Inhibited and Retarded Behavior by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process
Abstract
1. Introduction
2. Materials and Methods
2.1. NO-A10 Strains
2.2. Reactive Agents
2.3. Rcv Test
2.4. Methods
3. Results and Discussion
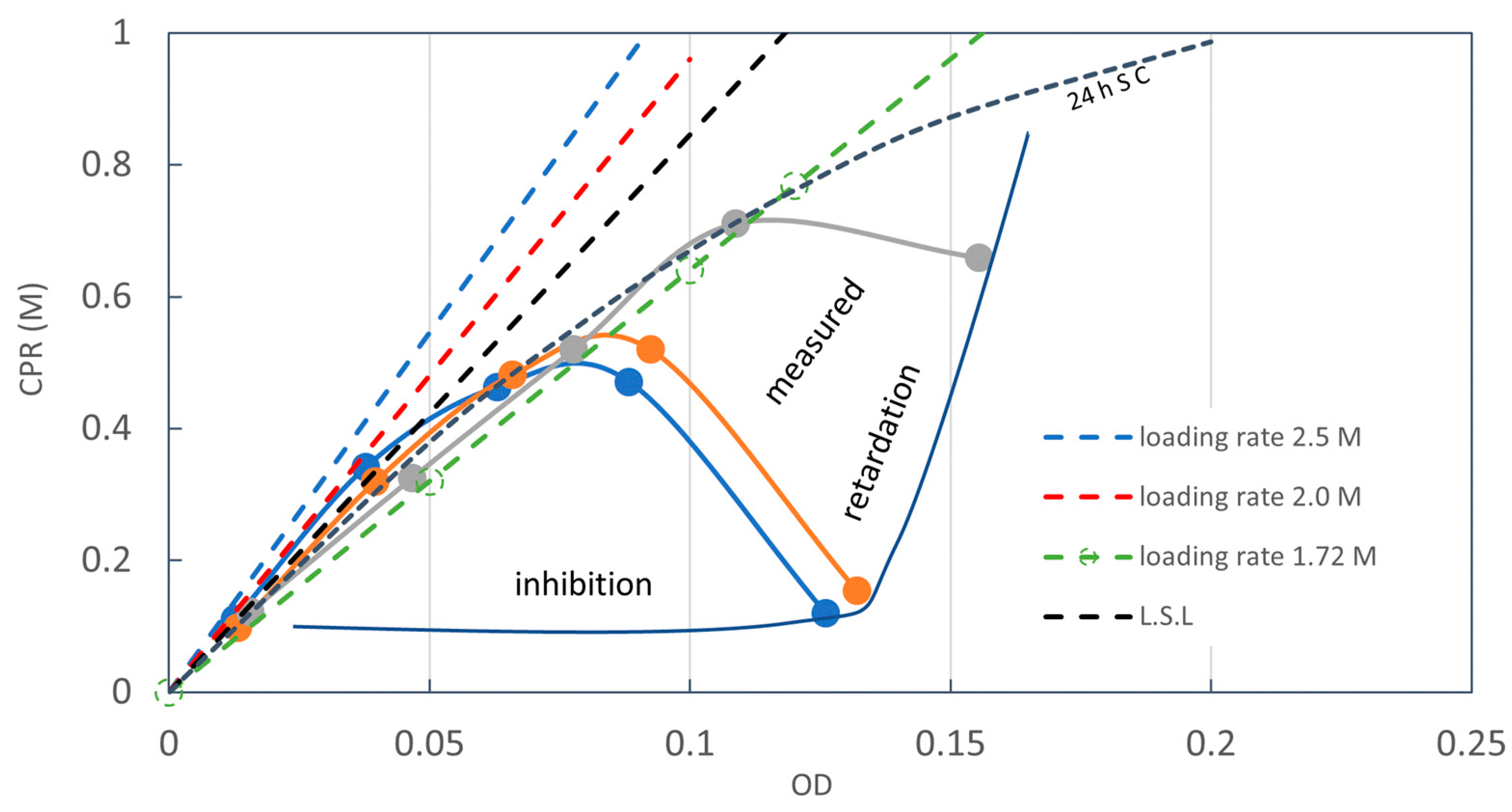
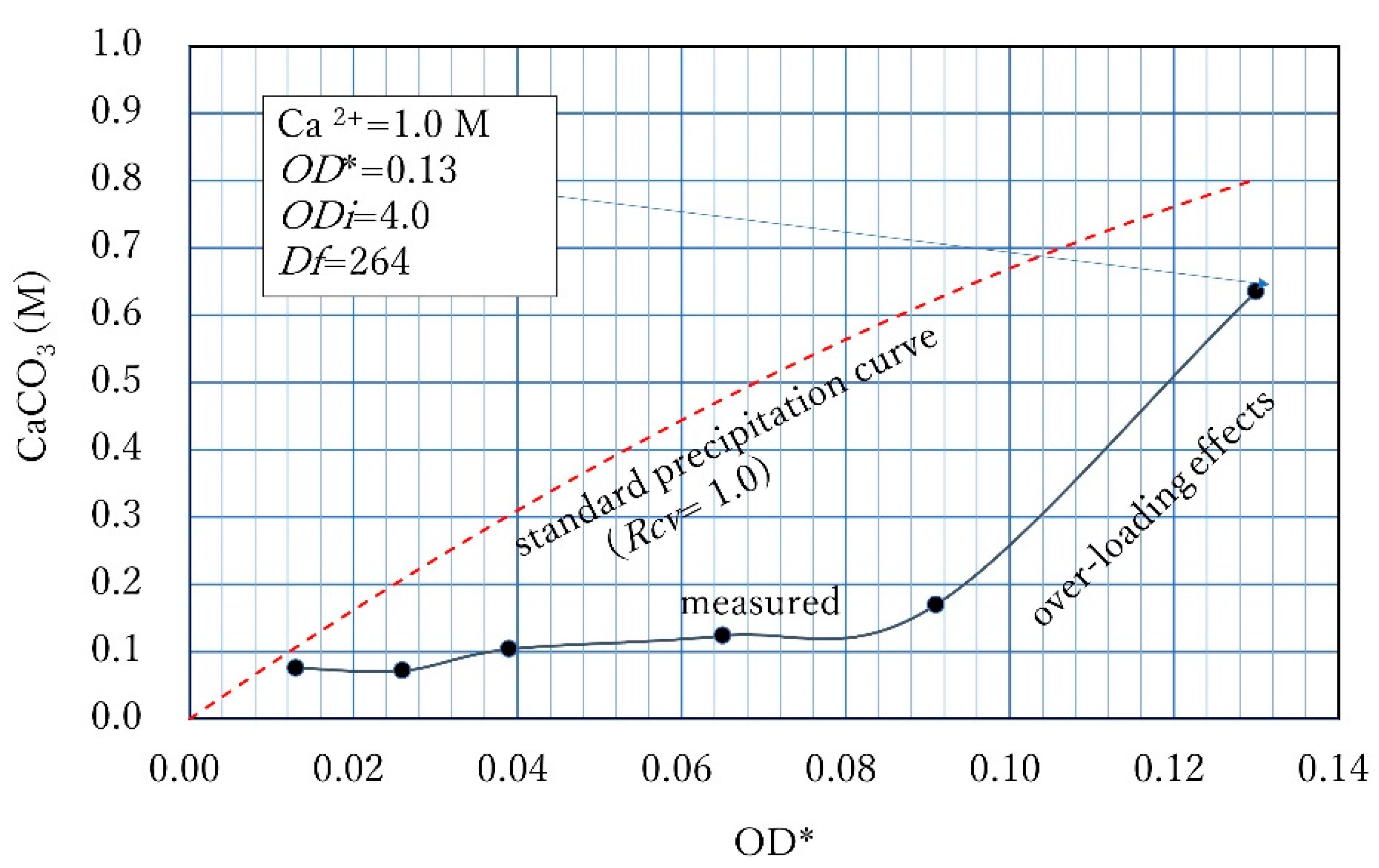
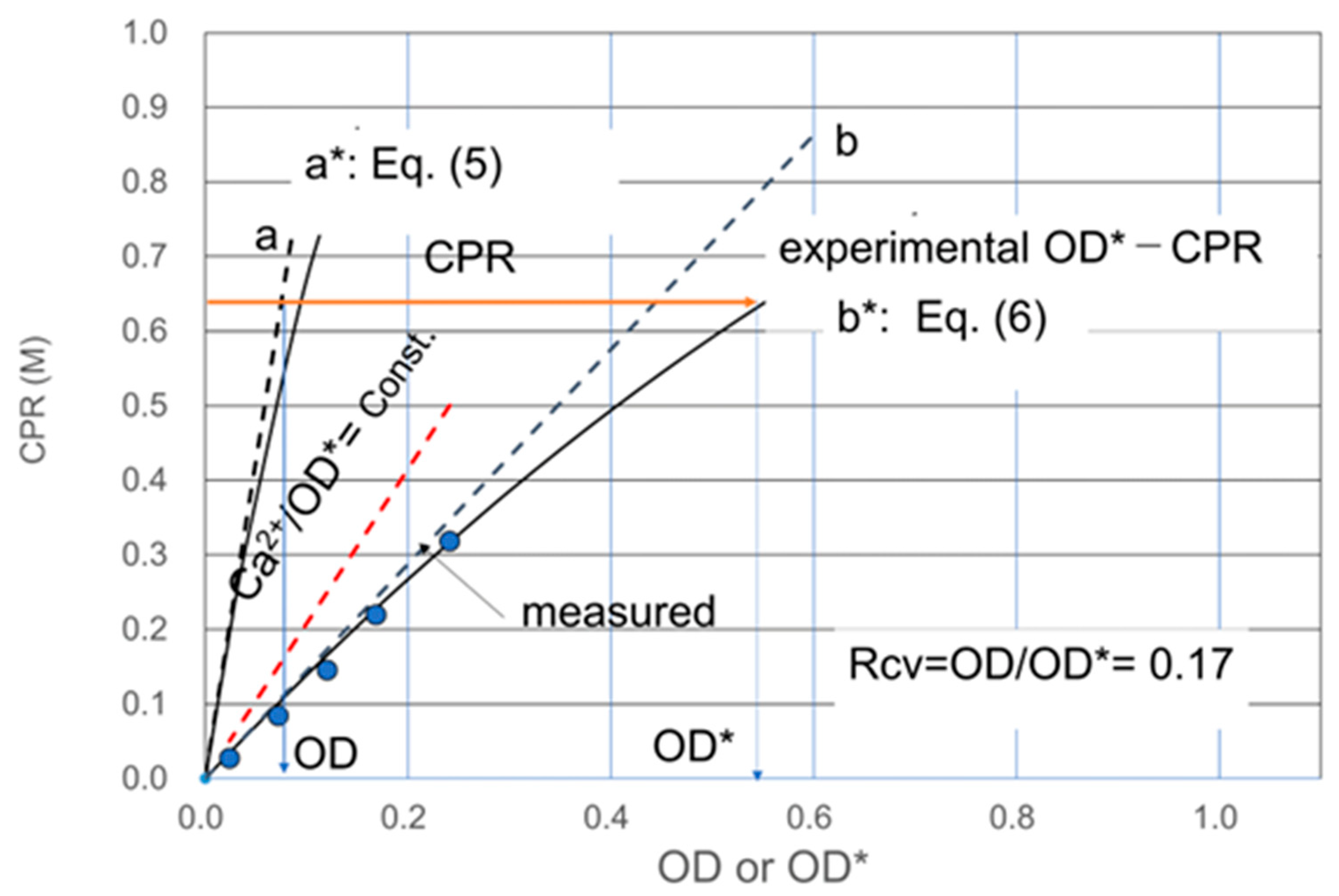
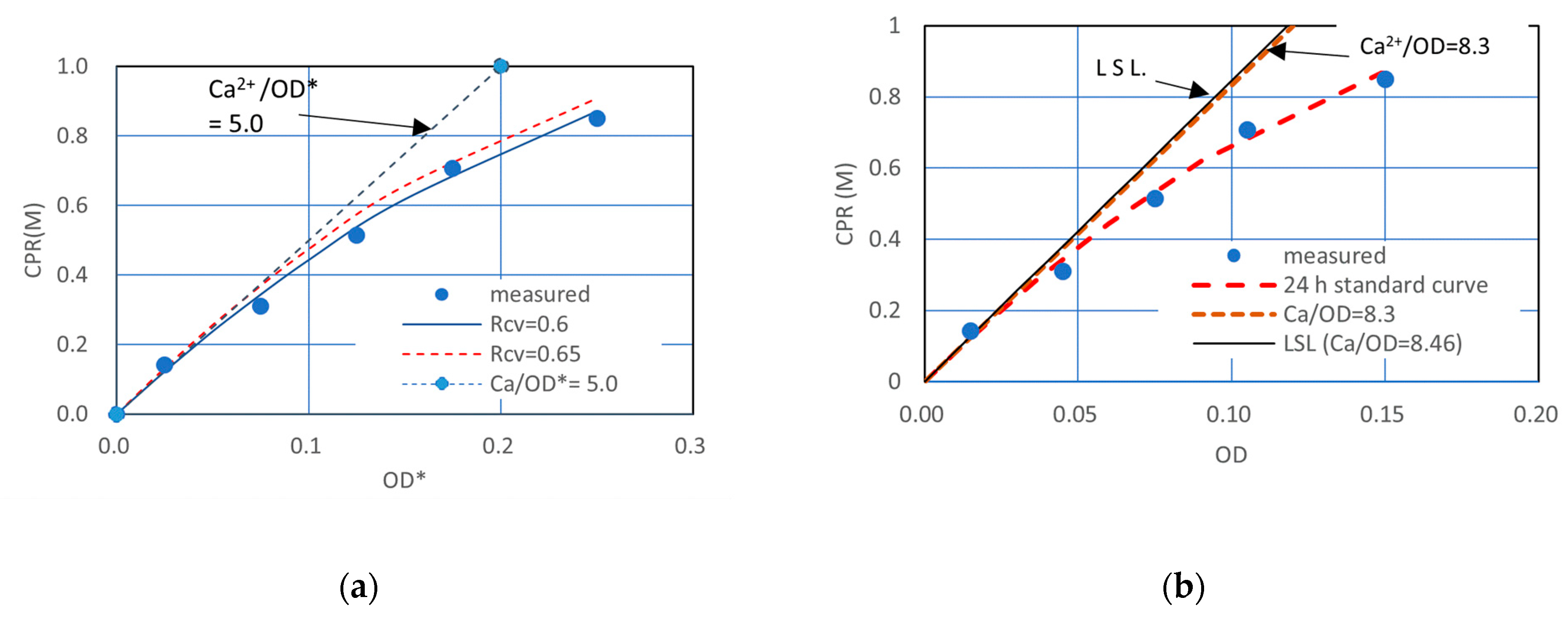
3.1. Rcv Determination Method of One Point
3.2. Constant Ca2+ Loading
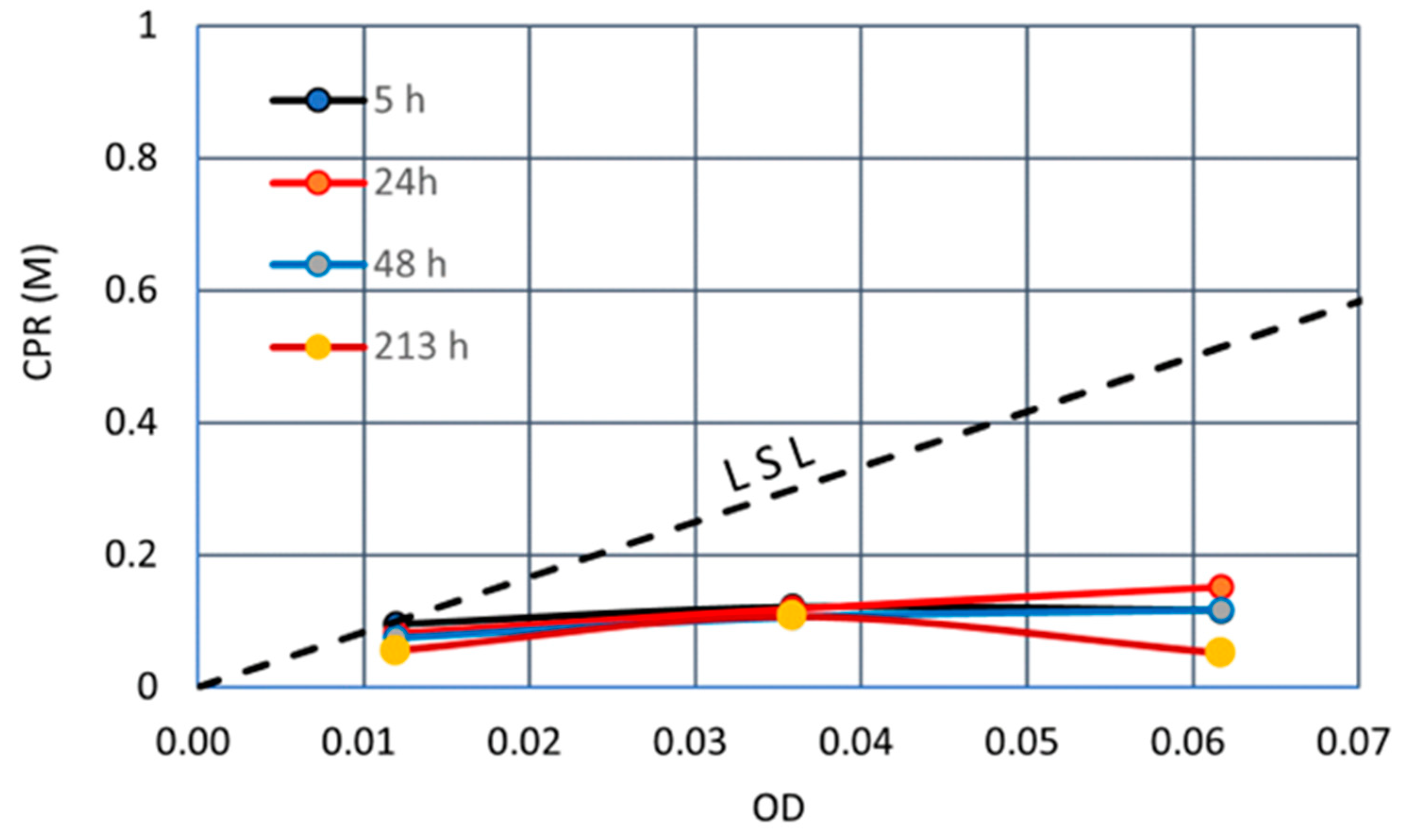
3.2.1. Ca2+ of 0.5 M
3.2.2. Ca2+ of 1.0 M
3.2.3. Low Ca2+ Loading (0.3 M)
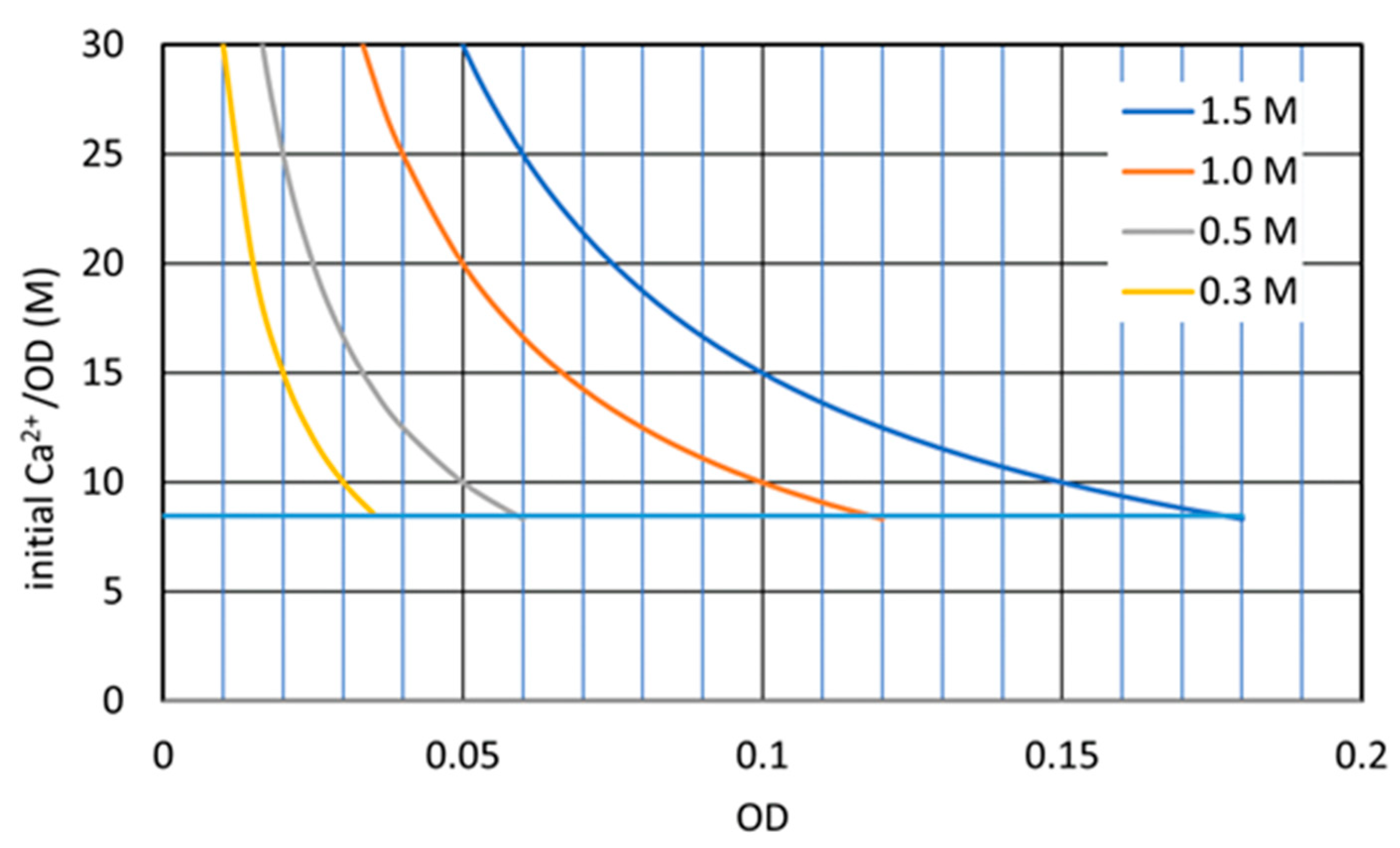
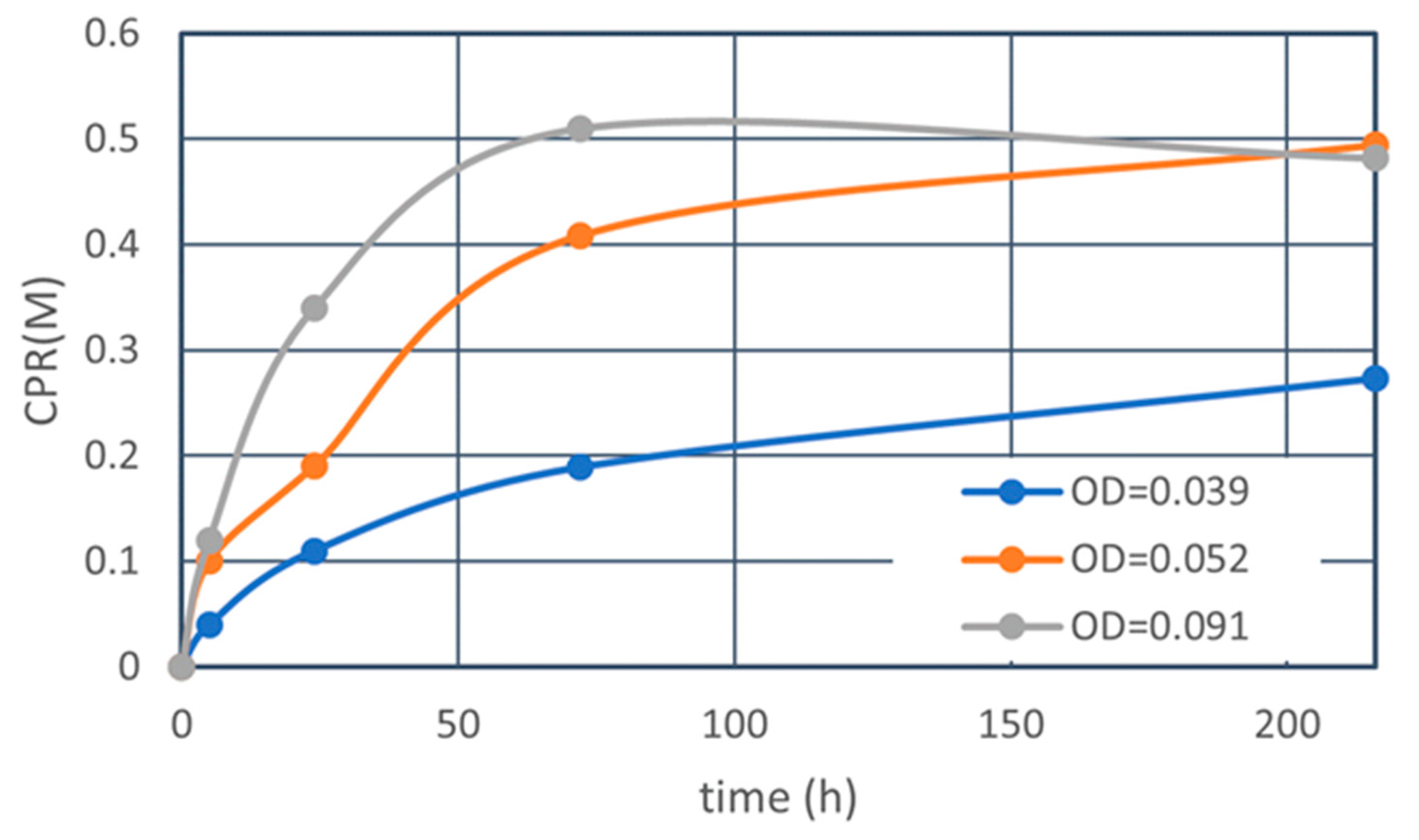
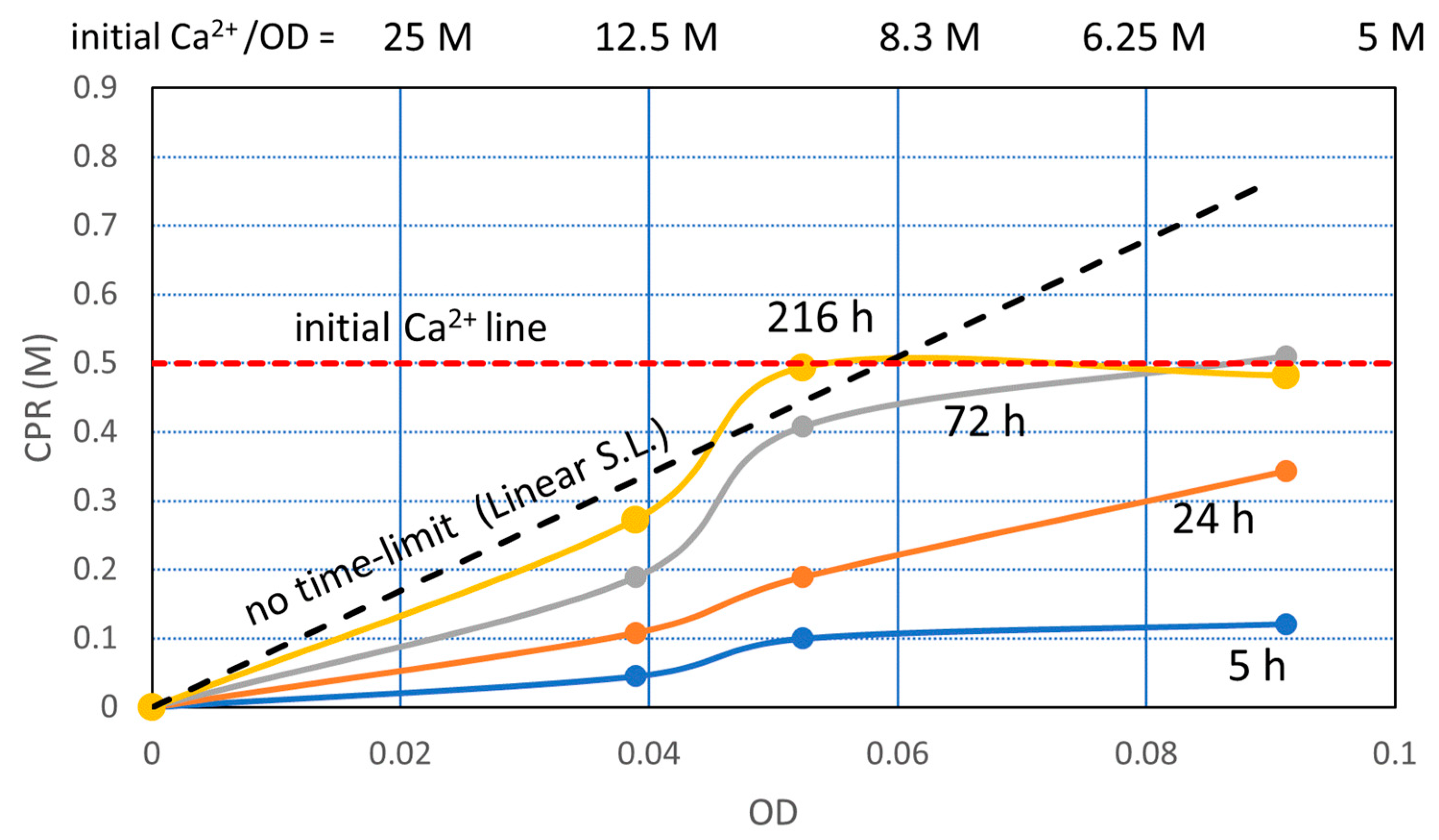
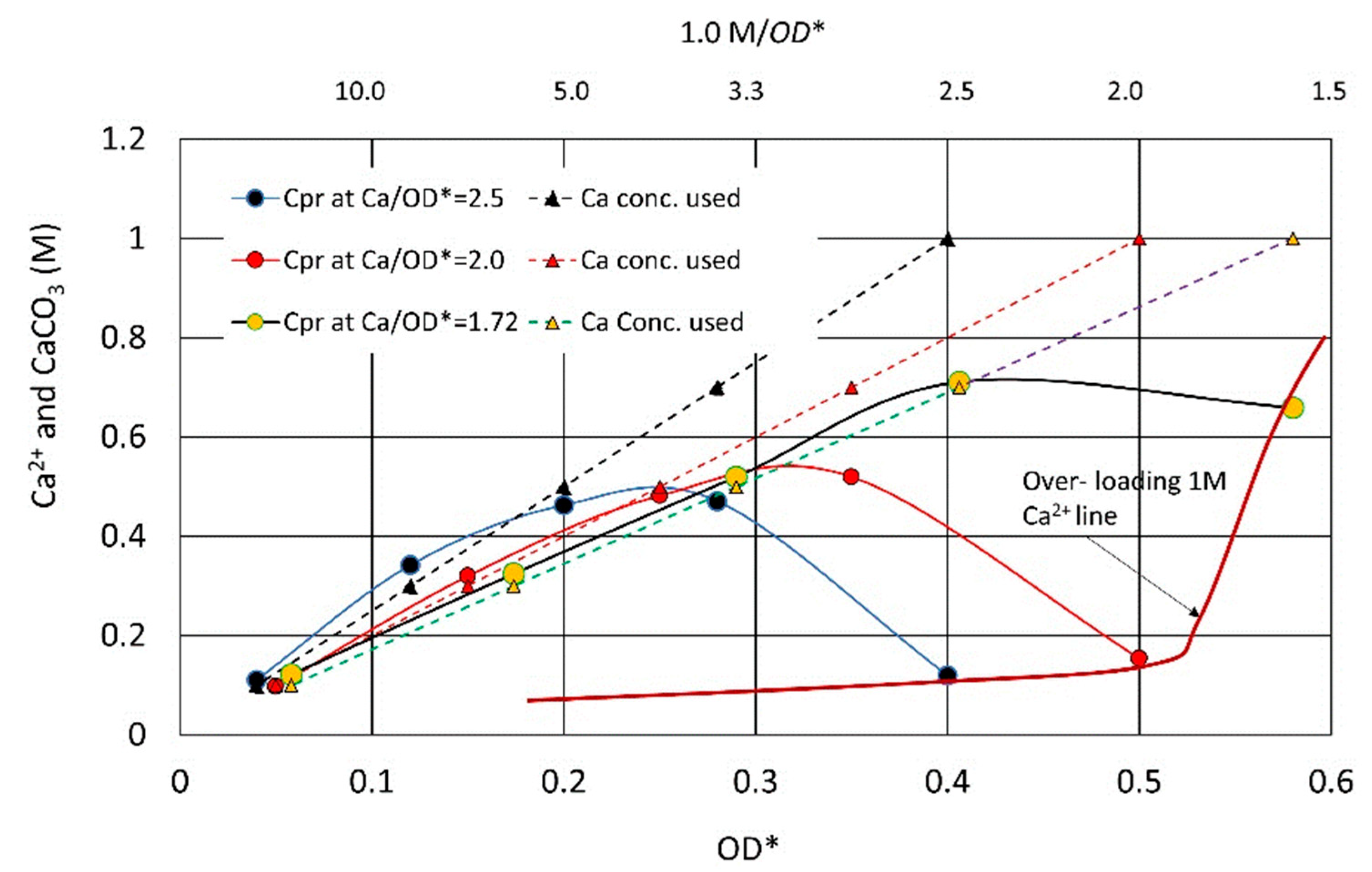
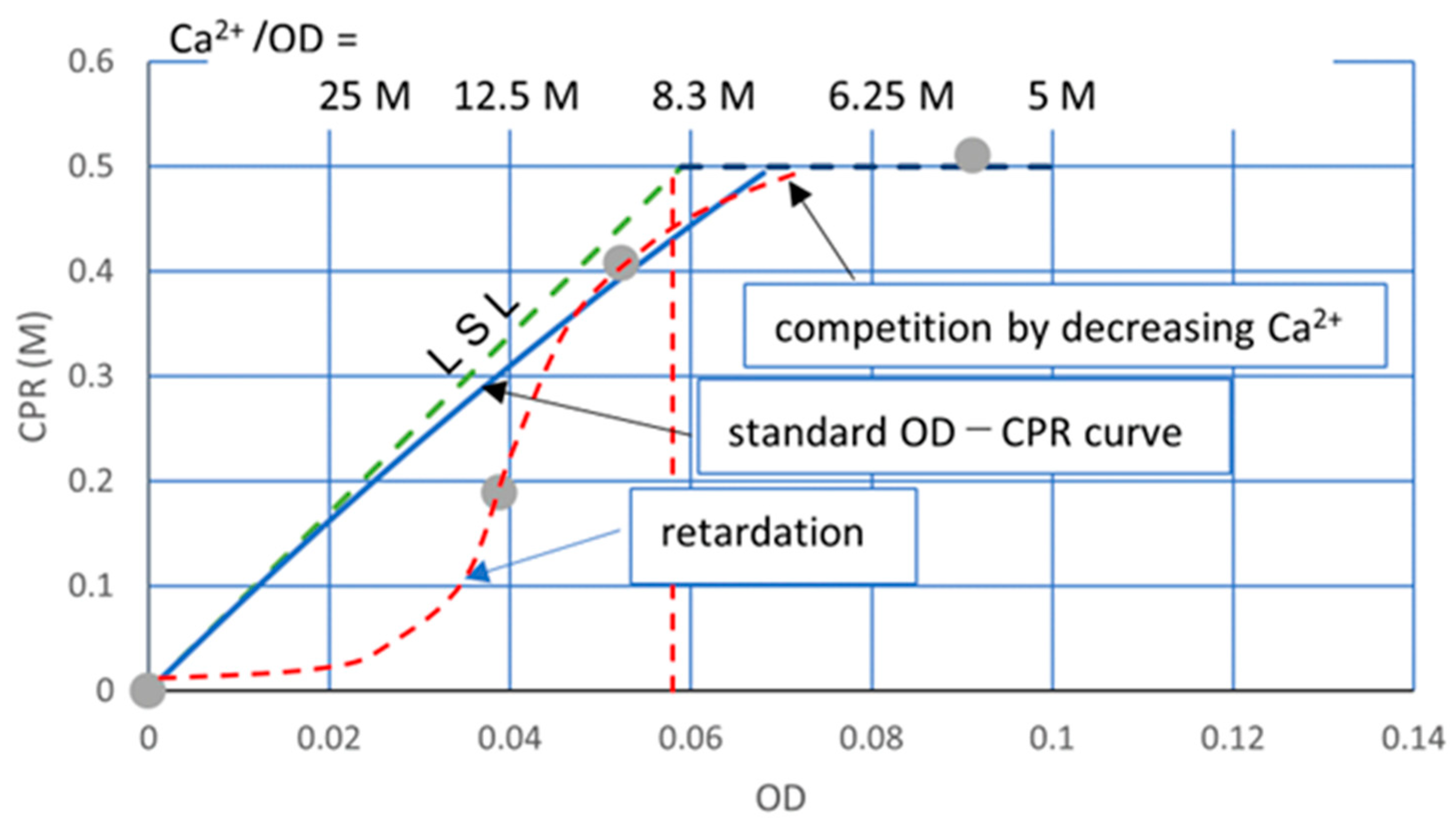
3.2.4. Constant Ca2+/OD Ratio
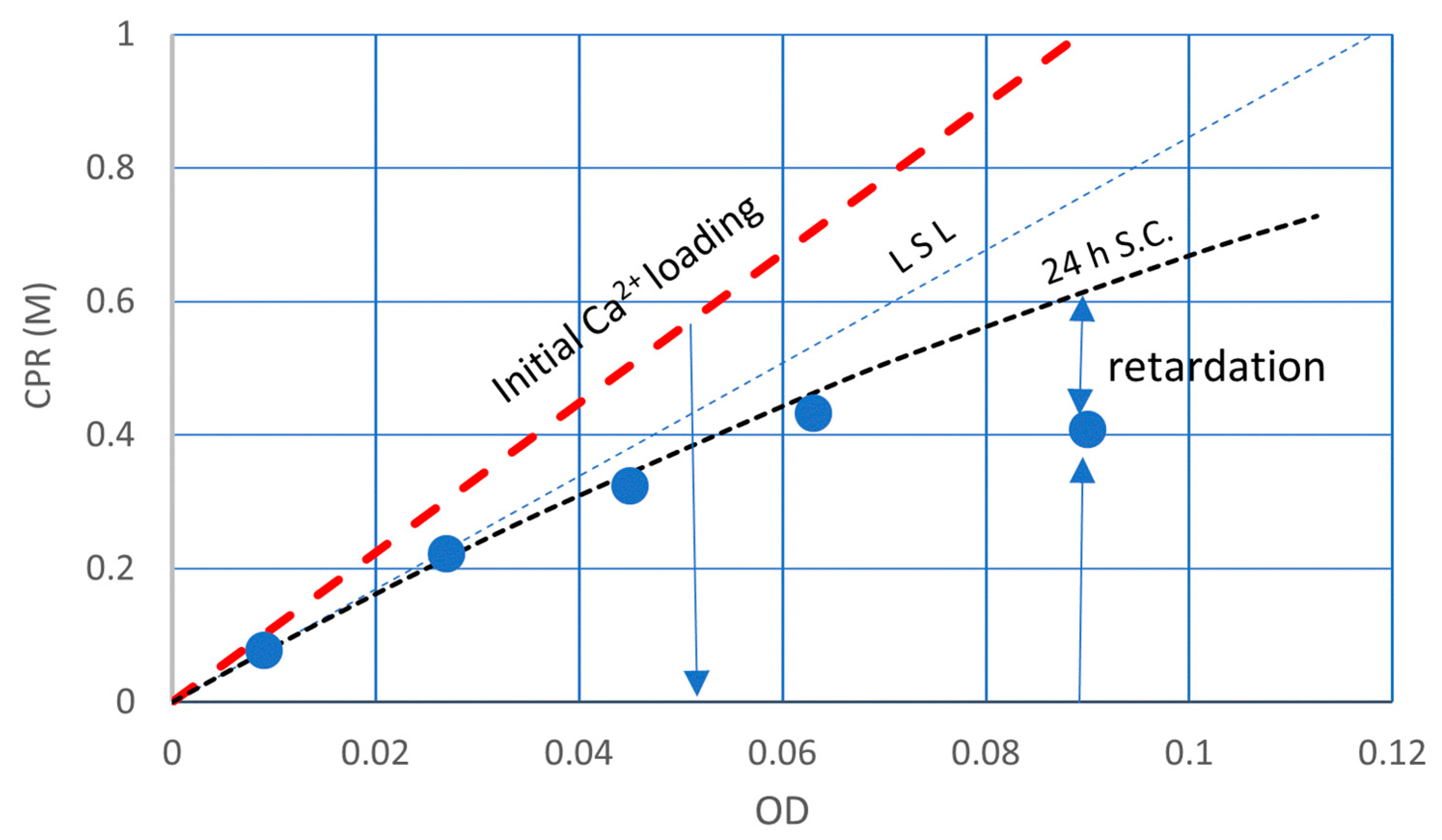
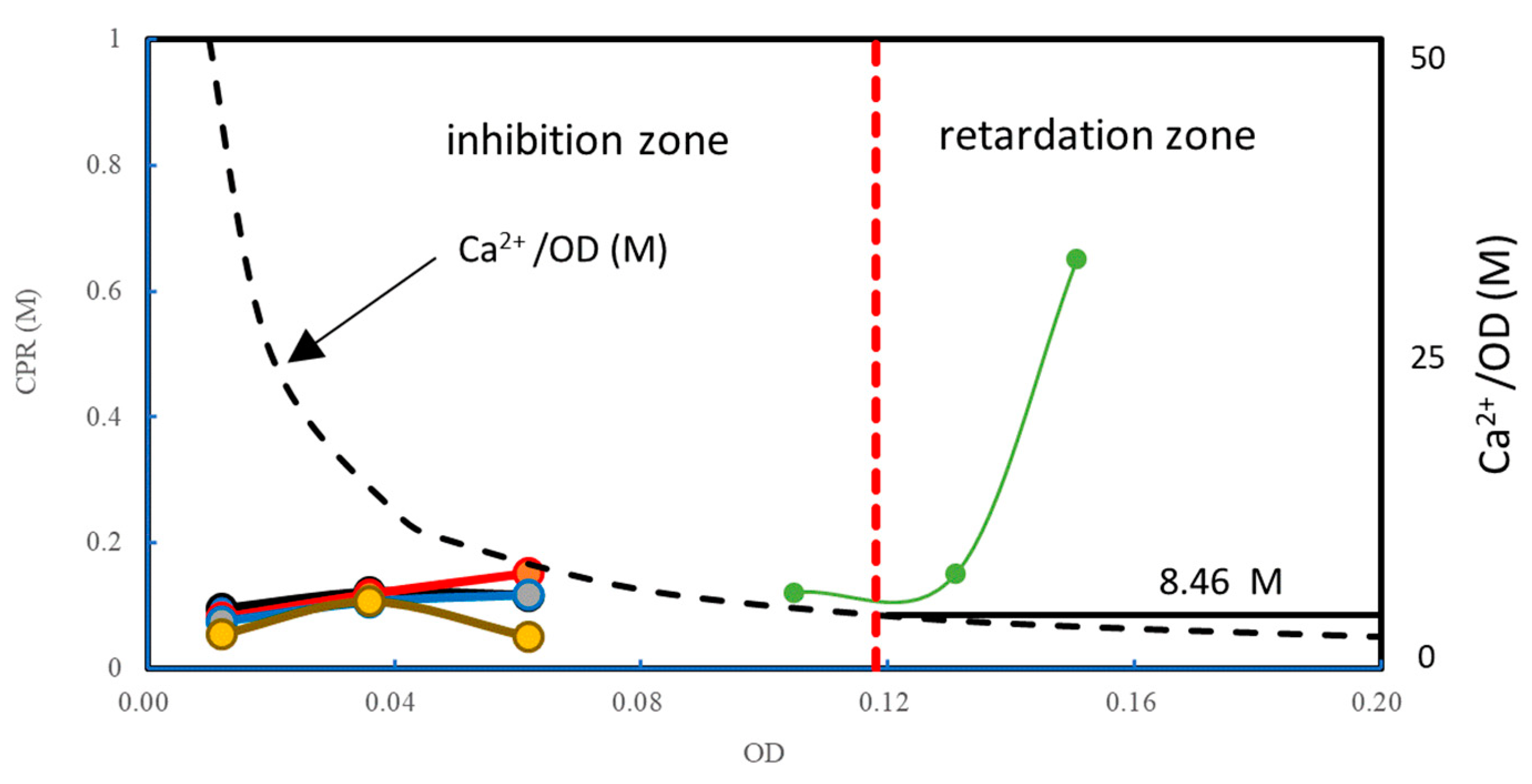
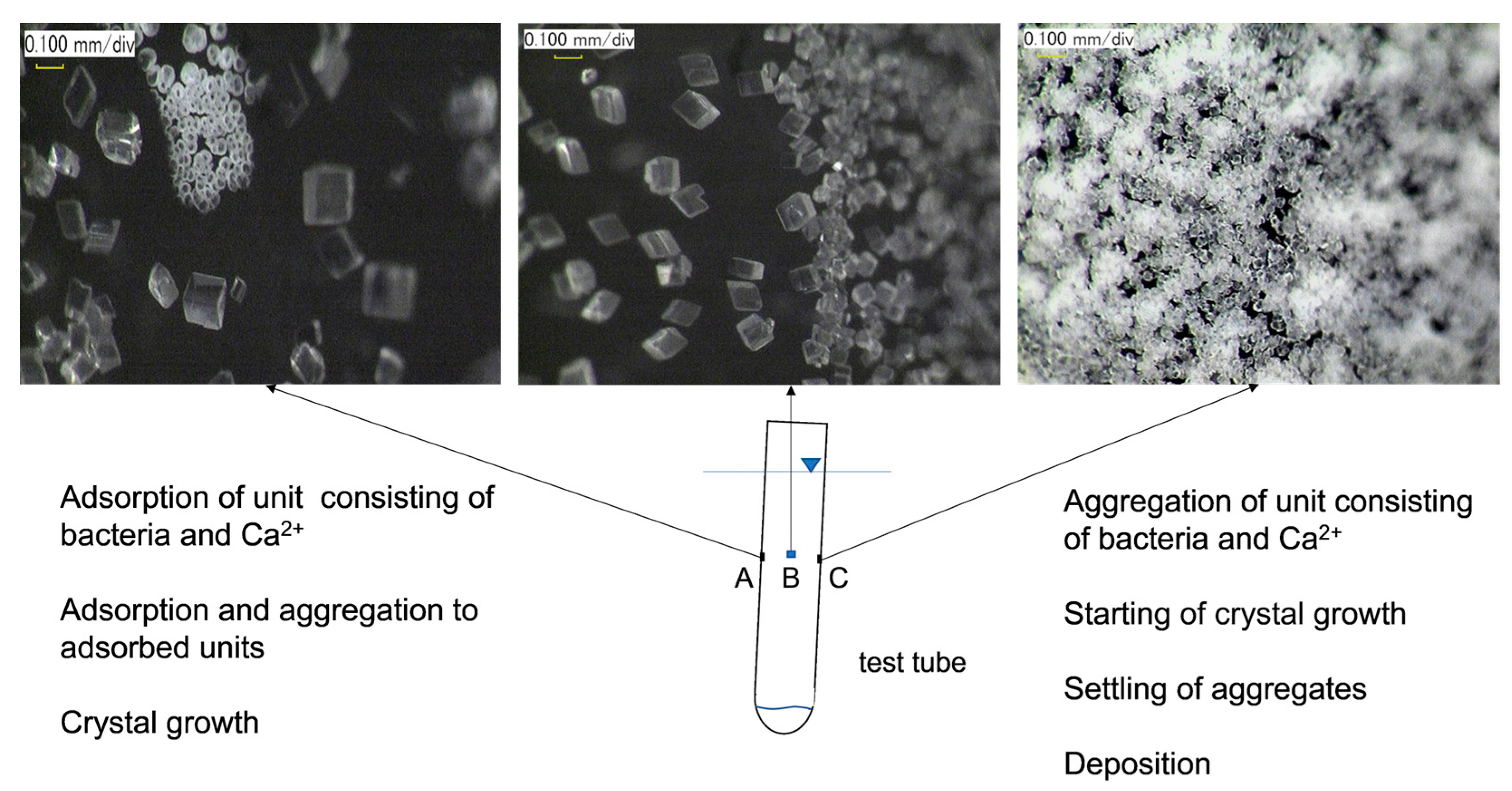
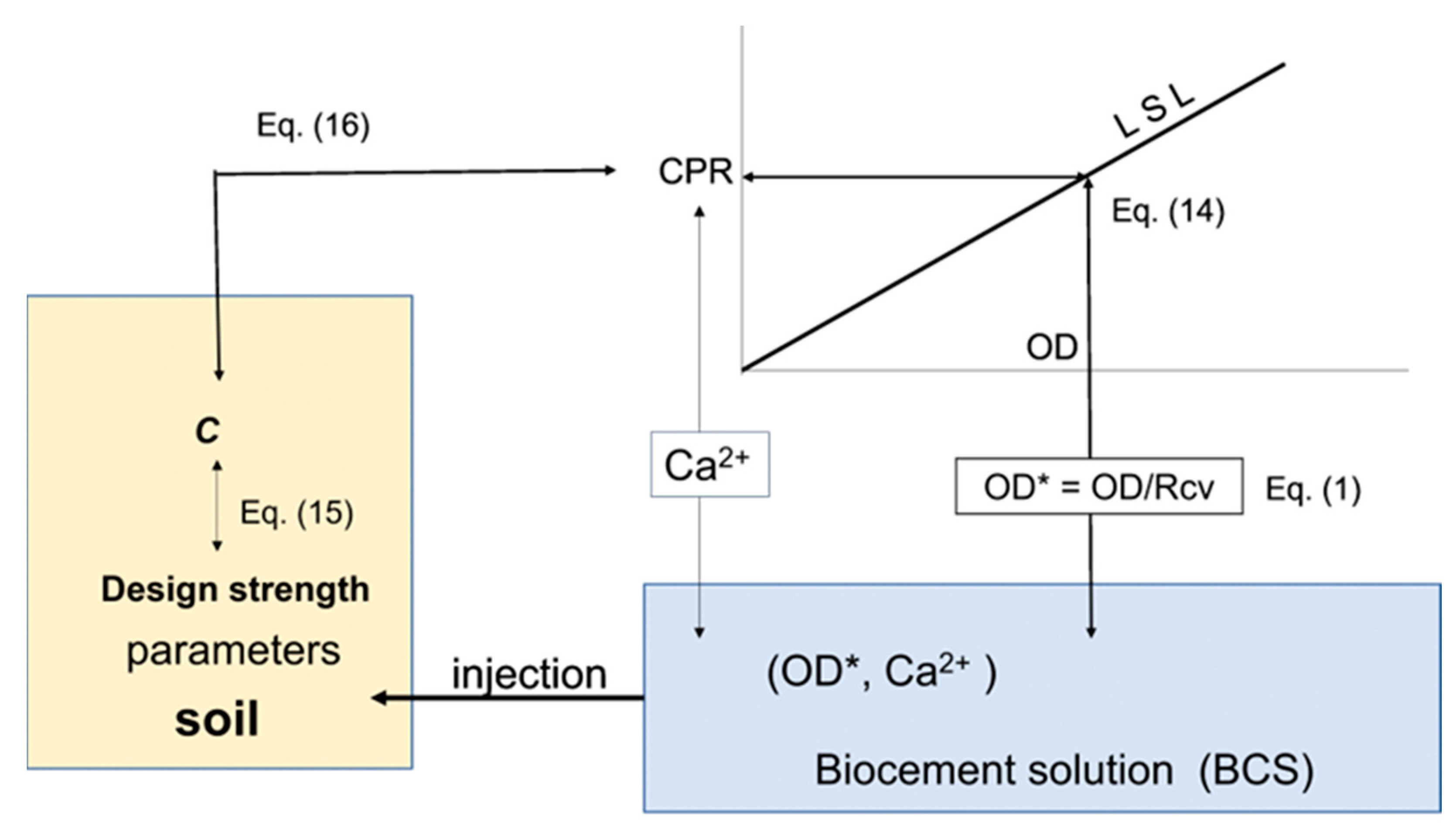
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstantinou, C.; Biscontin, G.; Logothetis, F. Tensile Strength of Artificially Cemented Sandstone Generated via Microbially Induced Carbonate Precipitation. Materials 2021, 14, 4735. [Google Scholar] [CrossRef]
- Menga, H.; Gaob, H.; Heb, J.; Qia, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Saridhe, S.P.; Selvaraj, T. Microbial precipitation of calcium carbonate in cementitious materials—A critical review. Mater. Today Proc. 2021, 43, 1232–1240. [Google Scholar] [CrossRef]
- Yu, X.; Rong, H. Seawater based MICP cements two/one-phase cemented sand blocks. Appl. Ocean Res. 2022, 118, 102972. [Google Scholar] [CrossRef]
- Sohail, M.G.; Disi, A.A.; Zouaari, N.; Nuaimi, N.A.; Kahraman, R.; Gencturk, B.; Rorigues, D.F.; Yildirim, Y. Bio self-healing concrete using MICP by an indigenous Bacillus cereus strain isolated from Qatari soil. Constr. Build. Mater. 2022, 328, 126943. [Google Scholar] [CrossRef]
- Martinez, A.; DeJong, J.; Akin, I.; Aleali, A.; Arson, C.; Atkinson, J.; Bandini, P.; Baser, T.; Borela, R.; Boulanger, R.; et al. Bio-inspired geotechnical engineering: Principles, current work, opportunities and challenges. Géotechnique 2022, 72, 687–705. [Google Scholar] [CrossRef]
- DeJong, J.T.; Gomez, M.G.; San Pablo, A.C.M.; Graddy, C.M.R.; Nelson, D.C.; Lee, M.; Ziotopoulou, K.; El Kortbawi, M.; Montoya, B.; Kwon, T.-H. State of the Art: MICP soil improvement and its application to liquefaction hazard mitigation. In Proceedings of the 20th International Conference on Soil Mechanics and Geotechnical Engineering, Sydney, Australia, 1–5 May 2022; Volume 1, pp. 405–508, ISBN 978-0-9946261-6-5. [Google Scholar]
- Khan, N.H.; Amarakoon, G.G.N.N.; Shimazaki, S.; Kawasaki, S. Coral Sand Solidification Test Based on microbially induced carbonate precipitation using ureolytic bacteria. Mater. Trans. 2015, 56, 1725–1732. [Google Scholar] [CrossRef]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Cheng, L.; Shahin, M.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotechnol. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Soga, K. Erosion behavior of gravel-sand mixtures stabilized by microbially induced calcite. Soils Found. 2019, 59, 699–709. [Google Scholar] [CrossRef]
- Choi, S.-G.; Chang, I.; Lee, M.; Lee, J.-H.; Han, J.-T.; Kwon, T.-H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Graddy, C.M.R.; Gomez, M.G.; DeJong, J.T.; Nelson, D.C. Native bacterial community convergence in augmented and stimulated ureolytic MICP biocementation. Environ. Sci. Technol. 2021, 55, 10784–10793. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Wu, S.-F.; Yang, Y.; Chu, J. Retarding effect of concentration of cementation solution on biocementation of soil. Acta Geotech. 2021, 16, 1457–1472. [Google Scholar] [CrossRef]
- Cui, M.-J.; Teng, A.; Chu, J.; Cai, M.A. quantitative, high-throughput urease activity assay for comparison and rapid screening of ureolytic bacteria. Environ. Res. 2022, 208, 112738. [Google Scholar] [CrossRef] [PubMed]
- Fukue, M.; Lechowicz, Z.; Fujimori, Y.; Emori, K.; Mulligan, C.N. Incorporation of optical density into the blending design for a biocement solution. Materials 2022, 15, 1951. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. The iGEM Interlab Study Contributors, Robust estimation of bacterial cell count from optical density. bioRxiv, 2019; preprint. [Google Scholar]
- Wen, K.; Li, Y.; Amin, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Francois, K.; Devlieghere, F.; Standaert, A.R.; Geeraerd, A.H.; Cools, I.; Van Impe, J.F.; Debevere, J. Environmental factors influencing the relationship between optical density and cell count for Listeria monocytogenes. J. Appl. Microbiol. 2005, 99, 1503–1515. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ginjm, R.H.; Nisson, P.M. Microbially induced carbonate precipitation via ureolysis process: A Mini-Review. Trans. Sci. Technol. 2018, 5, 245–256. [Google Scholar]
- Heveran, M.; Liang, L.; Nagarajan, A.; Hubler, M.H.; Gill, R.; Cameron, J.C.; Cook, S.M.; Srubar, W.V., III. Engineered ureolytic microorganisms can tailor the morphology and nanomechanical properties of microbial-precipitated calcium carbonate. Sci. Rep. 2019, 9, 14721. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Hausinger, R.P. Microbial, urease: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev. Environ. Sci. BioTechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Rivadeneyra, A.; Vilchez, J.I.; Martin-Ramos, D.; González-López, J.; Rivadeneyra, M.A. Carbonate Precipitation of Bacterial Strains Isolated from Sediments and Seawater: Formation Mechanisms. Geomicrobiol. J. 2013, 30, 840–850. [Google Scholar] [CrossRef]
- Lian, B.; Hu, Q.; Chen, J.; Ji, J.; Teng, H.H. Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Okwadha, G.D.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Poortingar, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 1–32. [Google Scholar] [CrossRef]
- Sanchez-Roman, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Fukue, M.; Ono, S.; Sato, Y. Cementation of sands due to microbiologically induced carbonate. Soils Found 2011, 51, 83–93. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Sand, K.; Benning, L.G. Chapter 5, ACC and vaterite as intermediates in the solution-based crystallization of CaCO3. In New Perspectives on Mineral Ucleation and Growth; Van Driessche, A., Kellermeier, M., Benning, L., Gebauer, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–111. [Google Scholar]
- Radha, A.V.; Forbesa, T.Z.; Killianb, C.E.; Gilbertb, P.U.P.A.; Navrotskya, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2010, 38, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- Zambare, N.M.; Naser, N.Y.; Gerlach, R.; Chang, C.B. Mineralogy of microbially induced calcium carbonate precipitates formed using single cell drop-based microfluidics. Sci. Rep. 2020, 10, 17535. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, Q.; Lishuang, C.; Wang, L.; Niu, S.; Zheng, J.; Yang, M.; Yan, Y. Morphological Changes of Calcium Carbonate and Mechanical Properties of Samples during Microbially Induced Carbonate Precipitation (MICP). Materials 2022, 15, 7754. [Google Scholar] [CrossRef]
- Imran, M.A.; Shinmura, M.; Nakashima, K.; Kawasaki, S. Factors affecting sand solidification using ureolytic bacteria. Mater. Trans. 2018, 59, 72–81. [Google Scholar]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Lee, M.L.; Ng, W.S.; Tan, C.K.; Hii, S.L. Bio-mediated soil improvement under various concentration of reagents. Appl. Mech. Mater. 2012, 204–208, 326–329. [Google Scholar]
- Mujah, D.; Cheng, L.; Shahin, M.A. Microstructural and geomechanical study on biocemented sand for optimization of MICP process. J. Mater. Civ. Eng. 2019, 31, 04019025. [Google Scholar] [CrossRef]
- Fukue, M.; Lechowicz, Z. Strength of biocemented sandy soils using simple devices. In Proceedings of the International Congress Natural Sciences and Engineering, Kuala Lumpur, Malaysia, 6–8 July 2018; pp. 17–28, ISBN 978-986-87417-8-2. [Google Scholar]
- Fukue, M.; Nakamura, T.; Kato, Y. Cementation of soils due to calcium carbonate. Soils Found. 1999, 39, 55–64. [Google Scholar] [CrossRef] [PubMed]
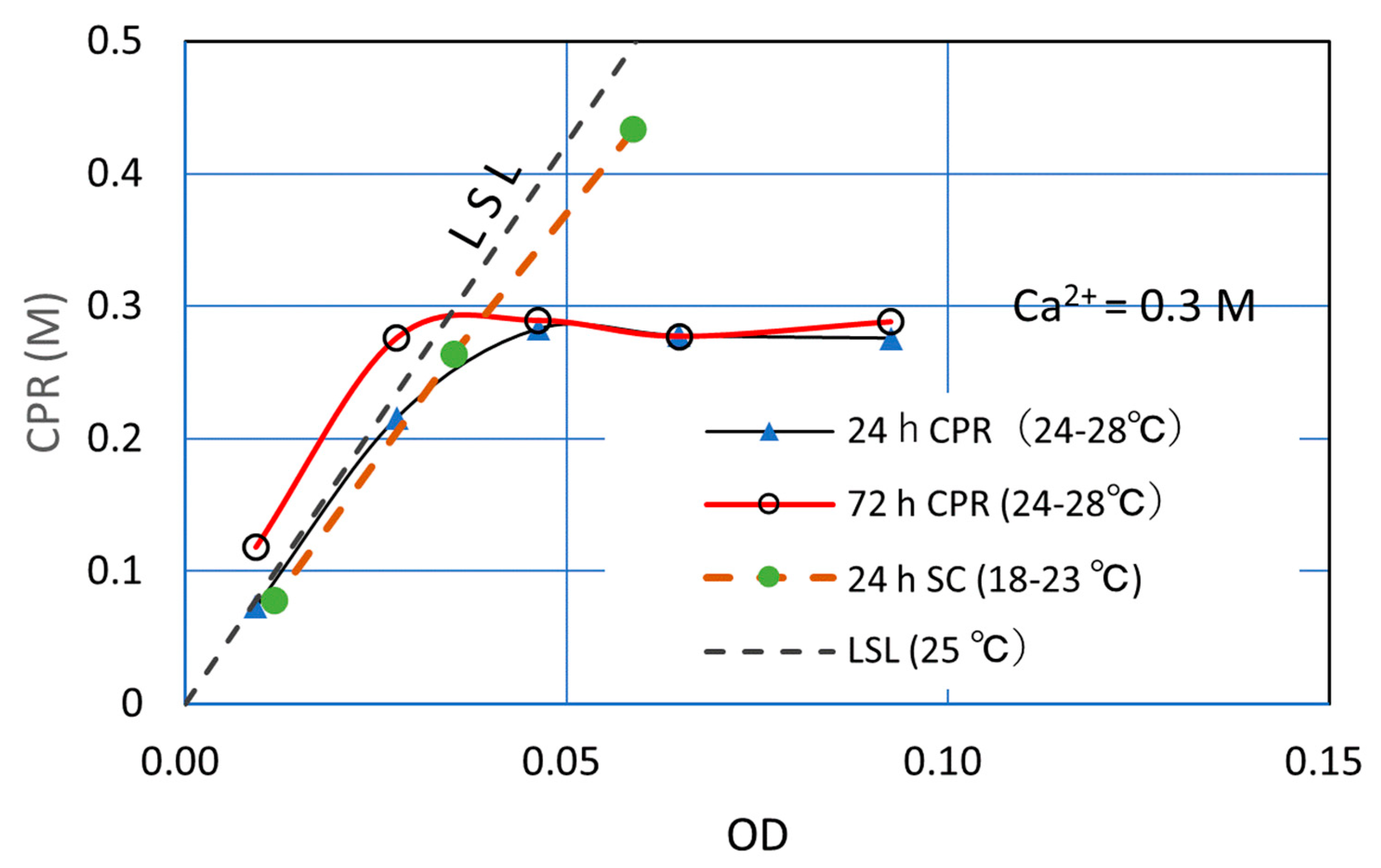

| Ca2+ | OD* | OD | 24 h CPR | 72 h CPR |
|---|---|---|---|---|
| (M) | (M) | (M) | ||
| 0.3 | 0.370 | 0.093 | 0.276 | 0.288 |
| 0.3 | 0.259 | 0.065 | 0.278 | 0.277 |
| 0.3 | 0.185 | 0.046 | 0.283 | 0.289 |
| 0.3 | 0.111 | 0.028 | 0.216 | 0.276 |
| 0.3 | 0.037 | 0.009 | 0.073 | 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukue, M.; Lechowicz, Z.; Fujimori, Y.; Emori, K.; Mulligan, C.N. Inhibited and Retarded Behavior by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process. Materials 2023, 16, 3357. https://doi.org/10.3390/ma16093357
Fukue M, Lechowicz Z, Fujimori Y, Emori K, Mulligan CN. Inhibited and Retarded Behavior by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process. Materials. 2023; 16(9):3357. https://doi.org/10.3390/ma16093357
Chicago/Turabian StyleFukue, Masaharu, Zbigniew Lechowicz, Yuichi Fujimori, Kentaro Emori, and Catherine N. Mulligan. 2023. "Inhibited and Retarded Behavior by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process" Materials 16, no. 9: 3357. https://doi.org/10.3390/ma16093357
APA StyleFukue, M., Lechowicz, Z., Fujimori, Y., Emori, K., & Mulligan, C. N. (2023). Inhibited and Retarded Behavior by Ca2+ and Ca2+/OD Loading Rate on Ureolytic Bacteria in MICP Process. Materials, 16(9), 3357. https://doi.org/10.3390/ma16093357







