Comparative Verification of the Accuracy of Implant Models Made of PLA, Resin, and Silicone
Abstract
1. Introduction
2. Materials and Methods
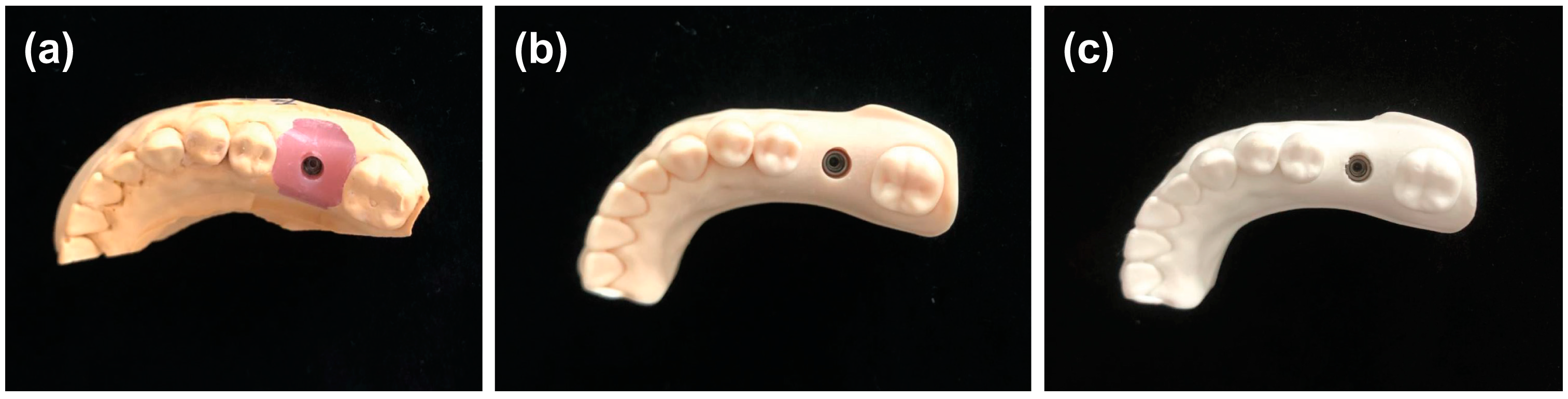
2.1. STL Data Acquisition for Plaster Implant Models
2.2. STL Date Acquisition for Resin Implant Models
2.3. STL Data Acquisition for PLA Implant Models
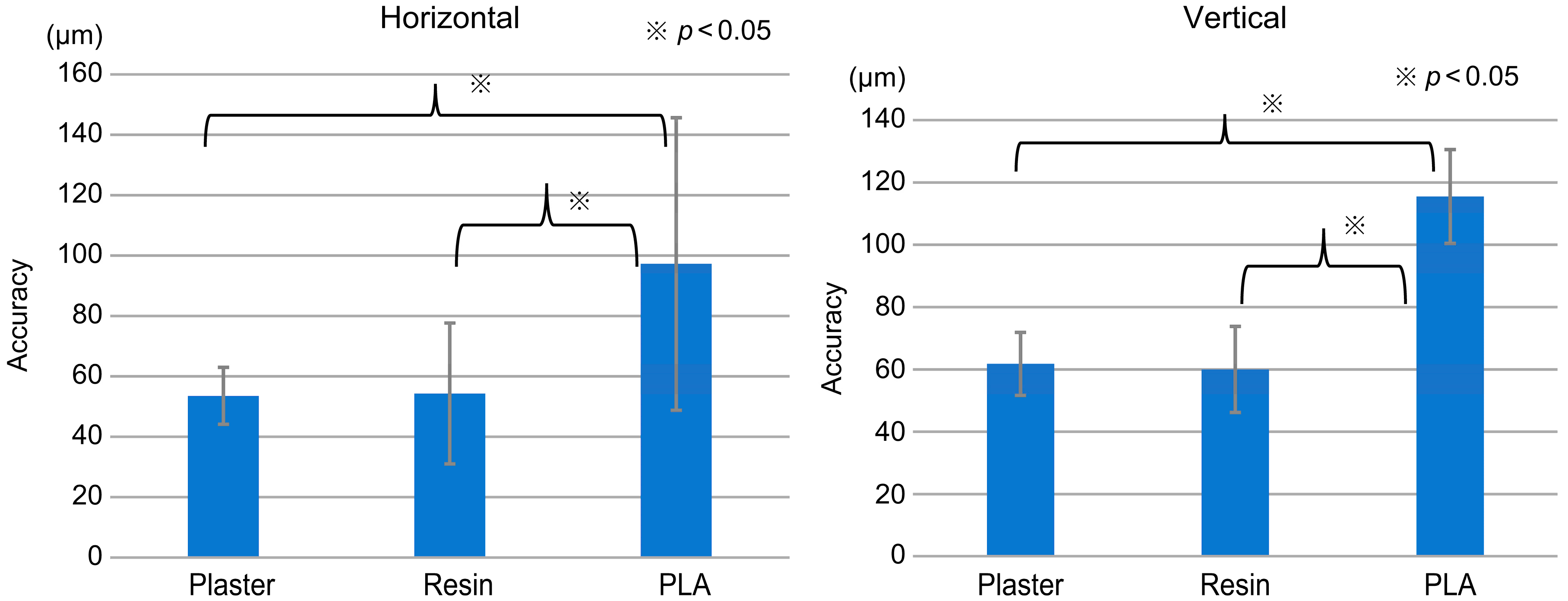
2.4. Measurement of Accuracy
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 16, 50–55. [Google Scholar] [CrossRef]
- Glauser, R.; Ree, A.; Lundgren, A.; Gottlow, J.; Hammerle, C.H.; Scharer, P. Immediate Occlusal Loading of Brånemark Implants Applied in Various Jawbone Regions: A Prospective, 1-Year Clinical Study. Clin. Implant. Dent. Relat. Res. 2001, 3, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Schmid, B.; Belser, U.C.; Lussi, A.; Buser, D. Early loading of non-submerged titanium implants with a sandblasted and acid-etched surface. 5-year results of a prospective study in partially edentulous patients. Clin. Oral Implant. Res. 2005, 16, 631–638. [Google Scholar] [CrossRef]
- Bergkvist, G.; Koh, K.-J.; Sahlholm, S.; Klintström, E.; Lindh, C. Bone density at implant sites and its relationship to assessment of bone quality and treatment outcome. Int. J. Oral Maxillofac. Implant. 2010, 25, 321–328. [Google Scholar]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implant. 2007, 22, 755–760. [Google Scholar]
- Cheng, Y.-C.; Ewers, R.; Morgan, K.; Hirayama, M.; Murcko, L.; Morgan, J.; Bergamo, E.T.P.; Bonfante, E.A. Antiresorptive therapy and dental implant survival: An up to 20-year retrospective cohort study in women. Clin. Oral Investig. 2022, 26, 6569–6582. [Google Scholar] [CrossRef]
- Andrade, C.A.S.; Paz, J.L.C.; de Melo, G.S.; Mahrouseh, N.; Januário, A.L.; Capeletti, L.R. Survival rate and peri-implant evaluation of immediately loaded dental implants in individuals with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Chackartchi, T.; Romanos, G.E.; Parkanyi, L.; Schwarz, F.; Sculean, A. Reducing errors in guided implant surgery to optimize treatment outcomes. Periodontology 2000 2022, 88, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Derksen, W.; Wismeijer, D.; Flügge, T.; Hassan, B.; Tahmaseb, A. The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin. Oral Implant. Res. 2019, 30, 1005–1015. [Google Scholar] [CrossRef]
- Wang, X.; Shaheen, E.; Shujaat, S.; Meeus, J.; Legrand, P.; Lahoud, P.; Gerhardt, M.D.N.; Politis, C.; Jacobs, R. Influence of experience on dental implant placement: An in vitro comparison of freehand, static guided and dynamic navigation approaches. Int. J. Implant. Dent. 2022, 8, 42. [Google Scholar] [CrossRef]
- Russo, L.L.; Caradonna, G.; Biancardino, M.; De Lillo, A.; Troiano, G.; Guida, L. Digital versus conventional workflow for the fabrication of multiunit fixed prostheses: A systematic review and meta-analysis of vertical marginal fit in controlled in vitro studies. J. Prosthet. Dent. 2019, 122, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Özcan, N.; Aydin, D.H.; Şahin, E.; Akça, K. Effect of digitizing techniques on the fit of implant-retained crowns with different antirotational abutment features. J. Prosthet. Dent. 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Nagata, K.; Fuchigami, K.; Okuhama, Y.; Wakamori, K.; Tsuruoka, H.; Nakashizu, T.; Hoshi, N.; Atsumi, M.; Kimoto, K.; Kawana, H. Comparison of digital and silicone impressions for single-tooth implants and two- and three-unit implants for a free-end edentulous saddle. BMC Oral Health 2021, 21, 464. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Levi, N. Poor performance in municipal recycling: The case of Chile. Waste Manag. 2021, 133, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Varzakas, T.; Tzoutzas, I. Circular Economy in Conjunction with Treatment Methodologies in the Biomedical and Dental Waste Sectors. Circ. Econ. Sustain. 2021, 1, 563–592. [Google Scholar] [CrossRef]
- Mandalidis, A.; Topalidis, A.; Voudrias, E.A.; Iosifidis, N. Composition, production rate and characterization of Greek dental solid waste. Waste Manag. 2018, 75, 124–130. [Google Scholar] [CrossRef]
- Koolivand, A.; Gholami-Borujeni, F.; Nourmoradi, H. Investigation on the characteristics and management of dental waste in Urmia, Iran. J. Mater. Cycles Waste Manag. 2015, 17, 553–559. [Google Scholar] [CrossRef]
- Sabbahi, D.A.; El-Naggar, H.M.; Zahran, M.H. Management of dental waste in dental offices and clinics in Jeddah, Saudi Arabia. J. Air Waste Manag. Assoc. 2020, 70, 1022–1029. [Google Scholar] [CrossRef]
- Papi, P.; Di Murro, B.; Penna, D.; Pompa, G. Digital prosthetic workflow during COVID-19 pandemic to limit infection risk in dental practice. Oral Dis. 2020, 27, 723–726. [Google Scholar] [CrossRef]
- Frahdian, T.; Hasratiningsih, Z.; Karlina, E.; Herdiyantoro, D.; Takarini, V. Dental alginate impression waste as additional fertiliser for plant yields and soil quality. Padjadjaran J. Dent. 2018, 30, 12. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of build direction on the mechanical properties of 3D-printed complete coverage interim dental restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef]
- Ishida, Y.; Miura, D.; Miyasaka, T.; Shinya, A. Dimensional Accuracy of Dental Casting Patterns Fabricated Using Consumer 3D Printers. Polymers 2020, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Shin, Y.-S.; Jung, H.-D.; Hwang, C.-J.; Baik, H.-S.; Cha, J.-Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Borrello, J.; Nasser, P.; Iatridis, J.C.; Costa, K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit. Manuf. 2018, 23, 374–380. [Google Scholar] [CrossRef]
- Maspero, C.; Tartaglia, G.M. 3D Printing of Clear Orthodontic Aligners: Where We Are and Where We Are Going. Materials 2020, 13, 5204. [Google Scholar] [CrossRef]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery—An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Arifin, Z. A review on the fused deposition modeling (FDM) 3D printing: Filament processing, materials, and printing parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Perales, E.; Huraibat, K.; Martínez-Verdú, F.M.; Viqueira, V. Maximization of FDM-3D-Objects Gonio-Appearance Effects Using PLA and ABS Filaments and Combining Several Printing Parameters: “A Case Study”. Materials 2019, 12, 1423. [Google Scholar] [CrossRef]
- D’anna, A.; Arrigo, R.; Frache, A. PLA/PHB Blends: Biocompatibilizer Effects. Polymers 2019, 11, 1416. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Gai, M.; Li, W.; Frueh, J.; Sukhorukov, G.B. Polylactic acid sealed polyelectrolyte complex microcontainers for controlled encapsulation and NIR-Laser based release of cargo. Colloids Surf. B Biointerfaces 2018, 173, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, T.; Chen, Y.; Gao, X.; Ye, J.; Jin, Y.; Chen, B. Oriented homo-epitaxial crystallization of polylactic acid displaying a biomimetic structure and improved blood compatibility. J. Biomed. Mater. Res. A 2021, 110, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.; Kumari, N.; Srivastava, A.; Bhati, P.; Vashisth, P.; Yadav, P.; Jacob, T.; Narang, R.; Bhatnagar, N. Biocompatibility analysis of PLA based candidate materials for cardiovascular stents in a rat subcutaneous implant model. Acta Histochem. 2020, 122, 151615. [Google Scholar] [CrossRef] [PubMed]
- Benli, M.; Eker-Gümüş, B.; Kahraman, Y.; Huck, O.; Özcan, M. Can polylactic acid be a CAD/CAM material for provisional crown restorations in terms of fit and fracture strength? Dent. Mater. J. 2021, 40, 772–780. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Canals, S.; Gómez-Polo, M.; Solá-Ruiz, M.; Highsmith, J.D.R.; Viñuela, A. Polylactic Acid as a Material for Three-Dimensional Printing of Provisional Restorations. Int. J. Prosthodont. 2018, 31, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Crenn, M.-J.; Rohman, G.; Fromentin, O.; Benoit, A. Polylactic acid as a biocompatible polymer for three-dimensional printing of interim prosthesis: Mechanical characterization. Dent. Mater. J. 2022, 41, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Park, J.-M.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y. Flexural Strength of 3D-Printing Resin Materials for Provisional Fixed Dental Prostheses. Materials 2020, 13, 3970. [Google Scholar] [CrossRef]
- Koske, D.; Ehrmann, A. Advanced Infill Designs for 3D Printed Shape-Memory Components. Micromachines 2021, 12, 1225. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Zhang, L.; Tang, Z.; Zhang, R.; Zhu, J. Isosorbide dioctoate as a “green” plasticizer for poly(lactic acid). Mater. Des. 2016, 91, 262–268. [Google Scholar] [CrossRef]
- Muta, S.; Ikeda, M.; Nikaido, T.; Sayed, M.; Sadr, A.; Suzuki, T.; Tagami, J. Chairside fabrication of provisional crowns on FDM 3D-printed PVA model. J. Prosthodont. Res. 2020, 64, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, J. Evaluation of Precision of Custom Edentulous Trays Fabricated with 3D Printing Technologies. Int. J. Prosthodont. 2021, 34, 109–117. [Google Scholar] [CrossRef]
- Lagazzo, A.; Moliner, C.; Bosio, B.; Botter, R.; Arato, E. Evaluation of the Mechanical and Thermal Properties Decay of PHBV/Sisal and PLA/Sisal Biocomposites at Different Recycle Steps. Polymers 2019, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Maniadi, A.; Velidakis, E.; Mountakis, N.; Kechagias, J.D. Sustainable Additive Manufacturing: Mechanical Response of Polyamide 12 over Multiple Recycling Processes. Materials 2021, 14, 466. [Google Scholar] [CrossRef]
- Anderson, I. Mechanical Properties of Specimens 3D Printed with Virgin and Recycled Polylactic Acid. 3D Print. Addit. Manuf. 2017, 4, 110–115. [Google Scholar] [CrossRef]
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical Recycling of Post-Consumer PLA Waste for Sustainable Production of Ethyl Lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Hegedus, T.; Kreuter, P.; Kismarczi-Antalffy, A.A.; Demeter, T.; Banyai, D.; Vegh, A.; Geczi, Z.; Hermann, P.; Payer, M.; Zsembery, A.; et al. User Experience and Sustainability of 3D Printing in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 1921. [Google Scholar] [CrossRef]
- Nagata, K.; Muromachi, K.; Kouzai, Y.; Inaba, K.; Inoue, E.; Fuchigami, K.; Nihei, T.; Atsumi, M.; Kimoto, K.; Kawana, H. Fit accuracy of resin crown on a dental model fabricated using fused deposition modeling 3D printing and a polylactic acid filament. J. Prosthodont. Res. 2023, 67, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, V.; Trovalusci, F.; Guarino, S.; Venettacci, S. Environmental and Economic Analysis of FDM, SLS and MJF Additive Manufacturing Technologies. Materials 2019, 12, 4161. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Brägger, U. Time-efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clin. Oral Implant. Res. 2016, 27, 1401–1406. [Google Scholar] [CrossRef]
- Mühlemann, S.; Greter, E.A.; Park, J.-M.; Hämmerle, C.H.F.; Thoma, D.S. Precision of digital implant models compared to conventional implant models for posterior single implant crowns: A within-subject comparison. Clin. Oral Implant. Res. 2018, 29, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Chen, Y.-W.; Hayashi, J.; Sadr, A. Accuracy of CAD/CAM Digital Impressions with Different Intraoral Scanner Parameters. Sensors 2020, 20, 1157. [Google Scholar] [CrossRef]
- Ren, S.; Jiang, X.; Lin, Y.; Di, P. Crown Accuracy and Time Efficiency of Cement-Retained Implant-Supported Restorations in a Complete Digital Workflow: A Randomized Control Trial. J. Prosthodont. 2021, 31, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Al Hamad, K.Q.; Al-Rashdan, R.B.; Al-Rashdan, B.A.; Baba, N.Z. Effect of Milling Protocols on Trueness and Precision of Ceramic Crowns. J. Prosthodont. 2021, 30, 171–176. [Google Scholar] [CrossRef]
- Hanon, M.M.; Zsidai, L.; Ma, Q. Accuracy investigation of 3D printed PLA with various process parameters and different colors. Mater. Today 2021, 42, 3089–3096. [Google Scholar] [CrossRef]
- Kamio, T.; Hayashi, K.; Onda, T.; Takaki, T.; Shibahara, T.; Yakushiji, T.; Shibui, T.; Kato, H. Utilizing a low-cost desktop 3D printer to develop a “one-stop 3D printing lab” for oral and maxillofacial surgery and dentistry fields. 3D Print. Med. 2018, 4, 6. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.; Yang, Y.; Niu, S.; Ren, H. The research status and development trend of additive manufacturing technology. Int. J. Adv. Manuf. Technol. 2016, 89, 3651–3660. [Google Scholar] [CrossRef]
- George, E.; Liacouras, P.; Rybicki, F.J.; Mitsouras, D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics 2017, 37, 1424–1450. [Google Scholar] [CrossRef]
- Rybachuk, M.; Mauger, C.A.; Fiedler, T.; Öchsner, A. Anisotropic mechanical properties of fused deposition modeled parts fabricated by using acrylonitrile butadiene styrene polymer. J. Polym. Eng. 2017, 37, 699–706. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Modeling, analysis, and optimization of dimensional accuracy of FDM-fabricated parts using definitive screening design and deep learning feedforward artificial neural network. Adv. Manuf. 2021, 9, 115–129. [Google Scholar] [CrossRef]
- Katsoulis, J.; Mericske-Stern, R.; Rotkina, L.; Zbären, C.; Enkling, N.; Blatz, M.B. Precision of fit of implant-supported screw-retained 10-unit computer-aided-designed and computer-aided-manufactured frameworks made from zirconium dioxide and titanium: An in vitro study. Clin. Oral Implant. Res. 2014, 25, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Meraikhi, H.; Yilmaz, B.; McGlumphy, E.; Brantley, W.; Johnston, W.M. In vitro fit of CAD-CAM complete arch screw-retained titanium and zirconia implant prostheses fabricated on 4 implants. J. Prosthet. Dent. 2017, 119, 409–416. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kale, E.; Johnston, W.M. Marginal discrepancy of CAD-CAM complete-arch fixed implant-supported frameworks. J. Prosthet. Dent. 2018, 120, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Presotto, A.G.C.; Bhering, C.L.B.; Mesquita, M.F.; Barão, V.A.R. Marginal fit and photoelastic stress analysis of CAD-CAM and overcast 3-unit implant-supported frameworks. J. Prosthet. Dent. 2017, 117, 373–379. [Google Scholar] [CrossRef]
- Yilmaz, B.; Alshahrani, F.A.; Kale, E.; Johnston, W.M. Effect of feldspathic porcelain layering on the marginal fit of zirconia and titanium complete-arch fixed implant-supported frameworks. J. Prosthet. Dent. 2018, 120, 71–78. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Highsmith, J.D.R.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of CAD/CAM Cobalt–Chrome and Zirconia Abutments on a Conical Connection Implant: An In Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef]
- Gedrimiene, A.; Adaskevicius, R.; Rutkunas, V. Accuracy of digital and conventional dental implant impressions for fixed partial dentures: A comparative clinical study. J. Adv. Prosthodont. 2019, 11, 271–279. [Google Scholar] [CrossRef]
- De La Cruz, J.E.; Funkenbusch, P.; Ercoli, C.; Moss, M.E.; Graser, G.N.; Tallents, R.H. Verification jig for implant-supported prostheses: A comparison of standard impressions with verification jigs made of different materials. J. Prosthet. Dent. 2002, 88, 329–336. [Google Scholar] [CrossRef]
- Krechmer, J.E.; Phillips, B.; Chaloux, N.; Shomberg, R.; Daube, C.; Manchanda, G.; Murray, S.; McCarthy, A.; Fonseca, R.; Thakkar, J.; et al. Chemical Emissions from Cured and Uncured 3D-Printed Ventilator Patient Circuit Medical Parts. ACS Omega 2021, 6, 30726–30733. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Hon, C.-Y.; Tarlo, S.M.; Rajaram, N.; House, R. Emissions and health risks from the use of 3D printers in an occupational setting. J. Toxicol. Environ. Health A 2020, 83, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wojtyła, S.; Klama, P.; Baran, T. Is 3D printing safe? Analysis of the thermal treatment of thermoplastics: ABS, PLA, PET, and nylon. J. Occup. Environ. Hyg. 2017, 14, D80–D85. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Kondej, D.; Kowalska, J.; Szewczyńska, M. State of the art in additive manufacturing and its possible chemical and particle hazards—Review. Indoor Air 2021, 31, 1733–1758. [Google Scholar] [CrossRef]
- Qiu, Q.Q.; Sun, W.Q.; Connor, J. Sterilization of Biomaterials of Synthetic and Biological Origin; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [PubMed]
- Davila, S.P.; Rodríguez, L.G.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]

| 3D Printing Technique | 3D Printer Used | Specifications |
|---|---|---|
| DLP † | cara Print 4.0 pro (Kulzer Japan Co., Ltd., Tokyo, Japan) | Pixel size: XY 65.0 μm Laminating pitch: 30–150 μm Modeling size: 127 mm × 70 mm × 130 mm |
| FFF ‡ | Moment M350 (Moment Co., Ltd., Seoul, Republic of Korea) | XYZ accuracy: XY 12 μm, Z 0.625 μm Laminating pitch: 0.05–0.3 mm Modeling size: 350 mm × 190 mm × 196 mm Nozzle: 0.4 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamori, K.; Nagata, K.; Nakashizu, T.; Tsuruoka, H.; Atsumi, M.; Kawana, H. Comparative Verification of the Accuracy of Implant Models Made of PLA, Resin, and Silicone. Materials 2023, 16, 3307. https://doi.org/10.3390/ma16093307
Wakamori K, Nagata K, Nakashizu T, Tsuruoka H, Atsumi M, Kawana H. Comparative Verification of the Accuracy of Implant Models Made of PLA, Resin, and Silicone. Materials. 2023; 16(9):3307. https://doi.org/10.3390/ma16093307
Chicago/Turabian StyleWakamori, Kana, Koudai Nagata, Toshifumi Nakashizu, Hayato Tsuruoka, Mihoko Atsumi, and Hiromasa Kawana. 2023. "Comparative Verification of the Accuracy of Implant Models Made of PLA, Resin, and Silicone" Materials 16, no. 9: 3307. https://doi.org/10.3390/ma16093307
APA StyleWakamori, K., Nagata, K., Nakashizu, T., Tsuruoka, H., Atsumi, M., & Kawana, H. (2023). Comparative Verification of the Accuracy of Implant Models Made of PLA, Resin, and Silicone. Materials, 16(9), 3307. https://doi.org/10.3390/ma16093307







