Systematic Investigation on Supported Gold Catalysts Prepared by Fluorine-Free Basic Etching Ti3AlC2 in Selective Oxidation of Aromatic Alcohols to Aldehydes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ti3AlxC2Ty Supports
2.3. Preparation of Aupre/Ti3AlxC2Ty Catalysts
2.4. Thermal Treatment Procedures
2.5. Preparation of Control Catalysts by the Chemical Reduction (cr) Method
2.6. Preparation of Control Catalysts by the Colloidal Deposition (col) Method
2.7. Preparation of Control Catalysts by the Wet Impregnation (wi) Method
2.8. Characterizations
2.9. Catalytic Reactions
3. Results and Discussion
3.1. Crystal Structures of Catalysts by XRD
3.2. Oxidation States of Gold by XPS Analyses
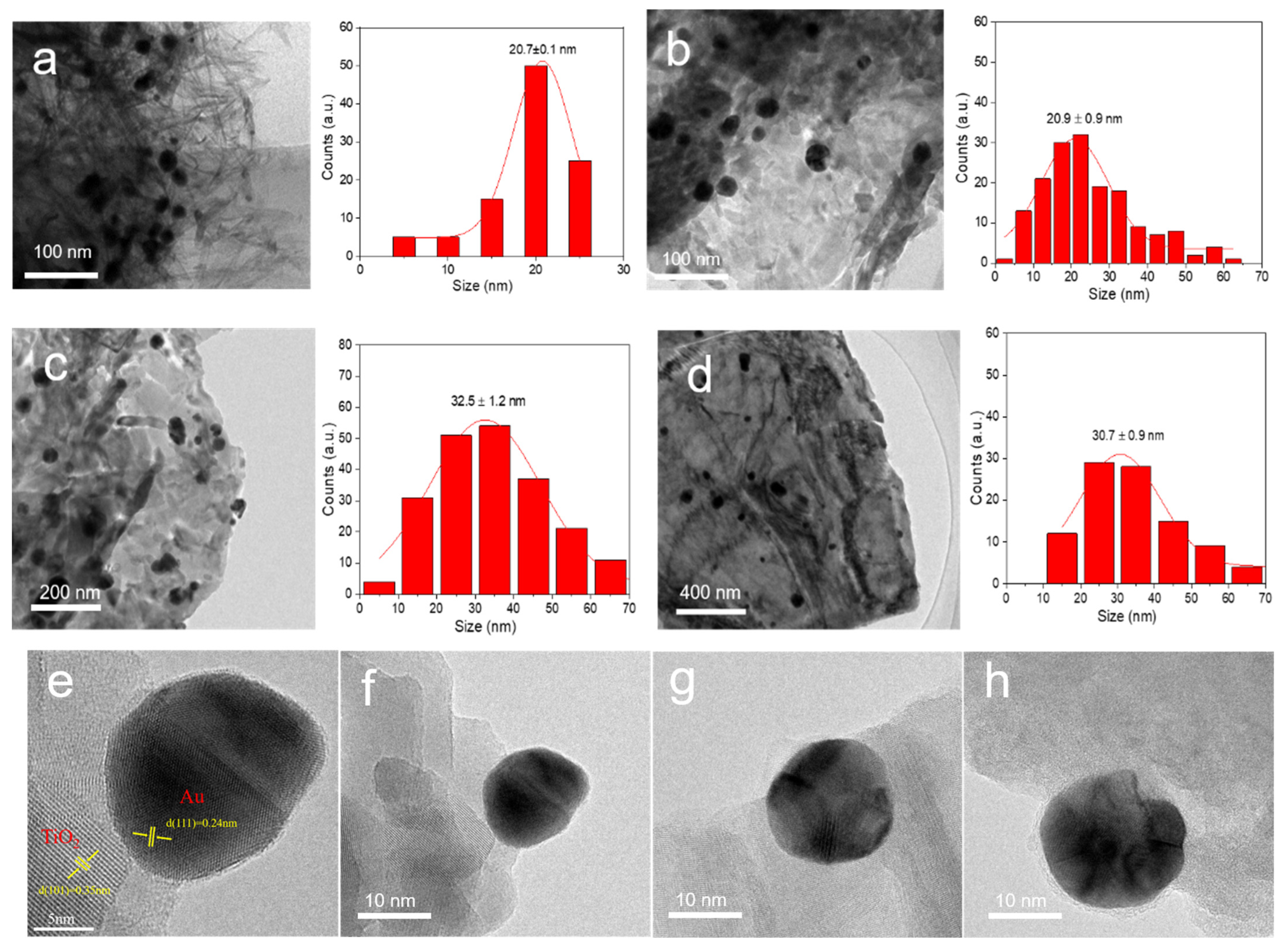
3.3. Size and Morphological Analyses
3.4. Catalytic Tests and Catalyst Screenings
3.5. Effect of Different Reactants
3.6. Stability Evaluation by Tests of Reusability and Hot Filtration
3.7. Synergistic Effect and Mechanistic Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar]
- Bhan, A.; Delgass, W.N. Best practices in catalysis: A perspective. J. Catal. 2022, 405, 419–429. [Google Scholar] [CrossRef]
- Prats, H.; Posada-Pérez, S.; Rodriguez, J.A.; Sayós, R.; Illas, F. Kinetic Monte Carlo Simulations Unveil Synergic Effects at Work on Bifunctional Catalysts. ACS Catal. 2019, 9, 9117–9126. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Mejia, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.Z.; Xu, B. Advances in the Synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef]
- Ng, W.H.K.; Gnanakumar, E.S.; Batyrev, E.; Sharma, S.K.; Pujari, P.K.; Greer, H.F.; Zhou, W.; Sakidja, R.; Rothenberg, G.; Barsoum, M.W.; et al. The Ti3AlC2 MAX Phase as an Efficient Catalyst for Oxidative Dehydrogenation of n-Butane. Angew. Chem. Int. Ed. 2018, 57, 1485–1490. [Google Scholar] [CrossRef]
- Trandafir, M.M.; Neaţu, F.; Chirica, I.M.; Neaţu, Ş.; Kuncser, A.C.; Cucolea, E.I.; Natu, V.; Barsoum, M.W.; Florea, M. Highly Efficient Ultralow Pd Loading Supported on MAX Phases for Chemoselective Hydrogenation. ACS Catal. 2020, 10, 5899–5908. [Google Scholar] [CrossRef]
- Slot, T.K.; Oulego, P.; Sofer, Z.; Bai, Y.; Rothenberg, G.; Raveendran Shiju, N. Ruthenium on Alkali-Exfoliated Ti3(Al0.8Sn0.2)C2 MAX Phase Catalyses Reduction of 4-Nitroaniline with Ammonia Borane. ChemCatChem 2021, 13, 3470–3478. [Google Scholar] [CrossRef]
- Chirica, I.M.; Mirea, A.G.; Neaţu, Ş.; Florea, M.; Barsoum, M.W.; Neaţu, F. Applications of MAX phases and MXenes as catalysts. J. Mater. Chem. A 2021, 9, 19589–19612. [Google Scholar]
- Ronda-Lloret, M.; Marakatti, V.S.; Sloof, W.G.; Delgado, J.J.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Rothenberg, G.; Shiju, N.R. Butane Dry Reforming Catalyzed by Cobalt Oxide Supported on Ti2AlC MAX Phase. ChemSusChem 2020, 13, 6401–6408. [Google Scholar] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Tahir, K.; Kim, J.; Lim, Y.; Kim, B.; Jang, J.; Lee, D.S. Exfoliation of Titanium Aluminum Carbide (211 MAX Phase) to Form Nanofibers and Two-Dimensional Nanosheets and Their Application in Aqueous-Phase Cadmium Sequestration. ACS Appl. Mater. Interfaces 2019, 11, 19156–19166. [Google Scholar] [CrossRef]
- Li, T.F.; Yao, L.L.; Liu, Q.L.; Gu, J.J.; Luo, R.C.; Li, J.H.; Yan, X.D.; Wang, W.Q.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Dong, X.S.; Wang, Y.Q.; Jia, M.M.; Niu, Z.Y.; Cai, J.M.; Yu, X.; Ke, X.B.; Yao, J.F.; Zhang, X.G. Sustainable and scalable in-situ synthesis of hydrochar-wrapped Ti3AlC2-derived nanofibers as adsorbents to remove heavy metals. Bioresour. Technol. 2019, 282, 222–227. [Google Scholar] [CrossRef]
- Gu, P.C.; Xing, J.L.; Wen, T.; Zhang, R.; Wang, J.; Zhao, G.X.; Hayat, T.; Ai, Y.J.; Lin, Z.; Wang, X.K. Experimental and theoretical calculation investigation on efficient Pb(ii) adsorption on etched Ti3AlC2 nanofibers and nanosheets. Environ. Sci. Nano 2018, 5, 946–955. [Google Scholar] [CrossRef]
- Shi, L.; Lu, K.; Kong, X.; Li, L.C.; Gu, X.L.; Cai, J.M.; Zhang, X.G. A new reduction method based on simultaneous Ti3AlC2 support etching and metal deposition to prepare Pt catalysts for aqueous-phase selective hydrogenation of furfural to furfuryl alcohol. New J. Chem. 2022, 46, 14958–14966. [Google Scholar] [CrossRef]
- Pincus, L.N.; Lounsbury, A.W.; Zimmerman, J.B. Toward Realizing Multifunctionality: Photoactive and Selective Adsorbents for the Removal of Inorganics in Water Treatment. Acc. Chem. Res. 2019, 52, 1206–1214. [Google Scholar] [CrossRef]
- Posada Pérez, S.; Solà, M.; Poater, A. Carbon Dioxide Conversion on Supported Metal Nanoparticles: A Brief Review. Catalysts 2023, 13, 305. [Google Scholar]
- Ahmad, F.; Salem Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [PubMed]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Chen, M.S.; Goodman, D.W. The Structure of Catalytically Active Gold on Titania. Science 2004, 306, 252–255. [Google Scholar]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar]
- Zhang, X.G.; Ke, X.B.; Zhu, H.Y. Zeolite-Supported Gold Nanoparticles for Selective Photooxidation of Aromatic Alcohols under Visible-Light Irradiation. Chem. Eur. J. 2012, 18, 8048–8056. [Google Scholar] [PubMed]
- Parmeggiani, C.; Matassini, C.; Cardona, F. A step forward towards sustainable aerobic alcohol oxidation: New and revised catalysts based on transition metals on solid supports. Green Chem. 2017, 19, 2030–2050. [Google Scholar]
- Bao, X.L.; Li, H.L.; Wang, Z.Y.; Tong, F.X.; Liu, M.; Zheng, Z.K.; Wang, P.; Cheng, H.F.; Liu, Y.Y.; Dai, Y.; et al. TiO2/Ti3C2 as an efficient photocatalyst for selective oxidation of benzyl alcohol to benzaldehyde. Appl. Catal. B 2021, 286, 119885. [Google Scholar] [CrossRef]
- Zaarour, M.; Cazemier, J.; Ruiz-Martínez, J. Recent developments in the control of selectivity in hydrogenation reactions by confined metal functionalities. Catal. Sci. Technol. 2020, 10, 8140–8172. [Google Scholar]
- Zhou, Y.H.; Wang, X.H.; Huang, X.; Deng, H.; Hu, Y.T.; Lu, L.F. Boosted photothermal hydrogenation of acetylene on an efficient Au–Fe alloy catalyst. Catal. Sci. Technol. 2023, 13, 41–46. [Google Scholar]
- Ke, X.B.; Zhang, X.G.; Zhao, J.; Sarina, S.; Barry, J.; Zhu, H.Y. Selective reductions using visible light photocatalysts of supported gold nanoparticles. Green Chem. 2013, 15, 236–244. [Google Scholar]
- Zhang, X.G.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Lee, A.F.; Wilson, K. Platinum-Catalyzed Aqueous-Phase Hydrogenation of d-Glucose to d-Sorbitol. ACS Catal. 2016, 6, 7409–7417. [Google Scholar]
- Lin, H.; Wang, X.G.; Yu, L.D.; Chen, Y.; Shi, J.L. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Du, X.; Huang, Y.; Pan, X.; Han, B.; Su, Y.; Jiang, Q.; Li, M.; Tang, H.; Li, G.; Qiao, B. Size-dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, L.; Yun, R.P.; Pu, M.; Zhang, B.; Xiang, X. Increasing the Activity and Selectivity of TiO2-Supported Au Catalysts for Renewable Hydrogen Generation from Ethanol Photoreforming by Engineering Ti3+ Defects. ACS Sustain. Chem. Eng. 2019, 7, 13856–13864. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gómez, S.A.; Zepeda, T.A.; Fierro-González, J.C.; Fuentes, G.A. Insight into the Deactivation of Au/CeO2 Catalysts Studied by In Situ Spectroscopy during the CO-PROX Reaction. ACS Catal. 2015, 5, 4003–4012. [Google Scholar] [CrossRef]
- Pollitt, S.; Truttmann, V.; Haunold, T.; Garcia, C.; Olszewski, W.; Llorca, J.; Barrabés, N.; Rupprechter, G. The Dynamic Structure of Au38(SR)24 Nanoclusters Supported on CeO2 upon Pretreatment and CO Oxidation. ACS Catal. 2020, 10, 6144–6148. [Google Scholar] [CrossRef]
- Peng, C.; Yang, X.F.; Li, Y.H.; Yu, H.; Wang, H.J.; Peng, F. Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 6051–6060. [Google Scholar] [CrossRef]
- Li, D.F.; Wang, J.G.; Xu, F.X.; Zhang, N.C.; Men, Y. Mesoporous (001)-TiO2 nanocrystals with tailored Ti3+ and surface oxygen vacancies for boosting photocatalytic selective conversion of aromatic alcohols. Catal. Sci. Technol. 2021, 11, 2939–2947. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.Y.; Liu, F.Y.; Zhang, Q.; Yao, S.; Liu, X.L.; Huang, F. Ti3C2: An Ideal Co-catalyst? Angew. Chem. Int. Ed. 2020, 59, 1914–1918. [Google Scholar] [CrossRef]
- Wang, K.; Lu, J.B.; Lu, Y.; Lau, C.H.; Zheng, Y.; Fan, X.F. Unravelling the CC coupling in CO2 photocatalytic reduction with H2O on Au/TiO2-x: Combination of plasmonic excitation and oxygen vacancy. Appl. Catal. B 2021, 292, 120147. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, Y.Y.; Zhu, X.J.; Liu, Y.; Wu, Z.B. Fluorine-induced oxygen vacancies on TiO2 nanosheets for photocatalytic indoor VOCs degradation. Appl. Catal. B 2022, 316, 121610. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Borau, V.; Colmenares, J.C.; Marinas, A.; Marinas, J.M.; Navío, J.A.; Urbano, F.J. Modification of the photocatalytic activity of Pd/TiO2 and Zn/TiO2 systems through different oxidative and reductive calcination treatments. Appl. Catal. B 2008, 80, 88–97. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef]
- Wu, Y.M.; Zhang, J.L.; Xiao, L.; Chen, F. Preparation and characterization of TiO2 photocatalysts by Fe3+ doping together with Au deposition for the degradation of organic pollutants. Appl. Catal. B 2009, 88, 525–532. [Google Scholar] [CrossRef]
- Qin, L.; Yi, H.; Zeng, G.M.; Lai, C.; Huang, D.L.; Xu, P.; Fu, Y.; He, J.F.; Li, B.S.; Zhang, C.; et al. Hierarchical porous carbon material restricted Au catalyst for highly catalytic reduction of nitroaromatics. J. Hazard. Mater. 2019, 380, 120864. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moemen, A.; Abdel-Mageed, A.M.; Bansmann, J.; Parlinska-Wojtan, M.; Behm, R.J.; Kučerová, G. Deactivation of Au/CeO2 catalysts during CO oxidation: Influence of pretreatment and reaction conditions. J. Catal. 2016, 341, 160–179. [Google Scholar] [CrossRef]
- Najafishirtari, S.; Friedel Ortega, K.; Douthwaite, M.; Pattisson, S.; Hutchings, G.J.; Bondue, C.J.; Tschulik, K.; Waffel, D.; Peng, B.; Deitermann, M.; et al. A Perspective on Heterogeneous Catalysts for the Selective Oxidation of Alcohols. Chem. Eur. J. 2021, 27, 16809–16833. [Google Scholar] [CrossRef] [PubMed]
- Yurdakal, S.; Palmisano, G.; Loddo, V.; Augugliaro, V.; Palmisano, L. Nanostructured Rutile TiO2 for Selective Photocatalytic Oxidation of Aromatic Alcohols to Aldehydes in Water. J. Am. Chem. Soc. 2008, 130, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Su, Y.; Zhang, B.S.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Li, L.; Ren, Y.G.; Huang, J.H.; Haruta, M.; et al. Classical strong metal–support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef]
- Qiu, J.h.; Zhang, X.G.; Xie, K.L.; Zhang, X.F.; Feng, Y.; Jia, M.M.; Yao, J.F. Noble metal nanoparticle-functionalized Zr-metal organic frameworks with excellent photocatalytic performance. J. Colloid Interf. Sci. 2019, 538, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, D.N.; Liao, Y.L.; Li, F.; Zhang, H.W.; Xiang, Q.J. Constructing functionalized plasmonic gold/titanium dioxide nanosheets with small gold nanoparticles for efficient photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2019, 555, 94–103. [Google Scholar] [CrossRef]
- Wang, Z.M.; Qi, J.; Zhao, K.; Zong, L.B.; Tang, Z.Y.; Wang, L.Z.; Yu, R.B. Controlled synthesis of highly active Au/CeO2 nanotubes for CO oxidation. Mater. Chem. Front. 2017, 1, 1629–1634. [Google Scholar] [CrossRef]
- Meher, S.; Rana, R.K. A rational design of a Pd-based catalyst with a metal–metal oxide interface influencing molecular oxygen in the aerobic oxidation of alcohols. Green Chem. 2019, 21, 2494–2503. [Google Scholar] [CrossRef]
- Wang, R.Y.; Lu, K.; Zhang, J.; Li, X.C.; Zheng, Z.F. Regulation of the Co–Nx Active Sites of MOF-Templated Co@NC Catalysts via Au Doping for Boosting Oxidative Esterification of Alcohols. ACS Catal. 2022, 12, 14290–14303. [Google Scholar] [CrossRef]
- Banerjee, B.; Singuru, R.; Kundu, S.K.; Dhanalaxmi, K.; Bai, L.Y.; Zhao, Y.L.; Reddy, B.M.; Bhaumik, A.; Mondal, J. Towards rational design of core–shell catalytic nanoreactor with high performance catalytic hydrogenation of levulinic acid. Catal. Sci. Technol. 2016, 6, 5102–5115. [Google Scholar] [CrossRef]
- Rucinska, E.; Miedziak, P.J.; Pattisson, S.; Brett, G.L.; Iqbal, S.; Morgan, D.J.; Sankar, M.; Hutchings, G.J. Cinnamyl alcohol oxidation using supported bimetallic Au–Pd nanoparticles: An investigation of autoxidation and catalysis. Catal. Sci. Technol. 2018, 8, 2987–2997. [Google Scholar] [CrossRef]
- Santra, C.; Rahman, S.; Bojja, S.; James, O.O.; Sen, D.; Maity, S.; Mohanty, A.K.; Mazumder, S.; Chowdhury, B. Barium, calcium and magnesium doped mesoporous ceria supported gold nanoparticle for benzyl alcohol oxidation using molecular O2. Catal. Sci. Technol. 2013, 3, 360–370. [Google Scholar] [CrossRef]
- Brindle, J.; Sufyan, S.A.; Nigra, M.M. Support, composition, and ligand effects in partial oxidation of benzyl alcohol using gold–copper clusters. Catal. Sci. Technol. 2022, 12, 3846–3855. [Google Scholar]
- Slot, T.K.; Eisenberg, D.; van Noordenne, D.; Jungbacker, P.; Rothenberg, G. Cooperative Catalysis for Selective Alcohol Oxidation with Molecular Oxygen. Chem. Eur. J. 2016, 22, 12307–12311. [Google Scholar]
- Santiago-Portillo, A.; Cabrero-Antonino, M.; Álvaro, M.; Navalón, S.; García, H. Tuning the Microenvironment of Gold Nanoparticles Encapsulated within MIL-101(Cr) for the Selective Oxidation of Alcohols with O2: Influence of the Amino Terephthalate Linker. Chem. Eur. J. 2019, 25, 9280–9286. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.F.; Zhao, W.N.; Liu, Z.P.; Xu, B.Q. NaOH alone can be a homogeneous catalyst for selective aerobic oxidation of alcohols in water. J. Catal. 2017, 353, 37–43. [Google Scholar] [CrossRef]
- Huang, J.W.; He, S.; Goodsell, J.L.; Mulcahy, J.R.; Guo, W.X.; Angerhofer, A.; Wei, W.D. Manipulating Atomic Structures at the Au/TiO2 Interface for O2 Activation. J. Am. Chem. Soc. 2020, 142, 6456–6460. [Google Scholar] [PubMed]
- Inoue, H.; Naya, S.-i.; Akita, A.; Sugime, H.; Tada, H. Photothermal Oxidation of Cinnamyl Alcohol with Hydrogen Peroxide Catalyzed by Gold Nanoparticle/Antimony-Doped Tin Oxide Nanocrystals. Chem. Eur. J. 2022, 28, e202201653. [Google Scholar] [CrossRef]





| Catalysts | ICP/wt% | Conversion/% | Selectivity/% | Yield/% | Reaction Rate/mmol·gAu−1·h−1 |
|---|---|---|---|---|---|
| No catalysts | null | 0.8 | 58.3 | 0.5 | null |
| Ti3AlC2 | null | 0.6 | 63.0 | 0.4 | null |
| Ti3AlxC2Ty | null | 3.6 | 42.5 | 1.5 | null |
| 0.25wt%Aupre/Ti3AlxC2Ty | 0.17 | 4.5 | 61.4 | 2.8 | 54.9 |
| 0.5wt%Aupre/Ti3AlxC2Ty | 0.32 | 12.1 | 68.8 | 8.3 | 86.5 |
| 1wt%Aupre/Ti3AlxC2Ty | 0.67 | 25.2 | 88.2 | 22.2 | 110.4 |
| 1.5wt%Aupre/Ti3AlxC2Ty | 1.06 | 32.8 | 86.8 | 28.5 | 89.6 |
| 2wt%Aupre/Ti3AlxC2Ty | 1.32 | 36.0 | 90.2 | 32.5 | 82.1 |
| 1wt%Aupre/Ti3AlxC2Ty-Air200 | 0.73 | 19.5 | 99.0 | 19.5 | 89.0 |
| 1wt%Aupre/Ti3AlxC2Ty-Air400 | 0.67 | 23.9 | 99.0 | 23.9 | 118.9 |
| 1wt%Aupre/Ti3AlxC2Ty-Air600 | 0.52 | 38.5 | 94.7 | 36.5 | 234.0 |
| 1wt%Aupre/Ti3AlxC2Ty-Ar200 | 0.41 | 14.2 | 99.0 | 14.2 | 115.4 |
| 1wt%Aupre/Ti3AlxC2Ty-Ar400 | 0.76 | 5.0 | 99.0 | 5.0 | 21.9 |
| 1wt%Aupre/Ti3AlxC2Ty-Ar600 | 0.68 | 1.6 | 99.0 | 1.6 | 7.8 |
| 1wt%Aupre/Ti3AlxC2Ty-H200 | 0.67 | 12.2 | 89.8 | 11.0 | 60.7 |
| 1wt%Aupre/Ti3AlxC2Ty-H400 | 0.55 | 10.4 | 91.1 | 9.5 | 57.6 |
| 1wt%Aupre/Ti3AlxC2Ty-H600 | 0.71 | 1.6 | 52.6 | 0.8 | 3.8 |
| Catalysts | Conversion/% | Selectivity/% | Yield/% |
|---|---|---|---|
| Aupre/Ti3AlxC2Ty | 25.2 | 88.2 | 22.2 |
| Aucol/Ti3AlxC2Ty | 16.6 | 96.3 | 16.0 |
| Aucr/Ti3AlxC2Ty | 7.7 | 94.1 | 7.3 |
| Auwi/Ti3AlxC2Ty | 10.2 | 93.1 | 9.5 |
| Aupre/Ti3AlxC2Ty-Air600 | 38.5 | 94.7 | 36.5 |
| Aucol/Ti3AlxC2Ty-Air600 | 30.2 | 88.4 | 26.7 |
| Aucr/Ti3AlxC2Ty-Air600 | 9.1 | 99.9 | 9.1 |
| Auwi/Ti3AlxC2Ty-Air600 | 8.6 | 89.7 | 7.7 |
| Reagent | Target Product | Conversion/% | Selectivity/% | Yield/% | Reaction Rate/mol·gAu−1·h−1 |
|---|---|---|---|---|---|
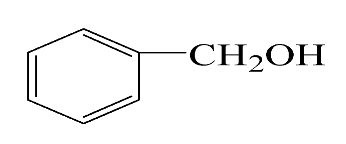 |  | 38.5 | 94.7 | 36.5 | 234.0 |
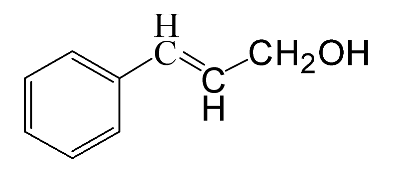 | 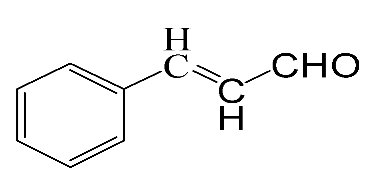 | 3.4 | >99 | 3.4 | 21.8 |
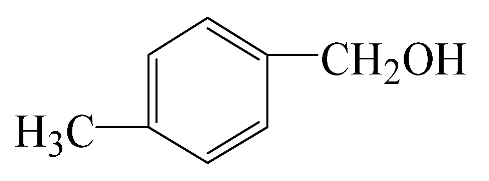 |  | 49.3 | 88.0 | 43.3 | 277.6 |
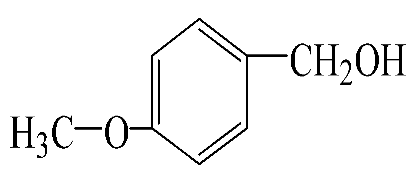 | 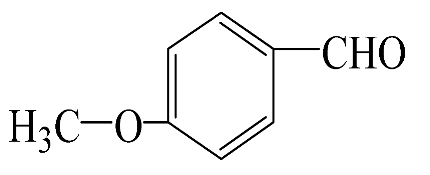 | 55.3 | 96.5 | 53.4 | 342.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Chen, X.; Cui, D.; Lu, K.; Kong, X.; Zhang, X. Systematic Investigation on Supported Gold Catalysts Prepared by Fluorine-Free Basic Etching Ti3AlC2 in Selective Oxidation of Aromatic Alcohols to Aldehydes. Materials 2023, 16, 3139. https://doi.org/10.3390/ma16083139
Jiang H, Chen X, Cui D, Lu K, Kong X, Zhang X. Systematic Investigation on Supported Gold Catalysts Prepared by Fluorine-Free Basic Etching Ti3AlC2 in Selective Oxidation of Aromatic Alcohols to Aldehydes. Materials. 2023; 16(8):3139. https://doi.org/10.3390/ma16083139
Chicago/Turabian StyleJiang, Hangwei, Xiya Chen, Danlan Cui, Kun Lu, Xiao Kong, and Xingguang Zhang. 2023. "Systematic Investigation on Supported Gold Catalysts Prepared by Fluorine-Free Basic Etching Ti3AlC2 in Selective Oxidation of Aromatic Alcohols to Aldehydes" Materials 16, no. 8: 3139. https://doi.org/10.3390/ma16083139
APA StyleJiang, H., Chen, X., Cui, D., Lu, K., Kong, X., & Zhang, X. (2023). Systematic Investigation on Supported Gold Catalysts Prepared by Fluorine-Free Basic Etching Ti3AlC2 in Selective Oxidation of Aromatic Alcohols to Aldehydes. Materials, 16(8), 3139. https://doi.org/10.3390/ma16083139





