Effects of Sodium Tripolyphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Preparation of Experimental Samples and Test Methods
2.2.1. Determination of STPP Adsorption
2.2.2. Determination of the Rheological Properties of PCAC Slurries
2.2.3. Determination of STPP Adsorption
2.2.4. Analysis of PCAC Hydration Behaviour Using XRD and SEM
3. Results and Discussion
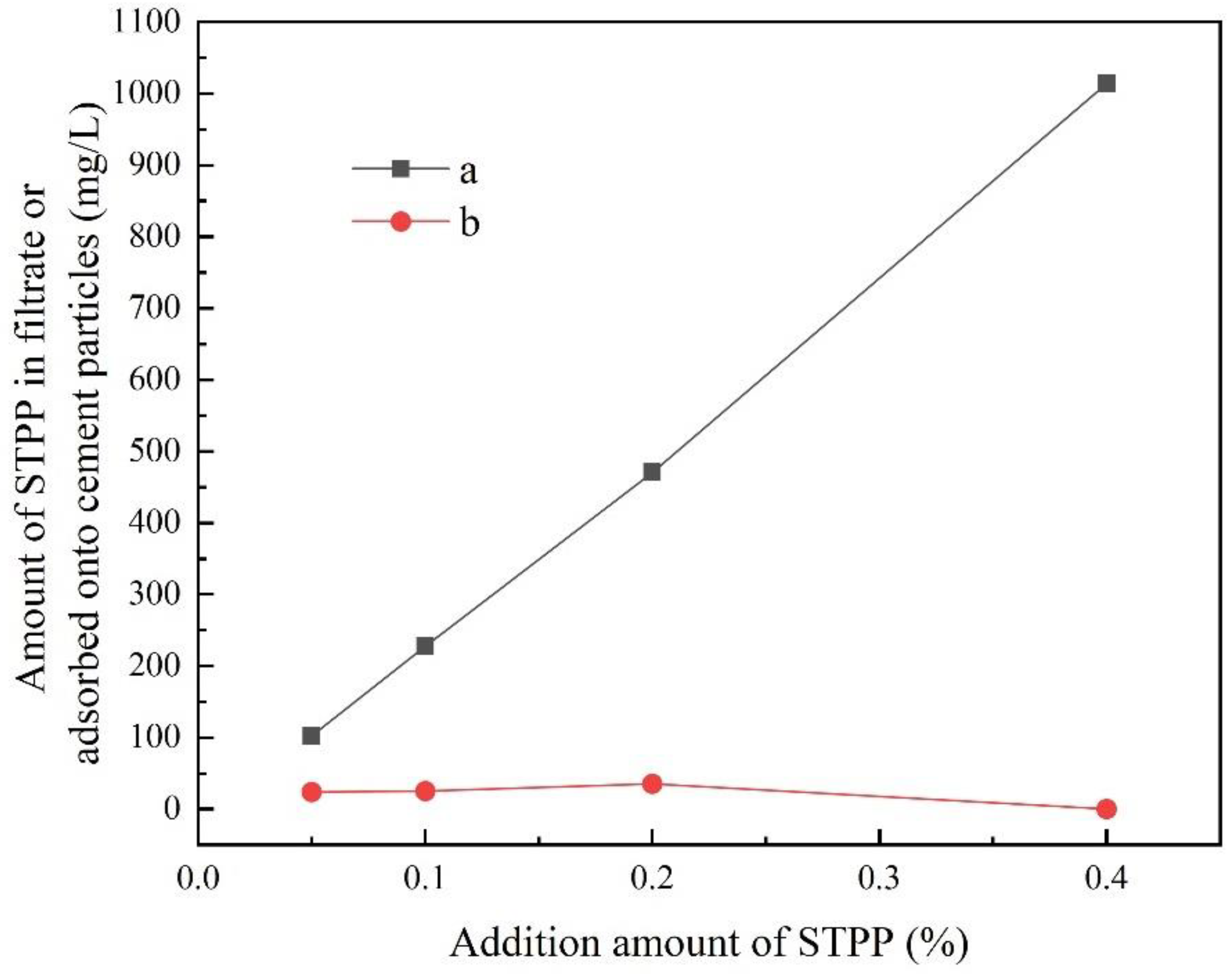
3.1. Adsorption Behaviour of STPP
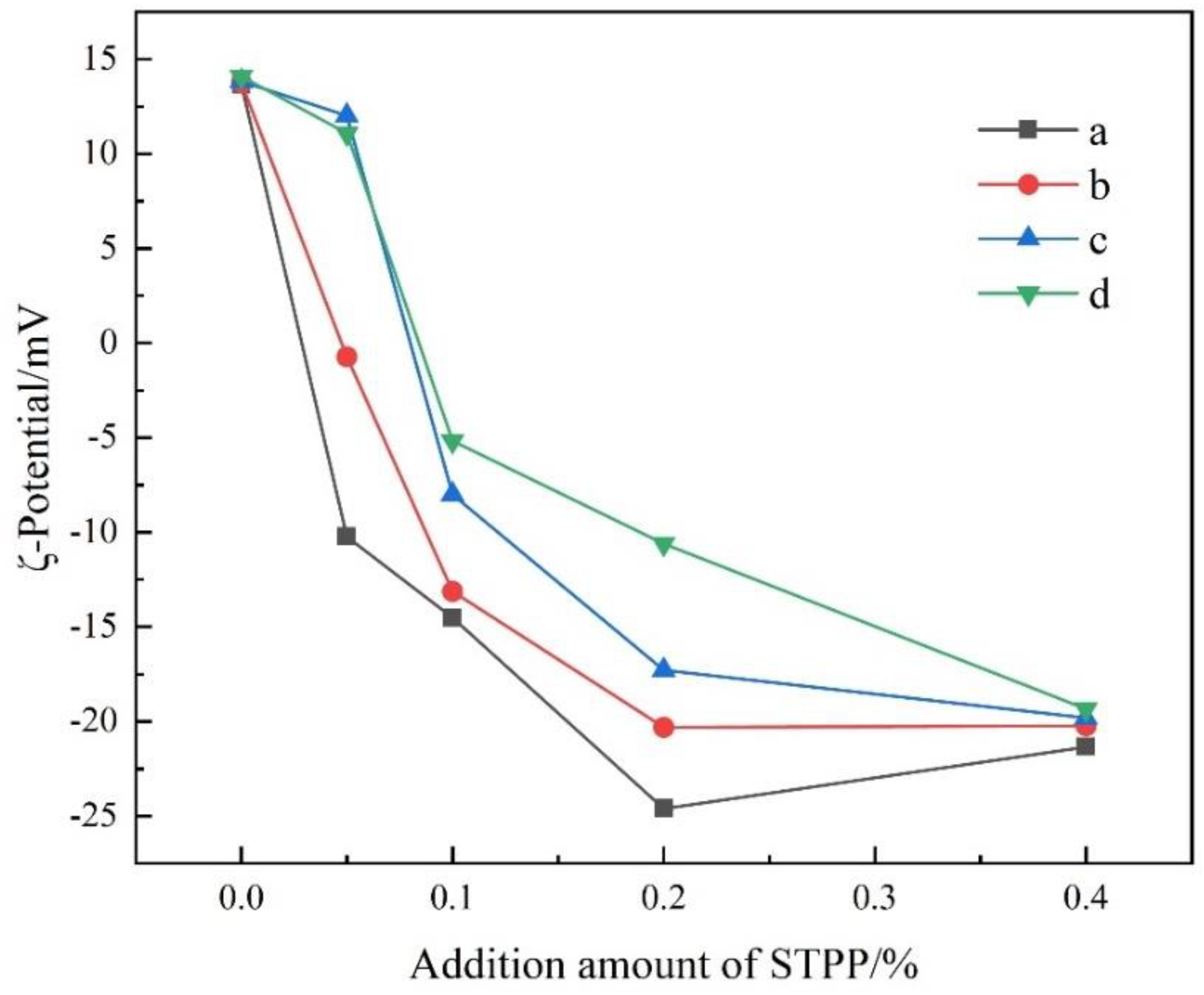
3.2. ζ-Potential of Cement Particles
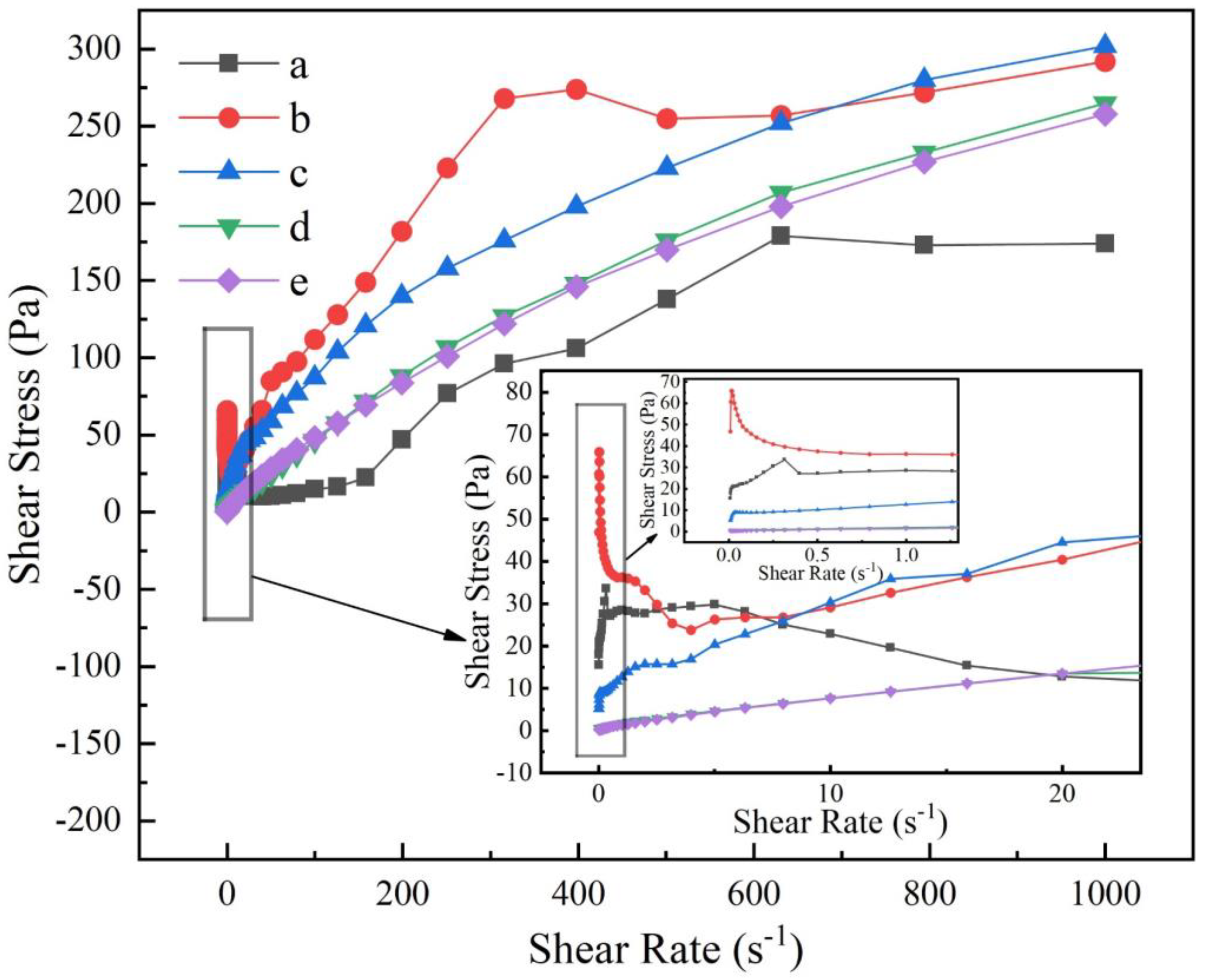
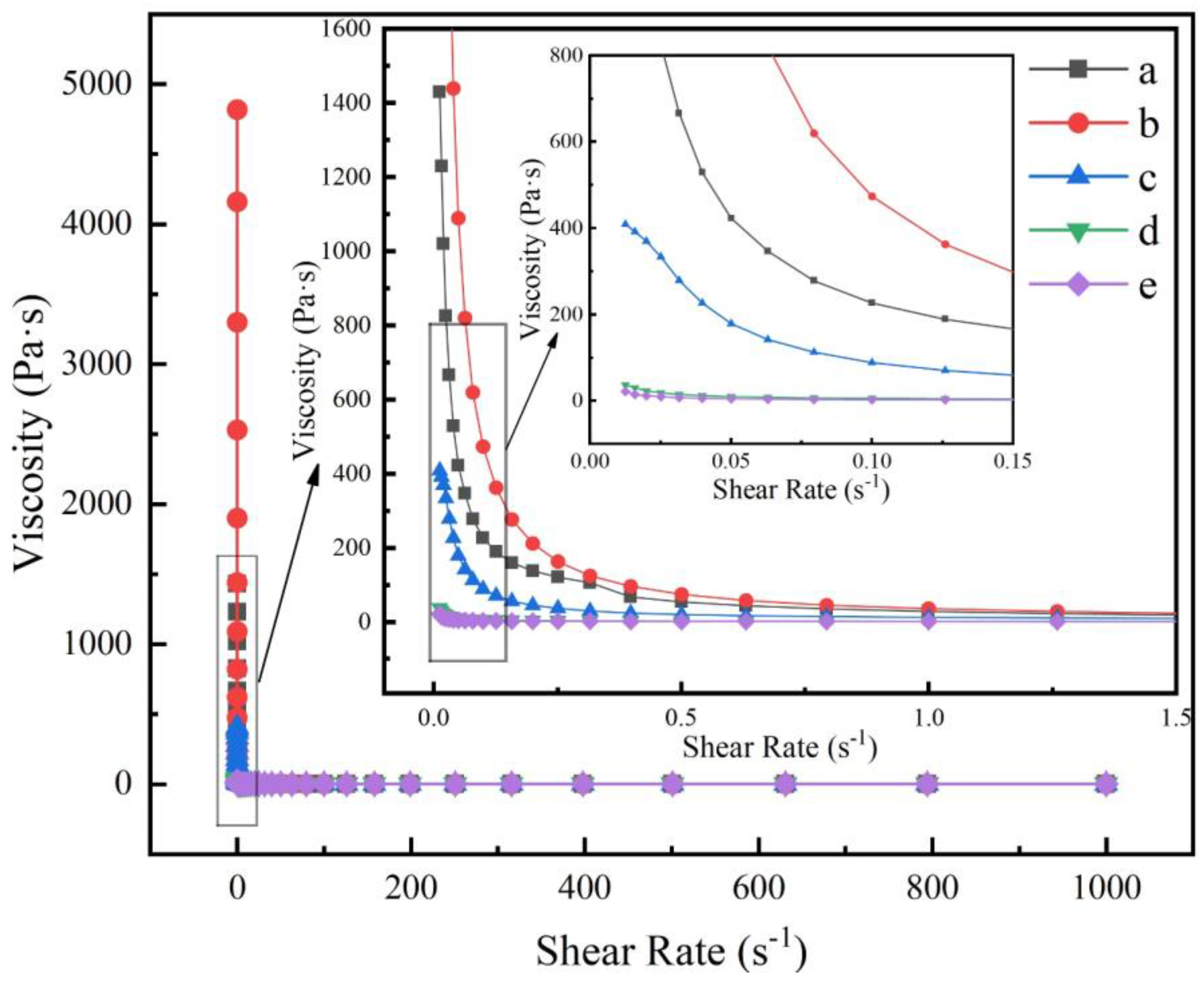
3.3. Rheological Properties
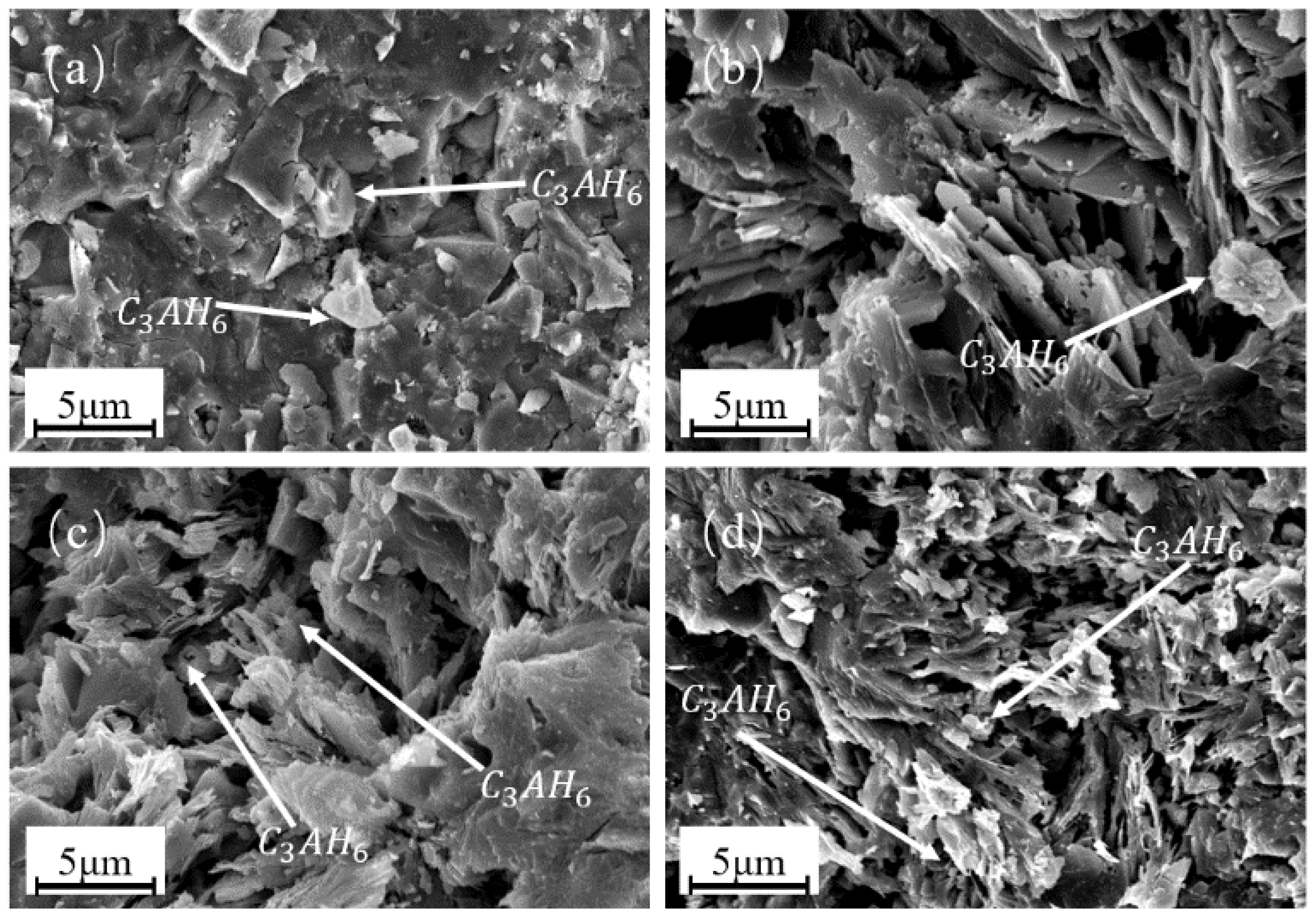
3.4. Effect of SHMP Addition on Hydration Behaviour of Calcium Aluminate Cement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, W.E.; Vieira, W.; Zhang, S. Castable refractory concretes. Int. Mater. Rev. 2001, 46, 145–167. [Google Scholar] [CrossRef]
- Oliveiair, I.R.; Pandolfellivc, V.C. Does a tiny amount of dispersant make any change to refractory castable properties? Ceram. Int. 2010, 36, 79–85. [Google Scholar] [CrossRef]
- Parker, K.M.; Sharp, J.H. Refractory calcium aluminate cements. Trans. Br. Ceram. Soc. 1982, 81, 35–42. [Google Scholar]
- Tchamba, A.; Sofack, J.; Yongue, R.; Melo, U.C. Formulation of calcium dealuminate (CaO·2Al2O3) refractory cement from local bauxite. J. Asian Ceram. Soc. 2015, 3, 164–172. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ye, G.T.; Shang, X.J.; Li, H.; Chen, L. Effect of B2O3 on hydration behavior of calcium aluminate cement. J. Chin. Ceram. Soc. 2016, 44, 1161–1165. [Google Scholar]
- Wang, Y.; Zhu, B.; Li, X.; Chen, P. Effect of dispersants on the hydrate morphologies of spinel-containing calcium aluminate cement and on the properties of refractory castables. Ceram. Int. 2016, 42, 711–720. [Google Scholar] [CrossRef]
- Bensed, J.; Barnes, P. Structure and Properties of Cement; Chemical Industry Press: Beijing, China, 2009; p. 103. [Google Scholar]
- Wang, C.H.; Ye, G.T. On the anomalous setting behavior of calcium aluminate cement. Naihuo Cailiao 1997, 31, 235–237. [Google Scholar]
- Yang, C.R.; Xiao, H.H. Preparation, properties and uses of sodium tripolyphosphate. Chemistry 1965, 5, 35–40. [Google Scholar]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Gaspar, C.; Silvestre, S.; Martinezde-Oliveira, J.; Amaral, M.H.; Breitenfeld, L. Sodium Tripolyphosphate: An excipient with intrinsic in vitro anti-Candida activity. Int. J. Pharm. 2011, 421, 130–134. [Google Scholar] [CrossRef]
- Recktenwald, J.M.; Norrlow, O.; Dey, E.S. The function of cationbinding agents in the enzymatic treatment of municipal sludge. Water Res. 2008, 42, 1555–1562. [Google Scholar]
- Rashchi, F.; Finch, J.A. Polyphosphates: A review their chemistry and application with particular reference to mineral processing. Miner. Eng. 2000, 13, 1019–1035. [Google Scholar] [CrossRef]
- Ma, B.G.; Tan, H.B.; Xu, Y.H. Study on the mechanism of action of different water reducing agents on cement hydration. China Concr. Cem. Prod. 2007, 34, 495. [Google Scholar]
- Shang, X.J.; Hou, D.; Tang, W. Effect of alumina micropowder on anomalous hydration behaviour of calcium aluminate cement. Naihuo Cailiao 2016, 50, 252–255. [Google Scholar]
- Wang, Y.H.; Sun, D.X.; Wang, L.G.; Zhou, Y. Effects of sodium tripolyphosphate and sodium carbonate on the selective flocculation of diasporic-bauxite in the presence of calcium and magnesium ions. Miner. Eng. 2011, 24, 1031–1037. [Google Scholar]
- Papo, A.; Piani, L.; Ricceri, R. Sodium tripolyphosphate and polyphosphate as dispersing agents for kaolin suspensions: Rheological characterization. Colloids Surf. A Physicochem. Eng. Asp. 2002, 201, 219–223. [Google Scholar] [CrossRef]
- Zhou, J.H.; Kuang, Z.Y.; Lei, L.L. Testing the ability of sodium tripolyphosphate to chelate calcium. China Surfactant Soap Deterg. 2008, 3, 79–80. [Google Scholar]
- Zhou, J.H.; Kuang, Z.Y.; Lei, L.L. Influencing factors of calcium ion chelating power of sodium tripolyphosphate. Inorg. Chem. Industry. 2009, 41, 38–39. [Google Scholar]
- Luo, M.; Fu, J.X.; Jiang, L.W.; Zang, L. Study of the effect of sodium tripolyphosphate on the properties of concrete. Henan Build. Mater. 2017, 2, 30–32. [Google Scholar]
- Wu, G.L.; Zhou, J.H.; Lu, Y.L. Hydrolysis and content measurement of sodium tripolyphosphate by titration method. Inorg. Chem. Ind. 2014, 2, 47–50. [Google Scholar]
- Zhou, J.H.; Zhang, L.P.; Yan, P. Determination of calcium c-helating capacity of sodium tri-polyphosphate. China Suffactant Deterg. C-Osmetics 2009, 39, 210–212. [Google Scholar]
- Yang, P. Adsorption Behavior on Cement Particles and Retarder Mechanism of Sodium Tripolyphosphate. Bull. Chin. Ceram. Soc. 2013, 32, 1212–1216. [Google Scholar]
- Li, C.; Lü, Y. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. II. The mechanism of the interaction between phosphate modifiers and minerals. Int. J. Miner. Process. 1983, 10, 219–235. [Google Scholar]
- Li, Z.-H.; Han, Y.-X.; Li, Y.-J.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferrous Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Rong, J. Properties and applications of sodium tripolyphosphate. Sichuan Silk 1996, 4, 39–41. [Google Scholar]
- Tang, S.; Chen, E.; Shao, H.; Li, Z. A fractal approach to determine thermal conductivity in cement pastes. Constr. Build. Mater. 2015, 74, 73–82. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Lv, Y.; Zhou, M.; He, C.; Jiang, W.; Liu, Z.; Li, C. Utilization of limestone powder and fly ash in blended cement: Rheology, strength and hydration characteristics. Constr. Build. Mater. 2020, 232, 117228. [Google Scholar] [CrossRef]
- Hu, C.; Li, C.; Yan, B.; Chen, G. Effect of water reducing agents on the rheology of fine-grained tailing sand pastes. Chin. J. Nonferrous Met. 2022, 32, 2458–2468. [Google Scholar]
- Zhu, B.; Wang, Y.; Li, X. Effect of Phase Distribution on Rheological Behavior of Calcium Aluminate Cement with Built-in Alumina-Magnesia Spinel. J. Chin. Ceram. Soc. 2014, 42, 1383–1388. [Google Scholar]
- Yang, Z.Q.; Ding, Y.; Yang, Y.; Zhu, Y.; Zhang, J.; Guo, Y. Hydration-time-dependent rheological behaviors of Newtonian cement grouts with different water cement ratios. Trans. Chin. Soc. Agric. Eng. 2020, 36, 161–167. [Google Scholar]
- Idrees, M.; Ekincioglu, O.; Sonyal, M.S. Hydration behavior of calcium aluminate cement mortars with mineral admixtures at different curing temperatures. Constr. Build. Mater. 2021, 285, 122839. [Google Scholar] [CrossRef]
- Zhang, S.J.; Jiang, Y.Q.; Kong, X.Z. Effects of Superplasticizers on Early Hydration of C3A. J. Build. Mater. 2014, 17, 887–891. [Google Scholar]
- Sereewatthanawut, I.; Prasittisopin, L. Effects of accelerating and retarding agents on nucleation and crystal growth of calcium aluminate cement. Open Ceram. 2022, 11, 100290. [Google Scholar] [CrossRef]
- Xu, W.; Dai, J.-G.; Ding, Z.; Wang, Y. Polyphosphate-modified calcium aluminate cement under normal and elevated temperatures: Phase evolution, microstructure, and mechanical properties. Ceram. Int. 2017, 43, 15525–15536. [Google Scholar] [CrossRef]
- Samanta, B.; Anand Kumar, P. Experimental investigation of the phase equilibria, phase stability, and defect structure in the Cr–Zr system by using DSC, XRD, and SEM. Intermetallics 2022, 150, 150687. [Google Scholar]



| Al2O3 | CaO | SiO2 | Fe2O3 | MgO | TiO2 | SO3 | Others | |
|---|---|---|---|---|---|---|---|---|
| w/% | 68.7 | 28.5 | 0.4 | 0.2 | 0.25 | 0.2 | 0.15 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Feng, H.; Armia, E.; Guo, H.; Zhang, S.; Zhang, H. Effects of Sodium Tripolyphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement. Materials 2023, 16, 3141. https://doi.org/10.3390/ma16083141
Cheng B, Feng H, Armia E, Guo H, Zhang S, Zhang H. Effects of Sodium Tripolyphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement. Materials. 2023; 16(8):3141. https://doi.org/10.3390/ma16083141
Chicago/Turabian StyleCheng, Benjun, Hao Feng, Erbolat Armia, Hongli Guo, Shaowei Zhang, and Haijun Zhang. 2023. "Effects of Sodium Tripolyphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement" Materials 16, no. 8: 3141. https://doi.org/10.3390/ma16083141
APA StyleCheng, B., Feng, H., Armia, E., Guo, H., Zhang, S., & Zhang, H. (2023). Effects of Sodium Tripolyphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement. Materials, 16(8), 3141. https://doi.org/10.3390/ma16083141







