Up to 100% Replacement of Natural Materials from Residues: Recycling Blast Furnace Slag and Fly Ash as Self-Leveling Cementitious Building Materials
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Methods
2.2.1. Chemical and Mineralogical Characterization
2.2.2. Mechanical Strength Measurements
3. Results and Discussion
3.1. Mineralogical Characterization
3.1.1. Development of Hydrated Phases
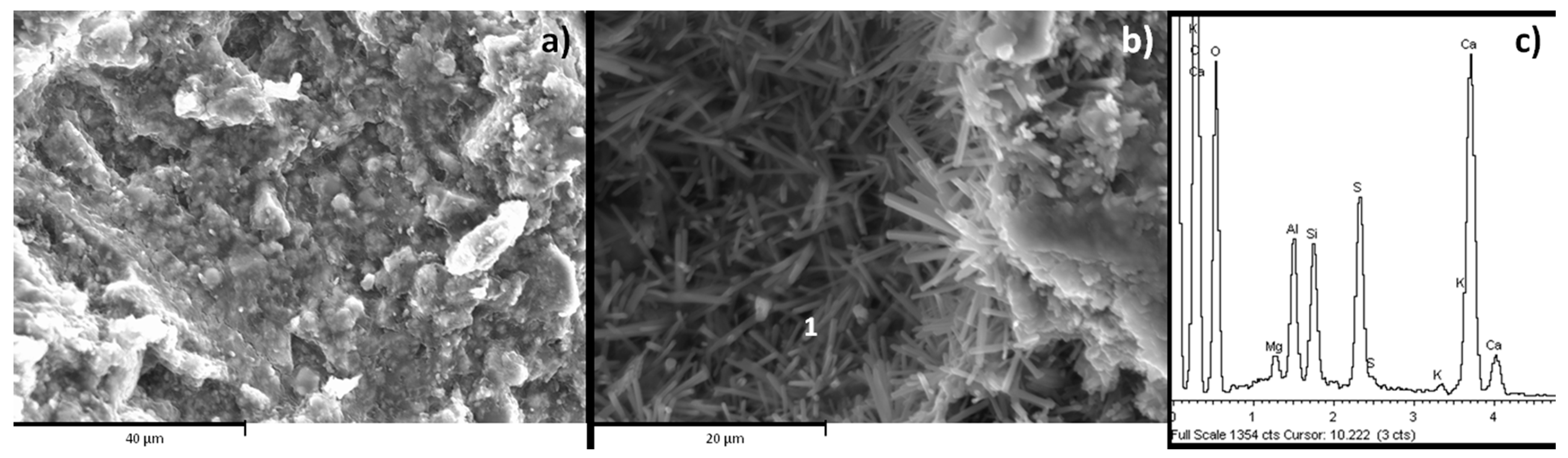
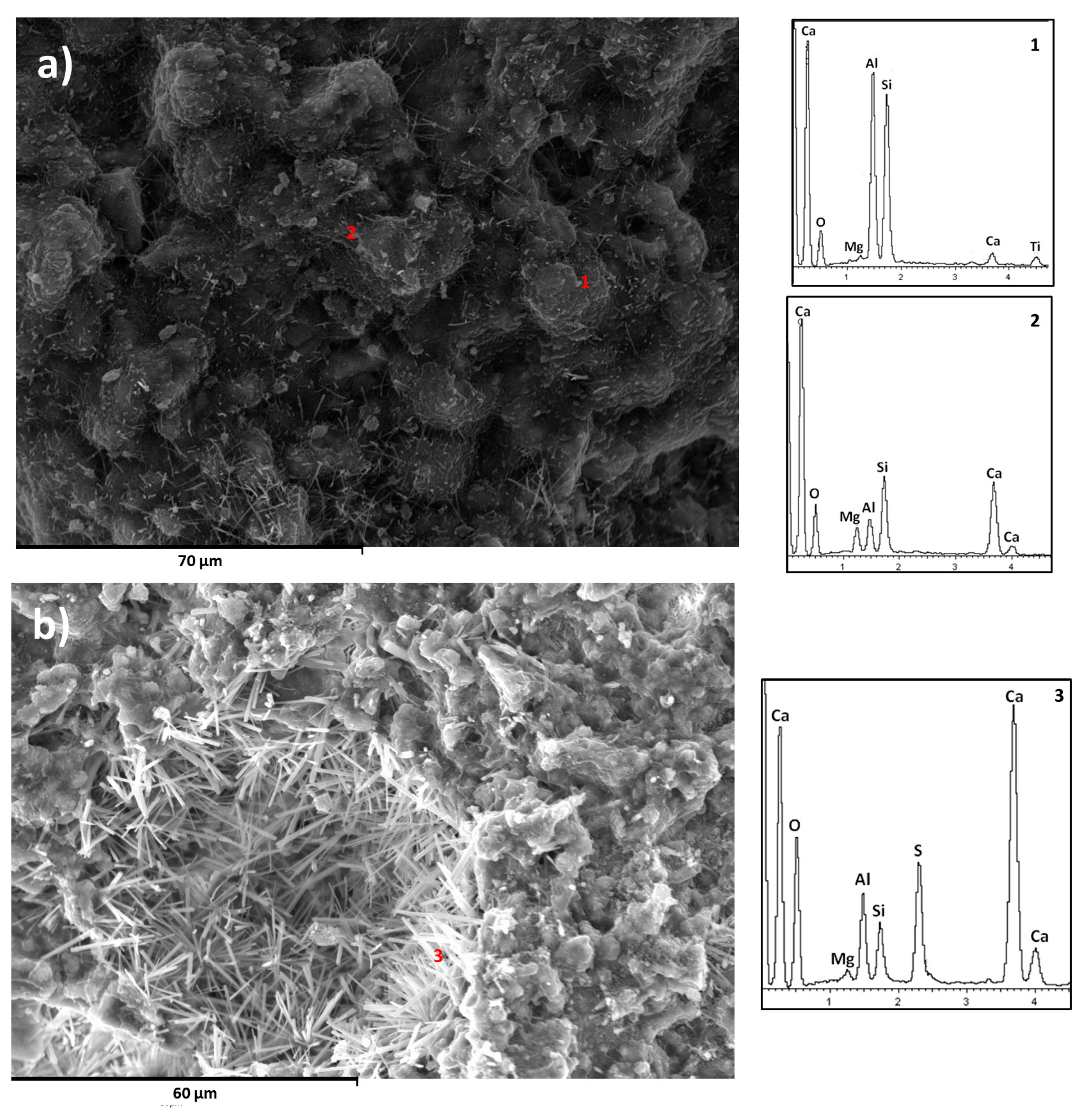
3.1.2. Morphologies and Element Composition of the Developed Phases
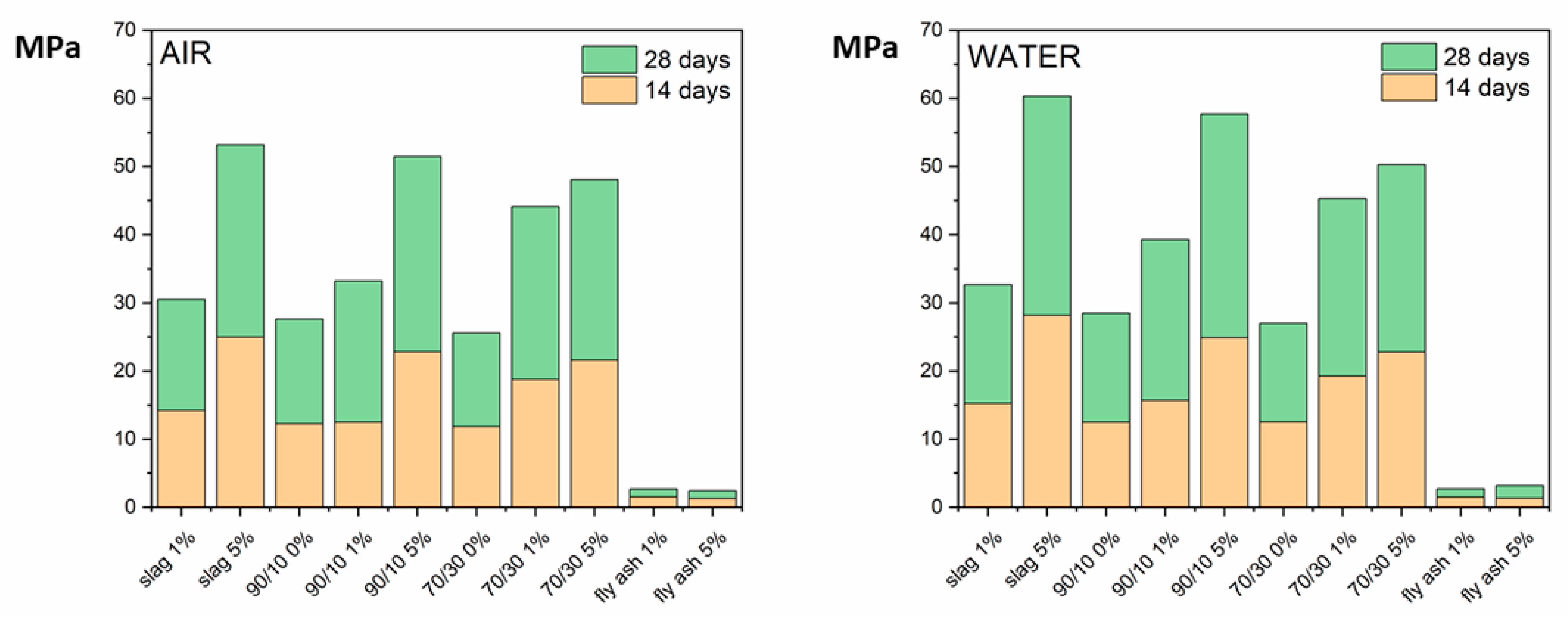
3.2. Mechanical Strength Behavior and Setting Times
3.3. Comparison of Results with PC/CAC/C Systems from Natural Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torréns-Martín, D. Mezclas Ternarias de Cemento Portland, Cemento de Aluminato de Calcio y Sulfato Cálcico: Mecanismos de Expansión. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2013. [Google Scholar]
- Bayoux, J.; Bonin, A.; Verschaeve, M. Study of the hydration properties of aluminous cement and calcium sulphate mixes. In Calcium Aluminate Cements; E&FN SPON: London, UK, 2001. [Google Scholar]
- WRI. New Climate CA, State of Climate Action. 2022. Available online: https://gccassociation.org/concretefuture/getting-to-net-zero/ (accessed on 3 April 2023).
- WBCSD. Cement Sustainability Initiative Right Getting the Numbers. Available online: http://www.wbcsdcement.org/index.php/key-issues/climate-protection/gnr-database (accessed on 3 May 2022).
- Scrivener, K.; John, V.M.; Gartner, E.M.; Environment UN. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 26. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022. [Google Scholar]
- Torréns-Martín, D.; Fernandez-Carrasco, L.; Blanco-Varela, M.T. Conduction calorimetric studies of ternary binders based on Portland cement, calcium aluminate cement and calcium sulphate. J. Therm. Anal. Calorim. 2013, 114, 799–807. [Google Scholar] [CrossRef]
- Torréns-Martín, D.; Fernandez-Carrasco, L. Effect of sulfate content on cement mixtures. Constr. Build. Mater. 2013, 48, 144–150. [Google Scholar] [CrossRef]
- Macphee, D.E. Sustainable cementiteous binders: New chemistries, new performance. Advanced in Cement and Concrete X. Sustainability. In Proceedings of the ECI Engineering Conferences International, Davos, Switherland, 7–12 September 2006. [Google Scholar]
- Moranville-Regourd, M. Cements Made from Blastfurnace Slag. In Lea’s Chemistry of Cement and Concrete; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2004; pp. 471–635. [Google Scholar]
- Massazza, F. Pozzolana and Pozzolanic Cements. In Lea’s Chemistry of Cement and Concrete; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2004; pp. 637–678. [Google Scholar]
- Jackson, I. Portland Cement: Classification and Manufacture. In Lea’s Chemistry of Cement and Concrete; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2004; pp. 27–94. [Google Scholar]
- Hwang, C.; Shen, D. The effects of blast-furnace slag and fly ash on the hydration of Portland cement. Cem. Concr. Res. 1991, 21, 410–425. [Google Scholar] [CrossRef]
- Douglas, E.; Pouskouleli, G. Prediction of compressive strength of mortars made with Portland cement-blast-furnace slag-fly ash blends. Cem. Concr. Res. 1991, 21, 523–534. [Google Scholar] [CrossRef]
- Hale, W.; Freyne, S.; Bush, T.; Russell, B. Properties of concrete mixtures containing slag cement and fly ash for use in transportation structures. Constr. Build. Mater. 2008, 22, 1990–2000. [Google Scholar] [CrossRef]
- Rupnow, T.; Wang, K.; Schaefer, V.; Tikalsky, P. A simple method for characterizing and predicting temperature behavior of ternary cementitious systems. Constr. Build. Mater. 2011, 25, 2290–2297. [Google Scholar] [CrossRef]
- Wang, X.; Cho, H.; Lee, H. Prediction of temperature distribution in concrete incorporating fly ash or slag using a hydration model. Compos. Part B: Eng. 2011, 42, 27–40. [Google Scholar] [CrossRef]
- Majumdar, A.; Edmonds, R.; Singh, B. Hydration of calcium aluminates cements in presence of granulated blastfurnace slag. In Calcium Aluminate Cement; E&FN SPON: London, UK, 1990; pp. 259–271. [Google Scholar]
- Fentiman, C.; Rashid, S.; Bayoux, J.; Bonin, A.; Testud, M. The effect of curing conditions on the hydration and strength development in Fondu: Slag. In Calcium Aluminate Cement; E&FN SPON: London, UK, 1990; pp. 272–281. [Google Scholar]
- Richardson, G.; Groves, G. The microstructure of blastfurnace slag/high alumina cement pastes. In Calcium Aluminate Cement; E&FN SPON: London, UK, 1990; pp. 282–293. [Google Scholar]
- Lamberet, S. Durability of Ternary Binders Based on Portland Cement, Calcium Aluminate Cement and Calcium Sulphate. Ph.D. Thesis, Ècole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2005. These no. 3151. [Google Scholar]
- Fernández-Carrasco, L. Calcium Aluminate Cement with Supplementary Cementitious Materials: Fly Ashes. In Proceedings of the Calcium Aluminate Cements: Centenary Conference, Avignon, France, 30 June 2008. [Google Scholar]
- Fernández-Carrasco, L. Reactions of fly ash with calcium aluminate cement and calcium sulphate. Fuel 2009, 88, 1533–1538. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cement Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Li, D.; Shen, J.; Chen, Y.; Cheng, L.; Wu, X. Study of properties on fly ash-slag complex cement. Cem. Concr. Res. 2000, 30, 1381–1387. [Google Scholar] [CrossRef]
- Frigione, R.; Sersale, R. Influence of gypsum content on properties of high-slag blast furnace cements. Am. Ceram. Soc. Bull. 1976, 55, 407. [Google Scholar]
- Frigione, R.; Sersale, R. Gypsum blast-furnace slag cements. Am. Ceram. Soc. Bull. 1983, 62, 1275–1279. [Google Scholar]
- Singh, M.; Garg, M. Activation of gypsum anhydrite-slag mixtures. Cem. Concr. Res. 1995, 25, 332–338. [Google Scholar] [CrossRef]
- Singh, M. Influence of blended gypsum on the properties of Portland cement and Portland slag cement. Cem. Concr. Res. 2000, 30, 1185–1188. [Google Scholar] [CrossRef]
- Aly, T.; Sanjayan, J. Effect of gypsum on free and restrained shrinkage behaviour of slag-concretes subjected to various curing conditions. Mater. Struct. 2008, 41, 1393–1403. [Google Scholar] [CrossRef]
- Melo-Neto, A.; Cincotto, M.; Repette, W. Mechanical properties, drying and autogenous shrinkage of blast furnace slag activated with hydrated lime and gypsum. Cem. Concr. Compos. 2010, 32, 312–318. [Google Scholar] [CrossRef]
- Michel, M.; Georgin, J.; Ambroise, J.; Péra, J. The influence of gypsum ratio on the mechanical performance of slag cement accelerated by calcium sulfoaluminate cement. Constr. Build. Mater. 2011, 25, 1298–1304. [Google Scholar] [CrossRef]
- Poon, C.; Kou, S.; Lam, L.; Lin, Z. Activation of fly ash/cement systems using calcium sulfate anhydrite (CaSO4). Cem. Concr. Res. 2001, 31, 873–881. [Google Scholar] [CrossRef]
- Sivapullaiah, P.; Moghal, A. Role of gypsum in the strength development of fly ash with lime. J. Mater. Civ. Eng. 2011, 23, 197–206. [Google Scholar] [CrossRef]
- Torrens-Martín, D.; Winnefeld, F.; Fernández-Carrasco, L.J. Thermodynamic model for ternary OPC/CAC/Calcium Sulfate binders. Constr. Build. Mater. 2021, 302, 124120. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Koch, A.; Steinegger, H. A rapid method for testing the resistance of cements to sulphate attack. Zem Kalk Gips 1960, 7, 317–324. [Google Scholar]
- Schilling, P.J.; Butler, L.G.; Roy, A.; Eaton, H.C. 29Si and 27Al MAS-NMR of NaOH activated Blast-Furnace Slag. J. Am. Ceram. Soc. 1994, 77, 2363–2368. [Google Scholar] [CrossRef]
- Standard UNE-EN 196-3:2005 + A1:2009; Methods of Testing Cement-Part 3: Determination of Setting Times and Soundness. UNE-EN: Madrid, Spain, 2009.
- Mandal, P.; Mandal, T. Anion water in gypsum (CaSO4·2H2O) and hemihydrate (CaSO4·1/2H2O). Cem. Concr. Res. 2002, 32, 313–316. [Google Scholar] [CrossRef]
- Fernandez-Carrasco, L.; Torrens, D.; Morales, L.; Martinez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy; IntechOpen: London, UK, 2012. [Google Scholar]
- Fu, X.; Hou, W.; Yang, C.; Li, D.; Wu, X. Studies on high-strength slag and fly ash compound cement. Cem. Concr. Res. 2000, 30, 1239–1243. [Google Scholar] [CrossRef]
- Zhao, F.; Ni, W.; Wang, H.; Liu, H. Activated fly ash/slag blended cement. Resour. Conserv. Recycl. 2007, 52, 303–313. [Google Scholar] [CrossRef]
- Ben Haha, M.; De Weerdt, K.; Lothenbach, B. Quantification of the degree of reaction of fly ash. Cem. Concr. Res. 2010, 40, 1620–1629. [Google Scholar] [CrossRef]
- Morales-Florez, V.; Rosa-Fox, N. Structure of supercritically dried calcium silicate hydrates (C-S-H) and structural changes induced by weathering. J. Mater. Sci. 2013, 48, 5022–5028. [Google Scholar] [CrossRef]
- Scrivener, K.; Capmas, A. Calcium aluminate cements. In Lea’s Chemistry of Cement and Concrete; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2004; pp. 713–782. [Google Scholar]
- Gawlicki, M.; Nocun-Wczelik, W.; Bak, Ł. Calorimetry in the studies of cement hydration setting and hardening. J. Therm. Anal. Calorim. 2010, 100, 571–576. [Google Scholar] [CrossRef]
- Mathur, P.C. Study of Cementitious Materials Using Transmission Electron Microscopy. Ph.D. Thesis, Ècole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2007. [Google Scholar]
- Xu, L.; Wang, P.; Zhang, G. Formation of ettringite in Portland cement/calcium aluminate cement/calcium sulfate ternary system hydrates at lower temperatures. Constr. Build. Mater. 2012, 31, 347–352. [Google Scholar] [CrossRef]
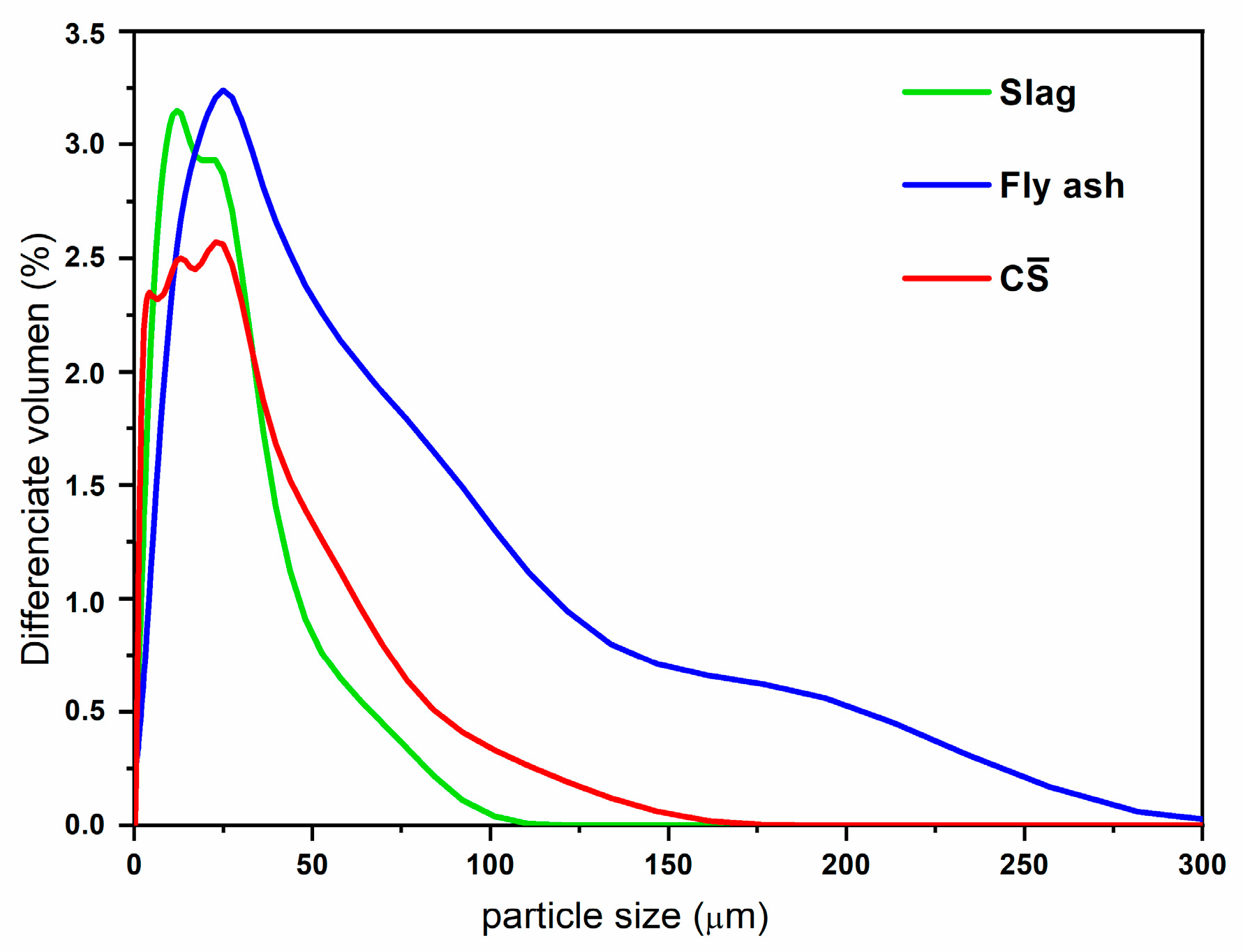
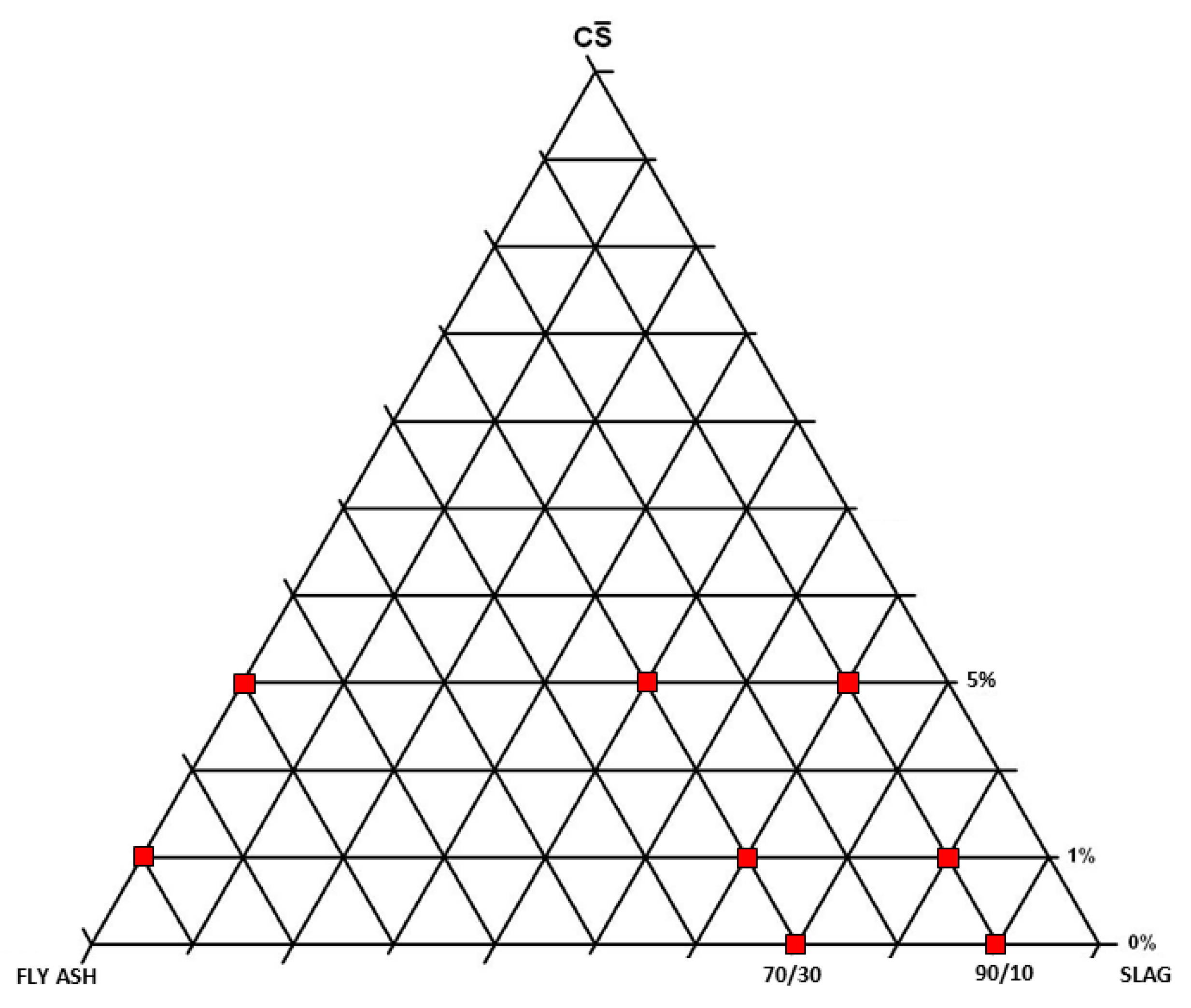


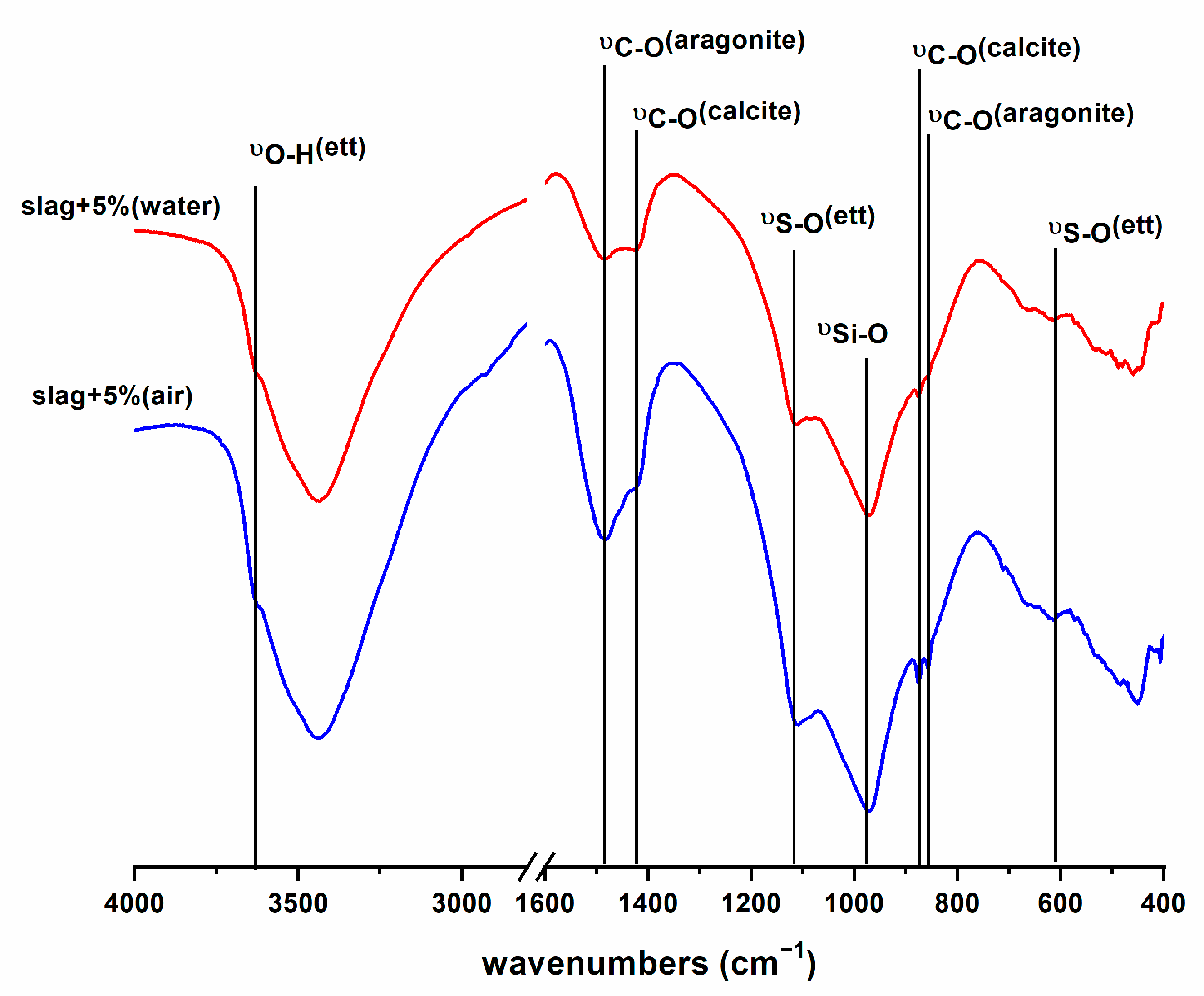

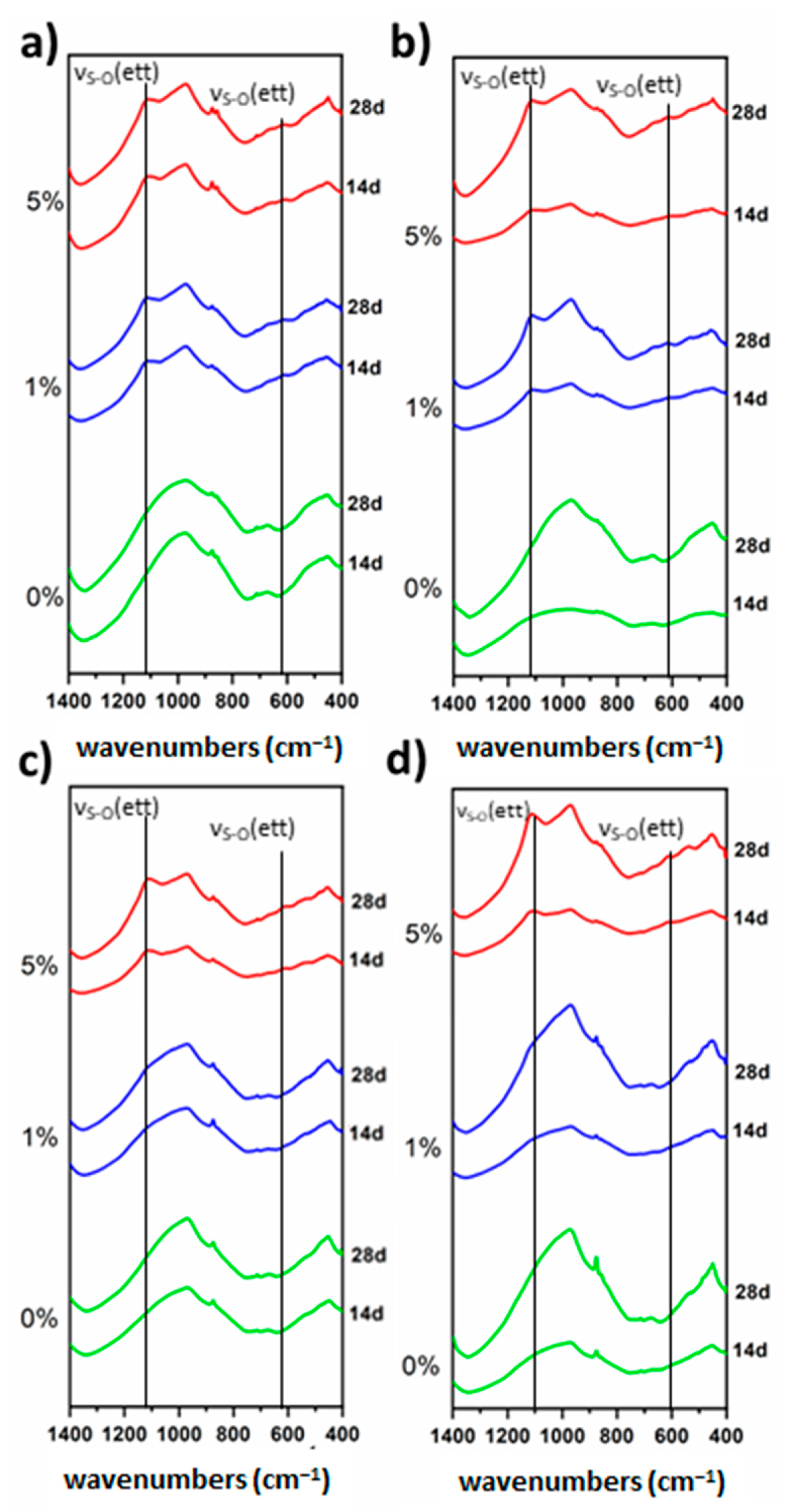


| SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | MgO | TiO2 | MnO | K2O | SrO | P2O5 | Na2O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 35.01 | 11.35 | 41.27 | 0.51 | - | 8.68 | 0.53 | 0.22 | 0.39 | - | 0.04 | <1 |
| Fly ash | 48.12 | 30.36 | 7.91 | 3.24 | 0.39 | 1.9 | 1.45 | - | 0.59 | 0.36 | 1.81 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torréns-Martín, D.; Fernández-Carrasco, L.J.; Blanco-Varela, M.T. Up to 100% Replacement of Natural Materials from Residues: Recycling Blast Furnace Slag and Fly Ash as Self-Leveling Cementitious Building Materials. Materials 2023, 16, 3350. https://doi.org/10.3390/ma16093350
Torréns-Martín D, Fernández-Carrasco LJ, Blanco-Varela MT. Up to 100% Replacement of Natural Materials from Residues: Recycling Blast Furnace Slag and Fly Ash as Self-Leveling Cementitious Building Materials. Materials. 2023; 16(9):3350. https://doi.org/10.3390/ma16093350
Chicago/Turabian StyleTorréns-Martín, David, Lucía J. Fernández-Carrasco, and María Teresa Blanco-Varela. 2023. "Up to 100% Replacement of Natural Materials from Residues: Recycling Blast Furnace Slag and Fly Ash as Self-Leveling Cementitious Building Materials" Materials 16, no. 9: 3350. https://doi.org/10.3390/ma16093350
APA StyleTorréns-Martín, D., Fernández-Carrasco, L. J., & Blanco-Varela, M. T. (2023). Up to 100% Replacement of Natural Materials from Residues: Recycling Blast Furnace Slag and Fly Ash as Self-Leveling Cementitious Building Materials. Materials, 16(9), 3350. https://doi.org/10.3390/ma16093350






