Mechanical Alloying Behavior and Thermal Stability of CoCrCuFeMnNix High-Entropy Alloy Powders Prepared via MA
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
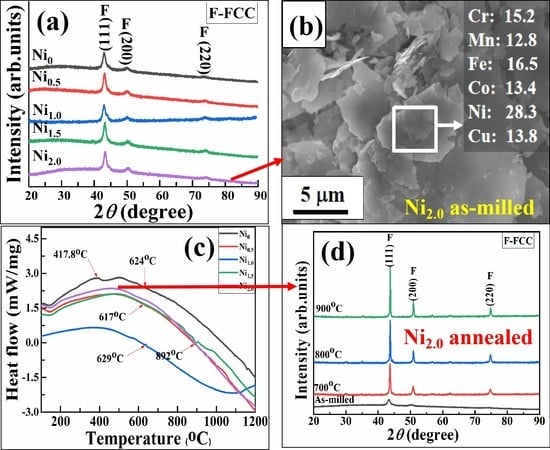
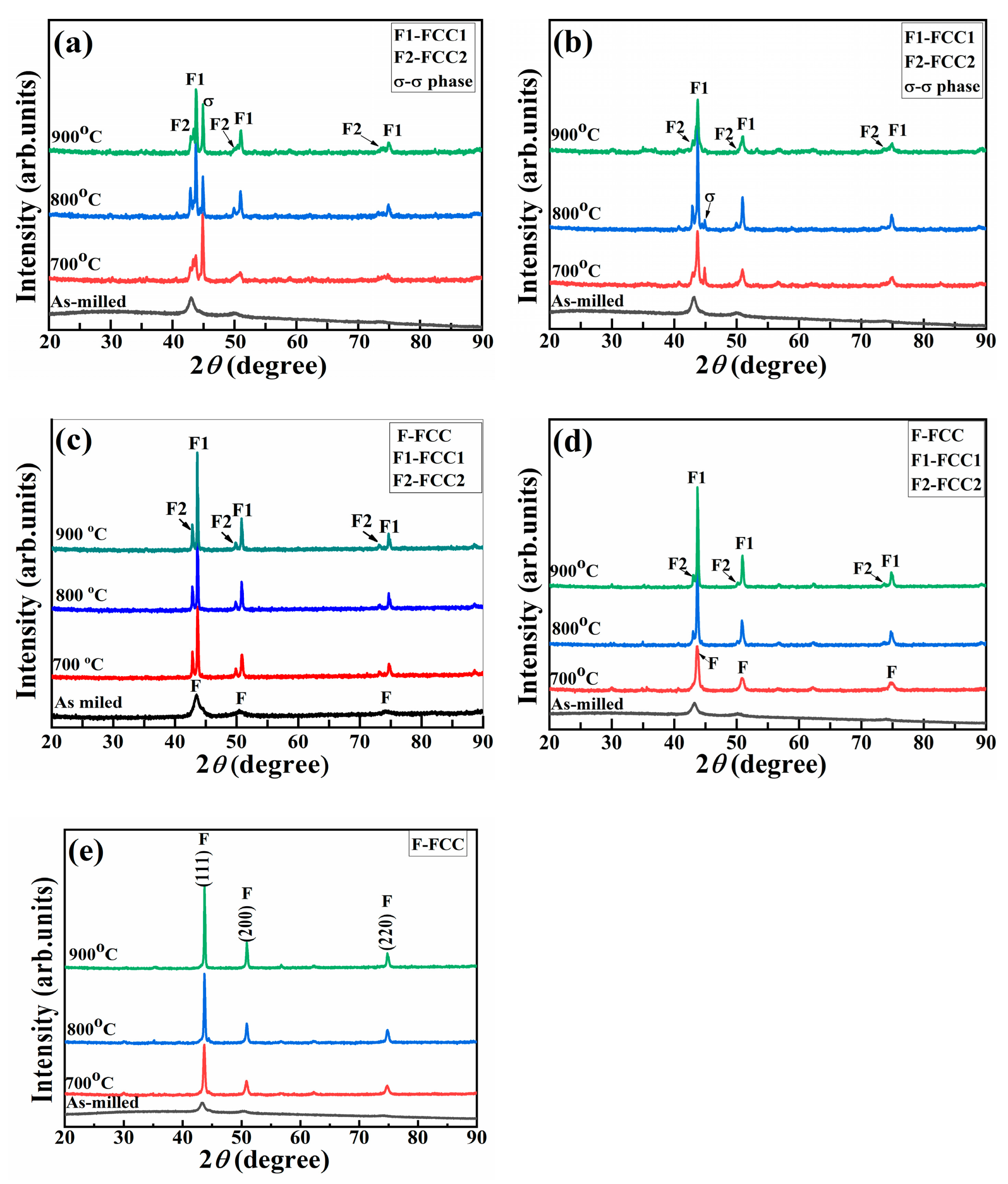
3.1. XRD Analysis of HEAPs
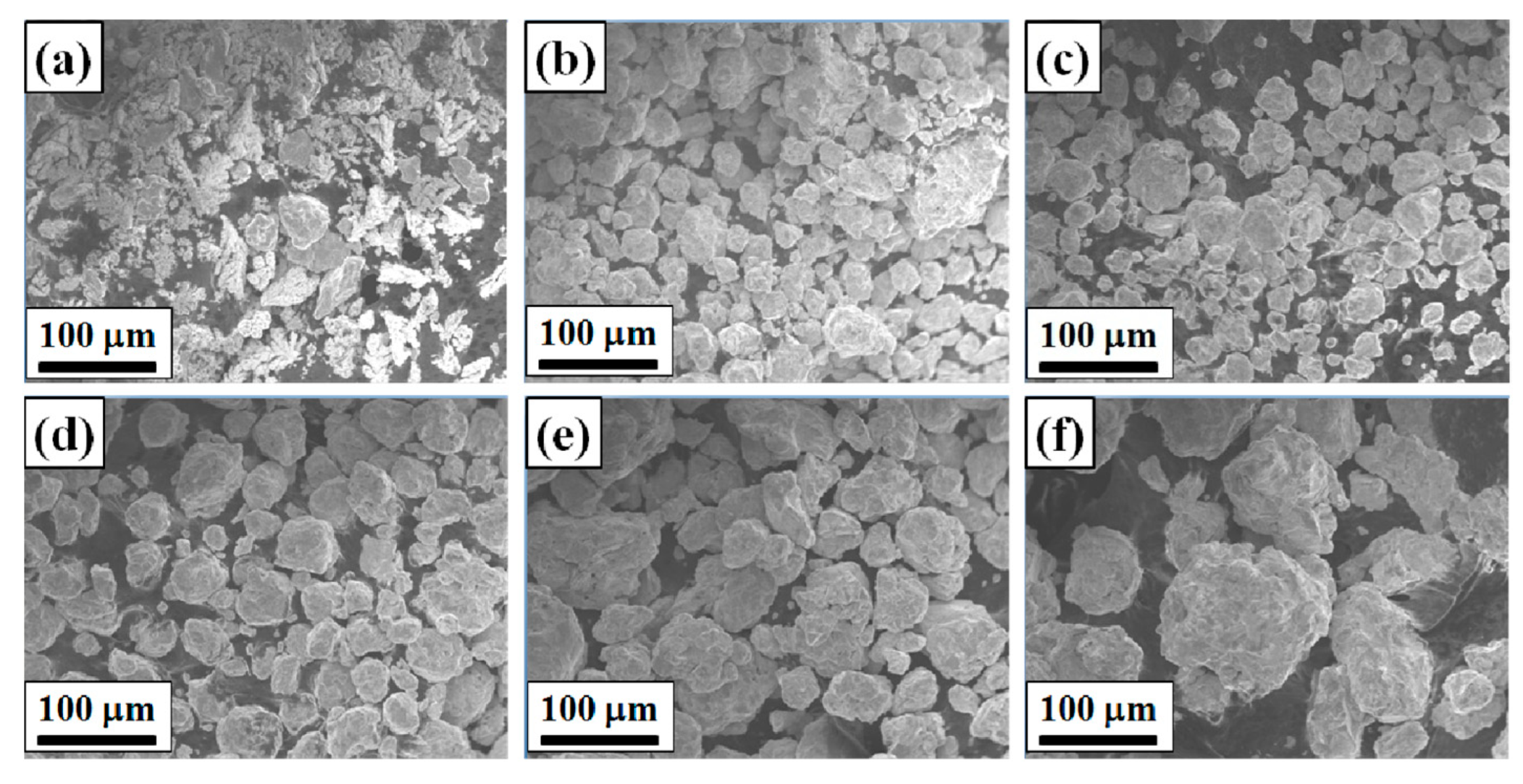
3.2. Microstructure of HEAPs
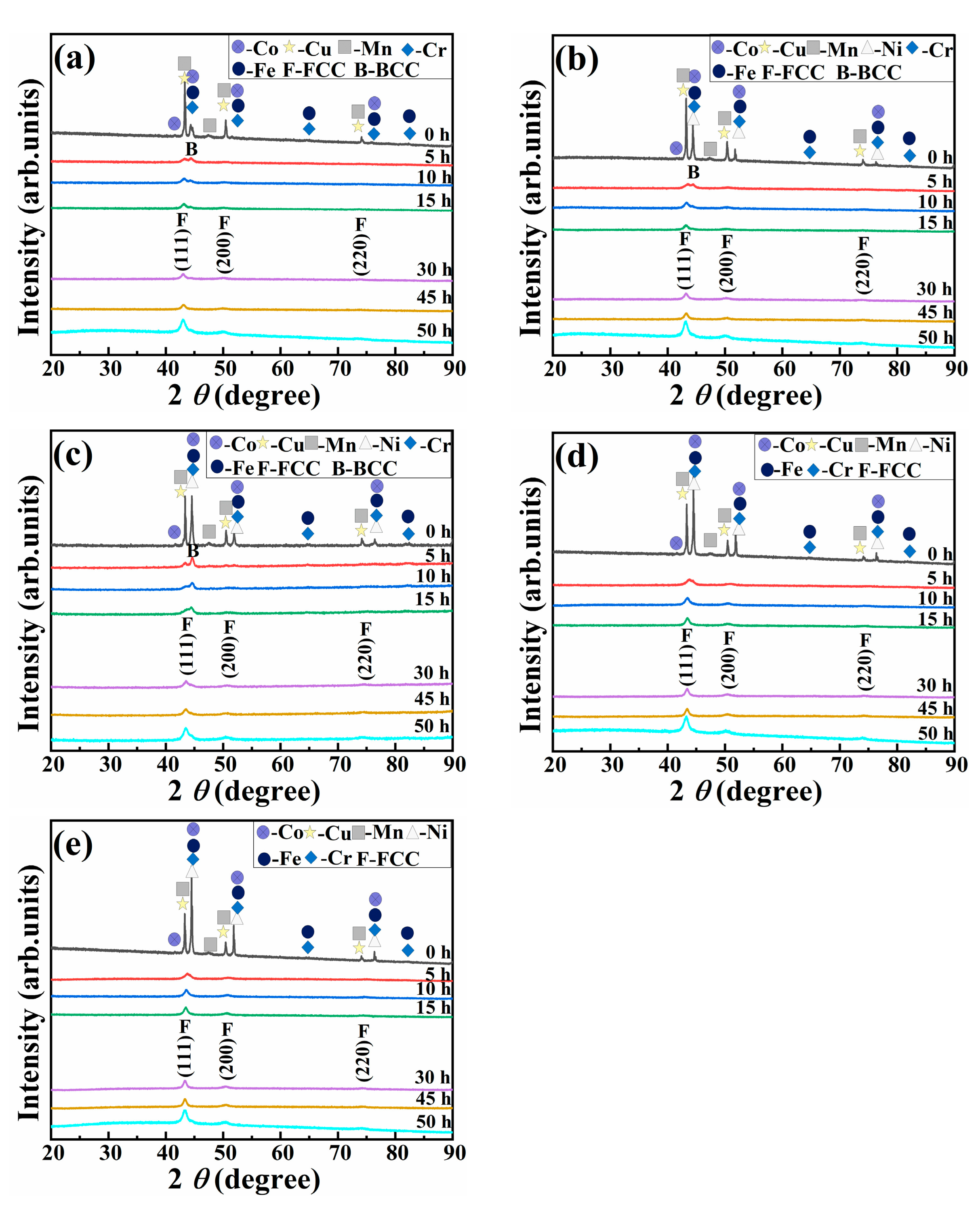
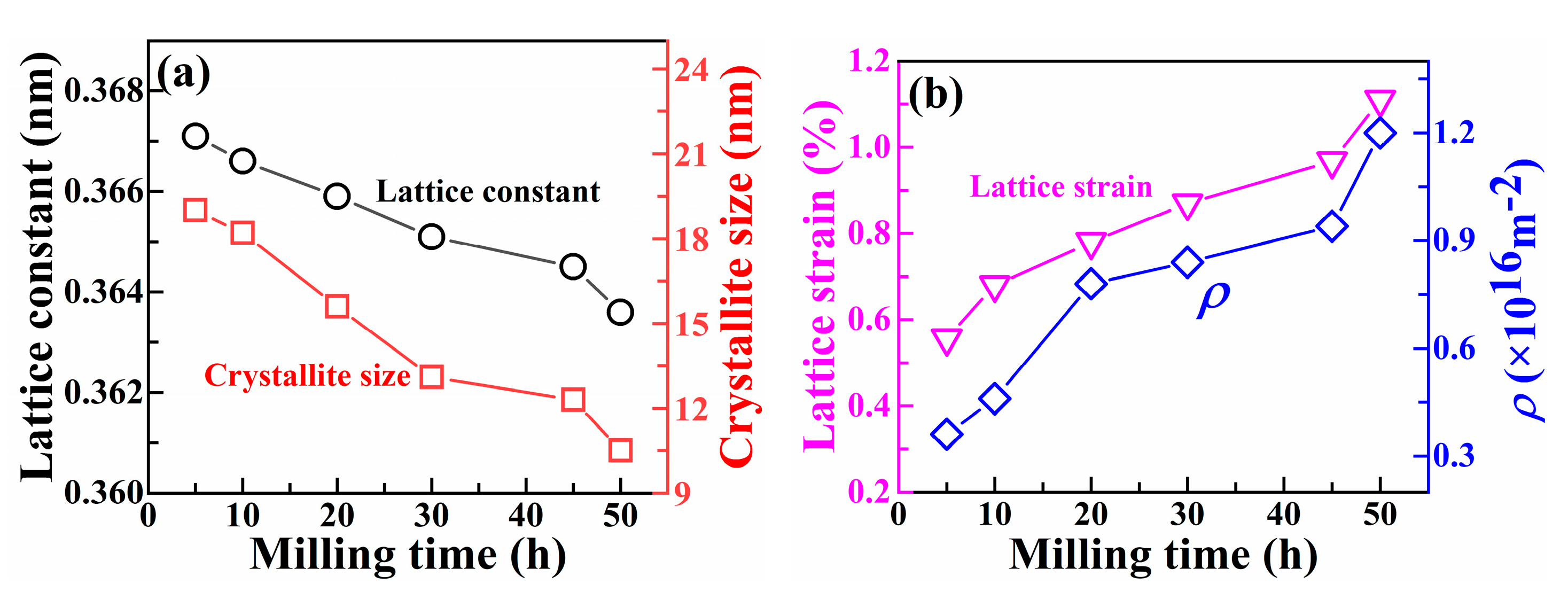
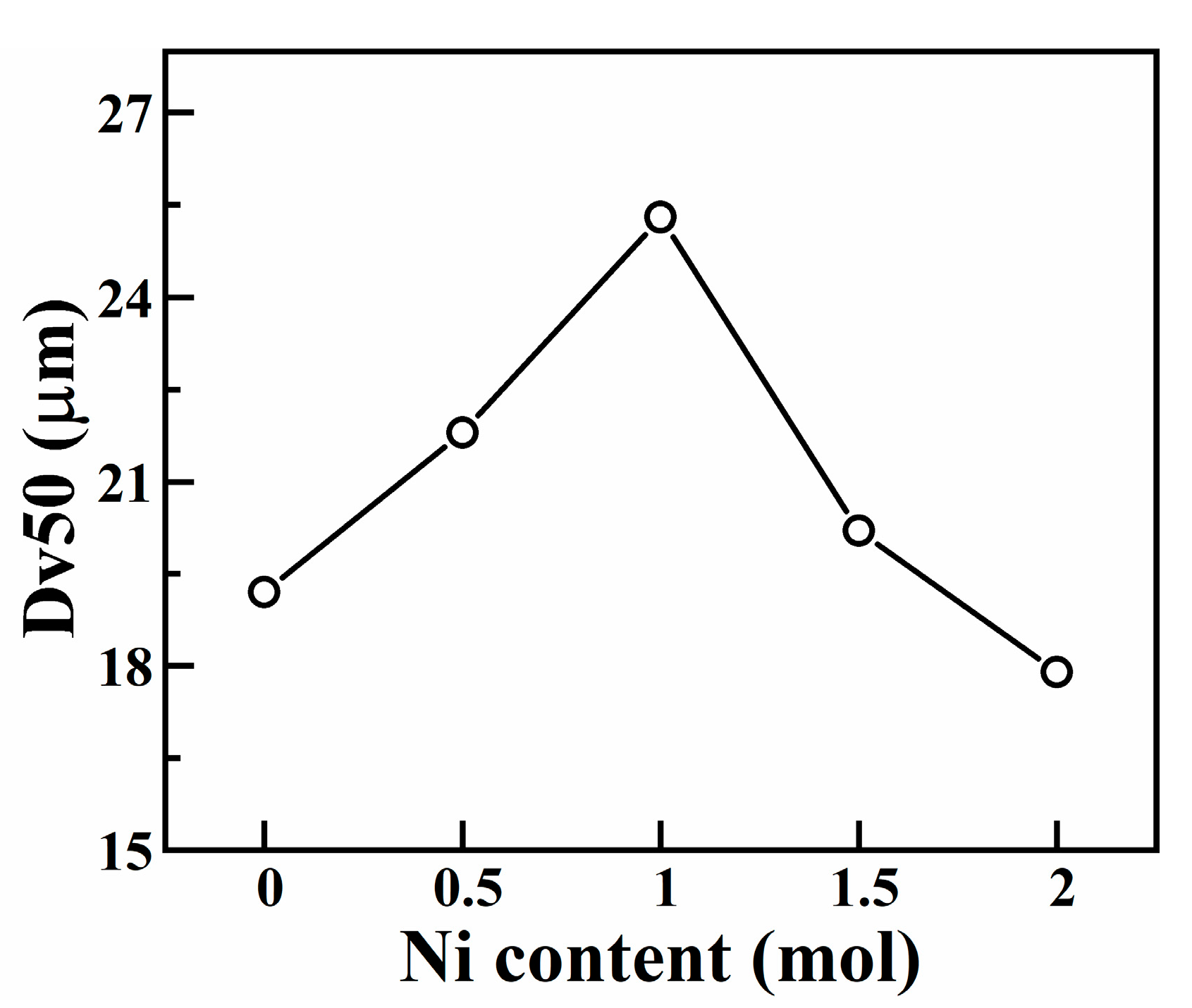
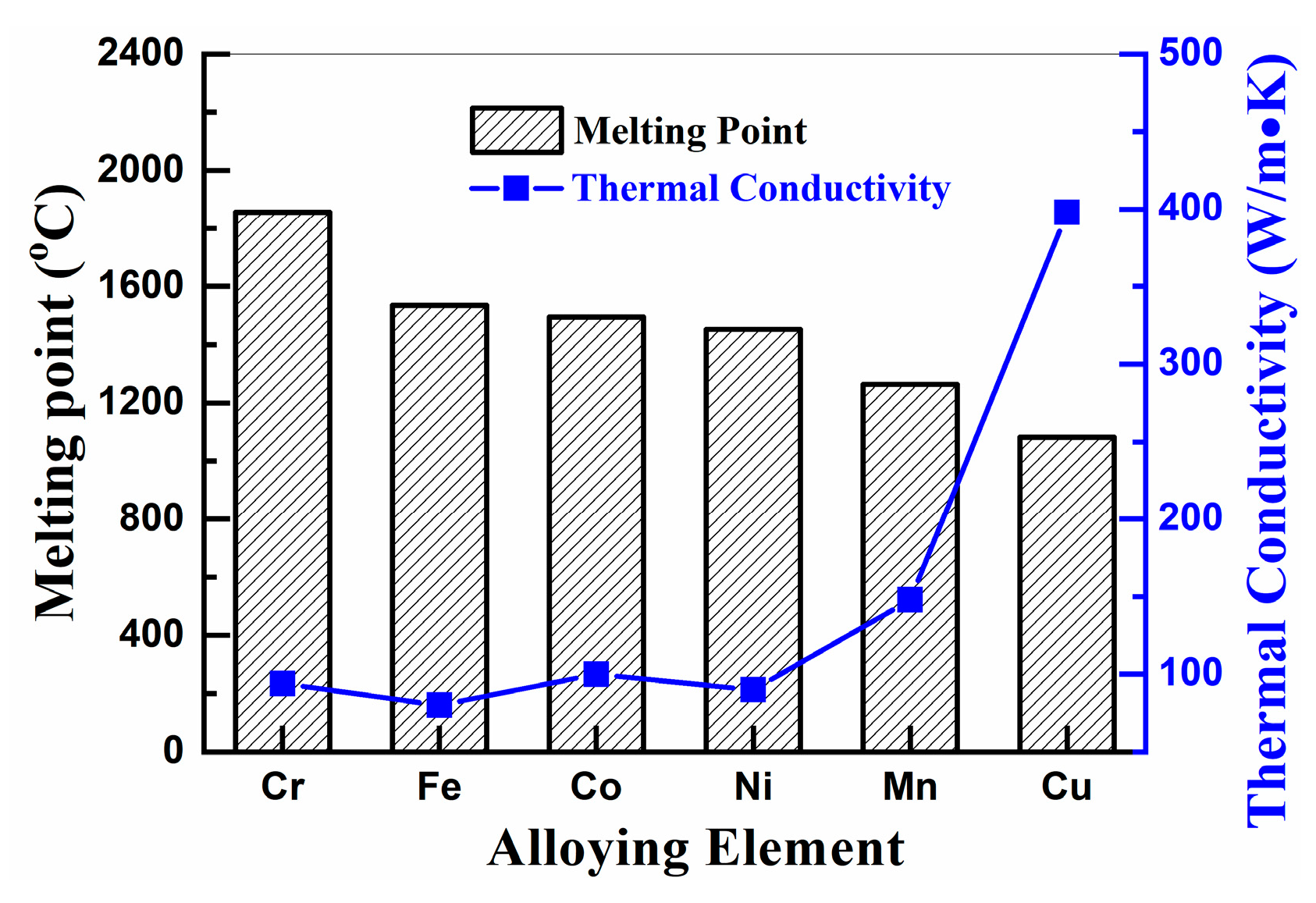
3.3. Mechanical Alloying Behavior of HEAPs
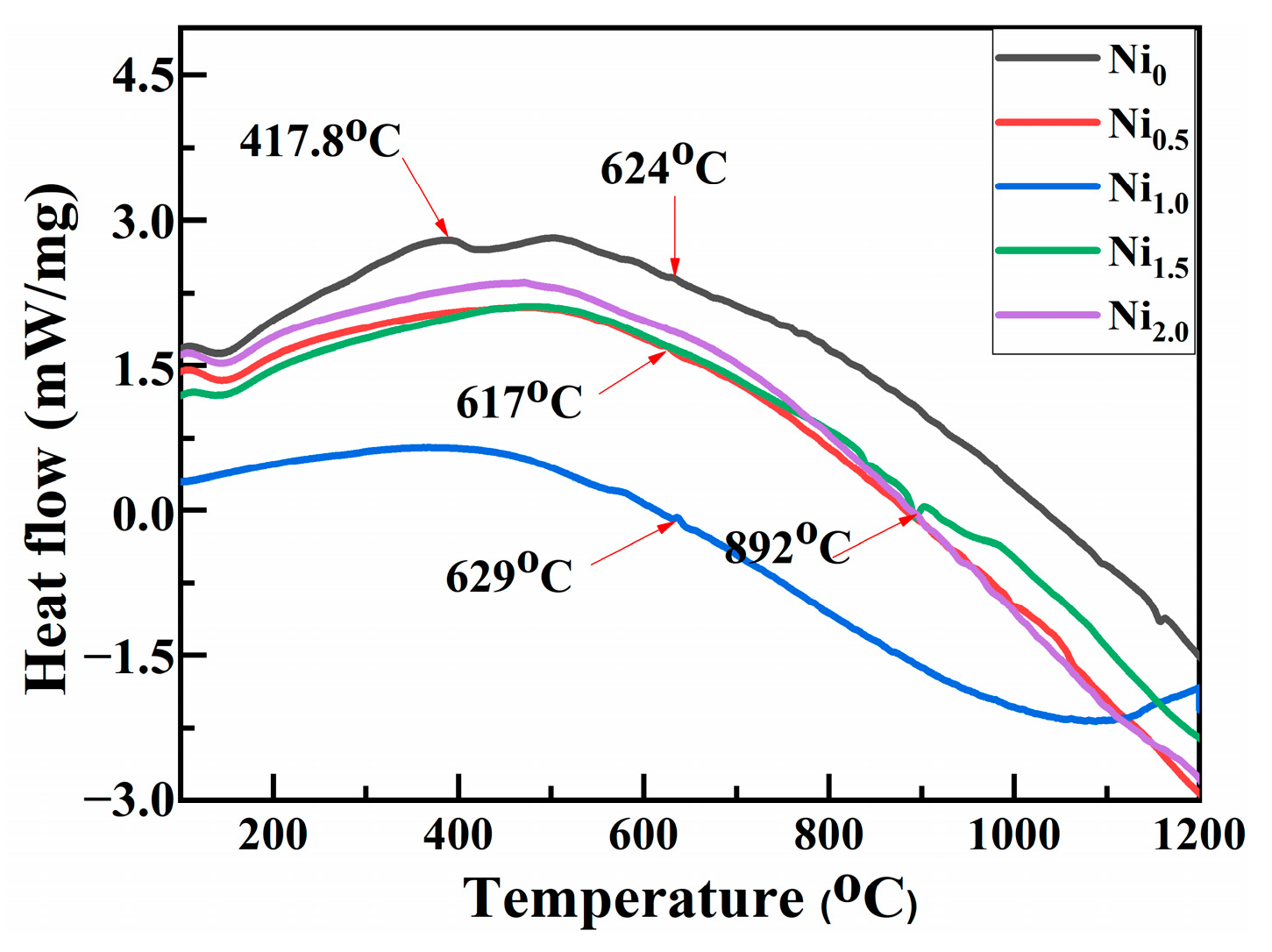
3.4. Thermal Stability of HEAPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructure development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Can, J.Y.; Chin, T.S.; Shun, T.T. Nanostructured high entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Xing, Y.; Li, C.J.; Mu, Y.K.; Jia, Y.D.; Song, K.K.; Tan, J.; Wang, G.; Zhang, Z.Q.; Yi, J.H.; Eckert, J. Strengthening and deformation mechanism of high-strength CrMnFeCoNi high entropy alloy prepared by powder metallurgy. J. Mater. Sci. Technol. 2023, 132, 119–131. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mangish, A.; Kumar, S.; Sharma, A.; Ahn, B.; Kumar, V. A review on high-temperature applicability: A milestone for high entropy alloys. Eng. Sci. Technol. 2022, 35, 101211. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Pan, Z.M.; Wang, X.F.; Luo, H.; Liu, Y.; Li, X.G. Corrosion and passive behavior of AlxCrFeNi3−x (x = 0.6, 0.8, 1.0) eutectic high entropy alloys in chloride environment. Corros. Sci. 2022, 208, 110666. [Google Scholar] [CrossRef]
- Yang, D.N.; Liu, Y.; Han, T.Y.; Zhou, F.; Qu, N.; Liao, M.Q.; Lai, Z.H.; Zhu, J.C. High thermal stability and oxidation behavior of FeCrNiAl-based medium-entropy alloys prepared by powder metallurgy. J. Alloys Compd. 2022, 918, 165562. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, A.K.; Mishra, R.K.; Ahmad, M.S.; Shahi, R.R. A comprehensive review: Recent progress on magnetic high entropy alloys and oxides. J. Magn. Magn. Mater. 2022, 554, 169142. [Google Scholar] [CrossRef]
- Duan, S.C.; Kang, J.; Cho, J.; Lee, M.; Mu, W.Z.; Park, J.H. Manufacturing an ultra-low-sulfur CoCrFeMnNi high-entropy alloy by slagging through induction melting with ferroalloys feedstock. J. Alloys Compd. 2022, 928, 167080. [Google Scholar] [CrossRef]
- Sathiaraj, G.D.; Ahmed, M.Z.; Bhattacharjee, P.P. Microstructure and texture of heavily cold-rolled and annealed fcc equiatomic medium to high entropy alloys. J. Alloys Compd. 2016, 664, 109–119. [Google Scholar] [CrossRef]
- Gludovatz, B.; George, E.P.; Ritchie, R.O. Processing, microstructure and mechanical properties of the CrMnFeCoNi high-entropy alloy. JOM 2015, 67, 2262–2270. [Google Scholar] [CrossRef]
- Yim, D.; Jang, M.J.; Bae, J.W.; Moon, J.; Lee, C.-H.; Hong, S.-J.; Hong, S.I.; Kim, H.S. Compaction behavior of water-atomized CoCrFeMnNi high-entropy alloy powders. Mater. Chem. Phys. 2018, 210, 95–102. [Google Scholar] [CrossRef]
- Gao, F.; Sun, Y.; Hu, L.; Shen, J.; Liu, W.C.; Ba, M.Y.; Deng, C. Microstructural evolution and thermal stability in a nanocrystalline lightweight TiAlV0.5CrMo refractory high-entropy alloy synthesized by mechanical alloying. Mater. Letter. 2022, 329, 133179. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ren, B.; Zhao, R.F.; Liu, Z.X.; Cai, B.; Zhang, G.P. Effect of Co on the microstructure and oxidation behavior of CoxCrCuFeMnNi high entropy alloy powders. Micron 2021, 142, 102995. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, R.F.; Jiang, A.Y.; Yu, Y. Microstructure and oxidation behavior of CoCrxCuFeMnNi high-entropy alloys fabricated by vacuum hot-pressing sintering. Micron 2022, 158, 103291. [Google Scholar] [CrossRef]
- Alvaredo, P.; Torralba, J.M.; García-Junceda, A. Sintering of high entropy alloys: Processing and properties. Encycl. Mater. Met. Alloys 2022, 3, 362–371. [Google Scholar] [CrossRef]
- Toroghinejad, M.R.; Pirmoradian, H.; Shabani, A. Synthesis of FeCrCoNiCu high entropy alloy through mechanical alloying and spark plasma sintering processes. Mater. Chem. Phys. 2022, 289, 126433. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Qin, Z.; Dong, P.; Mi, Y.J.; Gong, D.Q.; Li, W.G. Microstructure and corrosion properties of FeCoNiCrMn high entropy alloy coatings prepared by high speed laser cladding and ultrasonic surface mechanical rolling treatment. Mater. Lett. 2020, 259, 126769. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile properties of high- and medium- entropy alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Mao, M.M.; Wang, J.W.; Gludovatz, B.; Zhang, Z.; Mao, S.X.; George, E.P.; Yu, Q.; Qitchie, R.O. Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- He, J.Y.; Zhu, C.; Zhou, D.Q.; Liu, W.H.; Nieh, T.G.; Lu, Z.P. Steady state flow of the FeCoNiCrMn high entropy alloy at elevated temperatures. Intermetallics 2014, 55, 9–14. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- Stepanov, N.; Tikhonovsky, M.; Yurchenko, N.; Zyabkin, Y.D.; Klimova, M.; Zherebtsov, S.; Efimov, A.; Salishchev, G. Effect of cryo-deformation on structure and properties of CoCrFeNiMn high-entropy alloy. Intermetallics 2015, 59, 8–17. [Google Scholar] [CrossRef]
- Park, N.; Li, X.; Tsuji, N. Microstructure and mechanical properties of Co21Cr22Cu22Fe21Ni14 processed by high pressure torsion and annealing. JOM 2015, 67, 2303–2309. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Oh, S.M.; Hong, S.I. Microstructural stability and mechanical properties of equiatomic CoCrCuFeNi, CrCuFeMnNi, CoCrCuFeMn alloys. Mater. Chem. Phys. 2018, 210, 120–125. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Li, D.M.; Shi, L.; Cai, B.; Wang, M.X. Effect of elemental interaction on microstructure of CuCrFeNiMn high entropy alloy system. J. Alloys Compd. 2010, 493, 148–153. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.J.; Huang, B.X.; Zhao, X.C.; Chen, H.; Wang, C.Z. Effect of Cu segregation on the phase transformation and properties of AlCrFeNiTiCux high-entropy alloys. Intermetallics 2022, 140, 107397. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Wang, W.M.; Lee, S.W.; Niihara, K. Characterization of nanocrystalline CoCrFeNiTiAl high-entropy solid solution processed by mechanical alloying. J. Alloys Compd. 2010, 495, 33–38. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Velasco, F.; Robert, M.H.; Torralba, J.M. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Zhao, R.F.; Ren, B.; Zhang, G.P.; Liu, Z.X.; Zhang, J.J. Effect of Co content on the phase transition and magnetic properties of CoxCrCuFeMnNi high-entropy alloy powders. J. Magn. Magn. Mater. 2018, 468, 14–24. [Google Scholar] [CrossRef]
- Zhao, R.F.; Ren, B.; Zhang, G.P.; Liu, Z.-X.; Cai, B.; Zhang, J.J. CoCrxCuFeMnNi high-entropy alloy powders with superior soft magnetic properties. J. Magn. Magn. Mater. 2019, 491, 165574. [Google Scholar] [CrossRef]
- Huang, S.R.; Wu, H.; Zhu, H.G.; Xie, Z.H. Effect of niobium addition upon microstructure and tensile properties of CrMnFeCoNix high entropy alloys. Mater. Sci. Eng. A 2021, 809, 140959. [Google Scholar] [CrossRef]
- Wei, R.; Jiang, Z.; Gao, Q.Y.; Chen, C.; Zhang, K.S.; Zhang, S.; Han, Z.H.; Wang, T.; Wu, S.J.; Li, F.S. The effect of Co substitutions for Ni on microstructure, mechanical properties and corrosion resistance of Fe50Mn25Cr15Ni10 medium-entropy alloy. Intermetallics 2022, 149, 107654. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous decrease in X-ray diffraction intensities of CuNi–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Wang, N.R.; Wang, S.R.; Gou, X.X.; Shi, Z.C.; Lin, J.X.; Liu, G.Q.; Wang, Y. Alloying behavior and characterization of (CoCrFeNiMn)90M10 (M=Al, Hf) high-entropy materials fabricated by mechanical alloying. Trans. Nonferrous. Met. Soc. China 2022, 32, 2253–2265. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically alloyed high entropy alloys: Existing challenges and opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; John Wiley&-Songs, Inc.: New York, NY, USA, 1996; p. 673. [Google Scholar]
- Cantor, B. Stable and metastable multicomponent alloys. Ann. Chim. Sci. Mat. 2007, 32, 245–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloy. J. Appl. Phys. 2011, 109, 103–105. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Chen, W.P.; Xiao, H.Q.; Zhou, L.W.; Zhu, D.Z.; Yang, S.F. Fabrication and properties of nanocrystalline Co0.5FeNiCrTi0.5 high entropy alloy by MA-SPS technique. Mater. Des. 2013, 44, 535–539. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Processing and properties of nanocrystalline CuNiCoZnAlTi high entropy alloys by mechanical alloying. Mater. Sci. Eng. A 2010, 527, 1027–1030. [Google Scholar] [CrossRef]
- Praveen, S.; Murty, B.S.; Kottada, R.S. Alloying behavior in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys. Mater. Sci. Eng. A 2012, 534, 83–89. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Chen, W.P.; Wen, H.M.; Chen, Z.; Lavernia, E.J. Effects of Co and sintering method on microstructure and mechanical behavior of a high-entropy Al0.6NiFeCrCo alloy prepared by powder metallurgy. J. Alloys Compd. 2015, 646, 175–182. [Google Scholar] [CrossRef]




| Alloy | ΔHmix | ΔSmix | Ω | δ | VEC |
|---|---|---|---|---|---|
| Ni0 | 4.16 | 13.38 | 55.33 | 3.15 | 8.20 |
| Ni0.5 | 2.58 | 14.70 | 98.05 | 3.07 | 8.36 |
| Ni1.0 | 1.44 | 14.90 | 17.81 | 3.01 | 8.50 |
| Ni1.5 | 0.615 | 14.78 | 41.38 | 2.92 | 8.61 |
| Ni2.0 | 0 | 14.53 | - | 2.85 | 8.71 |
| Proponent | Empirical Rules |
|---|---|
| Hume-Rothery [43] | f ≥ 5, −40 < ΔHmix < 10 KJmol−1, d < 12% |
| Zhang [44] | −20 < ΔHmix < 5 KJmol−1, 12 < ΔSmix < 17.5 JK−1mol−1, δ < 6.4% |
| Yang [45] | Ω ≥ 1.1, δ ≤ 6.6% |
| Wang [46] | γ < 1.175 |
| Guo [47] | FCC (VEC < 6.87), FCC + BCC (6.87 ≤ VEC < 8), BCC (VEC ≥ 8) |
| Element | Co | Cr | Cu | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| Co | 0 | −4 | 6 | −1 | −5 | 0 |
| Cr | - | 0 | 12 | −1 | 2 | −7 |
| Cu | - | - | 0 | 13 | 4 | 4 |
| Fe | - | - | - | 0 | 0 | −2 |
| Mn | - | - | - | - | 0 | −8 |
| Ni | - | - | - | - | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhao, R.; Ren, B.; Jiang, A.; Chen, C.; Liu, J.; Zhou, Y. Mechanical Alloying Behavior and Thermal Stability of CoCrCuFeMnNix High-Entropy Alloy Powders Prepared via MA. Materials 2023, 16, 3179. https://doi.org/10.3390/ma16083179
Zhang B, Zhao R, Ren B, Jiang A, Chen C, Liu J, Zhou Y. Mechanical Alloying Behavior and Thermal Stability of CoCrCuFeMnNix High-Entropy Alloy Powders Prepared via MA. Materials. 2023; 16(8):3179. https://doi.org/10.3390/ma16083179
Chicago/Turabian StyleZhang, Baofeng, Ruifeng Zhao, Bo Ren, Aiyun Jiang, Chong Chen, Jianxiu Liu, and Yajun Zhou. 2023. "Mechanical Alloying Behavior and Thermal Stability of CoCrCuFeMnNix High-Entropy Alloy Powders Prepared via MA" Materials 16, no. 8: 3179. https://doi.org/10.3390/ma16083179
APA StyleZhang, B., Zhao, R., Ren, B., Jiang, A., Chen, C., Liu, J., & Zhou, Y. (2023). Mechanical Alloying Behavior and Thermal Stability of CoCrCuFeMnNix High-Entropy Alloy Powders Prepared via MA. Materials, 16(8), 3179. https://doi.org/10.3390/ma16083179