Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate
Abstract
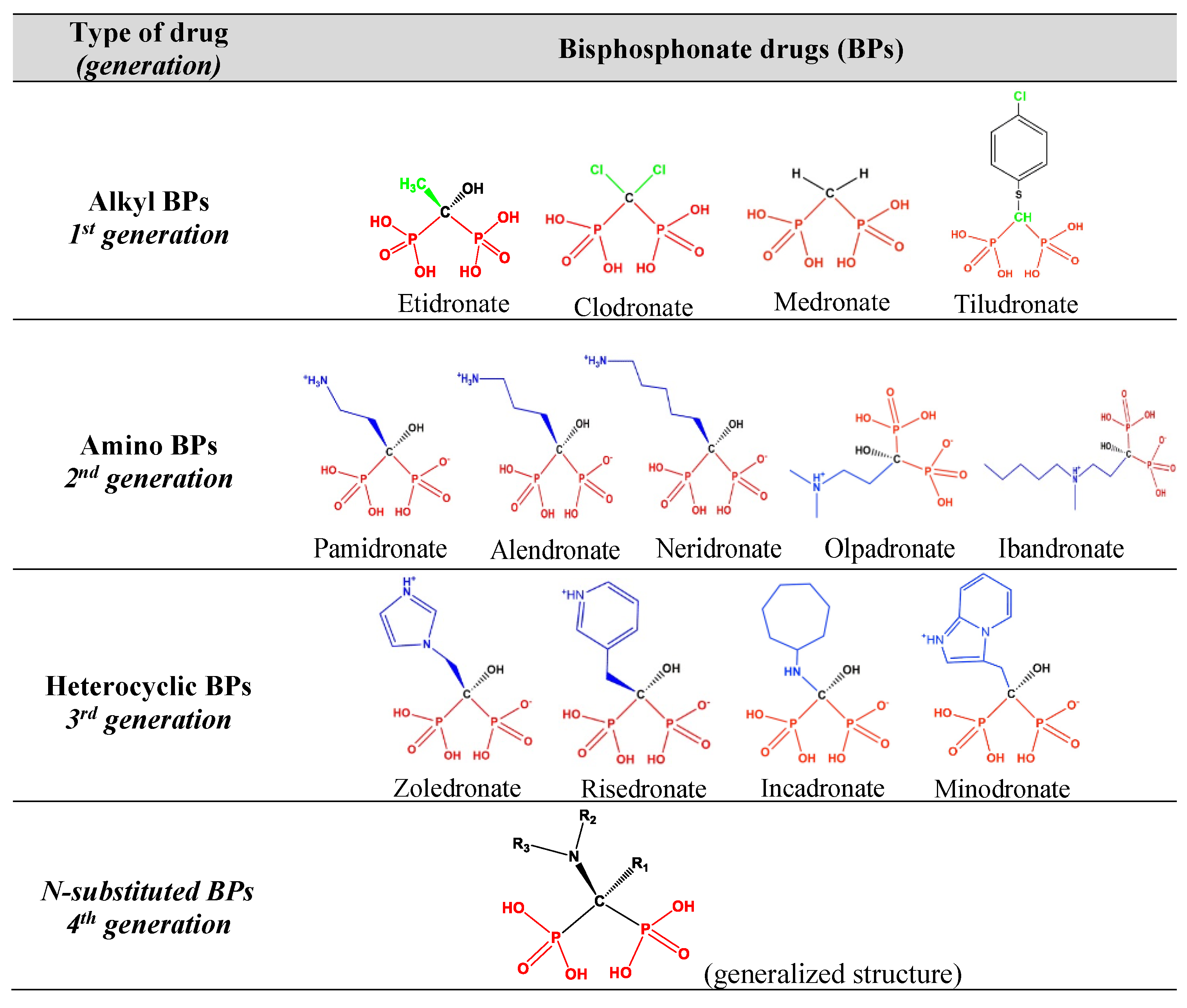
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Preparation of ETID-Loaded Silica Hydrogels
2.4. Preparation of Hydrogels Incorporating Ca2+ and Cu2+ Ions
2.5. Preparation of Silane-Grafted Hydrogels
2.6. General Drug Release Protocol
2.7. Kinetic and Statistical Analysis
2.7.1. Sink Conditions
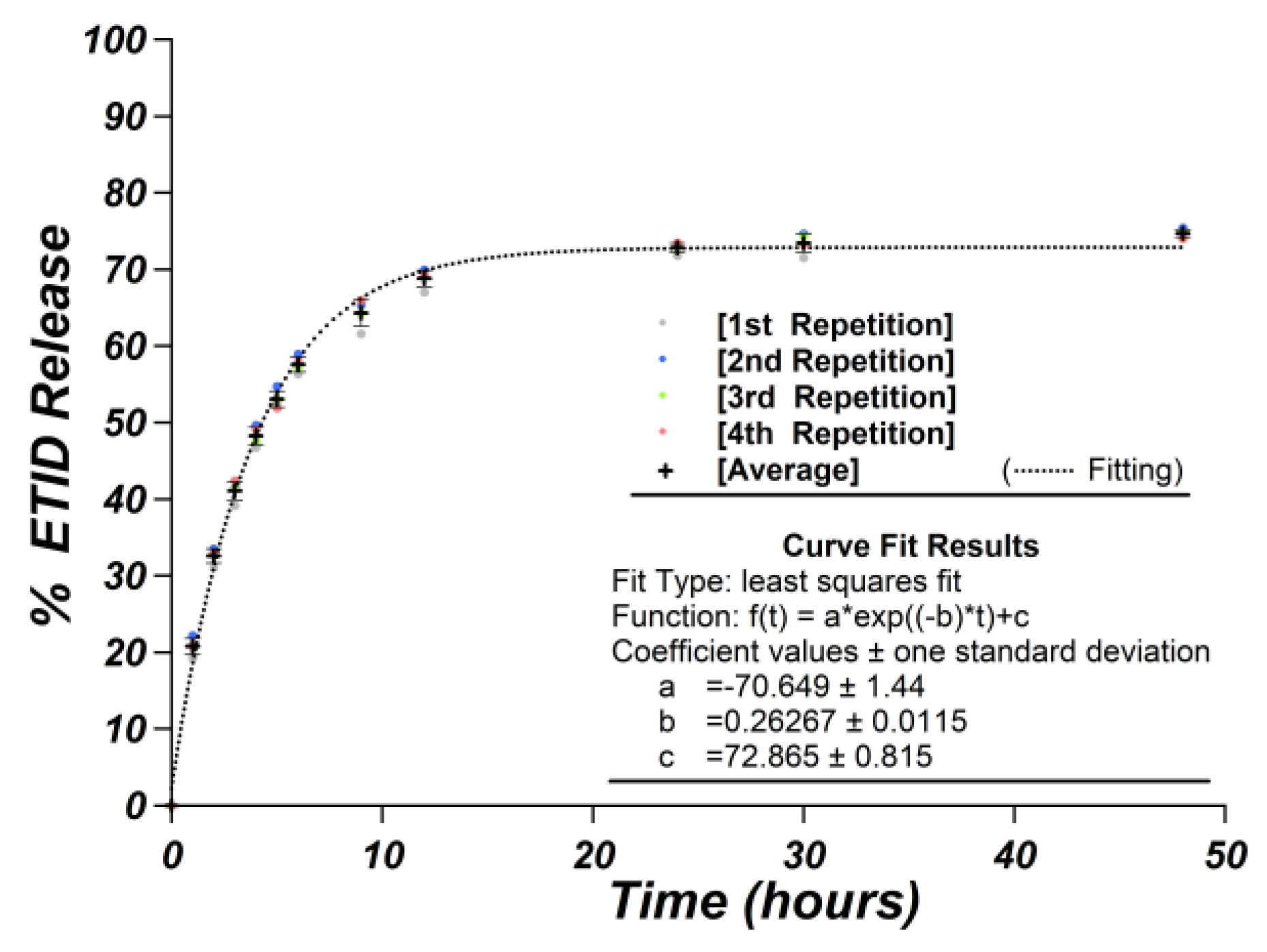
2.7.2. Repeatability and Reproducibility of the Release Experiments
2.7.3. Calculation of Initial Rates
2.7.4. Kinetic Models
3. Results
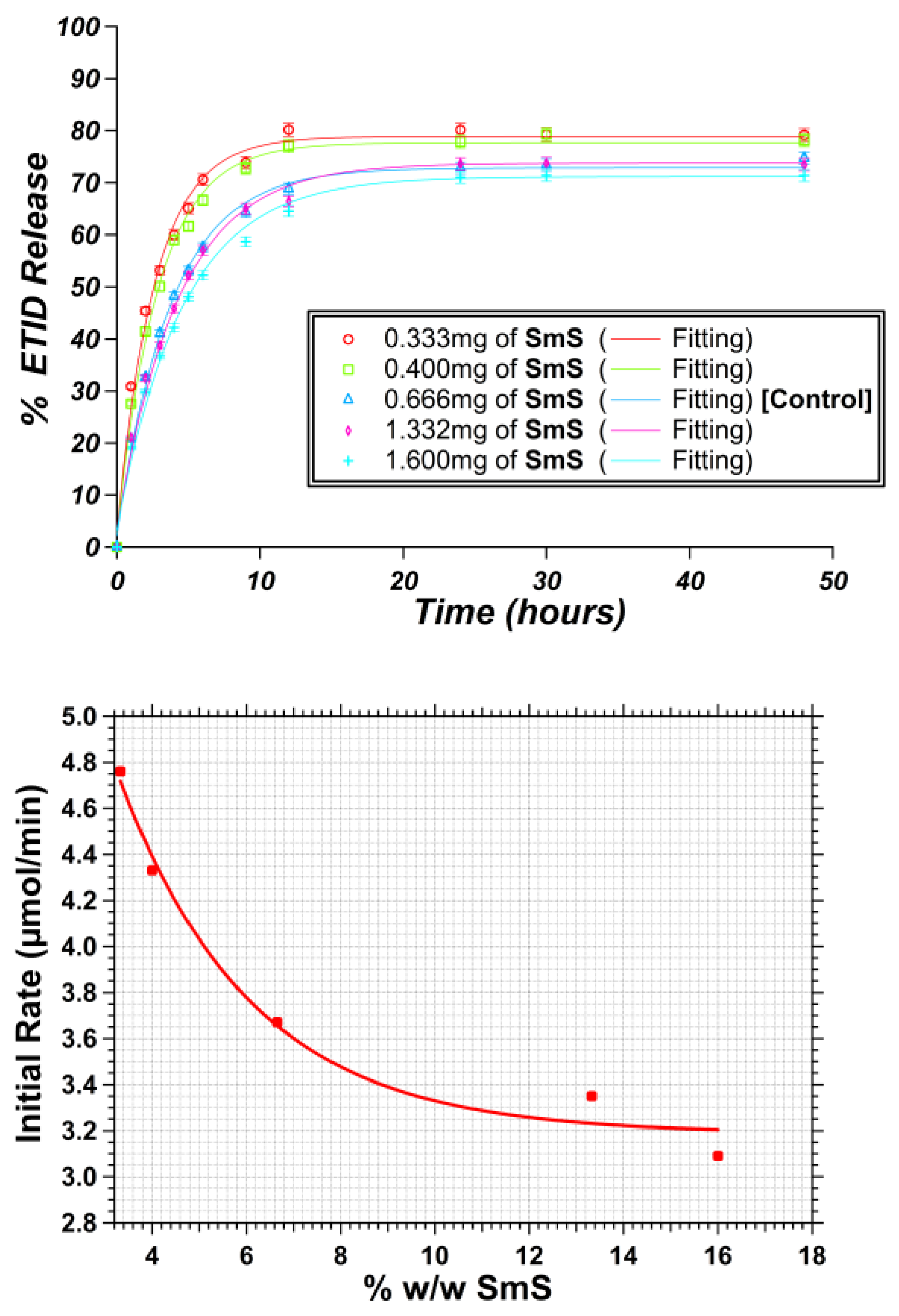
3.1. Gel Density: Impact of Silicate Concentration on the ETID Release Profile
3.2. Metal-Loaded Hydrogels
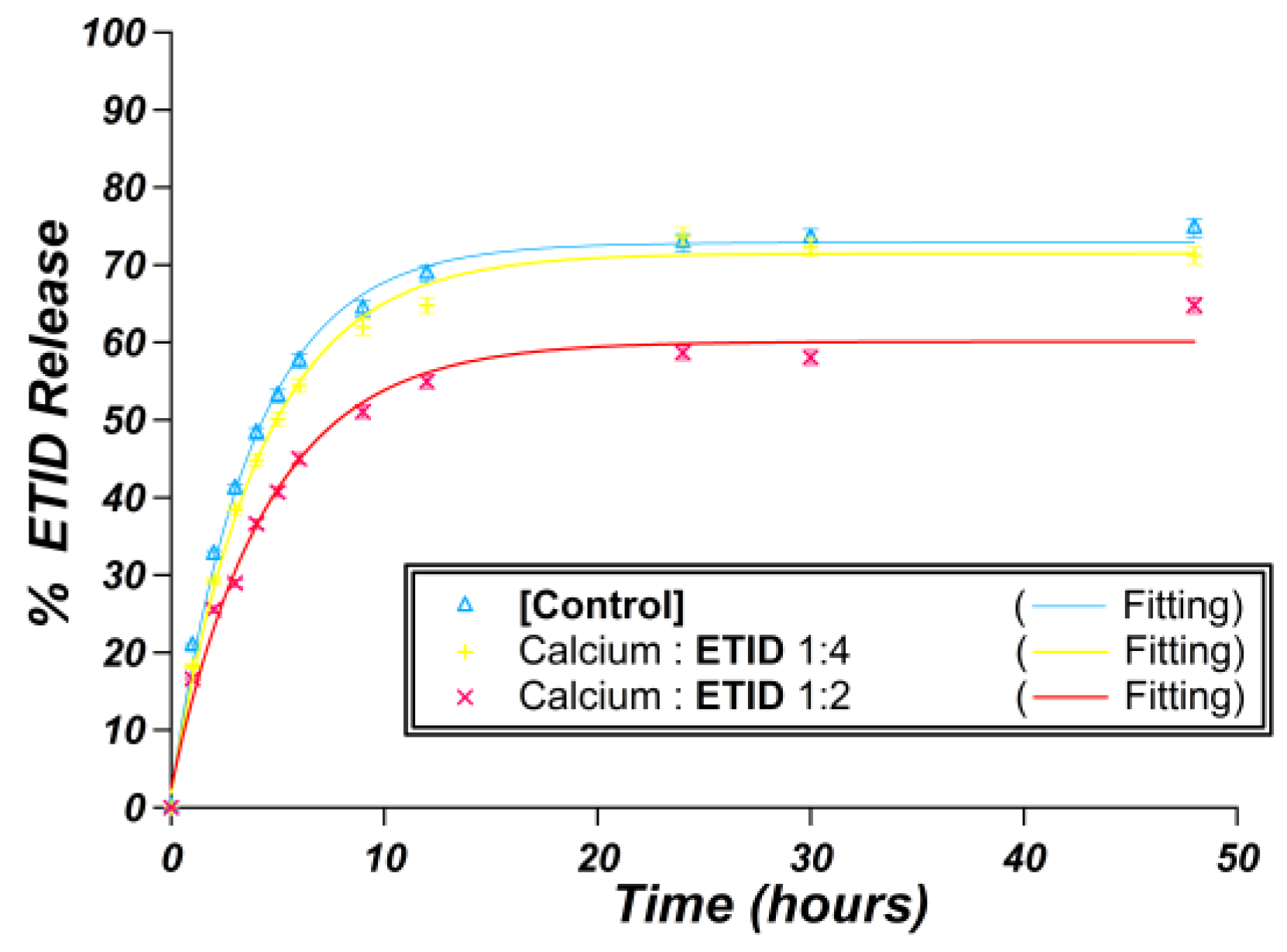
3.2.1. Ca2+-Loaded Hydrogels
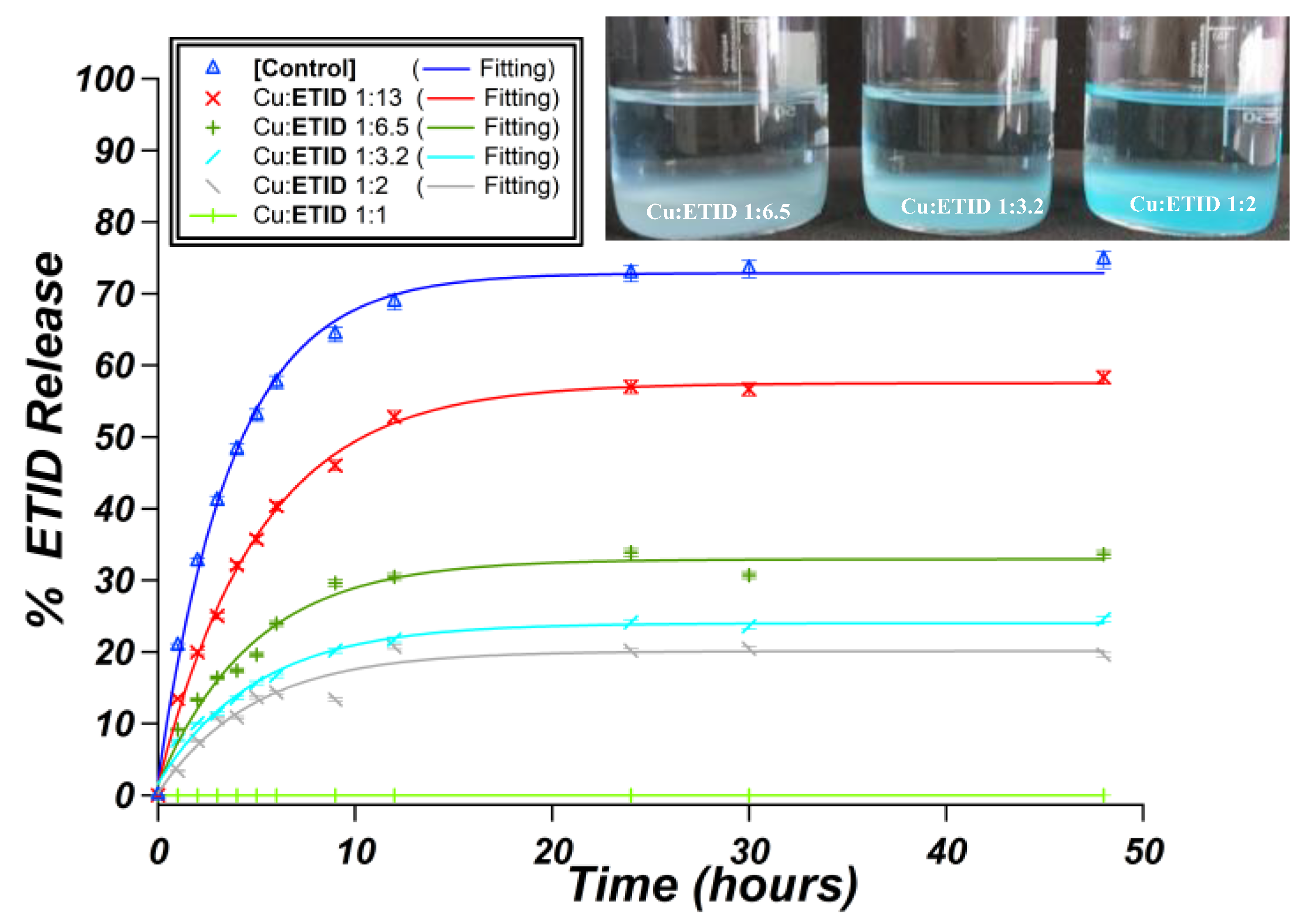
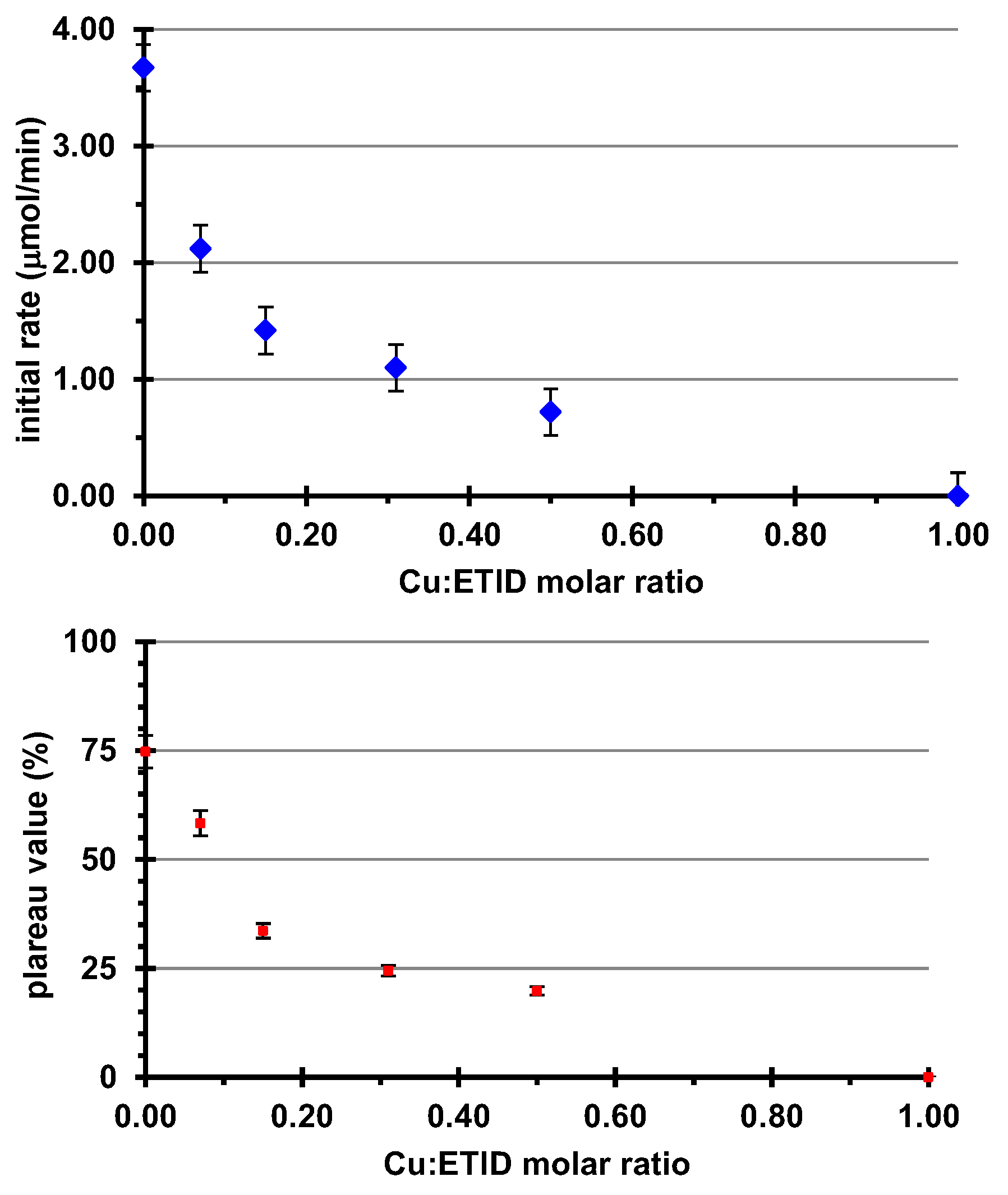
3.2.2. Cu2+-Loaded Hydrogels
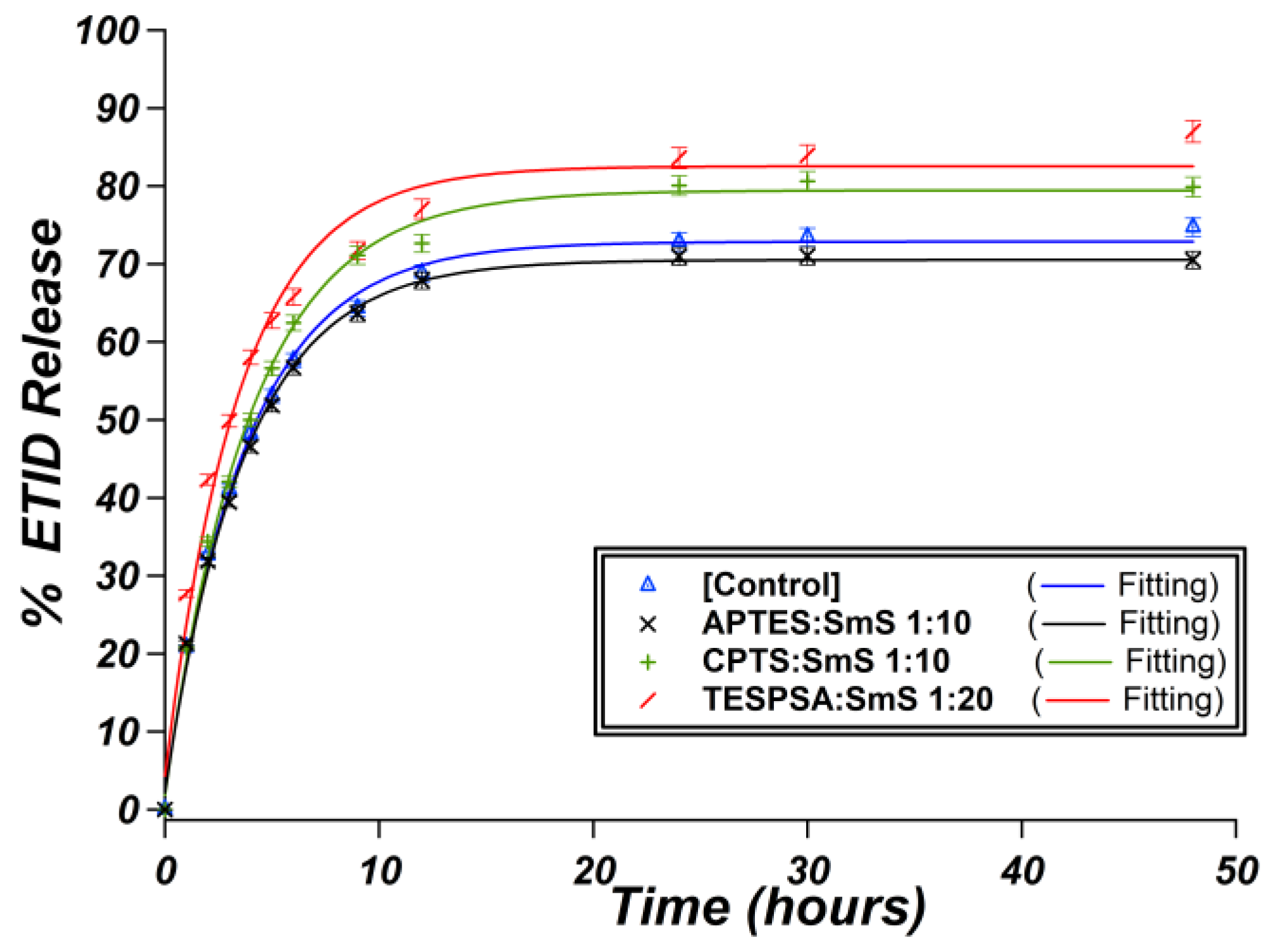
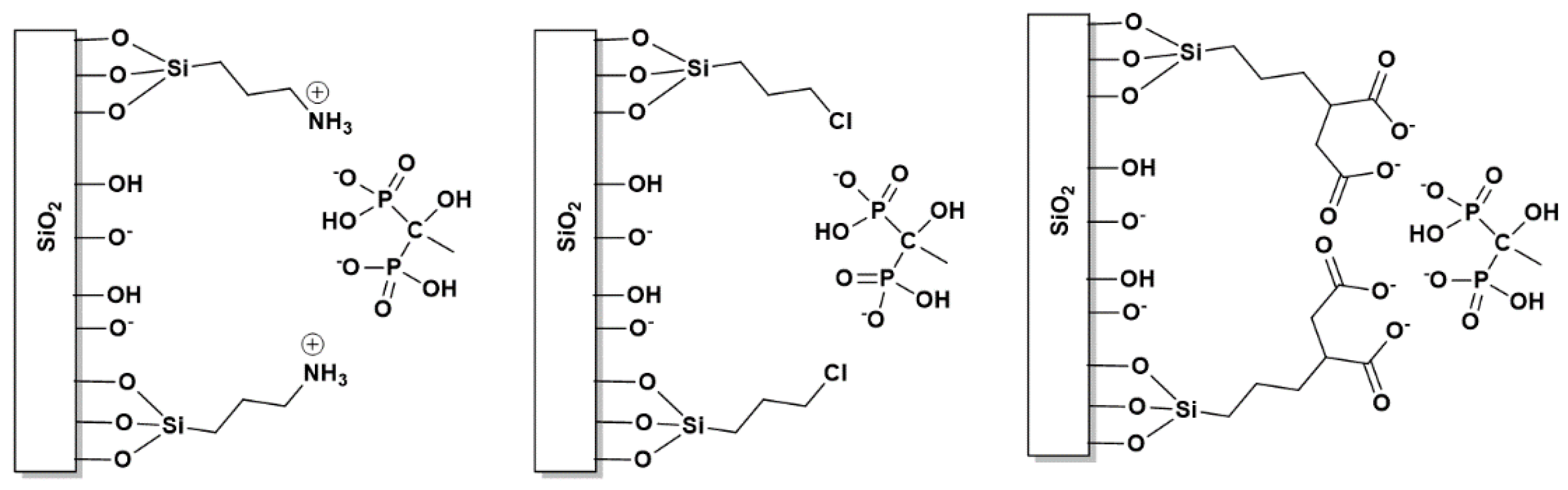
3.3. Surface Functionalization of ETID-Loaded Hydrogels by Silane Agents
3.3.1. The Case of (3-Aminopropyl)triethoxysilane (APTES)
3.3.2. The Case of (3-Chloropropyl)trimethoxysilane (CPTS)
3.3.3. The Case of (3-Triethoxysilyl)propylsuccinic Anhydride (TESPSA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, L.; Liu, J.; Li, F.; Wang, L.; Li, D.; Guo, B.; He, X.; Jiang, F.; Liang, C.; Liu, B.; et al. Targeted delivery systems for molecular therapy in skeletal disorders. Int. J. Mol. Sci. 2016, 17, 428. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Criseno, S. Osteoporosis. In Advanced Practice in Endocrinology Nursing; Llahana, S., Follin, C., Yedinak, C., Grossman, A., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1005–1035. [Google Scholar]
- Fuggle, N.R.; Curtis, E.M.; Ward, K.A.; Harvey, N.C.; Dennison, E.M.; Cooper, C. Fracture prediction, imaging and screening in osteoporosis. Nat. Rev. Endocrinol. 2019, 15, 535–547. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar]
- Poole, K.E.; Compston, J.E. Bisphosphonates in the treatment of osteoporosis. BMJ 2012, 344, e3211. [Google Scholar] [CrossRef]
- Karlic, H.; Varga, F. Mevalonate pathway. In Encyclopaedia of Cancer; Boffetta, P., Hainaut, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 445–457. [Google Scholar]
- Chen, C.-H.; Lin, S.-Y.; Lu, Y.-M.; Chou, S.-H.; Lee, T.-C. Medication for osteoporosis prevention and treatment. In Osteoporosis of the spine: Asian perspectives; Chen, P.-Q., Lin, R.-M., Tsai, K.-S., Eds.; Words Scientific: Singapore, 2021; pp. 125–156. [Google Scholar]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological management of osteoporosis in postmenopausal women: An Endocrine Society guideline update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, G.; Rizzati, M.; Salani, L.; Giganti, M. Postmenopausal osteoporosis: Risk evaluation and treatment options. Minerva Obstet. Gynecol. 2021, 73, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Chang, C.T.; Muo, C.H.; Tsai, C.H.; Shen, Y.F.; Wu, C.Z. Impact of bisphosphonate-related osteonecrosis of the jaw on osteoporotic patients after dental extraction: A population-based cohort study. PLoS ONE 2015, 10, e0120756. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, M.; Marcotte, M.; Laurin, D.; Furrer, D.; Vedel, I.; Tourigny, A.; Giguère, A.; Carmichael, P.-H.; Martines, R.; Morais, J.; et al. Discontinuation of bisphosphonates in seniors: A systematic review on health outcomes. Arch. Osteoporos. 2021, 16, 133. [Google Scholar] [CrossRef]
- Pazianas, M.; Abrahamsen, B.; Ferrari, S.; Russell, R.G.G. Eliminating the need for fasting with oral administration of bisphosphonates. Ther. Clin. Risk Manag. 2013, 9, 395–402. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Tsourdi, E.; Tabacco, G.; Naciu, A.M.; Napoli, N.; Vescini, F.; Palermo, A. The impact of antiosteoporotic drugs on glucose metabolism and fracture risk in diabetes: Good or bad news? J. Clin. Med. 2021, 10, 996. [Google Scholar]
- Weivoda, M.M.; Chew, C.K.; Monroe, D.G.; Farr, J.N.; Atkinson, E.J.; Geske, J.R.; Eckhardt, B.; Thicke, B.; Ruan, M.; Tweed, A.J.; et al. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 2020, 11, 87. [Google Scholar] [CrossRef]
- Wen, Q.; Dong, Y.; Demirci, U.; Khademhosseini, A. Gels Handbook: Fundamentals, Properties and Applications; Words Scientific: Singapore, 2016. [Google Scholar]
- Rao, K.M.; Rao, K.S.V.K.; Ha, C.-S. Stimuli responsive poly(vinyl caprolactam) gels for biomedical applications. Gels 2016, 2, 6. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Carvajal-Millan, E. Tailor-made polysaccharide-based hydrogels for biomedical applications. In Tailor-Made Polysaccharides in Biomedical Applications; Nayak, A., Hasnain, M.S., Aminabhavi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–132. [Google Scholar]
- Aydın, D.; Alipour, M.; Kizilel, S. Design of stimuli-responsive drug delivery hydrogels. In Functional Hydrogels in Drug Delivery: Key Features and Future Perspectives; Spizzirri, U.G., Cirillo, G., Eds.; CRS Press: Boca Raton, FL, USA, 2017; pp. 1–23. [Google Scholar]
- Wang, L.; Zhang, M.; Yang, Z.; Xu, B. The first pamidronate containing polymer and copolymer. Chem. Commun. 2006, 26, 2795–2797. [Google Scholar]
- Huang, W.; Wang, Y.; Ren, L.; Du, C.; Shi, X. A novel PHBV/HA microsphere releasing system loaded with alendronate. Mater. Sci. Eng. C 2009, 29, 2221–2225. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Kumar, A.; Khandelwal, V.K.M.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Yun, Y.-P.; Lee, H.J.; Hwang, Y.-S.; Kwon, I.K.; Lee, S.C. In situ fabrication of alendronate-loaded calcium phosphate microspheres: Controlled release for inhibition of osteoclastogenesis. J. Control. Release 2010, 147, 45–53. [Google Scholar] [CrossRef]
- Johnston, T.P.; Webb, C.L.; Schoen, F.J.; Levy, R.J. Site-specific delivery of ethanehydroxy diphosphonate from refillable polyurethane reservoirs to inhibit bioprosthetic tissue calcification. J. Control. Release 1993, 25, 227–240. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Pinto, M.; Barreiro, S.; Fernandes, C.; Mendes, R.F.; Lavrador, P.; Gaspar, V.M.; Mano, J.F.; Borges, F.; Remião, F.; et al. Coordination compounds as multi-delivery systems for osteoporosis. ACS Appl. Mater. Interfaces 2021, 13, 35469–35483. [Google Scholar] [CrossRef] [PubMed]
- Vassaki, M.; Papathanasiou, K.E.; Hadjicharalambous, C.; Chandrinou, D.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Self-sacrificial MOFs for ultra-long controlled release of bisphosphonate anti-osteoporotic drugs. Chem. Commun. 2020, 56, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Vassaki, M.; Kotoula, C.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Calcium and strontium coordination polymers as controlled delivery systems of the anti-osteoporosis drug risedronate and the augmenting effect of solubilizers. Appl. Sci. 2021, 11, 11383. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.-T.; Liao, R.; et al. Microfluidics-assisted fabrication of gelatin-silica core-shell microgels for injectable tissue constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Schneid, A.; Albuquerque, L.J.C.; Mondo, G.B.; Ceolin, M.; Picco, A.S.; Cardoso, M.B. Colloidal stability and degradability of silica nanoparticles in biological fluids: A review. J. Sol. Gel. Sci. Technol. 2022, 102, 41–62. [Google Scholar]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar]
- Clemments, A.M.; Botella, P.; Landry, C.C. Protein adsorption from biofluids on silica nanoparticles: Corona analysis as a function of particle diameter and porosity. ACS Appl. Mater. Interfaces 2015, 7, 21682–21689. [Google Scholar] [CrossRef]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; Maccuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous silica nanoparticles: Properties and strategies for enhancing clinical effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-property relationship for different mesoporous silica nanoparticles and its drug delivery applications: A review. Front. Chem. 2022, 10, 823785. [Google Scholar]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regi, M. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J. Amer. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef]
- Papathanasiou, K.E.; Turhanen, P.; Brückner, S.I.; Brunner, E.; Demadis, K.D. Smart, programmable and responsive injectable hydrogels for controlled release of cargo osteoporosis drugs. Sci. Rep. 2017, 7, 4743. [Google Scholar] [CrossRef]
- Papathanasiou, K.E.; Vassaki, M.; Spinthaki, A.; Alatzoglou, F.-E.G.; Tripodianos, E.; Turhanen, P.; Demadis, K.D. Phosphorus chemistry: From small molecules, to polymers, to pharmaceutical and industrial applications. Pure Appl. Chem. 2019, 91, 421–441. [Google Scholar] [CrossRef]
- Papathanasiou, K.E.; Moschona, A.; Spinthaki, A.; Vassaki, M.; Demadis, K.D. Silica-based polymeric gels as platforms for delivery of phosphonate pharmaceutics. In Polymer Gels: Synthesis and Characterization; Thakur, V.K., Thakur, M.K., Eds.; Springer: Cham, Switzerland, 2018; Chapter 5; pp. 127–140. [Google Scholar]
- Belmonte-Sánchez, J.R.; Aguilera-Sáez, L.M.; Romero-González, R.; Vidal, L.M.V.; Arrebola, F.J.; Frenich, A.G. Determination of etidronic acid in vegetable-washing water by a simple and validated quantitative 31P nuclear magnetic resonance method. Microchem. J. 2019, 150, 104083. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Popov, K.; Oshchepkov, M.; Tkachenko, S.; Sergienko, V.; Oshchepkov, A. Bisphosphonates: Synthesis, structures, properties, medical and industrial applications. J. Mol. Liq. 2022, 351, 118619. [Google Scholar]
- Popov, K.; Rönkkömäki, H.; Lajunen, L.H.J. Critical evaluation of stability constants of phosphonic acids (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 1641–1677. [Google Scholar] [CrossRef]
- Stavgianoudaki, N.; Papathanasiou, K.E.; Colodrero, R.M.P.; Choquesillo-Lazarte, D.; Garcia-Ruiz, J.M.; Cabeza, A.; Aranda, M.A.G.; Demadis, K.D. Crystal engineering in confined spaces. A novel method to grow crystalline metal phosphonates in alginate gel systems. Cryst. Eng. Comm. 2012, 14, 5385–5389. [Google Scholar] [CrossRef]
- Lodhia, S.; Turner, A.; Papadaki, M.; Demadis, K.D.; Hix, G.B. Polymorphism, composition, and structural variability in topology in ID, 2D, and 3D copper phosphonocarboxylate materials. Cryst. Growth Des. 2009, 9, 1811–1822. [Google Scholar] [CrossRef]
- Demadis, K.D.; Papadaki, M.; Aranda, M.A.G.; Cabeza, A.; Olivera-Pastor, P.; Sanakis, Y. Stepwise topotactic transformations (1D to 3D) in copper carboxyphosphonate materials: Structural correlations. Cryst. Growth Des. 2010, 10, 357–364. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, E.P.C. Chemical functionalities of 3-aminopropyltriethoxy-silane for surface modification of metal oxide nanoparticles. Silicon 2022, 14, 6535–6545. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Chen, J. A flexible dry electrode based on APTES-anchored PDMS substrate for portable ECG acquisition system. Microsyst. Technol. 2016, 22, 2027–2034. [Google Scholar] [CrossRef]
- Gang, A.; Gabernet, G.; Renner, L.D.; Baraban, L.; Cuniberti, G. A simple two-step silane-based (bio-) receptor molecule immobilization without additional binding site passivation. RSC Adv. 2015, 5, 35631–35634. [Google Scholar] [CrossRef]
- Cacopardo, L. Biomaterials and biocompatibility. In Human Orthopaedic Biomechanics: Fundamentals, Devices and Applications; Innocenti, B., Galbusera, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; Chapter 18; pp. 341–359. [Google Scholar]



| Kinetics Models * | Evaluation Criteria | Parameters | |||
|---|---|---|---|---|---|
| r2 | AIC | MSC | |||
| Zero-order F = k0 ∙ t | −1.086 | 115.292 | −1.454 | k0 2.430 | - |
| First-order | 0.632 | 94.467 | 0.282 | k1 0.130 | - |
| F = 100 ∙ [1 − Exp(−k1 ∙ t)] | |||||
| Higuchi F = kH ∙ t0.5 | 0.485 | 98.521 | −0.056 | kH 15.290 | - |
| Korsmeyer–Peppas | 0.918 | 78.504 | 1.612 | kKP 33.514 | n |
| F = kKP ∙ tn | 0.237 | ||||
| Hixson-Crowell F = 100 ∙ [1 − (1 − kHC ∙ t)3] | 0.380 | 100.734 | −0.240 | kHC 0.029 | - |
| Peppas–Sahlin F = kPS(1) ∙ tm + kKPS(2) ∙ t (2 ∙ m) | 0.983 | 61.512 | 3.028 | kPS(1), kPS(2) 28.899, −2.702 | m 0.494 |
| Hopfenberg F = 100 ∙ [1− (1 − kHB∙ t)n] | 0.632 | 96.469 | 0.115 | kHB 0 | n 5143.94 |
| Baker–Lonsdale [1 − (1 − )2/3] − = kBL ∙ t | 0.808 | 88.676 | 0.931 | kBL 0.008 | - |
| Weibull | 0.978 | 64.586 | 2.772 | α, β 2.175, 0.329 | Ti |
| F = 100 ∙ {1 − Exp[−((t − Ti)β)/α]} | 0.890 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatzoglou, F.-E.G.; Vassaki, M.; Nirgianaki, K.; Tripodianos, E.; Turhanen, P.; Demadis, K.D.; Papathanasiou, K.E. Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate. Materials 2023, 16, 3379. https://doi.org/10.3390/ma16093379
Alatzoglou F-EG, Vassaki M, Nirgianaki K, Tripodianos E, Turhanen P, Demadis KD, Papathanasiou KE. Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate. Materials. 2023; 16(9):3379. https://doi.org/10.3390/ma16093379
Chicago/Turabian StyleAlatzoglou, Fanouria-Eirini G., Maria Vassaki, Kalliopi Nirgianaki, Eleftherios Tripodianos, Petri Turhanen, Konstantinos D. Demadis, and Konstantinos E. Papathanasiou. 2023. "Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate" Materials 16, no. 9: 3379. https://doi.org/10.3390/ma16093379
APA StyleAlatzoglou, F.-E. G., Vassaki, M., Nirgianaki, K., Tripodianos, E., Turhanen, P., Demadis, K. D., & Papathanasiou, K. E. (2023). Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate. Materials, 16(9), 3379. https://doi.org/10.3390/ma16093379








