A New Microarchitecture-Based Parameter to Predict the Micromechanical Properties of Bone Allografts
Abstract
1. Introduction
2. Materials and Methods
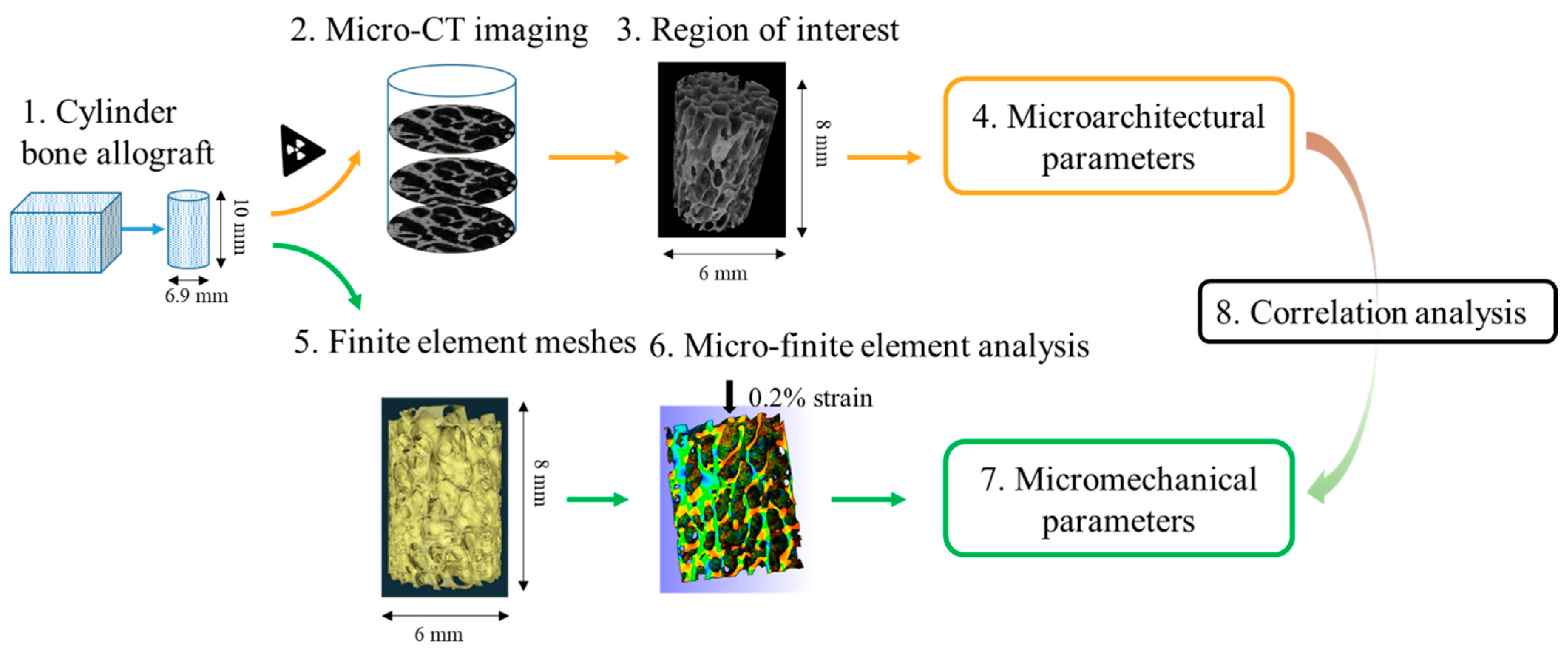
2.1. Experimental Design
2.2. Sample Preparation
2.3. X-ray Computed Tomography Analysis and Calculation of Bone Microarchitectural Parameters
2.4. Micro-Finite Element Meshing
2.5. Micro-Finite Element Analysis
2.6. Statistical Analysis
3. Results
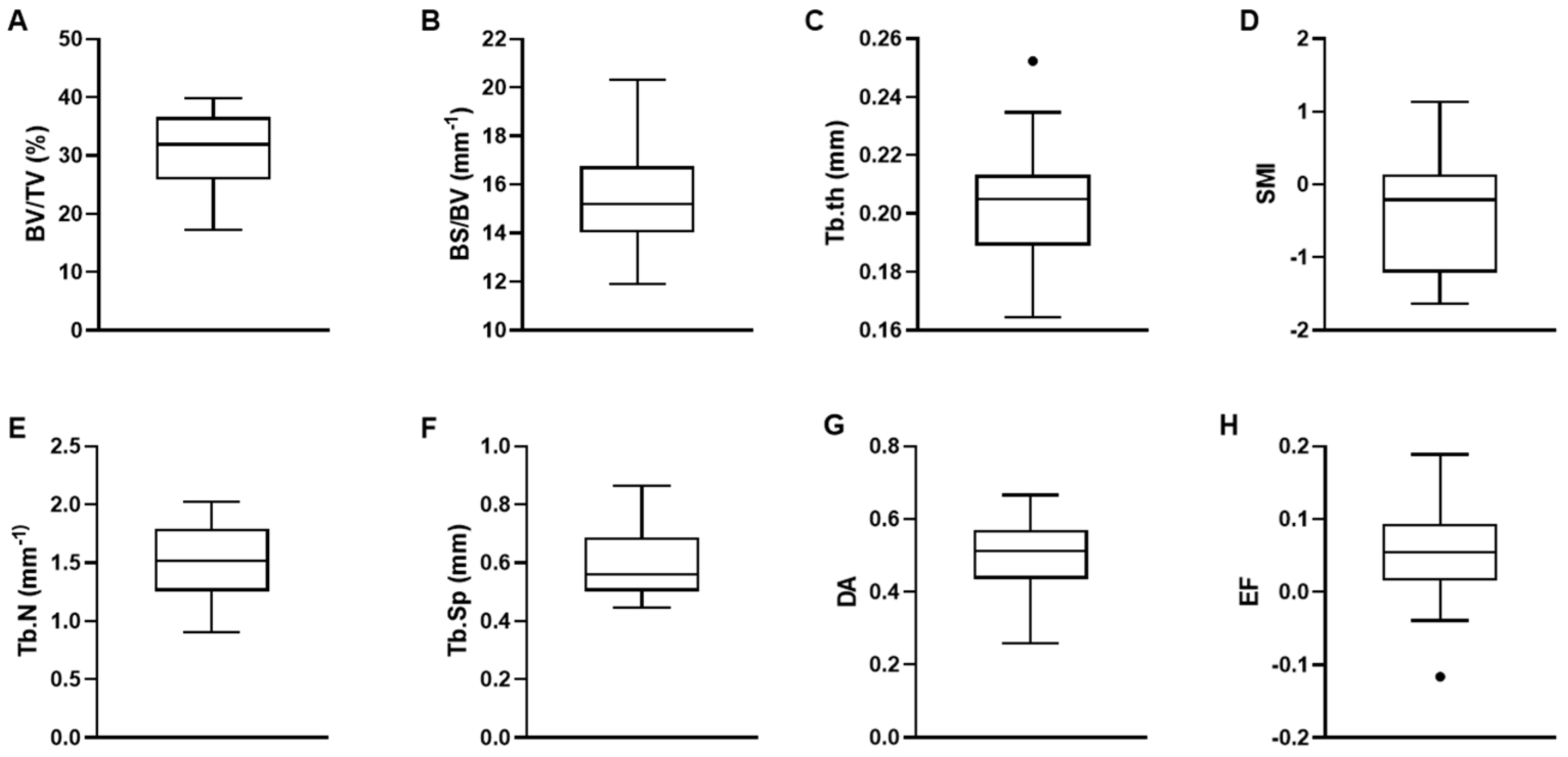
3.1. Trabecular Bone Microarchitectural Parameters
3.2. Micromechanical Parameters
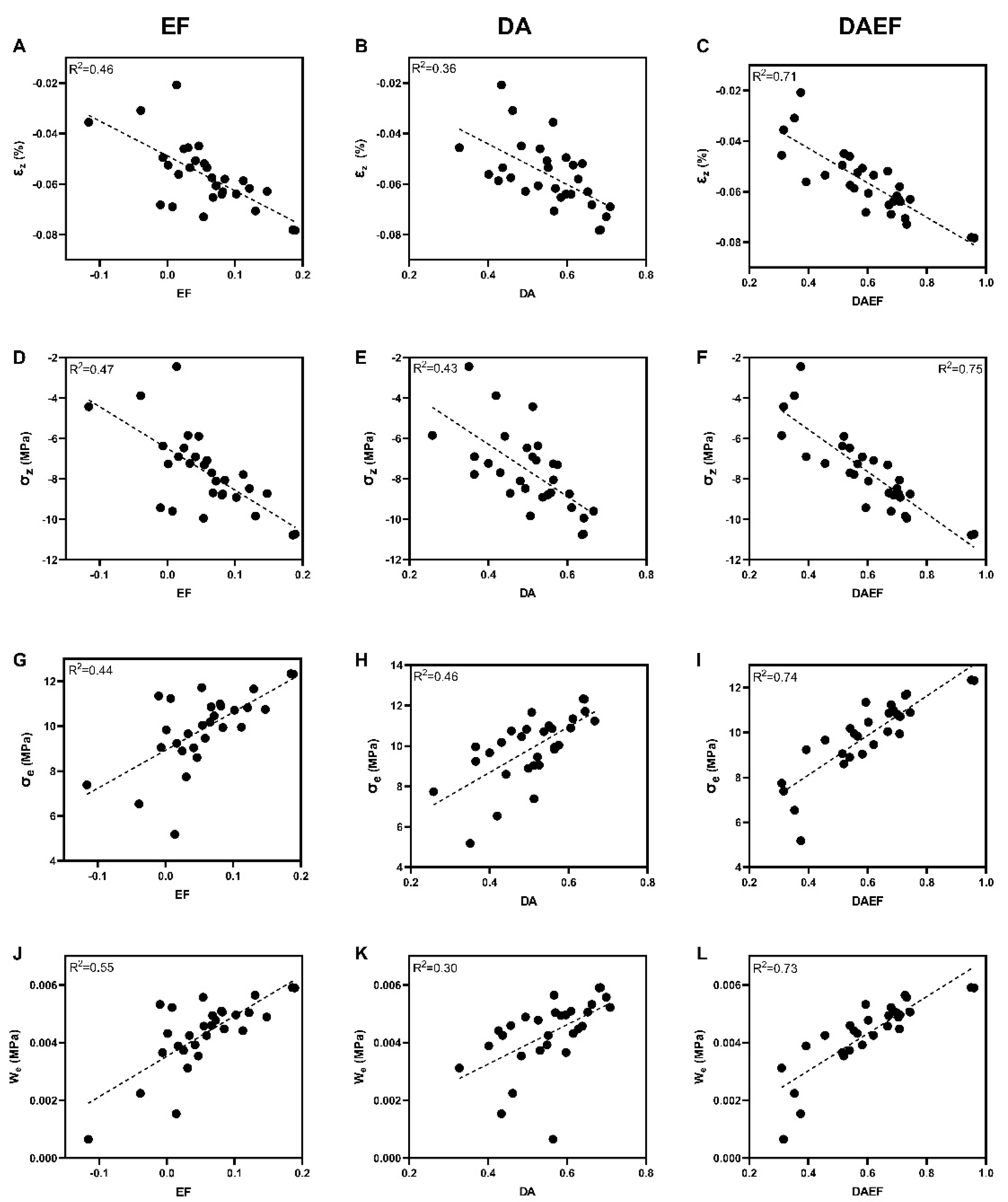
3.3. Relationship between Microarchitectural and Micromechanical Parameters
3.4. DAEF: An Index Derived to Predict Micromechanical Parameters
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Wildt, B.W.M.; Ansari, S.; Sommerdijk, N.A.J.M.; Ito, K.; Akiva, A.; Hofmann, S. From Bone Regeneration to Three-Dimensional in Vitro Models: Tissue Engineering of Organized Bone Extracellular Matrix. Curr. Opin. Biomed. Eng. 2019, 10, 107–115. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials: An Overview of the Basic Science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Almer, J.D.; Stock, S.R. Internal Strains and Stresses Measured in Cortical Bone via High-Energy X-Ray Diffraction. J. Struct. Biol. 2005, 152, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. Ueber die innere Architectur der Knochen und ihre Bedeutung für die Frage vom Knochenwachsthum. Arch. Pathol. Anat. 1870, 50, 389–450. [Google Scholar] [CrossRef]
- Potier, E.; Noailly, J.; Ito, K. Directing Bone Marrow-Derived Stromal Cell Function with Mechanics. J. Biomech. 2010, 43, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Xu, Z.; Wang, X.; Wang, Y.; Li, H.; Chen, T.; Hu, Y.; Chen, R.; Huang, K.; Chen, C.; et al. Biophysical Stimuli as the Fourth Pillar of Bone Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 790050. [Google Scholar] [CrossRef]
- Pereira, A.R.; Lipphaus, A.; Ergin, M.; Salehi, S.; Gehweiler, D.; Rudert, M.; Hansmann, J.; Herrmann, M. Modeling of the Human Bone Environment: Mechanical Stimuli Guide Mesenchymal Stem Cell–Extracellular Matrix Interactions. Materials 2021, 14, 4431. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Racine-Avila, J.; Pellicore, M.J.; Aaron, R. Biophysical Modulation of Mesenchymal Stem Cell Differentiation in the Context of Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 3919. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, T.; Rüegsegger, P. Quantification of Bone Microarchitecture with the Structure Model Index. Comput. Methods Biomech. Biomed. Eng. 1997, 1, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Odgaard, A. Three-Dimensional Methods for Quantification of Cancellous Bone Architecture. Bone 1997, 20, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Doube, M. The Ellipsoid Factor for Quantification of Rods, Plates, and Intermediate Forms in 3D Geometries. Front. Endocrinol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Müller, R.; Snyder, B.D. Bone Volume Fraction Explains the Variation in Strength and Stiffness of Cancellous Bone Affected by Metastatic Cancer and Osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Salmon, P.L.; Ohlsson, C.; Shefelbine, S.J.; Doube, M. Structure Model Index Does Not Measure Rods and Plates in Trabecular Bone. Front. Endocrinol. 2015, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Hemmatian, H.; Bakker, A.D.; Klein-Nulend, J.; van Lenthe, G.H. Alterations in Osteocyte Lacunar Morphology Affect Local Bone Tissue Strains. J. Mech. Behav. Biomed. Mater. 2021, 123, 104730. [Google Scholar] [CrossRef]
- Rubert, M.; Vetsch, J.R.; Lehtoviita, I.; Sommer, M.; Zhao, F.; Studart, A.R.; Müller, R.; Hofmann, S. Scaffold pore geometry guides gene regulation and bone-like tissue formation in dynamic cultures. Tissue Eng. Part A 2021, 27, 1192–1204. [Google Scholar] [CrossRef]
- Collins, C.J.; Atkins, P.R.; Ohs, N.; Blauth, M.; Lippuner, K.; Müller, R. Clinical Observation of Diminished Bone Quality and Quantity through Longitudinal HR-PQCT-Derived Remodeling and Mechanoregulation. Sci. Rep. 2022, 12, 17960. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Corté, L.; Allena, R.; Checa, S. A 3D in Silico Multi-Tissue Evolution Model Highlights the Relevance of Local Strain Accumulation in Bone Fracture Remodeling. Front. Bioeng. Biotechnol. 2022, 10, 835094. [Google Scholar] [CrossRef] [PubMed]
- Metzger, T.A.; Schwaner, S.A.; LaNeve, A.J.; Kreipke, T.C.; Niebur, G.L. Pressure and Shear Stress in Trabecular Bone Marrow during Whole Bone Loading. J. Biomech. 2015, 48, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Jeong, J.-Y.; Lee, T.-H.; Lee, H.S.; Shin, J.-W. Synergistic Integration of Mesenchymal Stem Cells and Hydrostatic Pressure in the Expansion and Maintenance of Human Hematopoietic/Progenitor Cells. Stem Cells Int. 2018, 2018, e4527929. [Google Scholar] [CrossRef]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone Remodelling in Humans Is Load-Driven but Not Lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, X.; Tao, C.; Dai, R.; Ni, J.; Liao, E. Association of Microstructural and Mechanical Properties of Cancellous Bone and Their Fracture Risk Assessment Tool Scores. Int. J. Clin. Exp. Med. 2015, 8, 3956–3964. [Google Scholar] [PubMed]
- Roux, J.-P.; Boutroy, S.; Bouxsein, M.L.; Chapurlat, R.; Wegrzyn, J. Local and Global Microarchitecture Is Associated with Different Features of Bone Biomechanics. Bone Rep. 2020, 13, 100716. [Google Scholar] [CrossRef] [PubMed]
- Prada, D.M.; Galvis, A.F.; Miller, J.; Foster, J.M.; Zavaglia, C. Multiscale Stiffness Characterisation of Both Healthy and Osteoporotic Bone Tissue Using Subject-Specific Data. J. Mech. Behav. Biomed. Mater. 2022, 135, 105431. [Google Scholar] [CrossRef]
- Kusins, J.; Knowles, N.; Targosinski, J.; Columbus, M.; Athwal, G.S.; Ferreira, L. 3D Strain Analysis of Trabecular Bone within the Osteoarthritic Humeral Head Subjected to Stepwise Compressive Loads. J. Mech. Behav. Biomed. Mater. 2022, 125, 104922. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, X.; Bullock, W.; Main, R.P. Adaptive Changes in Micromechanical Environments of Cancellous and Cortical Bone in Response to in Vivo Loading and Disuse. J. Biomech. 2019, 89, 85–94. [Google Scholar] [CrossRef]
- Mitton, D.; Rappeneau, J.; Bardonnet, R. Effect of a Supercritical CO2 Based Treatment on Mechanical Properties of Human Cancellous Bone. Eur. J. Orthop. Surg. Traumatol. 2005, 15, 264–269. [Google Scholar] [CrossRef]
- Chappard, C.; Arnaud, M.; Laurent, B. Interindividual and intraspecimen variability of 3-D bone microarchitectural parameters in iliac crest biopsies imaged by conventional micro-computed tomography. J. Bone Miner. Metab. 2008, 26, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Glüer, C.-C.; Blake, G.; Lu, Y.; Blunt1, B.A.; Jergas1, M.; Genant1, H.K. Accurate Assessment of Precision Errors: How to Measure the Reproducibility of Bone Densitometry Techniques. Osteoporos. Int. 1995, 5, 262–270. [Google Scholar] [CrossRef]
- Maas, S.A.; Ellis, B.J.; Ateshian, G.A.; Weiss, J.A. FEBio: Finite Elements for Biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.T.; Lanyon, L.E. Regulation of Bone Mass by Mechanical Strain Magnitude. Calcif. Tissue Int. 1985, 37, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.J.; Lanyon, L.E. Mechanical Strain and Bone Cell Function: A Review. Osteoporos. Int. 2002, 13, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Fritsch, A.; Lahayne, O.; Kropik, K.; Redl, H.; Noailly, J.; Lacroix, D.; Hellmich, C. Anisotropic Tissue Elasticity in Human Lumbar Vertebra, by Means of a Coupled Ultrasound-Micromechanics Approach. Mater. Lett. 2012, 78, 154–158. [Google Scholar] [CrossRef]
- Rieger, R.; Auregan, J.C.; Hoc, T. Micro-Finite-Element Method to Assess Elastic Properties of Trabecular Bone at Micro- and Macroscopic Level. Morphologie 2018, 102, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, T.; Laughrey, L.E.; Niroobakhsh, M.; Lara-Castillo, N. Multiscale Finite Element Modeling of Mechanical Strains and Fluid Flow in Osteocyte Lacunocanalicular System. Bone 2020, 137, 115328. [Google Scholar] [CrossRef] [PubMed]
- Chappard, C.; Peyrin, F.; Bonnassie, A.; Lemineur, G.; Brunet-Imbault, B.; Lespessailles, E.; Benhamou, C.-L. Subchondral Bone Micro-Architectural Alterations in Osteoarthritis: A Synchrotron Micro-Computed Tomography Study. Osteoarthr. Cartil. 2006, 14, 215–223. [Google Scholar] [CrossRef]
- Ryan, M.K.; Oliviero, S.; Costa, M.C.; Wilkinson, J.M.; Dall’Ara, E. Heterogeneous Strain Distribution in the Subchondral Bone of Human Osteoarthritic Femoral Heads, Measured with Digital Volume Correlation. Materials 2020, 13, 4619. [Google Scholar] [CrossRef]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Müller, R.; Link, T.M.; Lochmüller, E.-M. Sex Differences of Human Trabecular Bone Microstructure in Aging Are Site-Dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Arlot, M.E.; Burt-Pichat, B.; Roux, J.-P.; Vashishth, D.; Bouxsein, M.L.; Delmas, P.D. Microarchitecture Influences Microdamage Accumulation in Human Vertebral Trabecular Bone. J. Bone Miner. Res. 2008, 23, 1613–1618. [Google Scholar] [CrossRef]
- Ito, M.; Nishida, A.; Aoyagi, K.; Uetani, M.; Hayashi, K.; Kawase, M. Effects of Risedronate on Trabecular Microstructure and Biomechanical Properties in Ovariectomized Rat Tibia. Osteoporos. Int. 2005, 16, 1042–1048. [Google Scholar] [CrossRef]
- Skedros, J.G.; Knight, A.N.; Farnsworth, R.W.; Bloebaum, R.D. Do Regional Modifications in Tissue Mineral Content and Microscopic Mineralization Heterogeneity Adapt Trabecular Bone Tracts for Habitual Bending? Analysis in the Context of Trabecular Architecture of Deer Calcanei. J. Anat. 2012, 220, 242–255. [Google Scholar] [CrossRef]
- Homminga, J.; Mccreadie, B.R.; Weinans, H.; Huiskes, R. The Dependence of the Elastic Properties of Osteoporotic Cancellous Bone on Volume Fraction and Fabric. J. Biomech. 2003, 36, 1461–1467. [Google Scholar] [CrossRef]
- Barak, M.M.; Black, M.A. A Novel Use of 3D Printing Model Demonstrates the Effects of Deteriorated Trabecular Bone Structure on Bone Stiffness and Strength. J. Mech. Behav. Biomed. Mater. 2018, 78, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Pallua, J.D.; Putzer, D.; Jäger, E.; Degenhart, G.; Arora, R.; Schmölz, W. Characterizing the Mechanical Behavior of Bone and Bone Surrogates in Compression Using PQCT. Materials 2022, 15, 5065. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Libonati, F.; Rinaudo, L.; Bellazzi, M.; Ulivieri, F.M.; Vergani, L. A New Finite Element Based Parameter to Predict Bone Fracture. PLoS ONE 2019, 14, e0225905. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Libonati, F.; Böhm, C.; Rinaudo, L.; Cesana, B.M.; Ulivieri, F.M.; Vergani, L. Fatigue-Caused Damage in Trabecular Bone from Clinical, Morphological and Mechanical Perspectives. Int. J. Fatigue 2020, 133, 105451. [Google Scholar] [CrossRef]
- Tassani, S.; Pani, M.; Noailly, J.; Gonzalez Ballester, M.A. Trabecular Fracture Zone Might Not Be the Higher Strain Region of the Trabecular Framework. Front. Mater. 2018, 5, 6. [Google Scholar] [CrossRef]
- Peyroteo, M.M.A.; Belinha, J.; Natal Jorge, R.M. Load Adaptation through Bone Remodeling: A Mechanobiological Model Coupled with the Finite Element Method. Biomech. Model. Mechanobiol. 2021, 20, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Nikodem, A. Correlations between structural and mechanical properties of human trabecular femur bone. Acta Bioeng. Biomech. 2012, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.B.; Torres, A.M.; Palomino, P.M.; Marty, E.; Saiyed, R.; Cohn, M.; Jo, J.; Warner, S.; Sroga, G.E.; King, K.B.; et al. Altered Tissue Composition, Microarchitecture, and Mechanical Performance in Cancellous Bone From Men With Type 2 Diabetes Mellitus. J. Bone Miner. Res. 2019, 34, 1191–1206. [Google Scholar] [CrossRef]
- Yu, Y.E.; Hu, Y.J.; Zhou, B.; Wang, J.; Guo, X.E. Microstructure Determines Apparent-Level Mechanics Despite Tissue-Level Anisotropy and Heterogeneity of Individual Plates and Rods in Normal Human Trabecular Bone. J. Bone Miner. Res. 2021, 36, 1796–1807. [Google Scholar] [CrossRef] [PubMed]


| R2 | BV/TV (%) | BS/BV (mm−1) | Tb.Th (mm) | Tb.N (mm−1) | Tb.Sp (mm) | SMI | DA | EF |
|---|---|---|---|---|---|---|---|---|
| BV/TV (%) | 1 | |||||||
| BS/BV (mm−1) | 0.26 | 1 | ||||||
| Tb.Th (mm) | 0.08 | 0.81 | 1 | |||||
| Tb.N (mm−1) | 0.76 | <0.01 | 0.05 | 1 | ||||
| Tb.Sp (mm) | 0.76 | 0.01 | <0.01 | 0.88 | 1 | |||
| SMI | 0.83 | 0.32 | 0.06 | 0.63 | 0.47 | 1 | ||
| DA | <0.01 | 0.01 | 0.03 | 0.01 | <0.01 | 0.01 | 1 | |
| EF | 0.45 | 0.14 | 0.11 | 0.26 | 0.37 | 0.33 | 0.04 | 1 |
| R2 | εz (%) | σz (MPa) | σe (MPa) | We (MPa) |
|---|---|---|---|---|
| EF | 0.46 | 0.48 | 0.44 | 0.55 |
| DA | 0.36 | 0.40 | 0.42 | 0.30 |
| BV/TV (%) | 0.29 | 0.28 | 0.26 | 0.26 |
| SMI | 0.32 | 0.22 | 0.23 | 0.28 |
| Tb.Sp (mm) | 0.26 | 0.26 | 0.27 | 0.25 |
| Tb.N (mm−1) | 0.16 | 0.14 | 0.14 | 0.13 |
| BS/BV (mm−1) | 0.14 | 0.14 | 0.10 | 0.12 |
| Tb.Th (mm) | 0.11 | 0.12 | 0.09 | 0.11 |
| εz | −0.016 | −0.067 | −0.117 | 1.74 |
| σz | −1.3 | −10.6 | −17.3 | 1.63 |
| σe | 4.3 | 9.4 | 14.0 | 1.49 |
| We | 0.0009 | 0.0054 | 0.0120 | 2.29 |
| Refs. | Species | Sampling Site | Sample Number | Sex | Age | Pathology | Microarchitectural Parameters | Mechanical Parameters |
|---|---|---|---|---|---|---|---|---|
| Macroscopic scale | ||||||||
| [13] | Human | Femoral heads | 77 | 29 M, 48 F | 55–87 years old | OP | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, SMI, DA | Maximum stress, elasticity stress, modules of elasticity, microcrack surface density |
| 25 | 10 M, 15 F | 56–78 years old | OA | BV/TV, BS/BV, Tb.Th, Tb.Sp, Tb.N, SMI, DA | Maximum stress, elasticity stress, modules of elasticity, microcrack surface density | |||
| [14] | Human | Lumbar spines (L1–L5) | 21 | 11 M, 10 F | 65–86 years old | 11 normal, 10 OA | BV/TV, Tb.N, SMI | Initial failure load, initial stiffness, post-fracture load, post-fracture stiffness |
| [19] | Human | Spine, femur | 41 | 8 M, 7 F, | 36–83 years old | Metastasis cancer | BV/TV, BS/TV, BS/BV, SMI, Tb.N, Tb.Th, DA | Modulus of elasticity, yield strain, yield strength |
| 96 | 22 M, 21 F | 23–93 years old | Noncancer | BV/TV, BS/TV, BS/BV, SMI, Tb.N, Tb.Th, DA | Modulus of elasticity, yield strain, yield strength | |||
| [39] | Human | Femoral heads | 42 | 7 M, 35 F | 68–92 years old | OP | BV/TV | Modulus of elasticity, yield stress |
| [44] | Human | L2 vertebrae | 23 | 8 M, 15 F | 54–93 years old | / | BV/TV, Tb.Th, Tb.N, Tb.Sp, SMI, DA | Microcrack density, mean crack length, diffuse damage area |
| [45] | Rat | Left tibiae | 10 | F | 18 weeks old | Sham operated | BV/TV, Tb.Th, Tb.Sp, Tb.N, SMI, DA | Modulus of elasticity, Shear modulus |
| 40 | F | 18 weeks old | Ovariectomy | BV/TV, Tb.Th, Tb.Sp, Tb.N, SMI, DA | Modulus of elasticity, Shear modulus | |||
| [47] | Human | Femoral heads | 32 | F | 62–88 years old | / | BV/TV | Modulus of elasticity, stiffness |
| 26 | F | 73–87 years old | OP | BV/TV | Modulus of elasticity, stiffness | |||
| [51] | Porcine | Vertebrae | 30 | / | 12–18 months old | / | BV/TV, BS/BV, Tb.Th, DA | Elastic stiffness, stress amplitude, fatigue life, accumulated damage |
| [54] | Human | Femur epiphyses | 4 | / | 42–79 years old | Normal | BV/TV, Tb.N, Tb.Th, Tb.Sp, SMI, DA | Modulus of elasticity, ultimate stress, mechanical anisotropy |
| Femoral heads | 7 | / | 42–79 years old | OP | BV/TV, Tb.N, Tb.Th, Tb.Sp, SMI, DA | Modulus of elasticity, ultimate stress, mechanical anisotropy | ||
| Femoral heads | 12 | / | 42–79 years old | QA | BV/TV, Tb.N, Tb.Th, Tb.Sp, SMI, DA | Modulus of elasticity, ultimate stress, mechanical anisotropy | ||
| [55] | Human | Femoral heads and necks | 45 | M | 52–71 years old | OA, Type 1 diabetes | BV/TV | Modulus of elasticity, yield stress, ultimate stress, post-yield toughness, toughness |
| Microscopic scale | ||||||||
| [28] | Human | Humeral heads | 6 | 3 M, 3 F | 54–82 years old | OA | BV/TV, Tb.N, Tb.Sp | Third principal strain |
| [29] | Mouse | Left tibia | 7 | F | 16 weeks | / | BV/TV, Tb.Th, Tb.Sp, Tb.N | Maximal principal strain, minimal principal strain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Rouquier, L.; Chappard, C.; Bachy, M.; Huang, X.; Potier, E.; Bensidhoum, M.; Hoc, T. A New Microarchitecture-Based Parameter to Predict the Micromechanical Properties of Bone Allografts. Materials 2023, 16, 3349. https://doi.org/10.3390/ma16093349
Xiong Z, Rouquier L, Chappard C, Bachy M, Huang X, Potier E, Bensidhoum M, Hoc T. A New Microarchitecture-Based Parameter to Predict the Micromechanical Properties of Bone Allografts. Materials. 2023; 16(9):3349. https://doi.org/10.3390/ma16093349
Chicago/Turabian StyleXiong, Zhuang, Léa Rouquier, Christine Chappard, Manon Bachy, Xingrong Huang, Esther Potier, Morad Bensidhoum, and Thierry Hoc. 2023. "A New Microarchitecture-Based Parameter to Predict the Micromechanical Properties of Bone Allografts" Materials 16, no. 9: 3349. https://doi.org/10.3390/ma16093349
APA StyleXiong, Z., Rouquier, L., Chappard, C., Bachy, M., Huang, X., Potier, E., Bensidhoum, M., & Hoc, T. (2023). A New Microarchitecture-Based Parameter to Predict the Micromechanical Properties of Bone Allografts. Materials, 16(9), 3349. https://doi.org/10.3390/ma16093349







