Some Critical Reflections on the SEM-EDS Microanalysis of the Hydrotalcite-like Phase in Slag Cement Paste
Abstract
1. Introduction
2. Materials and Methodology
2.1. Sample Information
2.2. SEM-EDS Microanalysis
3. Results and Discussion
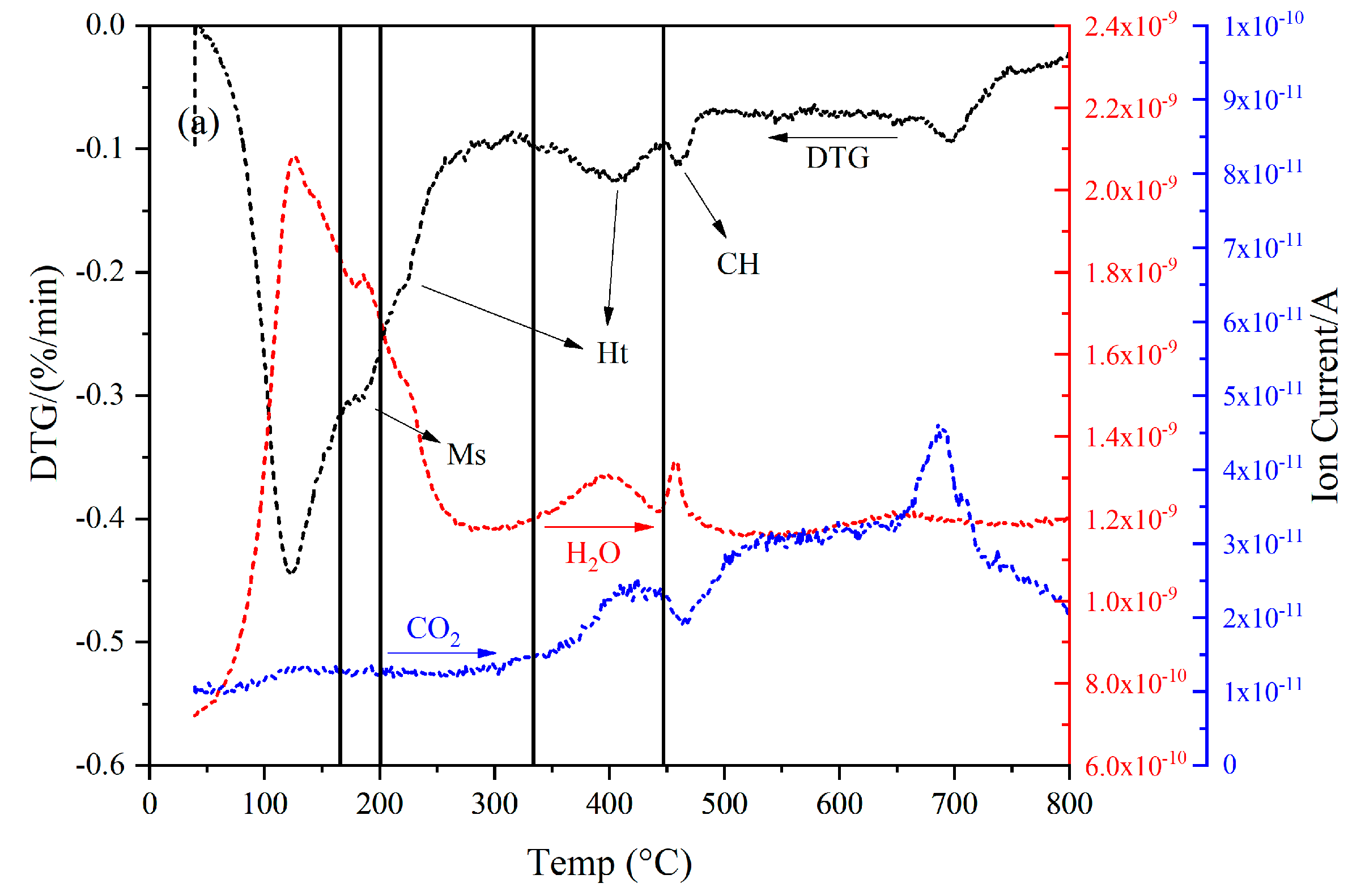
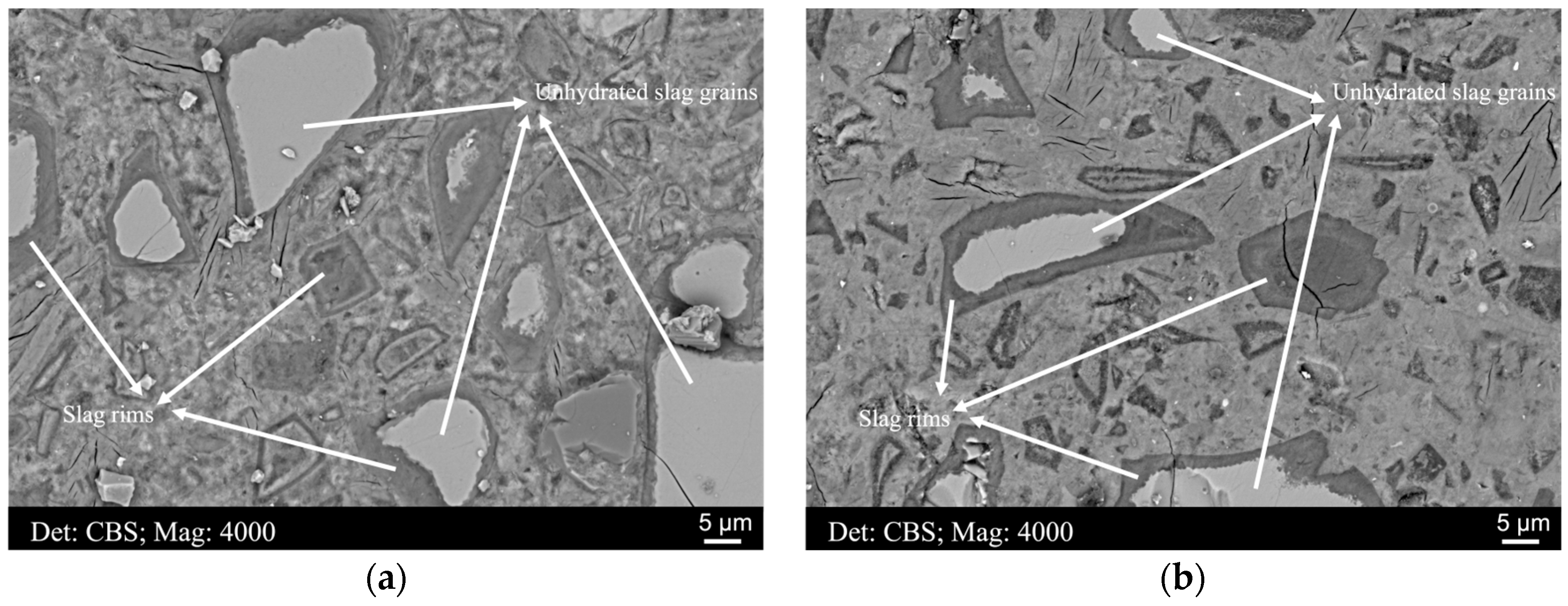
3.1. The Thickness of the Slag Rim and the Interaction Volume of the Electron
3.1.1. The Thickness of the Slag Rim
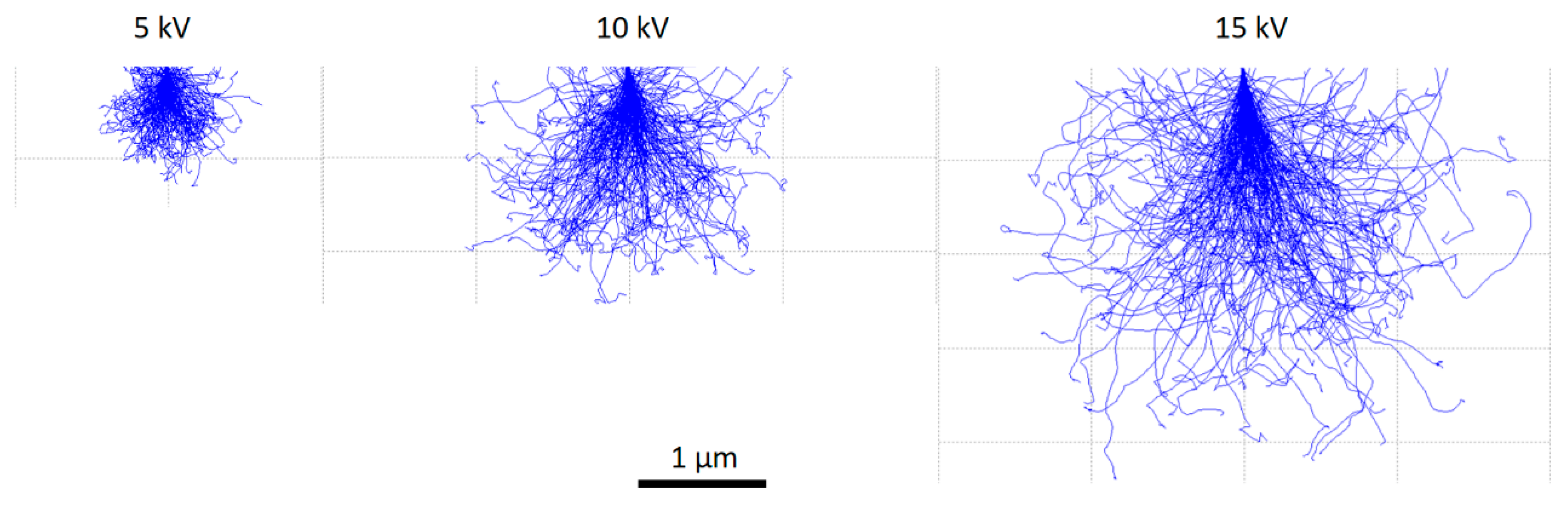
3.1.2. Accelerating Voltage and Interaction Volume
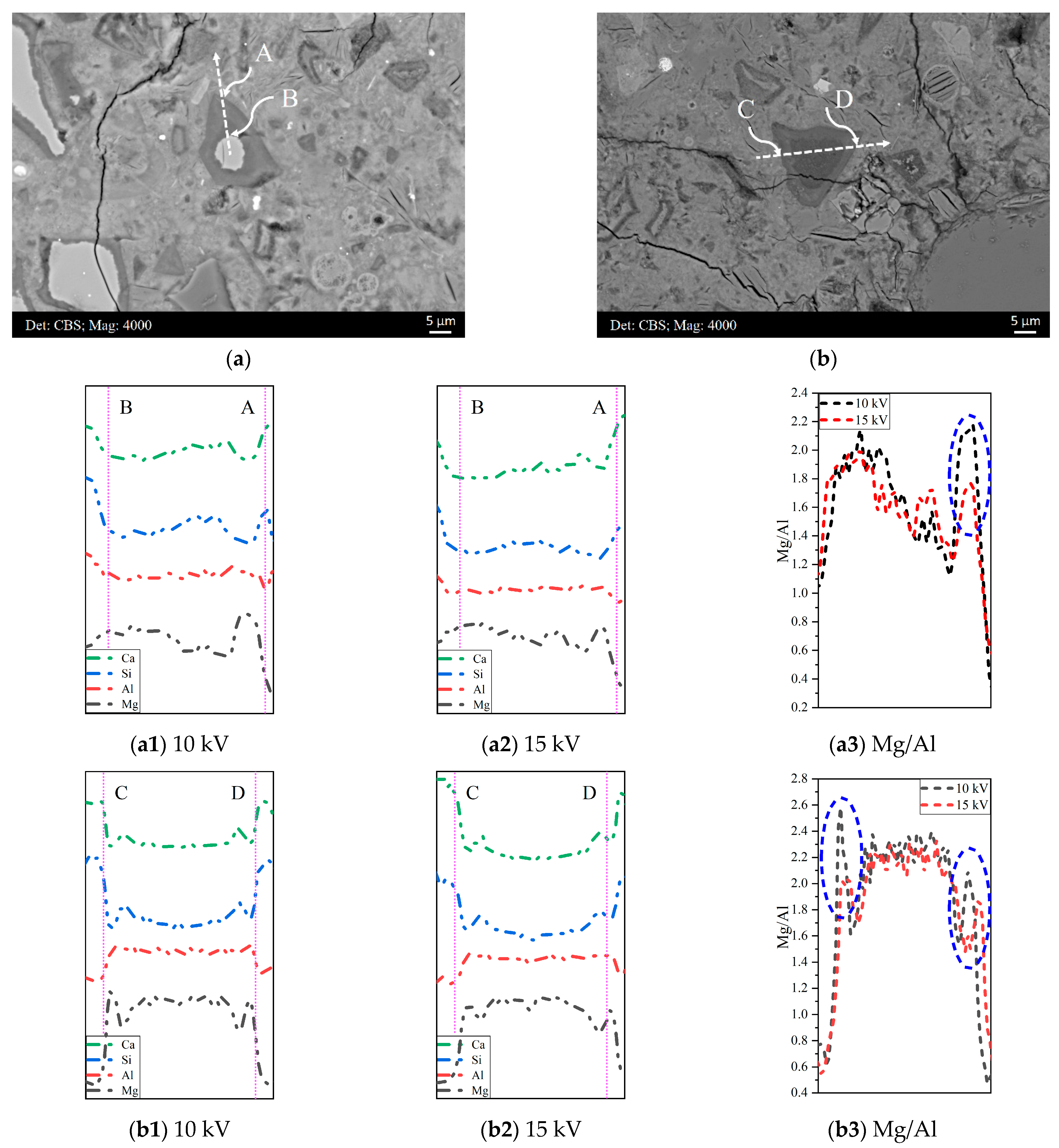
3.1.3. The Influence of the Accelerating Voltage on Determining the Mg/Al Ratio of the Hydrotalcite-like Phase
3.2. Std.-Based and Standardless Microanalysis
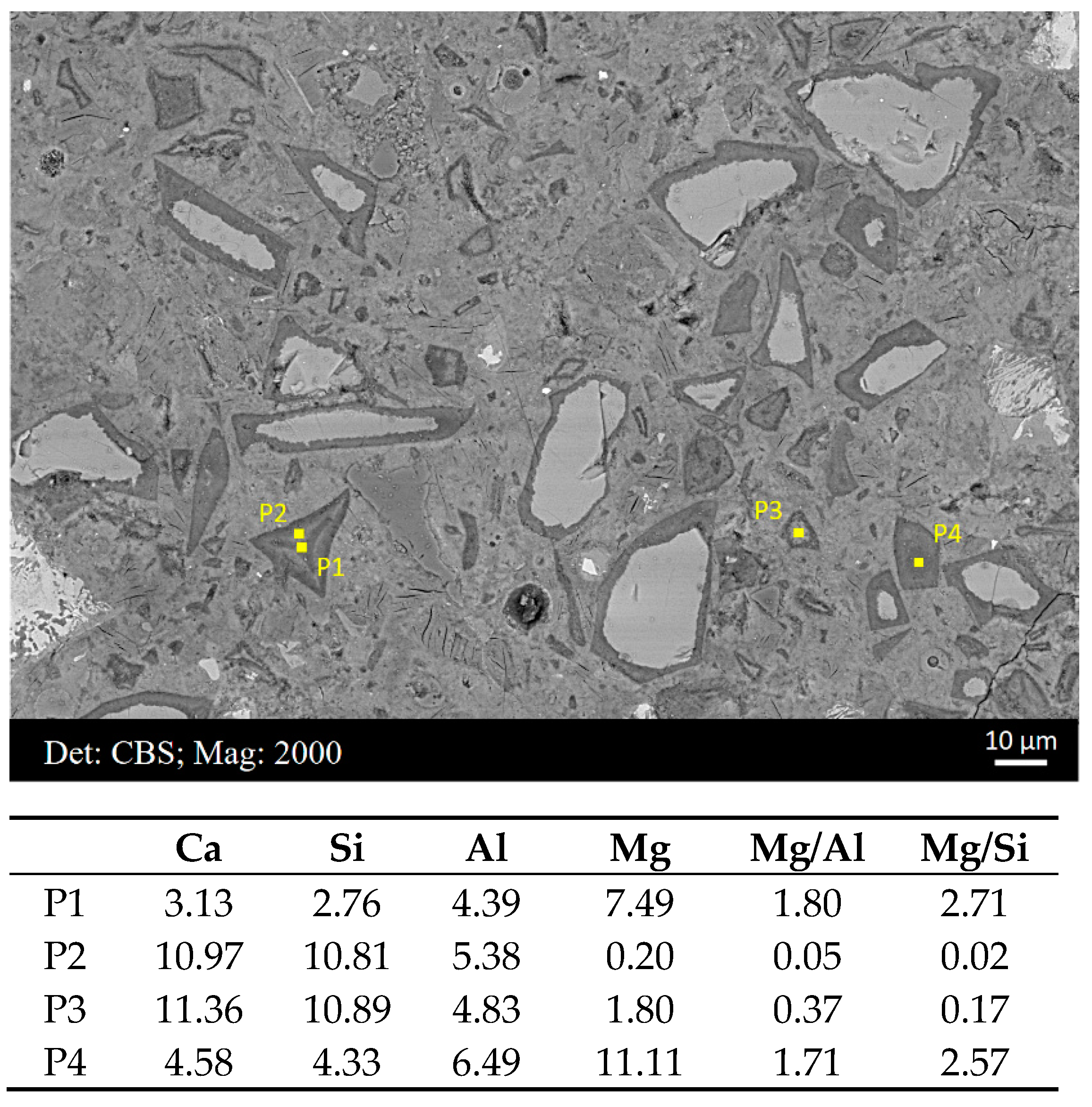
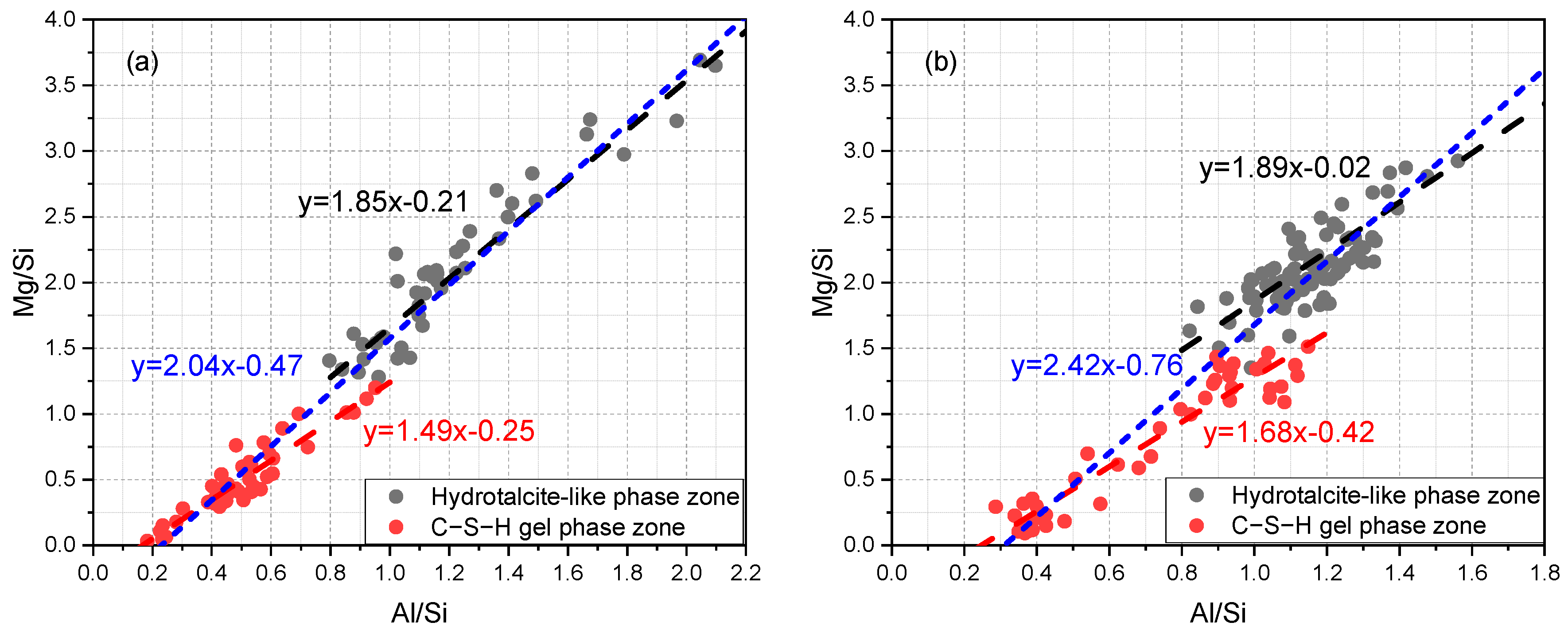
3.3. Compositional Zonation within Slag Rim
4. Conclusions
- During microanalysis, the beam energy of 10 kV was more appropriate than 15 kV for samples with a thin slag rim to compromise to meet the requirements of obtaining an adequate overvoltage ratio and minimize the interference.
- A lower Mg/Al ratio was obtained when a higher accelerating voltage was employed. The effect was especially distinct in the border of the slag rim where there was a significant fluctuation in the Mg content.
- The analysis total of the hydrates within the slag rim was in the range of 30–40%, lower than that in the cement matrix. Aside from the water chemically bound in the C−S−H gel phase, the hydrotalcite-like phase also contained a certain amount of hydroxide ions and chemically bound water.
- It was noted that the Mg/Al ratio decreased from zones rich in the hydrotalcite-like phase to zones rich in the C−S−H gel phase. Indiscriminately fitting scatter points selected from the slag rim would bias the Mg/Al ratio of the hydrotalcite-like phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bijen, J. Benefits of slag and fly ash. Constr. Build. Mater. 1996, 10, 309–314. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Crossin, E. The greenhouse gas implications of using ground granulated blast furnace slag as a cement substitute. J. Clean. Prod. 2015, 95, 101–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Schlangen, E.; Çopuroğlu, O. Effect of slags of different origins and the role of sulfur in slag on the hydration characteristics of cement-slag systems. Constr. Build. Mater. 2022, 316, 125266. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H. The hydration of slag, part 2: Reaction models for blended cement. J. Mater. Sci. 2007, 42, 444–464. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, C.; Zhou, H.; Zhang, Y. The characteristics and formation mechanism of the dark rim in alkali-activated slag. Cem. Concr. Compos. 2020, 112, 103682. [Google Scholar] [CrossRef]
- Ye, H. Nanoscale attraction between calcium-aluminosilicate-hydrate and Mg-Al layered double hydroxides in alkali-activated slag. Mater. Charact. 2018, 140, 95–102. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Chen, W. Spatial zonation of a hydrotalcite-like phase in the inner product of slag: New insights into the hydration mechanism. Cem. Concr. Res. 2021, 145, 106460. [Google Scholar] [CrossRef]
- Zhang, Y.; Çopuroğlu, O. Role of the grain size on the hydration characteristics of slag in an aged field concrete. Cem. Concr. Res. 2022, 162, 106985. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 119. [Google Scholar]
- Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Bernard, E.; Zucha, W.J.; Lothenbach, B.; Mäder, U. Stability of hydrotalcite (Mg-Al layered double hydroxide) in presence of different anions. Cem. Concr. Res. 2022, 152, 106674. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Mitchell, L. Layered double hydroxide-like materials: Nanocomposites for use in concrete. Cem. Concr. Res. 2004, 34, 1717–1724. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar]
- Whittaker, M.; Zajac, M.; Ben Haha, M.; Bullerjahn, F.; Black, L. The role of the alumina content of slag, plus the presence of additional sulfate on the hydration and microstructure of Portland cement-slag blends. Cem. Concr. Res. 2014, 66, 91–101. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.A.; Bernal, S.A.; van Deventer, J.S.; Provis, J.L. Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Richardson, I.; Groves, G. Microstructure and microanalysis of hardened cement pastes involving ground granulated blast-furnace slag. J. Mater. Sci. 1992, 27, 6204–6212. [Google Scholar] [CrossRef]
- Taylor, R.; Richardson, I.; Brydson, R. Composition and microstructure of 20-year-old ordinary Portland cement–ground granulated blast-furnace slag blends containing 0 to 100% slag. Cem. Concr. Res. 2010, 40, 971–983. [Google Scholar] [CrossRef]
- Richardson, I.; Li, S. Composition and structure of an 18-year-old 5M KOH-activated ground granulated blast-furnace slag paste. Constr. Build. Mater. 2018, 168, 404–411. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford London: London, UK, 1997; Volume 2. [Google Scholar]
- Arakcheeva, A.V.; Pushcharovskii, D.Y.; Rastsvetaeva, R.K.; Atencio, D.; Lubman, G.U. Crystal structure and comparative crystal chemistry of Al2Mg4(OH)12(CO3)·3H2O, a new mineral from the hydrotalcite-manasseite group. Crystallogr. Rep. 1996, 41, 972–981. [Google Scholar]
- Zhang, Y.; Saravanakumar, K.; Çopuroğlu, O. EDS Microanalysis of Unhydrated Blast Furnace Slag Grains in Field Concrete with Different Service Life. Microsc. Microanal. 2022, 28, 1493–1503. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W. Quantitative electron-excited X-ray microanalysis of borides, carbides, nitrides, oxides, and fluorides with scanning electron microscopy/silicon drift detector energy-dispersive spectrometry (SEM/SDD-EDS) and NIST DTSA-II. Microsc. Microanal. 2015, 21, 1327–1340. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Famy, C.; Scrivener, K.; Crumbie, A. What causes differences of CSH gel grey levels in backscattered electron images? Cem. Concr. Res. 2002, 32, 1465–1471. [Google Scholar] [CrossRef]
- Kjellsen, K.; Atlassi, E.H. X-ray microanalysis of hydrated cement: Is the analysis total related to porosity? Cem. Concr. Res. 1998, 28, 161–165. [Google Scholar]
- Newbury, D.E.; Ritchie, N.W. Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 2013, 35, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C.; Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; et al. Quantitative Analysis: From k-Ratio to Composition. In Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 289–307. [Google Scholar]
- Newbury, D.E.; Swyt, C.R.; Myklebust, R.L. “Standardless” quantitative electron probe microanalysis with energy-dispersive X-ray spectrometry: Is it worth the risk? Anal. Chem. 1995, 67, 1866–1871. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lachowski, E.; Glasser, F. Densification and migration of ions in blast furnace slag-portland cement pastes. MRS Online Proc. Libr. Arch. 1988, 136, 263. [Google Scholar] [CrossRef]
- Zhang, Y.; Çopuroğlu, O. The role of hydrotalcite-like phase and monosulfate in slag cement paste during atmospheric and accelerated carbonation. Cem. Concr. Compos. 2022, 132, 104642. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, M.; Gan, Y.; Çopuroğlu, O. Effect of MgO content on the quantitative role of hydrotalcite-like phase in a cement-slag system during carbonation. Cem. Concr. Compos. 2022, 134, 104765. [Google Scholar] [CrossRef]

| Sample | |
|---|---|
| A | The sample was sourced from a parking garage built around 1980. It was in Jupiterstraat, Hoofddorp. The cement was partially replaced by blast furnace slag. |
| B | The sample was collected from a wind deflection screen near Calandbrug, Europoort Rotterdam (Port of Rotterdam), which was built in 1985. The cement type was reported as CEM III/B. |
| Target Element | Mineral | Composition |
|---|---|---|
| Ca | Calcite | CaCO3 |
| Si | Quartz | SiO2 |
| Al | Albite | NaAlSi3O8 |
| Mg | Dolomite | MgCa(CO3)2 |
| Mg/Al | % Analysis Total | |||
|---|---|---|---|---|
| Std.-Based | Standardless | Std.-Based | Standardless | |
| Sample A | 1.74 | 1.86 | 33.4 | 100.0 |
| Sample B | 1.79 | 1.91 | 31.2 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Saravanakumar, K.; Çopuroğlu, O. Some Critical Reflections on the SEM-EDS Microanalysis of the Hydrotalcite-like Phase in Slag Cement Paste. Materials 2023, 16, 3143. https://doi.org/10.3390/ma16083143
Zhang Y, Saravanakumar K, Çopuroğlu O. Some Critical Reflections on the SEM-EDS Microanalysis of the Hydrotalcite-like Phase in Slag Cement Paste. Materials. 2023; 16(8):3143. https://doi.org/10.3390/ma16083143
Chicago/Turabian StyleZhang, Yu, Karthikeyan Saravanakumar, and Oğuzhan Çopuroğlu. 2023. "Some Critical Reflections on the SEM-EDS Microanalysis of the Hydrotalcite-like Phase in Slag Cement Paste" Materials 16, no. 8: 3143. https://doi.org/10.3390/ma16083143
APA StyleZhang, Y., Saravanakumar, K., & Çopuroğlu, O. (2023). Some Critical Reflections on the SEM-EDS Microanalysis of the Hydrotalcite-like Phase in Slag Cement Paste. Materials, 16(8), 3143. https://doi.org/10.3390/ma16083143







