu2SnSe3 by (Ag, In)-Co-Doping. Adv. Funct. Mater. 2016, 26, 6025–6032. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, D.W.; Su, X.L.; Xie, H.Y.; Liu, W.; Zheng, Y.; Tang, X.F. Synergistically Enhanced Thermoelectric Performance of Cu2SnSe3-Based Composites via Ag Doping Balance. ACS Appl. Mater. Interfaces 2021, 13, 55178–55187. [Google Scholar] [CrossRef]
- Ming, H.W.; Zhu, G.F.; Zhu, C.; Qin, X.Y.; Chen, T.; Zhang, J.; Li, D.; Xin, H.X.; Jabar, B. Boosting Thermoelectric Performance of Cu2SnSe3 via Comprehensive Band Structure Regulation and Intensified Phonon Scattering by Multidimensional Defects. ACS Nano 2021, 15, 10532–10541. [Google Scholar] [CrossRef]
- Ming, H.W.; Zhu, C.; Chen, T.; Yang, S.H.; Chen, Y.; Zhang, J.; Li, D.; Xin, H.X.; Qin, X.Y. Creating High-Dense Stacking Faults and Endo-Grown Nanoneedles to Enhance Phonon Scattering and Improve Thermoelectric Performance of Cu2SnSe3. Nano Energy 2022, 100, 107510. [Google Scholar] [CrossRef]
- Ming, H.W.; Zhu, C.; Qin, X.Y.; Zhang, J.; Li, D.; Zhang, B.L.; Chen, T.; Li, J.M.; Lou, X.N.; Xin, H.X. Improved Figure of Merit of Cu2SnSe3 via Band Structure Modification and Energy-Dependent Carrier Scattering. ACS Appl. Mater. Interfaces 2020, 12, 19693–19700. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Q.; Ming, H.W.; Qin, X.Y.; Yang, Y.; Zhang, J.; Peng, D.; Chen, T.; Li, D.; Kawazoe, Y. Improved Thermoelectric Performance of Cu12Sb4S13 through Gd-Substitution Induced Enhancement of Electronic Density of States and Phonon Scattering. ACS Appl. Mater. Interfaces 2021, 13, 25092–25101. [Google Scholar] [CrossRef]
- Zhu, C.; Ming, H.; Huang, L.; Zhang, B.; Lou, X.; Li, D.; Jabar, B.; Xin, H.; Zhang, J.; Qin, X. Achieving High Power Factor and Thermoelectric Performance through Dual Substitution of Zn and Se in Tetrahedrites Cu12Sb4S13. Appl. Phys. Lett. 2019, 115, 182102. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, I.W.; Huang, F.Q.; Chen, L.D. Improved Thermoelectric Properties of Cu-Doped Quaternary Chalcogenides of Cu2CdSnSe4. Adv. Mater. 2009, 21, 3808–3812. [Google Scholar] [CrossRef]
- Chen, Q.F.; Yan, Y.C.; Zhan, H.; Yao, W.; Chen, Y.; Dai, J.Y.; Sun, X.N.; Zhou, X.Y. Enhanced Thermoelectric Performance of Chalcogenide Cu2CdSnSe4 by Ex-Situ Homogeneous Nanoinclusions. J. Mater. 2016, 2, 179–186. [Google Scholar]
- Fan, F.J.; Yu, B.; Wang, Y.X.; Zhu, Y.L.; Liu, X.J.; Yu, S.H.; Ren, Z.F. Colloidal Synthesis of Cu2CdSnSe4 Nanocrystals and Hot-Pressing to Enhance the Thermoelectric Figure-of-Merit. J. Am. Chem. Soc. 2011, 133, 15910–15913. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Wang, G.W.; Zhang, A.J.; Yang, D.F.; Yao, W.; Peng, K.L.; Yan, Y.C.; Sun, X.N.; Liu, A.P.; Wang, G.Y.; et al. Colloidal Synthesis of Cu2−xAgxCdSnSe4 Nanocrystals: Microstructures Facilitate High Performance Thermoelectricity. J. Mater. Chem. C 2015, 3, 12273–12280. [Google Scholar] [CrossRef]
- Basu, R.; Mandava, S.; Bohra, A.; Bhattacharya, S.; Bhatt, R.; Ahmad, S.; Bhattacharyya, K.; Samanta, S.; Debnath, A.K.; Singh, A.; et al. Improving the Thermoelectric Performance of Tetrahedrally Bonded Quaternary Selenide Cu2CdSnSe4 Using Cdse Precipitates. J. Electron. Mater. 2019, 48, 2120–2130. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Femi, O.E.; Chattopadhyay, K.; Mallik, R.C. Thermoelectric Properties of in-Doped Cu2ZnGeSe4. J. Electron. Mater. 2015, 45, 1625–1632. [Google Scholar] [CrossRef]
- Song, Q.F.; Qiu, P.F.; Hao, F.; Zhao, K.P.; Zhang, T.S.; Ren, D.D.; Shi, X.; Chen, L.D. Quaternary Pseudocubic Cu2TmSnSe4(Tm = Mn, Fe, Co) Chalcopyrite Thermoelectric Materials. Adv. Electron. Mater. 2016, 2, 1600312. [Google Scholar] [CrossRef]
- Song, Q.F.; Qiu, P.F.; Zhao, K.; Deng, T.T.; Shi, X.; Chen, L.D. Crystal Structure and Thermoelectric Properties of Cu2Fe1−xMnxSnSe4 Diamond-Like Chalcogenides. ACS Appl. Energy Mater. 2019, 3, 2137–2146. [Google Scholar] [CrossRef]
- Song, Q.F.; Qiu, P.F.; Chen, H.; Zhao, K.; Guan, M.; Zhou, Y.; Wei, T.R.; Ren, D.D.; Xi, L.; Yang, J.; et al. Enhanced Carrier Mobility and Thermoelectric Performance in Cu2FeSnSe4 Diamond-Like Compound via Manipulating the Intrinsic Lattice Defects. Mater. Today Phys. 2018, 7, 45–53. [Google Scholar] [CrossRef]
- Pavan Kumar, V.; Guilmeau, E.; Raveau, B.; Caignaert, V.; Varadaraju, U.V. A New Wide Band Gap Thermoelectric Quaternary Selenide Cu2MgSnSe4. J. Appl. Phys. 2015, 118, 155101. [Google Scholar] [CrossRef]
- Shen, J.W.; Zhang, X.Y.; Lin, S.Q.; Li, J.; Chen, Z.W.; Li, W.; Pei, Y.Z. Vacancy Scattering for Enhancing the Thermoelectric Performance of CuGaTe2 Solid Solutions. J. Mater. Chem. A 2016, 4, 15464–15470. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, J.; Liu, H.T.; Chen, Z.W.; Lin, L.; Ren, D.; Liu, B.; Ang, R. Optimized Strategies for Advancing N-Type PbTe Thermoelectrics: A Review. ACS Appl. Mater. Interfaces 2020, 12, 49323–49334. [Google Scholar] [CrossRef]
- Shi, H.N.; Qin, Y.X.; Qin, B.C.; Su, L.Z.; Wang, Y.P.; Chen, Y.J.; Gao, X.; Liang, H.; Ge, Z.H.; Hong, T.; et al. Incompletely Decomposed In4SnSe4 Leads to High-Ranged Thermoelectric Performance in N-Type PbTe. Adv. Energy Mater. 2022, 12, 2202539. [Google Scholar] [CrossRef]
- Jia, B.H.; Huang, Y.; Wang, Y.; Zhou, Y.; Zhao, X.D.; Ning, S.T.; Xu, X.; Lin, P.J.; Chen, Z.Q.; Jiang, B.B.; et al. Realizing High Thermoelectric Performance in Non-Nanostructured N-Type PbTe. Energy Environ. Sci. 2022, 15, 1920–1929. [Google Scholar] [CrossRef]
- Wang, S.Q.; Chang, C.; Bai, S.L.; Qin, B.C.; Zhu, Y.C.; Zhan, S.P.; Zheng, J.Q.; Tang, S.W.; Zhao, L.D. Fine Tuning of Defects Enables High Carrier Mobility and Enhanced Thermoelectric Performance of N-Type PbTe. Chem. Mater. 2023, 35, 755–763. [Google Scholar] [CrossRef]
- Xu, H.H.; Wan, H.; Xu, R.; Hu, Z.Q.; Liang, X.L.; Li, Z.; Song, J.M. Enhancing the Thermoelectric Performance of SnTe-CuSbSe2 with an Ultra-Low Lattice Thermal Conductivity. J. Mater. Chem. A 2023, 11, 4310–4318. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Guo, X.M.; Zhang, F.J.; Shi, Q.; Tang, M.J.; Ang, R. Routes for Advancing SnTe Thermoelectrics. J. Mater. Chem. A 2020, 8, 16790–16813. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.X.; Lin, S.Q.; Chen, Z.W.; Li, J.; Zhang, X.Y.; Zheng, L.L.; Pei, Y.Z. Advances in Environment-Friendly SnTe Thermoelectrics. ACS Energy Lett. 2017, 2, 2349–2355. [Google Scholar] [CrossRef]
- Tippireddy, S.; Azough, F.; Vikram; Tompkins, F.T.; Bhui, A.; Freer, R.; Grau-Crespo, R.; Biswas, K.; Vaqueiro, P.; Powell, A.V. Tin-Substituted Chalcopyrite: An N-Type Sulfide with Enhanced Thermoelectric Performance. Chem. Mater. 2022, 34, 5860–5873. [Google Scholar] [CrossRef]
- Xie, H.Y.; Su, X.L.; Hao, S.Q.; Zhang, C.; Zhang, Z.K.; Liu, W.; Yan, Y.G.; Wolverton, C.; Tang, X.F.; Kanatzidis, M.G. Large Thermal Conductivity Drops in the Diamondoid Lattice of CuFeS2 by Discordant Atom Doping. J. Am. Chem. Soc. 2019, 141, 18900–18909. [Google Scholar] [CrossRef]
- Bo, L.; Li, F.J.; Hou, Y.B.; Wang, L.; Wang, X.L.; Zhang, R.P.; Zuo, M.; Ma, Y.Z.; Zhao, D.G. Enhanced Thermoelectric Properties of Cu3SbSe4 via Configurational Entropy Tuning. J. Mater. Sci. 2022, 57, 4643–4651. [Google Scholar] [CrossRef]
- Fan, J.; Carrillo-Cabrera, W.; Antonyshyn, I.; Prots, Y.; Veremchuk, I.; Schnelle, W.; Drathen, C.; Chen, L.D.; Grin, Y. Crystal Structure and Physical Properties of Ternary Phases around the Composition Cu5Sn2Se7 with Tetrahedral Coordination of Atoms. Chem. Mater. 2014, 26, 5244–5251. [Google Scholar] [CrossRef]
- Adhikary, A.; Mohapatra, S.; Lee, S.H.; Hor, Y.S.; Adhikari, P.; Ching, W.Y.; Choudhury, A. Metallic Ternary Telluride with Sphalerite Superstructure. Inorg. Chem. 2016, 55, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Sturm, C.; Macario, L.R.; Mori, T.; Kleinke, H. Thermoelectric Properties of Zinc-Doped Cu5Sn2Se7 and Cu5Sn2Te7. Dalton Trans. 2021, 50, 6561–6567. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; San, S.; Baral, K.; Li, N.; Rulis, P.; Ching, W.Y. First-Principles Calculations of Thermoelectric Transport Properties of Quaternary and Ternary Bulk Chalcogenide Crystals. Materials 2022, 15, 2843–2871. [Google Scholar] [CrossRef] [PubMed]
- Pavan Kumar, V.; Passuti, S.; Zhang, B.; Fujii, S.; Yoshizawa, K.; Boullay, P.; Le Tonquesse, S.; Prestipino, C.; Raveau, B.; Lemoine, P.; et al. Engineering Transport Properties in Interconnected Enargite-Stannite Type Cu2+xMn1−xGeS4 Nanocomposites. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210600. [Google Scholar] [CrossRef]
- Shi, X.Y.; Huang, F.Q.; Liu, M.L.; Chen, L.D. Thermoelectric Properties of Tetrahedrally Bonded Wide-Gap Stannite Compounds Cu2ZnSn1−xInxSe4. Appl. Phys. Lett. 2009, 94, 122103. [Google Scholar] [CrossRef]
- Zou, D.F.; Nie, G.Z.; Li, Y.; Xu, Y.; Lin, J.; Zheng, H.; Li, J.G. Band Engineering via Biaxial Strain for Enhanced Thermoelectric Performance in Stannite-Type Cu2ZnSnSe4. RSC Adv. 2015, 5, 24908–24914. [Google Scholar] [CrossRef]
- Prem Kumar, D.S.; Chetty, R.; Rogl, P.; Rogl, G.; Bauer, E.; Malar, P.; Mallik, R.C. Thermoelectric Properties of Cd Doped Tetrahedrite: Cu12−xCdxSb4S13. Intermetallics 2016, 78, 21–29. [Google Scholar] [CrossRef]
- Chen, S.Y.; Gong, X.G.; Walsh, A.; Wei, S.H. Electronic Structure and Stability of Quaternary Chalcogenide Semiconductors Derived from Cation Cross-Substitution of II-VI And I-III-VI2 compounds. Phys. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Wang, Y.X.; Lai, W.; Xia, Y.; Ozolins, V. Phase Stability, Crystal Structure, and Thermoelectric Properties of Cu12Sb4S13−xSex Solid Solutions. Chem. Mater. 2016, 28, 1781–1786. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Mallik, R.C. Tetrahedrites as Thermoelectric Materials: An Overview. J. Mater. Chem. C 2015, 3, 12364–12378. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.Y.; Uher, C. High Performance Thermoelectricity in Earth-Abundant Compounds Based on Natural Mineral Tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Hu, H.H.; Zhuang, H.L.; Jiang, Y.L.; Shi, J.L.; Li, J.W.; Cai, B.W.; Han, Z.N.; Pei, J.; Su, B.; Ge, Z.H.; et al. Thermoelectric Cu12Sb4S13-Based Synthetic Minerals with a Sublimation-Derived Porous Network. Adv. Mater. 2021, 33, e2103633. [Google Scholar] [CrossRef]
- Bouyrie, Y.; Ohta, M.; Suekuni, K.; Kikuchi, Y.; Jood, P.; Yamamoto, A.; Takabatake, T. Enhancement in the Thermoelectric Performance of Colusites Cu26A2E6S32(A=Nb, Ta; E=Sn, Ge) Using E-Site Non-Stoichiometry. J. Mater. Chem. C 2017, 5, 4174–4184. [Google Scholar] [CrossRef]
- Bourgès, C.; Gilmas, M.; Lemoine, P.; Mordvinova, N.E.; Lebedev, O.I.; Hug, E.; Nassif, V.; Malaman, B.; Daou, R.; Guilmeau, E. Structural Analysis and Thermoelectric Properties of Mechanically Alloyed Colusites. J. Mater. Chem. C 2016, 4, 7455–7463. [Google Scholar] [CrossRef]
- Bourgès, C.; Bouyrie, Y.; Supka, A.R.; Al Rahal Al Orabi, R.; Lemoine, P.; Lebedev, O.I.; Ohta, M.; Suekuni, K.; Nassif, V.; Hardy, V.; et al. High-Performance Thermoelectric Bulk Colusite by Process Controlled Structural Disordering. J. Am. Chem. Soc. 2018, 140, 2186–2195. [Google Scholar] [CrossRef]
- Kim, F.S.; Suekuni, K.; Nishiate, H.; Ohta, M.; Tanaka, H.I.; Takabatake, T. Tuning the Charge Carrier Density in the Thermoelectric Colusite. J. Appl. Phys. 2016, 119, 175105. [Google Scholar] [CrossRef]
- Pavan Kumar, V.; Supka, A.R.; Lemoine, P.; Lebedev, O.I.; Raveau, B.; Suekuni, K.; Nassif, V.; Al Rahal Al Orabi, R.; Fornari, M.; Guilmeau, E. High Power Factors of Thermoelectric Colusites Cu26T2Ge6S32 (T= Cr, Mo, W): Toward Functionalization of the Conductive “Cu-S” Network. Adv. Energy Mater. 2018, 9, 1803249. [Google Scholar] [CrossRef]
- Guélou, G.; Lemoine, P.; Raveau, B.; Guilmeau, E. Recent Developments in High-Performance Thermoelectric Sulphides: An Overview of the Promising Synthetic Colusites. J. Mater. Chem. C 2021, 9, 773–795. [Google Scholar] [CrossRef]
- Bouyrie, Y.; Candolfi, C.; Dauscher, A.; Malaman, B.; Lenoir, B. Exsolution Process as a Route toward Extremely Low Thermal Conductivity in Cu12Sb4−xTexS13 Tetrahedrites. Chem. Mater. 2015, 27, 8354–8361. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Wei, T.R.; Wang, H.; Gibbs, Z.M.; Wu, C.F.; Snyder, G.J.; Li, J.F. Thermoelectric Properties of Sn-Doped P-Type Cu3SbSe4: A Compound with Large Effective Mass and Small Band Gap. J. Mater. Chem. A 2014, 2, 13527–13533. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The Science and technology of mechanical Alloying. Mater. Sci. Eng. A 2001, 304, 151–158. [Google Scholar]
- Zhang, D.; Yang, J.Y.; Jiang, Q.H.; Zhou, Z.W.; Li, X.W.; Xin, J.; Basit, A.; Ren, Y.Y.; He, X.; Chu, W.J.; et al. Combination of Carrier Concentration Regulation and High Band Degeneracy for Enhanced Thermoelectric Performance of Cu3SbSe4. ACS Appl. Mater. Interfaces 2017, 9, 28558–28565. [Google Scholar] [CrossRef]
- Chen, K.; Du, B.; Bonini, N.; Weber, C.; Yan, H.X.; Reece, M.J. Theory-Guided Synthesis of an Eco-Friendly and Low-Cost Copper Based Sulfide Thermoelectric Material. J. Phys. Chem. C 2016, 120, 27135–27140. [Google Scholar] [CrossRef]
- Nautiyal, H.; Lohani, K.; Mukherjee, B.; Isotta, E.; Malagutti, M.A.; Ataollahi, N.; Pallecchi, I.; Putti, M.; Misture, S.T.; Rebuffi, L.; et al. Mechanochemical Synthesis of Sustainable Ternary and Quaternary Nanostructured Cu2SnS3, Cu2ZnSnS4, and Cu2ZnSnSe4 Chalcogenides for Thermoelectric Applications. Nanomaterials 2023, 13, 366–387. [Google Scholar] [CrossRef]
- Wang, W.Y.; Bo, L.; Wang, Y.P.; Wang, L.; Li, F.J.; Zuo, M.; Zhao, D.G. Enhanced Thermoelectric Properties of Graphene /Cu3SbSe4 Composites. J. Electron. Mater. 2021, 50, 4880–4886. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xie, W.J.; Li, H.; Tang, X.F.; Zhang, Q.J. Effects of Cooling Rate on Thermoelectric Properties of N-Type Bi2(Se0.4Te0.6)3 Compounds. J. Electron. Mater. 2011, 40, 1150–1157. [Google Scholar] [CrossRef]
- Xie, W.J.; Tang, X.F.; Yan, Y.G.; Zhang, Q.J.; Tritt, T.M. Unique Nanostructures and Enhanced Thermoelectric Performance of Melt-Spun Bisbte Alloys. Appl. Phys. Lett. 2009, 94, 102111. [Google Scholar] [CrossRef]
- Zhao, D.G.; Wang, L.; Wu, D.; Bo, L. Thermoelectric Properties of CuSnSe3-Based Composites Containing Melt-Spun Cu-Te. Metals 2019, 9, 971–980. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, H.Y.; Zhang, Q.; Suwardi, A.; Cheng, X.; Zhang, Y.F.; Shu, W.; Wan, X.J.; Yang, Z.L.; Liu, Z.H.; et al. Unraveling the Critical Role of Melt-Spinning Atmosphere in Enhancing the Thermoelectric Performance of P-Type Bi0.52Sb1.48Te3 Alloys. ACS Appl. Mater. Interfaces 2020, 12, 36186–36195. [Google Scholar] [CrossRef]
- Ding, G.C.; Si, J.X.; Wu, H.F.; Yang, S.D.; Zhao, J.; Wang, G.W. Thermoelectric Properties of Melt Spun PbTe with Multi-Scaled Nanostructures. J. Alloys Compd. 2016, 662, 368–373. [Google Scholar] [CrossRef]
- Yang, B.; Li, S.M.; Li, X.; Liu, Z.P.; Zhong, H.; Feng, S.K. Ultralow Thermal Conductivity and Enhanced Thermoelectric Properties of SnTe Based Alloys Prepared by Melt Spinning Technique. J. Alloys Compd. 2020, 837, 155568. [Google Scholar] [CrossRef]
- Geng, H.Y.; Zhang, J.L.; He, T.H.; Zhang, L.X.; Feng, J.C. Microstructure Evolution and Mechanical Properties of Melt Spun Skutterudite-Based Thermoelectric Materials. Materials 2020, 13, 984–998. [Google Scholar] [CrossRef]
- Su, X.L.; Fu, F.; Yan, Y.G.; Zheng, G.; Liang, T.; Zhang, Q.; Cheng, X.; Yang, D.W.; Chi, H.; Tang, X.F.; et al. Self-Propagating High-Temperature Synthesis for Compound Thermoelectrics and New Criterion for Combustion Processing. Nat. Commun. 2014, 5, 4908–4915. [Google Scholar] [CrossRef]
- Cheng, X.; You, Y.H.; Fu, J.F.; Hu, T.Z.; Liu, W.; Su, X.L.; Yan, Y.G.; Tang, X.F. Self-Propagating High-Temperature Synthesis and Thermoelectric Performances of CuSnSe3. J. Alloys Compd. 2018, 750, 965–971. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, B.; Yang, D.W.; Su, X.L.; Liu, W.; Xie, H.Y.; Zheng, Y.; Tang, X.F. Enhanced Thermoelectric Properties of Cu2SnSe3-Based Materials with Ag2Se Addition. ACS Appl. Mater. Interfaces 2022, 14, 5439–5446. [Google Scholar] [CrossRef]
- Wei, S.T.; Wang, B.Y.; Zhang, Z.P.; Li, W.H.; Yu, L.; Wei, S.K.; Ji, Z.; Song, W.Y.; Zheng, S.Q. Achieving High Thermoelectric Performance through Carrier Concentration Optimization and Energy Filtering in Cu3SbSe4-Based Materials. J. Mater. 2022, 8, 929–936. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, e2106052. [Google Scholar] [CrossRef]
- Shi, X.L.; Tao, X.Y.; Zou, J.; Chen, Z.G. High-Performance Thermoelectric SnSe: Aqueous Synthesis, Innovations, and Challenges. Adv. Sci. 2020, 7, 1902923. [Google Scholar] [CrossRef]
- Balow, R.B.; Tomlinson, E.P.; Abu-Omar, M.M.; Boudouris, B.W.; Agrawal, R. Solution-Based Synthesis and Characterization of Earth Abundant Cu3(As,Sb)Se4 Nanocrystal Alloys: Towards Scalable Room-Temperature Thermoelectric Devices. J. Mater. Chem. A 2016, 4, 2198–2204. [Google Scholar] [CrossRef]
- Xiong, Q.H.; Xie, D.D.; Wang, H.; Wei, Y.Q.; Wang, G.W.; Wang, G.Y.; Liao, H.J.; Zhou, X.Y.; Lu, X. Colloidal Synthesis of Diamond-Like Compound Cu2SnTe3 and Thermoelectric Properties of (Cu0.96InTe2)1−x(Cu2SnTe3)x Solid Solutions. Chem. Eng. J. 2021, 422, 129985. [Google Scholar] [CrossRef]
- Wang, B.Y.; Wang, Y.L.; Zheng, S.Q.; Liu, S.C.; Li, J.; Chang, S.Y.; An, T.; Sun, W.L.; Chen, Y.X. Improvement of Thermoelectric Properties of Cu3SbSe4 Hierarchical with In-Situ Second Phase Synthesized by Microwave-Assisted Solvothermal Method. J. Alloys Compd. 2019, 806, 676–682. [Google Scholar] [CrossRef]
- Huang, L.L.; Zhang, J.; Zhu, C.; Ge, Z.H.; Li, Y.Y.; Li, D.; Qin, X.Y. Synergistically Optimized Electrical and Thermal Properties by Introducing Electron Localization and Phonon Scattering Centers in CuGaTe2 with Enhanced Mechanical Properties. J. Mater. Chem. C 2020, 8, 7534–7542. [Google Scholar] [CrossRef]
- Wang, W.L.; Feng, W.L.; Ding, T.; Yang, Q. Phosphine-Free Synthesis and Characterization of Cubic-Phase Cu2SnTe3 Nanocrystals with Optical and Optoelectronic Properties. Chem. Mater. 2015, 27, 6181–6184. [Google Scholar] [CrossRef]
- Zhang, D.W.; Lim, W.Y.S.; Duran, S.S.F.; Loh, X.J.; Suwardi, A. Additive Manufacturing of Thermoelectrics: Emerging Trends and Outlook. ACS Energy Lett. 2022, 7, 720–735. [Google Scholar] [CrossRef]
- Oztan, C.; Welch, R.; LeBlanc, S. Additive Manufacturing of Bulk Thermoelectric Architectures: A Review. Energies 2022, 15, 3121–3137. [Google Scholar] [CrossRef]
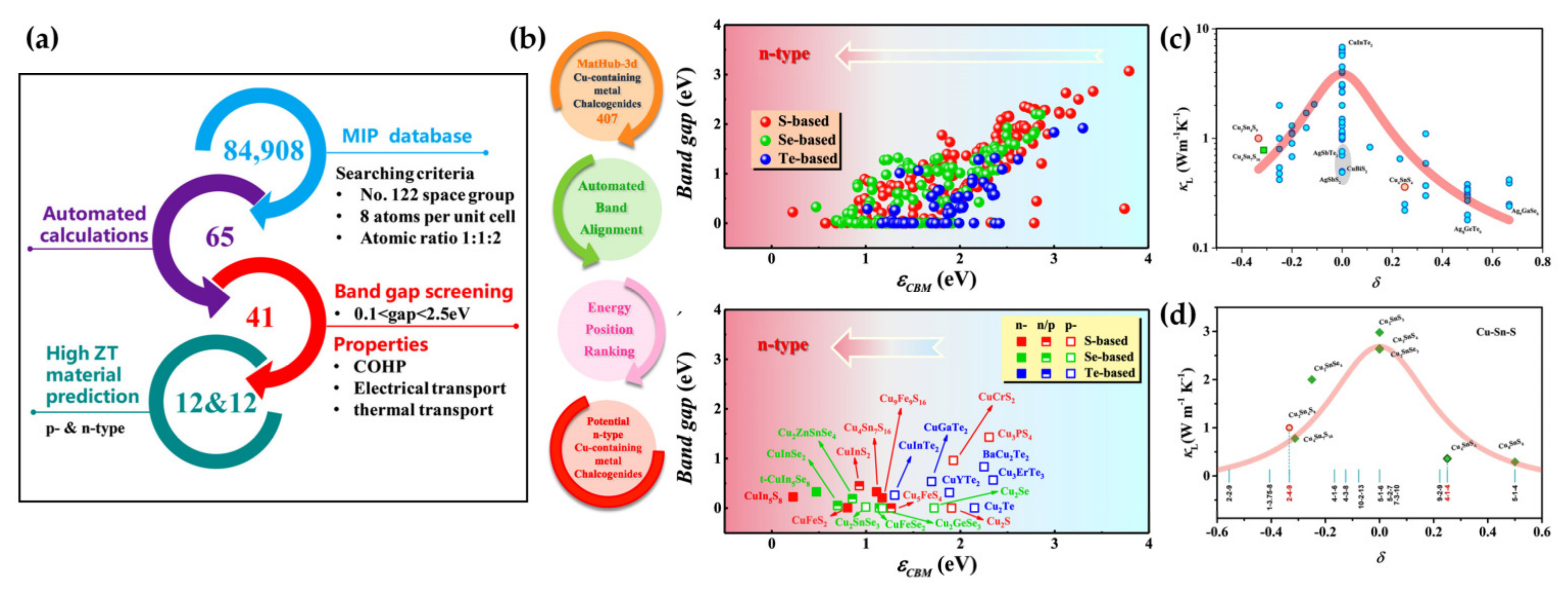
- Li, R.X.; Li, X.; Xi, L.L.; Yang, J.; Singh, D.J.; Zhang, W.Q. High-Throughput Screening for Advanced Thermoelectric Materials: Diamond-Like Abx(2) Compounds. ACS Appl. Mater. Interfaces 2019, 11, 24859–24866. [Google Scholar] [CrossRef]
- Xiong, Y.F.; Jin, Y.Q.; Deng, T.T.; Mei, K.L.; Qiu, P.F.; Xi, L.L.; Zhou, Z.Y.; Yang, J.; Shi, X.; Chen, L.D. High-Throughput Screening for Thermoelectric Semiconductors with Desired Conduction Types by Energy Positions of Band Edges. J. Am. Chem. Soc. 2022, 144, 8030–8037. [Google Scholar] [CrossRef]
- Xi, L.L.; Pan, S.S.; Li, X.; Xu, Y.L.; Ni, J.Y.; Sun, X.; Yang, J.; Luo, J.; Xi, J.; Zhu, W.H.; et al. Discovery of High-Performance Thermoelectric Chalcogenides through Reliable High-Throughput Material Screening. J. Am. Chem. Soc. 2018, 140, 10785–10793. [Google Scholar] [CrossRef]
- Recatala-Gomez, J.; Suwardi, A.; Nandhakumar, I.; Abutaha, A.; Hippalgaonkar, K. Toward Accelerated Thermoelectric Materials and Process Discovery. ACS Appl. Energy Mater. 2020, 3, 2240–2257. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Snoussi, H.; Zhang, G. Machine Learning Approaches for Thermoelectric Materials Research. Adv. Funct. Mater. 2019, 30, 1906041. [Google Scholar] [CrossRef]
- Sparks, T.D.; Gaultois, M.W.; Oliynyk, A.; Brgoch, J.; Meredig, B. Data Mining Our Way to the Next Generation of Thermoelectrics. Scr. Mater. 2016, 111, 10–15. [Google Scholar] [CrossRef]
- Deng, T.T.; Wei, T.R.; Huang, H.; Song, Q.F.; Zhao, K.P.; Qiu, P.F.; Yang, J.; Chen, L.D.; Shi, X. Number Mismatch between Cations and Anions as an Indicator for Low Lattice Thermal Conductivity in Chalcogenides. npj Comput. Mater. 2020, 6, 81. [Google Scholar] [CrossRef]
- Cheng, N.S.; Liu, R.H.; Bai, S.; Shi, X.; Chen, L.D. Enhanced Thermoelectric Performance in Cd Doped CuInTe2 Compounds. J. Appl. Phys. 2014, 115, 163705. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.Y.; Li, D.; Xin, H.X.; Song, C.J.; Li, L.L.; Wang, Z.M.; Guo, G.L.; Wang, L. Enhanced Thermoelectric Properties of Ag-Doped Compounds CuAgxGa1−xTe2 (0 ⩽ x ⩽ 0.05). J. Alloys Compd. 2014, 586, 285–288. [Google Scholar] [CrossRef]
- Ahmed, F.; Tsujii, N.; Mori, T. Thermoelectric Properties of CuGa1−xMnxTe2: Power Factor Enhancement by Incorporation of Magnetic Ions. J. Mater. Chem. A 2017, 5, 7545–7554. [Google Scholar] [CrossRef]
- Kucek, V.; Drasar, C.; Kasparova, J.; Plechacek, T.; Navratil, J.; Vlcek, M.; Benes, L. High-Temperature Thermoelectric Properties of Hg-Doped CuInTe2. J. Appl. Phys. 2015, 118, 125105. [Google Scholar] [CrossRef]
- Shen, J.W.; Chen, Z.W.; Lin, S.Q.; Zheng, L.L.; Li, W.; Pei, Y.Z. Single Parabolic Band Behavior of Thermoelectric P-Type CuGaTe2. J. Mater. Chem. C 2016, 4, 209–214. [Google Scholar] [CrossRef]
- Xie, H.Y.; Li, Z.; Liu, Y.; Zhang, Y.K.; Uher, C.; Dravid, V.P.; Wolverton, C.; Kanatzidis, M.G. Silver Atom Off-Centering in Diamondoid Solid Solutions Causes Crystallographic Distortion and Suppresses Lattice Thermal Conductivity. J. Am. Chem. Soc. 2023, 145, 3211–3220. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhang, T.S.; Qin, Y.T.; Day, T.; Jeffrey Snyder, G.; Shi, X.; Chen, L.D. Thermoelectric Transport Properties of Diamond-Like Cu1−xfe1+xS2 Tetrahedral Compounds. J. Appl. Phys. 2014, 116, 203705. [Google Scholar] [CrossRef]
- Liu, Y.; García, G.; Ortega, S.; Cadavid, D.; Palacios, P.; Lu, J.Y.; Ibáñez, M.; Xi, L.L.; De Roo, J.; López, A.M.; et al. Solution-Based Synthesis and Processing of Sn- and Bi-Doped Cu3SbSe4 nanocrystals, Nanomaterials and Ring-Shaped Thermoelectric Generators. J. Mater. Chem. A 2017, 5, 2592–2602. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, C.L.; Chiu, W.T.; Chen, Y.Y. Enhanced Thermoelectric Properties of Cu3SbSe4 by Germanium Doping. Mater. Lett. 2017, 186, 227–230. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Mallik, R.C. Thermoelectric Properties of Indium Doped Cu2CdSnSe4. Intermetallics 2016, 72, 17–24. [Google Scholar] [CrossRef]
- Ohta, M.; Jood, P.; Murata, M.; Lee, C.H.; Yamamoto, A.; Obara, H. An Integrated Approach to Thermoelectrics: Combining Phonon Dynamics, Nanoengineering, Novel Materials Development, Module Fabrication, and Metrology. Adv. Energy Mater. 2018, 9, 1801304. [Google Scholar] [CrossRef]
- Kucek, V.; Drasar, C.; Navratil, J.; Plechacek, T.; Benes, L. Thermoelectric Properties of Ni-Doped CuInTe2. J. Phys. Chem. Solids 2015, 83, 18–23. [Google Scholar] [CrossRef]
- Zhong, Y.H.; Wang, P.D.; Mei, H.Y.; Jia, Z.Y.; Cheng, N.P. Elastic, Vibration and Thermodynamic Properties of Cu1−xAgxInte2 (x = 0, 0.25, 0.5, 0.75 and 1) Chalcopyrite Compounds via First Principles. Semicond. Sci. Technol. 2018, 33, 065014. [Google Scholar] [CrossRef]
- Wei, T.R.; Li, F.; Li, J.F. Enhanced Thermoelectric Performance of Nonstoichiometric Compounds Cu3−xSbSe4 by Cu Deficiencies. J. Electron. Mater. 2014, 43, 2229–2238. [Google Scholar] [CrossRef]
- Heinrich, C.P.; Day, T.W.; Zeier, W.G.; Snyder, G.J.; Tremel, W. Effect of Isovalent Substitution on the Thermoelectric Properties of the Cu2ZnGeSe4−xSx Series of Solid Solutions. J. Am. Chem. Soc. 2014, 136, 442–448. [Google Scholar] [CrossRef]
- Li, J.H.; Tan, Q.; Li, J.F. Synthesis and Property Evaluation of CuFeS2−x as Earth-Abundant and Environmentally-Friendly Thermoelectric Materials. J. Alloys Compd. 2013, 551, 143–149. [Google Scholar] [CrossRef]
- Goto, Y.; Naito, F.; Sato, R.; Yoshiyasu, K.; Itoh, T.; Kamihara, Y.; Matoba, M. Enhanced Thermoelectric Figure of Merit in Stannite-Kuramite Solid Solutions Cu2+xFe1−xSnS4–y (x = 0–1) with Anisotropy Lowering. Inorg. Chem. 2013, 52, 9861–9866. [Google Scholar] [CrossRef]
- Huang, Y.L.; Shen, X.C.; Wang, G.W.; Zhang, B.; Zheng, S.K.; Yang, C.C.; Hu, X.; Gong, S.K.; Han, G.; Wang, G.Y.; et al. High Thermoelectric Performance and Compatibility in Cu3SbSe4–CuAlS2 Composites. Energy Environ. Sci. 2023, 16, 1763–1772. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Xi, L.L.; Wang, Y.W.; Zhang, J.W.; Zhang, P.H.; Zhang, W.Q. Electronic Properties of Energy Harvesting Cu-Chalcogenides: P-D Hybridization and D-Electron Localization. Comp. Mater. Sci. 2015, 108, 239–249. [Google Scholar] [CrossRef]
- Do, D.; Ozolins, V.; Mahanti, S.D.; Lee, M.S.; Zhang, Y.S.; Wolverton, C. Physics of Bandgap Formation in Cu-Sb-Se Based Novel Thermoelectrics: The Role of Sb Valency and Cu D Levels. J. Phys. Condens. Matter 2012, 24, 415502. [Google Scholar] [CrossRef]
- Ai, L.; Ming, H.W.; Chen, T.; Chen, K.; Zhang, J.H.; Zhang, J.; Qin, X.Y.; Li, D. High Thermoelectric Performance of Cu3SbSe4 Obtained by Synergistic Modulation of Power Factor and Thermal Conductivity. ACS Appl. Energy Mater. 2022, 5, 13070–13078. [Google Scholar] [CrossRef]
- Wang, B.Y.; Zheng, S.Q.; Chen, Y.; Wang, Q.; Li, Z.; Wu, Y.; Li, J.; Mu, Y.; Xu, S.; Liang, J. Realizing Ultralow Thermal Conductivity in Cu3SbSe4 via All-Scale Phonon Scattering by Co-Constructing Multiscale Heterostructure and Iiib Element Doping. Mater. Today Energy 2021, 19, 100620. [Google Scholar] [CrossRef]
- Li, C.; Song, H.L.; Cheng, Y.; Qi, R.J.; Huang, R.; Cui, C.Q.; Wang, Y.F.; Zhang, Y.; Miao, L. Highly Suppressed Thermal Conductivity in Diamond-Like Cu2SnS3 by Dense Dislocation. ACS Appl. Energy Mater. 2021, 4, 8728–8733. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, X.; Chen, L.; Zhang, Z.; Qi, N.; Wu, Y.; Chen, Z.; Tang, X. The Reduction of Thermal Conductivity in Cd and Sn Co-Doped Cu3SbSe4-Based Composites with a Secondary-Phase Cdse. J. Mater. Sci. 2020, 56, 4727–4740. [Google Scholar] [CrossRef]
- Sharma, S.D.; Bayikadi, K.; Raman, S.; Neeleshwar, S. Synergistic Optimization of Thermoelectric Performance in Earth-Abundant Cu2ZnSnS4 by Inclusion of Graphene Nanosheets. Nanotechnology 2020, 31, 365402. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yu, L.H.; Yang, J.; Wang, M.Y.; Shao, H.C.; Wang, J.L.; Shi, Z.Q.; Wan, N.; Hussain, S.; Qiao, G.J.; et al. Enhancing Thermoelectric and Mechanical Properties of P-Type Cu3SbSe4-Based Materials via Embedding Nanoscale Sb2Se3. Mater. Chem. Phys. 2022, 292, 126669. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Liang, X.L.; Dong, D.M.; Zhang, K.R.; Li, Z.; Song, J.M. To Improve the Thermoelectric Properties of Cu2GeSe3 via Gese Compensatory Compositing Strategy. J. Alloys Compd. 2022, 921, 166181. [Google Scholar] [CrossRef]
- Sun, F.H.; Dong, J.F.; Tang, H.; Zhuang, H.L.; Li, J.F. ZnO-Nanoparticle-Dispersed Cu11.5Ni0.5Sb4S13−δ Tetrahedrite Composites with Enhanced Thermoelectric Performance. J. Electron. Mater. 2018, 48, 1840–1845. [Google Scholar] [CrossRef]
- Sun, F.H.; Dong, J.F.; Tang, H.; Shang, P.P.; Zhuang, H.L.; Hu, H.; Wu, C.F.; Pan, Y.; Li, J.F. Enhanced Performance of Thermoelectric Nanocomposites Based on Cu12Sb4S13 Tetrahedrite. Nano Energy 2019, 57, 835–841. [Google Scholar] [CrossRef]
- Hu, H.H.; Sun, F.H.; Dong, J.F.; Zhuang, H.L.; Cai, B.; Pei, J.; Li, J.F. Nanostructure Engineering and Performance Enhancement in Fe2O3-Dispersed Cu12Sb4S13 Thermoelectric Composites with Earth-Abundant Elements. ACS Appl. Mater. Interfaces 2020, 12, 17852–17860. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, A.R.; Srinivasan, B.; Tonquesse, S.L.; Singh, S.; Soelami, N.; Yuliarto, B.; Berthebaud, D.; Mori, T. Physical Insights on the Lattice Softening Driven Mid-Temperature Range Thermoelectrics of Ti/Zr-Inserted SnTe-an Outlook Beyond the Horizons of Conventional Phonon Scattering and Excavation of Heikes’ Equation for Estimating Carrier Properties. Adv. Energy Mater. 2021, 11, 2101122. [Google Scholar] [CrossRef]
- Bo, L.; Zhang, R.P.; Zhao, H.Y.; Hou, Y.B.; Wang, X.L.; Zhu, J.L.; Zhao, L.H.; Zuo, M.; Zhao, D.G. Achieving High Thermoelectric Properties of Cu2Se via Lattice Softening and Phonon Scattering Mechanism. ACS Appl. Energy Mater. 2022, 5, 6453–6461. [Google Scholar] [CrossRef]
- Tan, G.J.; Hao, S.Q.; Hanus, R.C.; Zhang, X.M.; Anand, S.; Bailey, T.P.; Rettie, A.J.E.; Su, X.; Uher, C.; Dravid, V.P.; et al. High Thermoelectric Performance in SnTe-AgSbTe2 Alloys from Lattice Softening, Giant Phonon-Vacancy Scattering, and Valence Band Convergence. ACS Energy Lett. 2018, 3, 705–712. [Google Scholar] [CrossRef]
- Hanus, R.; Agne, M.T.; Rettie, A.J.E.; Chen, Z.W.; Tan, G.J.; Chung, D.Y.; Kanatzidis, M.G.; Pei, Y.Z.; Voorhees, P.W.; Snyder, G.J. Lattice Softening Significantly Reduces Thermal Conductivity and Leads to High Thermoelectric Efficiency. Adv. Mater. 2019, 31, e1900108. [Google Scholar] [CrossRef]
- Slade, T.J.; Anand, S.; Wood, M.; Male, J.P.; Imasato, K.; Cheikh, D.; Al Malki, M.M.; Agne, M.T.; Griffith, K.J.; Bux, S.K.; et al. Charge-Carrier-Mediated Lattice Softening Contributes to High zT in Thermoelectric Semiconductors. Joule 2021, 5, 1168–1182. [Google Scholar] [CrossRef]
- Pöhls, J.H.; MacIver, M.; Chanakian, S.; Zevalkink, A.; Tseng, Y.C.; Mozharivskyj, Y. Enhanced Thermoelectric Efficiency through Li-Induced Phonon Softening in CuGaTe2. Chem. Mater. 2022, 34, 8719–8728. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhao, K.P.; Chen, H.Y.; Ren, Q.Y.; Yue, Z.M.; Wei, T.R.; Qiu, P.F.; Chen, L.D.; Shi, X. Entropy Engineering Induced Exceptional Thermoelectric and Mechanical Performances in Cu2–yAgyTe1−2xSxSe. Acta Mater. 2022, 224, 117512. [Google Scholar] [CrossRef]
- Cai, J.F.; Yang, J.X.; Liu, G.Q.; Wang, H.X.; Shi, F.F.; Tan, X.J.; Ge, Z.H.; Jiang, J. Ultralow Thermal Conductivity and Improved Zt of CuInTe2 by High-Entropy Structure Design. Mater. Today Phys. 2021, 18, 100394. [Google Scholar] [CrossRef]
- Fan, Y.J.; Wang, G.Y.; Zhang, B.; Li, Z.; Wang, G.W.; Zhang, X.; Huang, Y.L.; Chen, K.S.; Gu, H.S.; Lu, X.; et al. Synergistic Effect of CuInSe2 Alloying on Enhancing the Thermoelectric Performance of CuSnSe3 Compounds. J. Mater. Chem. A 2020, 8, 21181–21188. [Google Scholar] [CrossRef]
- Qiu, P.F.; Qin, Y.T.; Zhang, Q.H.; Li, R.X.; Yang, J.; Song, Q.F.; Tang, Y.S.; Bai, S.Q.; Shi, X.; Chen, L.D. Intrinsically High Thermoelectric Performance in AgInSe2 N-Type Diamond-Like Compounds. Adv. Sci. 2018, 5, 1700727. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Bo, L.; Zhu, J.; Zhao, D. Copper-Based Diamond-like Thermoelectric Compounds: Looking Back and Stepping Forward. Materials 2023, 16, 3512. https://doi.org/10.3390/ma16093512
Wang W, Bo L, Zhu J, Zhao D. Copper-Based Diamond-like Thermoelectric Compounds: Looking Back and Stepping Forward. Materials. 2023; 16(9):3512. https://doi.org/10.3390/ma16093512
Chicago/Turabian StyleWang, Wenying, Lin Bo, Junliang Zhu, and Degang Zhao. 2023. "Copper-Based Diamond-like Thermoelectric Compounds: Looking Back and Stepping Forward" Materials 16, no. 9: 3512. https://doi.org/10.3390/ma16093512
APA StyleWang, W., Bo, L., Zhu, J., & Zhao, D. (2023). Copper-Based Diamond-like Thermoelectric Compounds: Looking Back and Stepping Forward. Materials, 16(9), 3512. https://doi.org/10.3390/ma16093512







