Synthesis of Ion-Exchange Catalysts by Introduction of Fluorinated Ponytails into Novel Mesoporous Polymers
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of Mesoporous Polydivinylbenzene
2.2. Acylation with Perfluorobutyryl Chloride
2.3. Sulfonation with Concentrated Sulfuric Acid
2.4. Esterification of Stearic Acid
2.5. Solid-State NMR
2.6. DFT Calculations
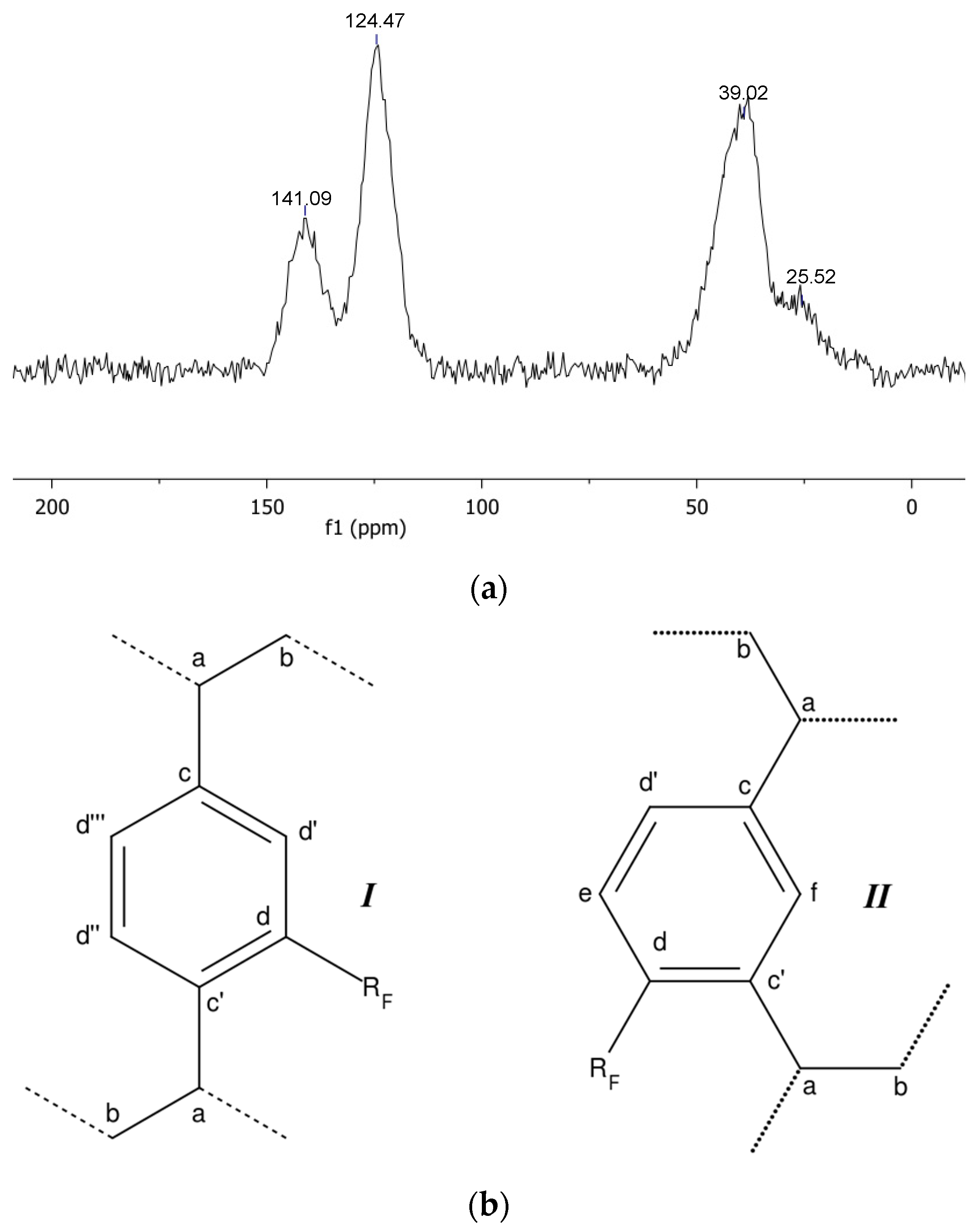
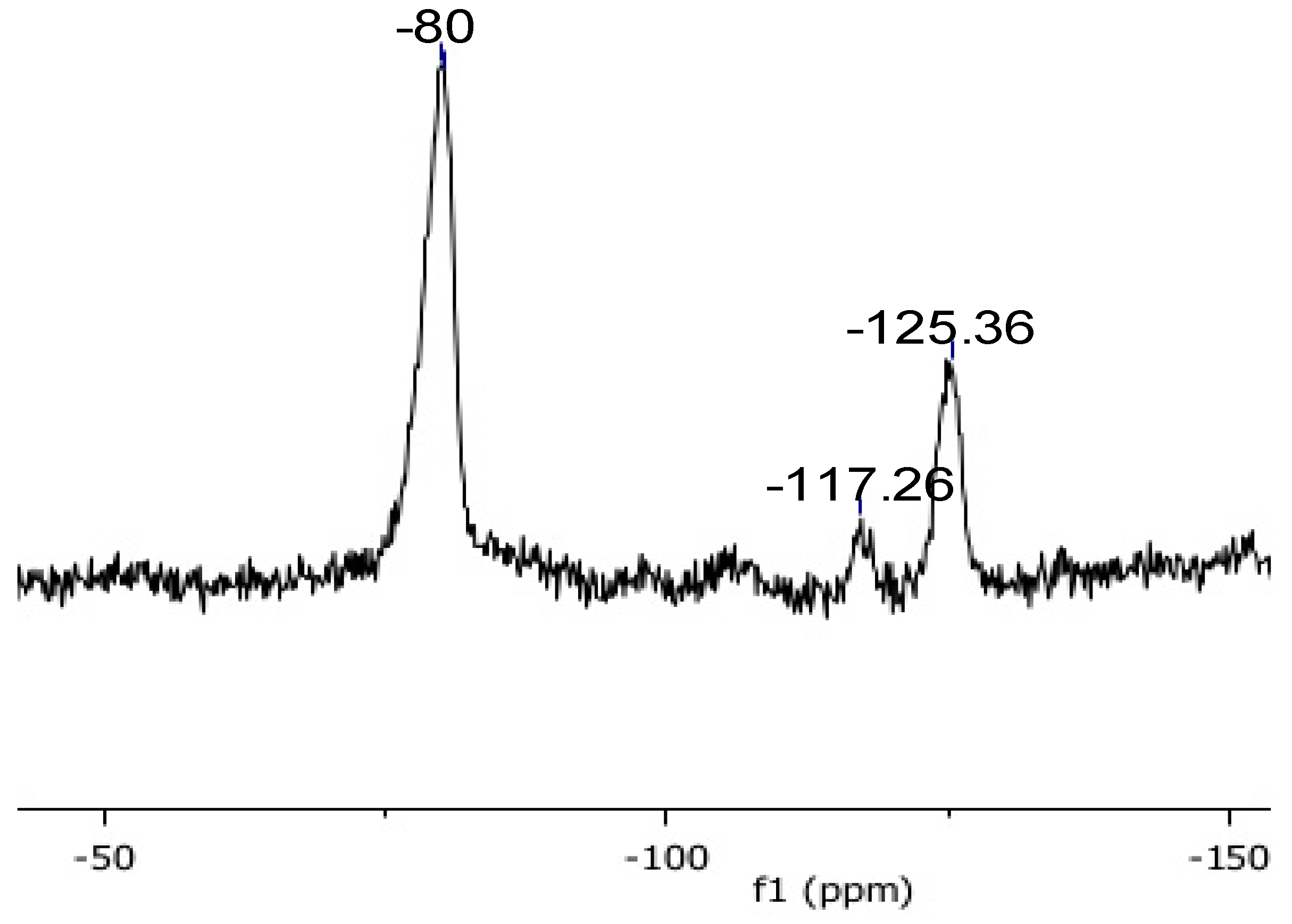
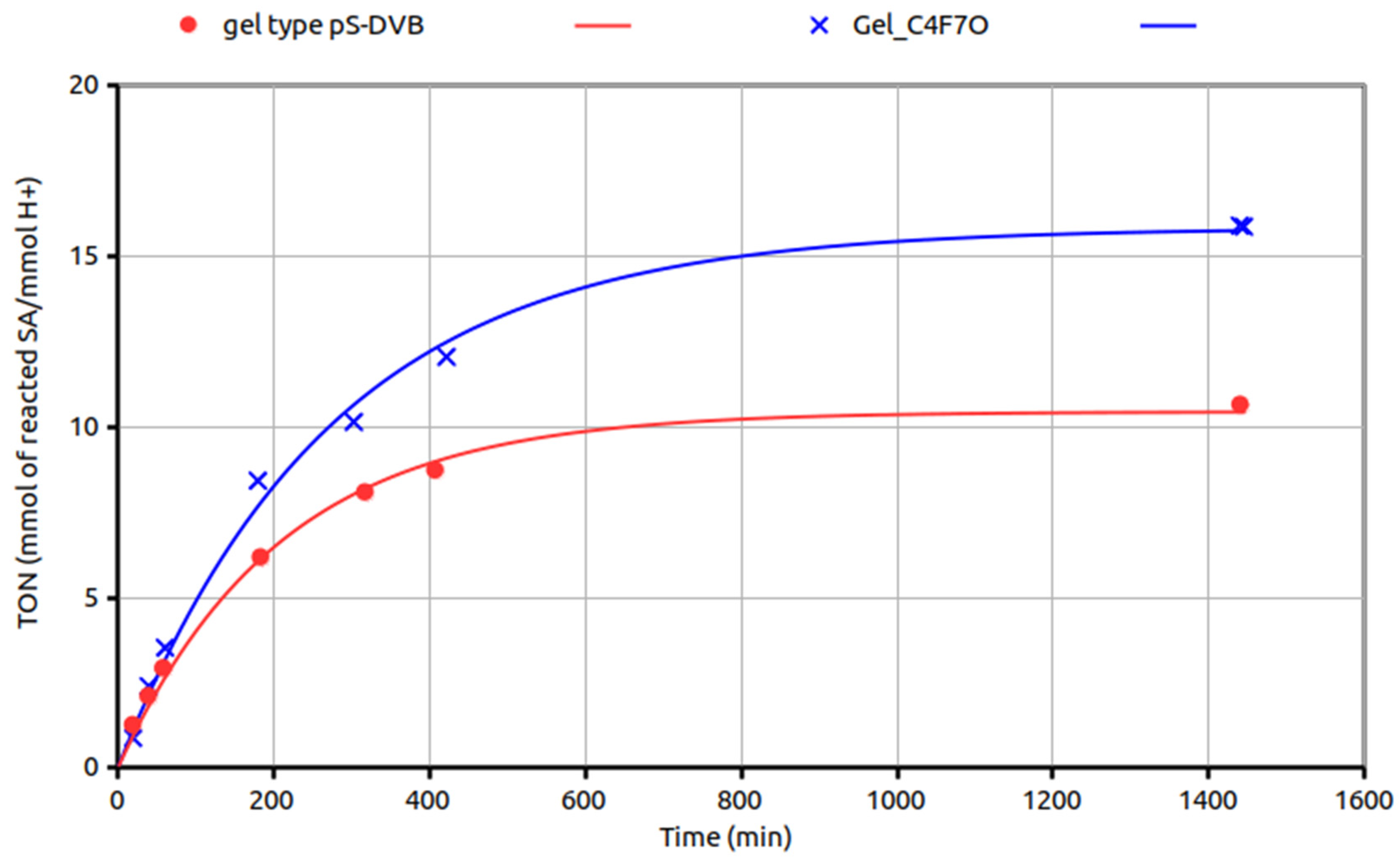
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorfner, K. Ion Exchangers; De Gruyter: Berlin, Germany; New York, NY, USA, 2011; ISBN 978-3-11-086243-0. [Google Scholar]
- Sherrington, D.C.; Hodge, P. Syntheses and Separations Using Functional Polymers; J. Wiley & Sons: Chichester, UK, 1988; ISBN 0-471-91848-2. [Google Scholar]
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.-H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; ISBN 978-3-527-30673-2. [Google Scholar]
- de Dardel, F.; Arden, T.V. Ion Exchangers. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; ISBN 978-3-527-30673-2. [Google Scholar]
- Corain, B.; Zecca, M.; Canton, P.; Centomo, P. Synthesis and Catalytic Activity of Metal Nanoclusters inside Functional Resins: An Endeavour Lasting 15 Years. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2010, 368, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Marco, Z.; Paolo, C.; Benedetto, C. CHAPTER 10—Metal Nanoclusters Supported on Cross-Linked Functional Polymers: A Class of Emerging Metal Catalysts. In Metal Nanoclusters in Catalysis and Materials Science; Corain, B., Schmid, G., Toshima, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 201–232. ISBN 978-0-444-53057-8. [Google Scholar]
- De Zan, L.; Gasparovicova, D.; Kralik, M.; Centomo, P.; Carraro, M.; Campestrini, S.; Jerabek, K.; Corain, B. Nanoclustered Palladium(0) Supported on a Gel-Type Poly-Acrylonitrile–N,N-Dimethylacrylamide–Ethylenedimethacrylate Resin: Nanostructural Aspects and Catalytic Behaviour. J. Mol. Catal. Chem. 2007, 265, 1–8. [Google Scholar] [CrossRef]
- Centomo, P.; Zecca, M.; Corain, B. Template Controlled Synthesis (TCS) of Size-Controlled Metal Nanoclusters: Preparation of Nanostructured Metals Supported by Inorganic Supports. J. Clust. Sci. 2007, 18, 947–962. [Google Scholar] [CrossRef]
- Centomo, P.; Jeřábek, K.; Canova, D.; Zoleo, A.; Maniero, A.L.; Sassi, A.; Canton, P.; Corain, B.; Zecca, M. Highly Hydrophilic Copolymers of N,N-Dimethylacrylamide, Acrylamido-2-Methylpropanesulfonic Acid, and Ethylenedimethacrylate: Nanoscale Morphology in the Swollen State and Use as Exotemplates for Synthesis of Nanostructured Ferric Oxide. Chem.—Eur. J. 2012, 18, 6632–6643. [Google Scholar] [CrossRef]
- Mbaraka, I.K.; Shanks, B.H. Design of Multifunctionalized Mesoporous Silicas for Esterification of Fatty Acid. J. Catal. 2005, 229, 365–373. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q. Solid Acid Catalysis Using Ion-Exchange Resins. Appl. Catal. Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Jerabek, K.; Hankova, L.; Holub, L.; Corain, B.; Zecca, M.; Centomo, P.; Bonato, I. Strongly Acidic Ion Exchanger Catalyst and Method of Preparing the Same. WO2012127414A1, 27 September 2012. [Google Scholar]
- Centomo, P.; Bonato, I.; Hanková, L.; Holub, L.; Jeřábek, K.; Zecca, M. Novel Ion-Exchange Catalysts for Reactions Involving Lipophilic Reagents: Perspectives in the Reaction of Esterifications of Fatty Acids with Methanol. Top. Catal. 2013, 56, 611–617. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Al-Resayes, S.I.; Nehdi, I.A. Recent Progress in Synthesis and Surface Functionalization of Mesoporous Acidic Heterogeneous Catalysts for Esterification of Free Fatty Acid Feedstocks: A Review. Energy Convers. Manag. 2017, 141, 183–205. [Google Scholar] [CrossRef]
- Riess, J.G.; Blanc, M.L. Solubility and Transport Phenomena in Perfluorochemicals Relevant to Blood Substitution and Other Biomedical Applications. Pure Appl. Chem. 1982, 54, 2383–2406. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Liu, F.; Du, Y.; Liu, S.; Ji, Y.; Yokoi, T.; Tatsumi, T.; Xiao, F.-S. Superhydrophobic Nanoporous Polymers as Efficient Adsorbents for Organic Compounds. Nano Today 2009, 4, 135–142. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bagno, A.; Rastrelli, F.; Saielli, G. Predicting 13C NMR Spectra by DFT Calculations. J. Phys. Chem. A 2003, 107, 9964–9973. [Google Scholar] [CrossRef]
- Bagno, A.; Rastrelli, F.; Saielli, G. Toward the Complete Prediction of the 1H and 13C NMR Spectra of Complex Organic Molecules by DFT Methods: Application to Natural Substances. Chem.—Eur. J. 2006, 12, 5514–5525. [Google Scholar] [CrossRef] [PubMed]
- Jerabek, K. Characterization of Swollen Polymer Gels Using Size Exclusion Chromatography. Anal. Chem. 1985, 57, 1598–1602. [Google Scholar] [CrossRef]
- Jeřábek, K.; Hanková, L.; Holub, L. Working-State Morphologies of Ion Exchange Catalysts and Their Influence on Reaction Kinetics. J. Mol. Catal. Chem. 2010, 333, 109–113. [Google Scholar] [CrossRef]
- Jeřábek, K.; Zecca, M.; Centomo, P.; Marchionda, F.; Peruzzo, L.; Canton, P.; Negro, E.; Di Noto, V.; Corain, B. Synthesis of Nanocomposites from Pd0 and a Hyper-Cross-Linked Functional Resin Obtained from a Conventional Gel-Type Precursor. Chem.—Eur. J. 2013, 19, 9381–9387. [Google Scholar] [CrossRef]
- Liu, F.; Meng, X.; Zhang, Y.; Ren, L.; Nawaz, F.; Xiao, F.-S. Efficient and Stable Solid Acid Catalysts Synthesized from Sulfonation of Swelling Mesoporous Polydivinylbenzenes. J. Catal. 2010, 271, 52–58. [Google Scholar] [CrossRef]
- Hanková, L.; Holub, L.; Jeřábek, K. Formation of Porous Polymer Morphology by Microsyneresis during Divinylbenzene Polymerization. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 774–781. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E.; Ando, I.; Korosu, H. Solid State 13C MAS NMR Studies of Anion Exchange Resins and Their Precursors. Ind. Eng. Chem. Res. 1995, 34, 2740–2749. [Google Scholar] [CrossRef]
- Ford, W.T.; Periyasamy, M.; Mohanraj, S.; McEnroe, F.J. Peak Area Measurements of Cross-Polarization Magic-Angle-Spinning 13C-NMR Spectra of Crosslinked Polystyrenes. J. Polym. Sci. Part Polym. Chem. 1989, 27, 2345–2355. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E. Quantitative Solid State 13C NMR Studies of Highly Cross-Linked Poly(Divinylbenzene) Resins. Macromolecules 1997, 30, 2868–2875. [Google Scholar] [CrossRef]
- Tanaka, S.; Matsumoto, M.; Goseki, R.; Ishizone, T.; Hirao, A. Living Anionic Polymerization of 1,4-Divinylbenzene and Its Isomers. Macromolecules 2013, 46, 146–154. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, R.; Pan, H.; Jin, X.; Qu, Y.; Wu, C.; Jiang, X. Thermal Decomposition of Some Perfluoro- and Polyfluorodiacyl Peroxides. J. Org. Chem. 1982, 47, 2009–2013. [Google Scholar] [CrossRef]


| Macro | Meso | Micro | Vp | |
|---|---|---|---|---|
| Pd-SpDVB | 10% | 81% | 9% | 0.85 |
| pDVB_C4F7O | 39% | 54% | 7% | 0.72 |
| C (%, w/w) | H (%, w/w) | Fraction of Acylated Rings (%) | ||
|---|---|---|---|---|
| b | a | |||
| Gel_C4F7O | 76.36 | 6.05 | 19 | 21 |
| pDVB_C4F7O | 78.69 | 7.05 | 10 | 13 |
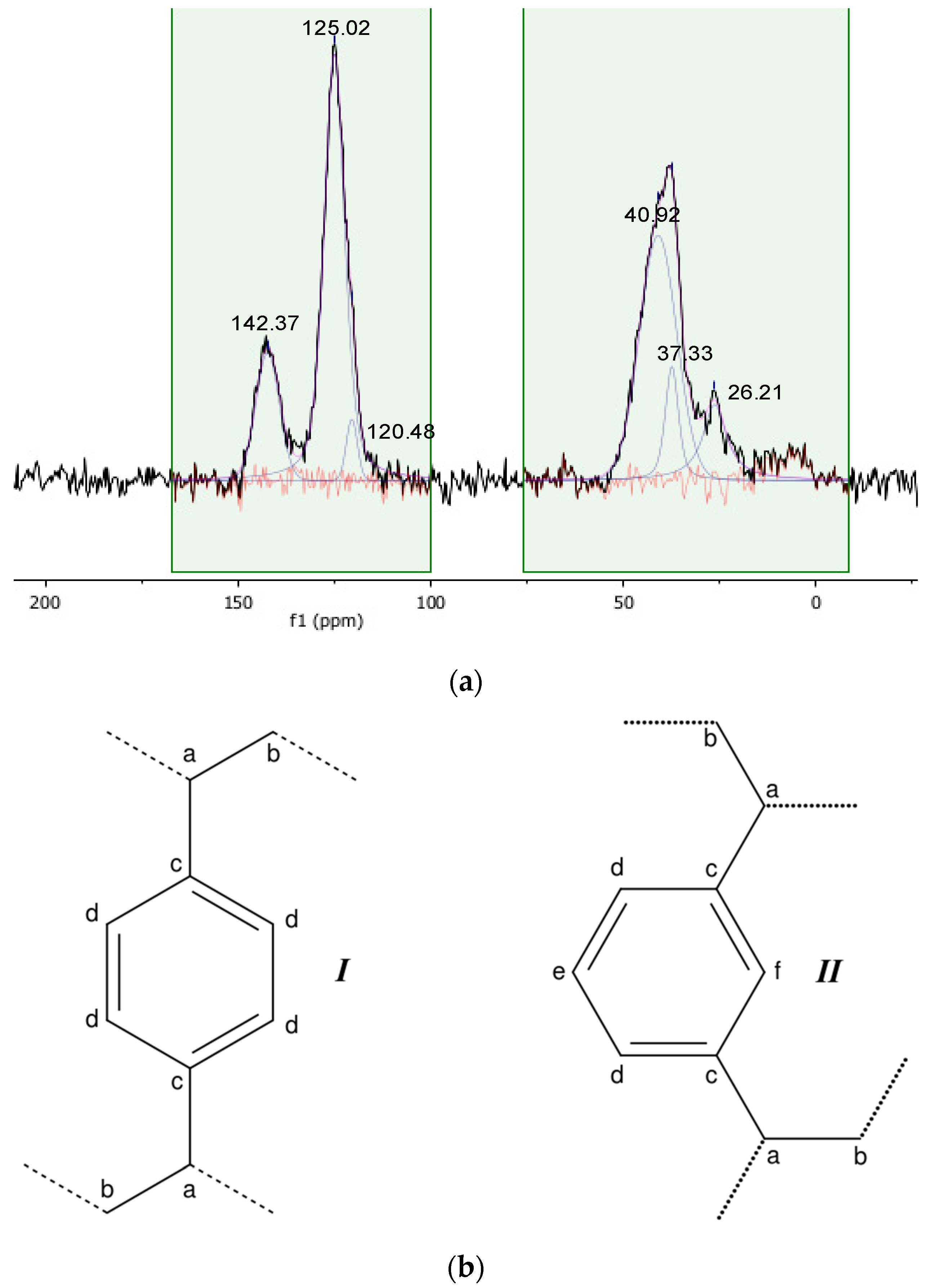
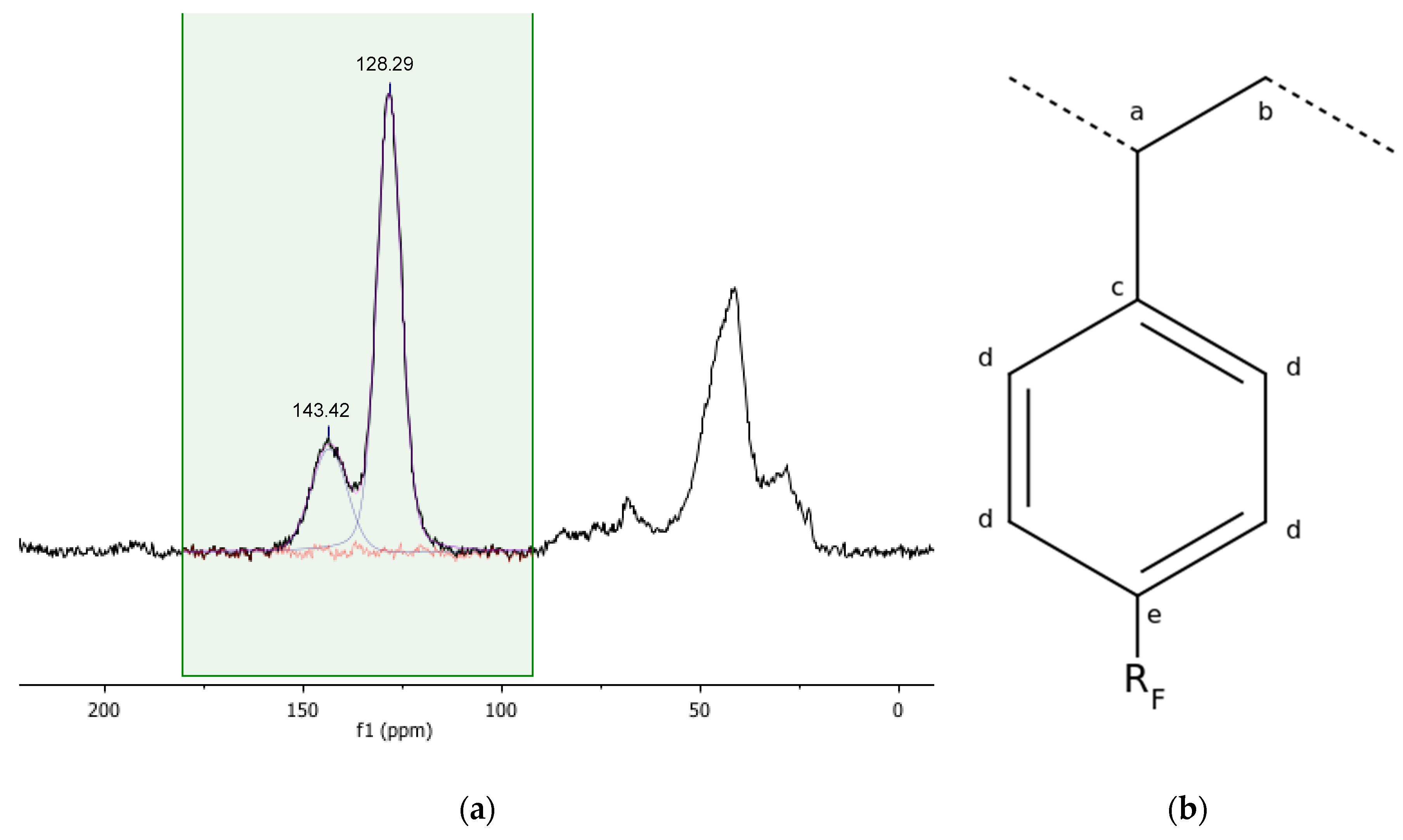
| Ca | Cb | Cc | Cd,e | |
|---|---|---|---|---|
| Gel_C4F7O | 43.03 ppm | 28.33 ppm | 143.42 ppm | 128.29 ppm |
| pDVB_C4F7O | 39.02 ppm | 25.52 ppm | 141.09 ppm | 124.47 ppm |
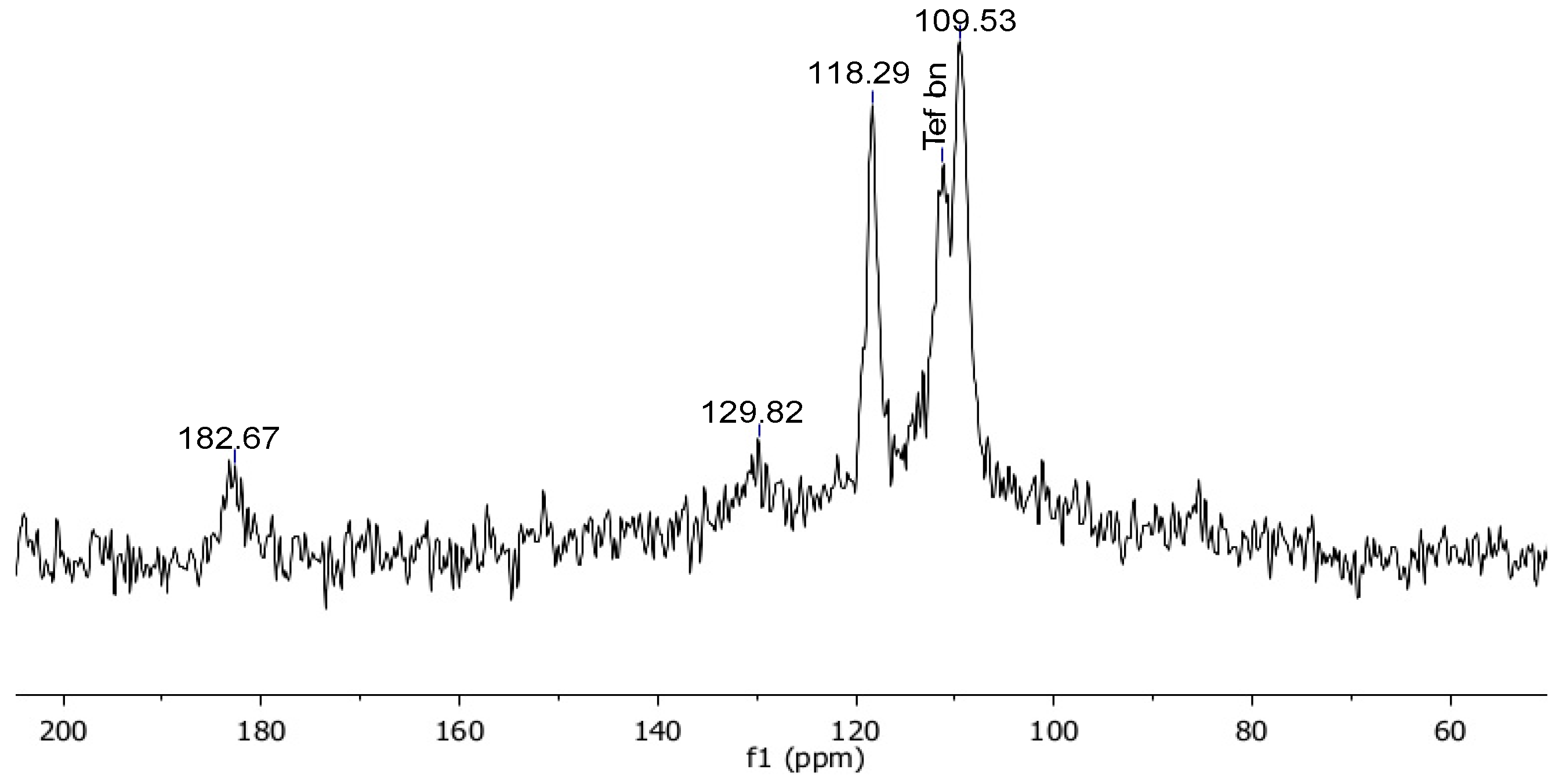
| Resin-CO-CF2-CF2-CF3 | 182.67 ppm |
| Resin-CO-CF2-CF2-CF3 | 129.82 ppm |
| Resin-CO-CF2-CF2-CF3 | 118.47 ppm |
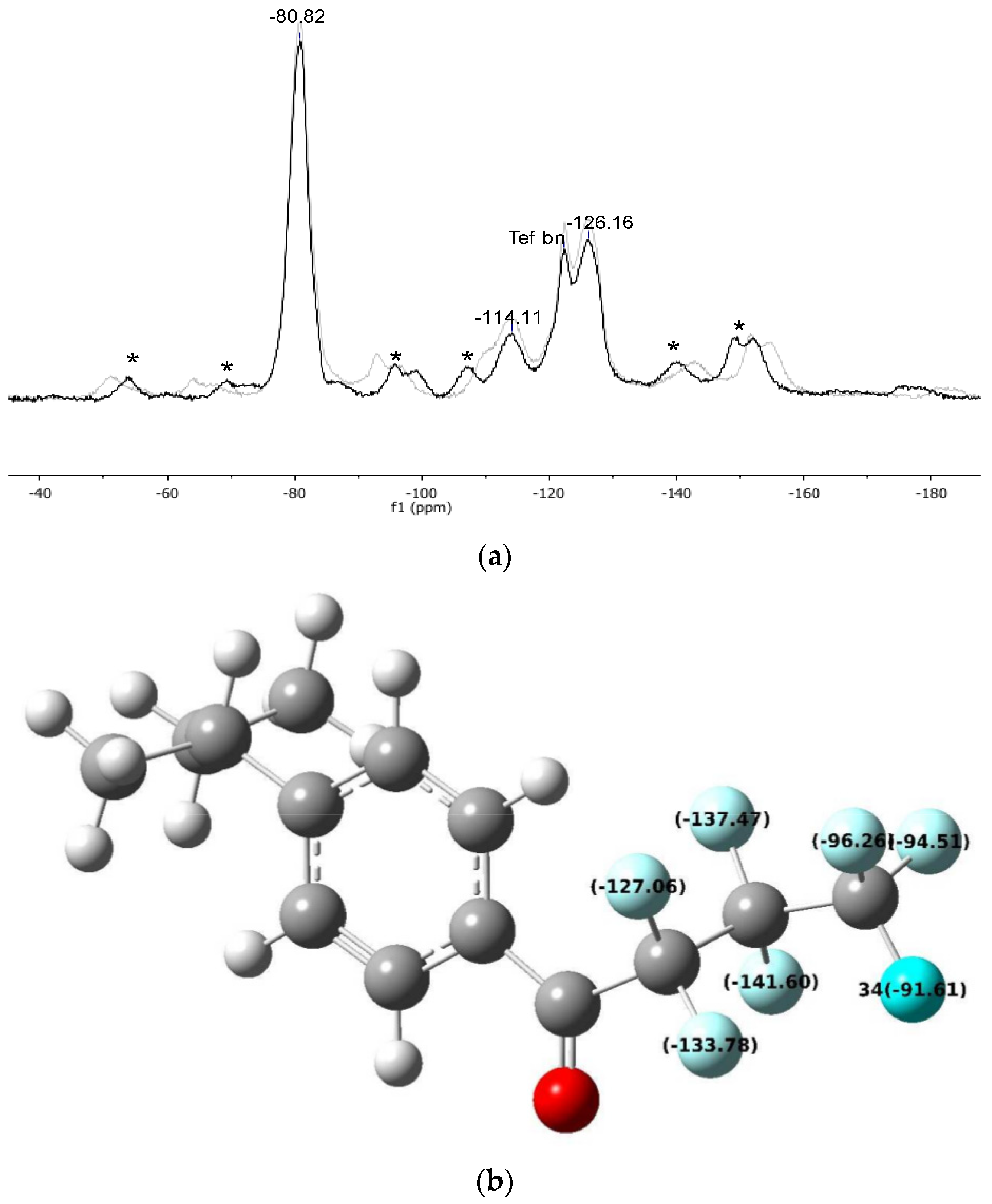
| Resin-CO-CF2-CF2-CF3 | Resin-CO-CF2-CF2-CF3 | Resin-CO-CF2-CF2-CF3 | |
|---|---|---|---|
| FBMPB a | −130.42 | −139.54 | −93.94 |
| Gel_C4F7O | −114.11 | −126.16 | −80.82 |
| pDVB_C4F7O | −117.26 | −125.36 | −80 |
| Δ (Gel_C4F7O) | 16.31 | 13.38 | 13.12 |
| Δ (pDVB_C4F7O) | 13.16 | 14.18 | 13.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Valle, C.; Sandri, F.; Zecca, M.; Rastrelli, F.; Campestrini, S.; Centomo, P. Synthesis of Ion-Exchange Catalysts by Introduction of Fluorinated Ponytails into Novel Mesoporous Polymers. Materials 2023, 16, 3808. https://doi.org/10.3390/ma16103808
Dalla Valle C, Sandri F, Zecca M, Rastrelli F, Campestrini S, Centomo P. Synthesis of Ion-Exchange Catalysts by Introduction of Fluorinated Ponytails into Novel Mesoporous Polymers. Materials. 2023; 16(10):3808. https://doi.org/10.3390/ma16103808
Chicago/Turabian StyleDalla Valle, Chiara, Francesco Sandri, Marco Zecca, Federico Rastrelli, Sandro Campestrini, and Paolo Centomo. 2023. "Synthesis of Ion-Exchange Catalysts by Introduction of Fluorinated Ponytails into Novel Mesoporous Polymers" Materials 16, no. 10: 3808. https://doi.org/10.3390/ma16103808
APA StyleDalla Valle, C., Sandri, F., Zecca, M., Rastrelli, F., Campestrini, S., & Centomo, P. (2023). Synthesis of Ion-Exchange Catalysts by Introduction of Fluorinated Ponytails into Novel Mesoporous Polymers. Materials, 16(10), 3808. https://doi.org/10.3390/ma16103808







