Three-Dimensional-Printed Molds from Water-Soluble Sulfate Ceramics for Biocomposite Formation through Low-Pressure Injection Molding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Mixtures Preparation
2.3. Ceramic Samples Preparation
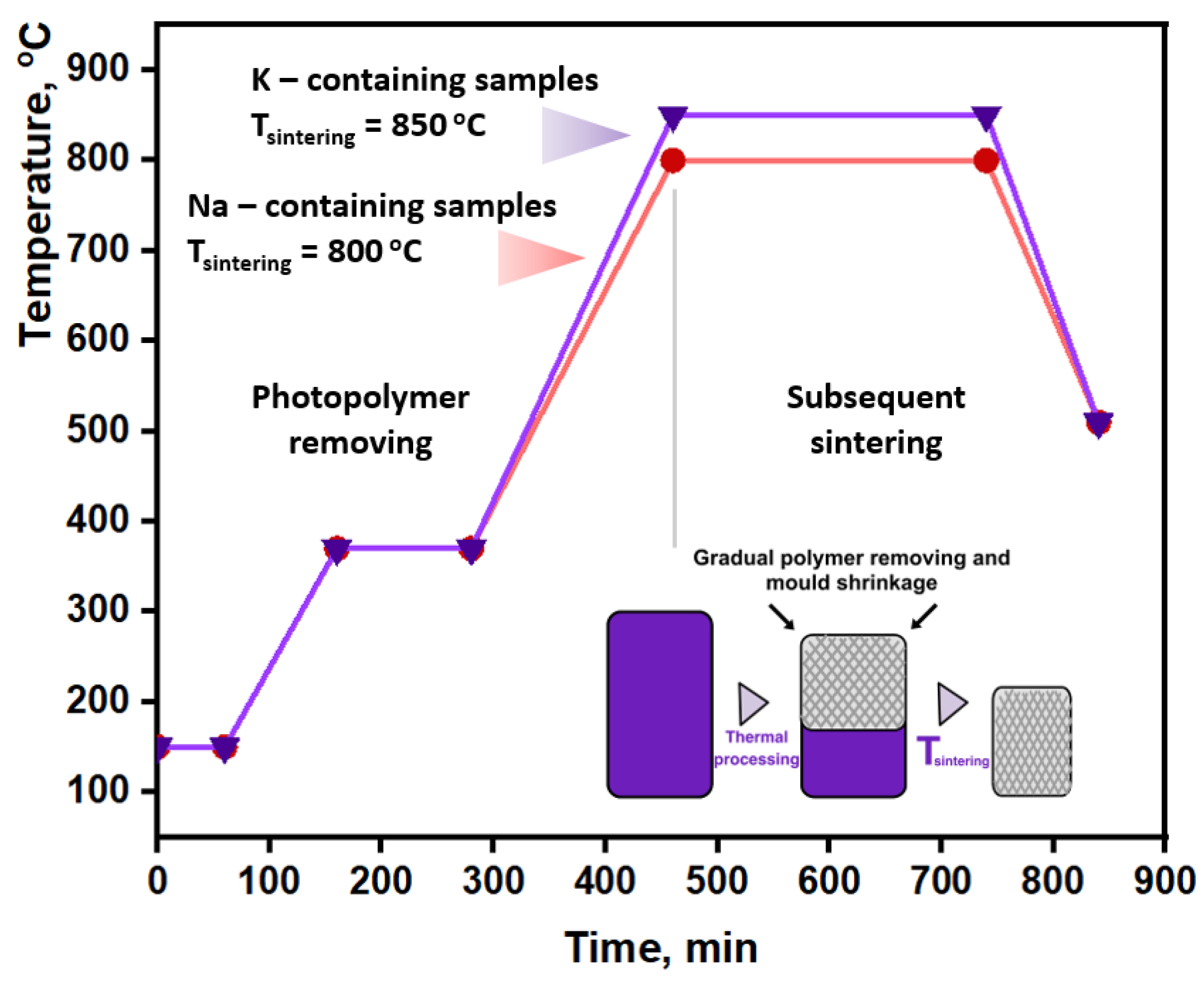
2.4. 3D Molds Preparation
2.5. Characterization Techniques
- ρ—density of the sample, g/cm3;
- m—weight of the sample, g;
- h—thickness of the sample, cm
- D—diameter of the sample, cm;
- ρn—density of the corresponding phase, g/cm3;
- nn—the content of the corresponding phase.
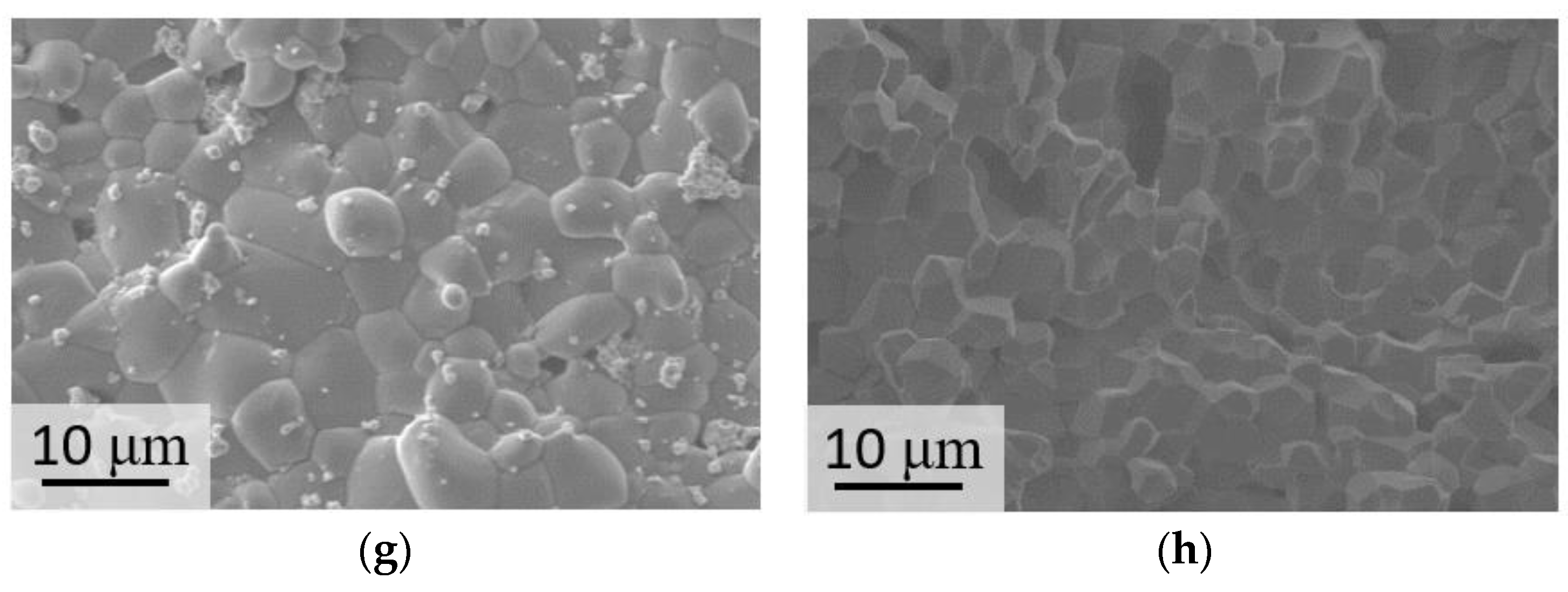
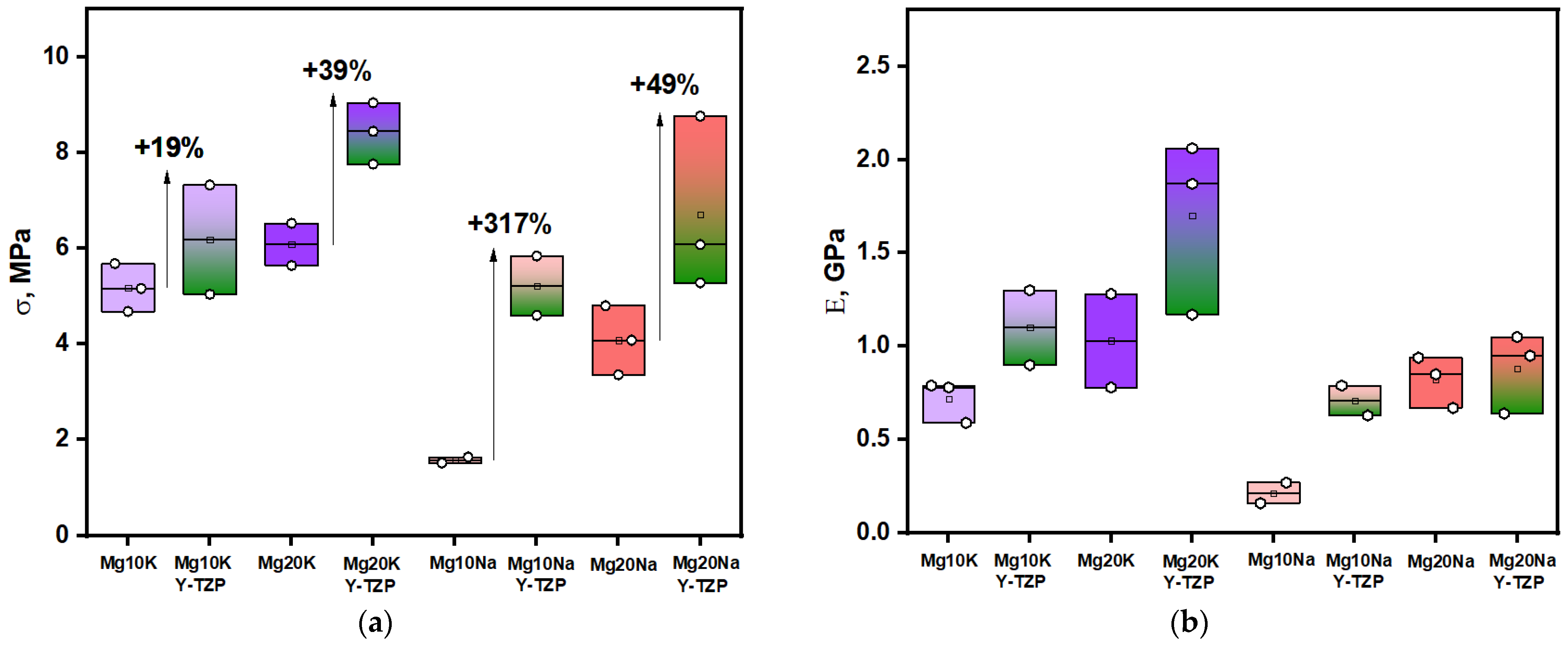
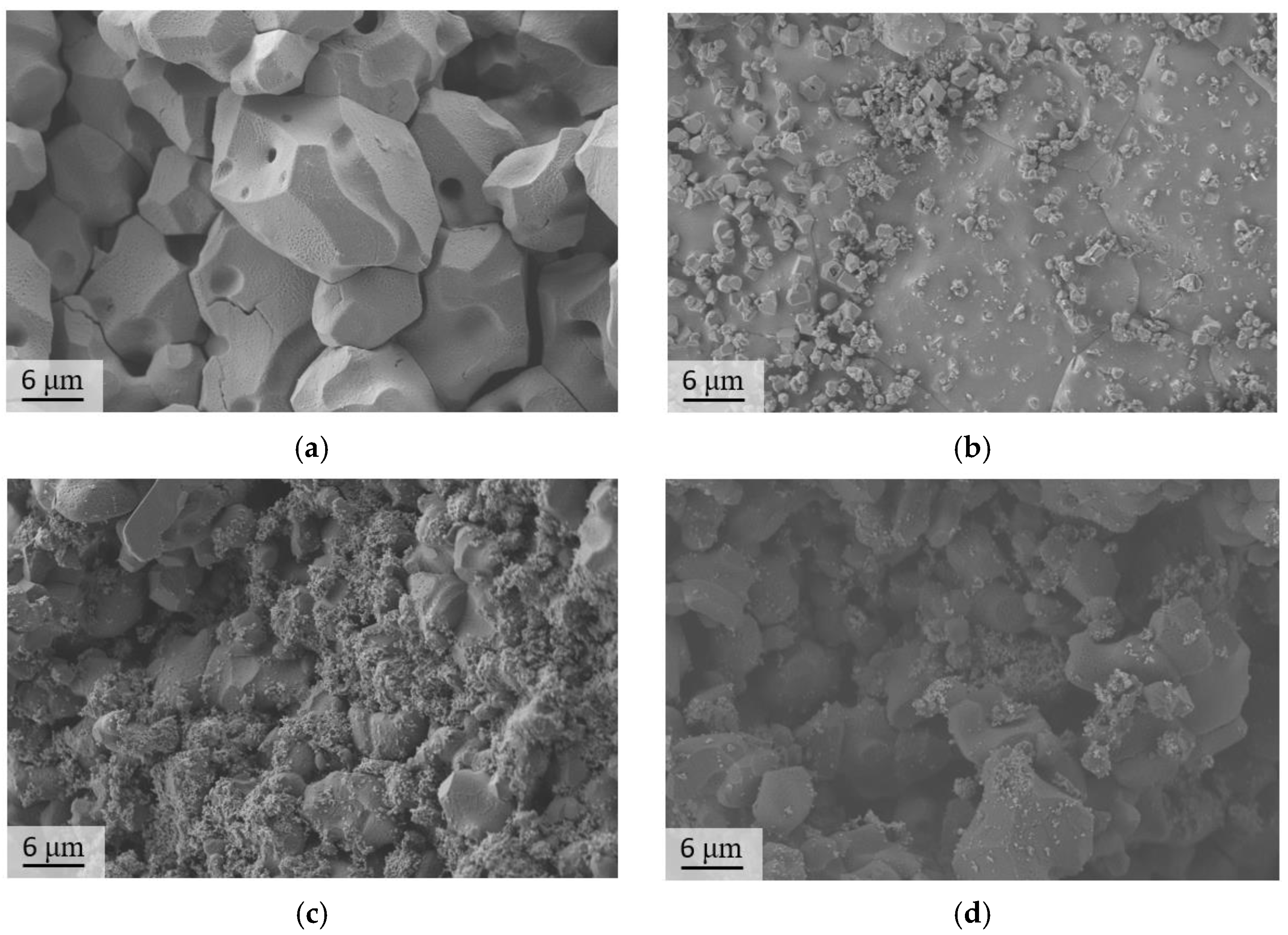
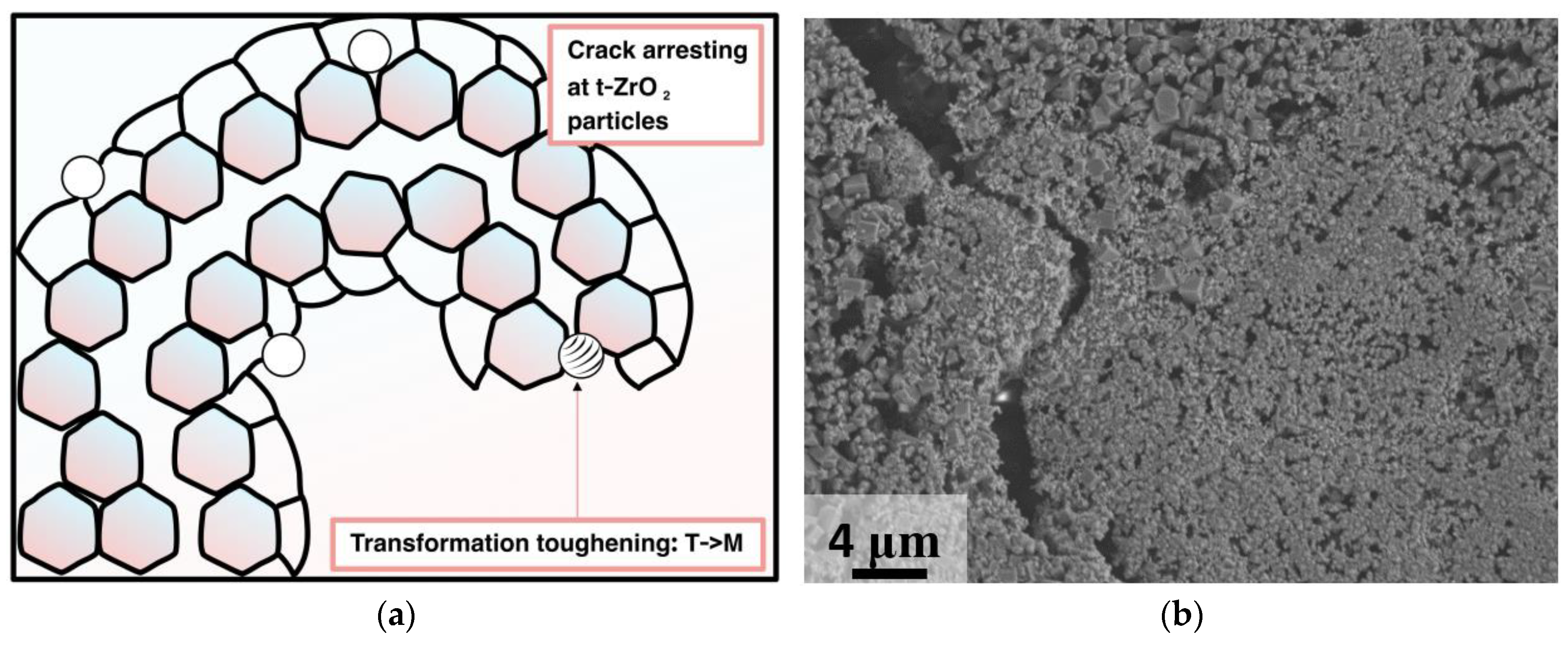
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juhasz, J.A.; Best, S.M. Bioactive Ceramics: Processing, Structures and Properties. J. Mater. Sci. 2012, 47, 610–624. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Epple, M. Composites of Calcium Phosphate and Polymers as Bone Substitution Materials. Eur. J. Trauma 2006, 32, 125–131. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone Tissue Engineering Graft Substitutes: What Is the Future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Kazakova, G.; Safronova, T.; Golubchikov, D.; Shevtsova, O.; Rau, J.V. Resorbable Mg2+—Containing Phosphates for Bone Tissue Repair. Materials 2021, 14, 4857. [Google Scholar] [CrossRef]
- Wang, N.; Thameem Dheen, S.; Fuh, J.Y.H.; Senthil Kumar, A. A Review of Multi-Functional Ceramic Nanoparticles in 3D Printed Bone Tissue Engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Kamboj, N.; Ressler, A.; Hussainova, I. Bioactive Ceramic Scaffolds for Bone Tissue Engineering by Powder Bed Selective Laser Processing: A Review. Materials 2021, 14, 5338. [Google Scholar] [CrossRef]
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on Current Limits and Potentialities of Technologies for Biomedical Ceramic Scaffolds Production. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 377–393. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, P.V.; Tikhonova, S.A.; Kiseleva, A.K.; Filippov, Y.Y.; Novoseletskaya, E.S.; Efimenko, A.Y.; Putlayev, V.I. Effect of the Pore Size on the Biological Activity of β-Ca3(PO4)2-Based Resorbable Macroporous Ceramic Materials Obtained by Photopolymerization. Russ. J. Inorg. Chem. 2021, 66, 1609–1615. [Google Scholar] [CrossRef]
- Tikhonov, A.; Putlayev, V. Synthesis and Thermal Behaviour of Calcium Alkyl Phosphates as Bioceramic Precursors. Ceramics 2022, 5, 362–371. [Google Scholar] [CrossRef]
- Kumar, A.; Mir, S.M.; Aldulijan, I.; Mahajan, A.; Anwar, A.; Leon, C.H.; Terracciano, A.; Zhao, X.; Su, T.L.; Kalyon, D.M.; et al. Load-Bearing Biodegradable PCL-PGA-Beta TCP Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 193–200. [Google Scholar] [CrossRef]
- Zhukova, P.A.; Senatov, F.S.; Zadorozhnyy, M.Y.; Chmelyuk, N.S.; Zaharova, V.A. Polymer Composite Materials Based on Polylactide with a Shape Memory Effect for “Self-Fitting” Bone Implants. Polymers 2021, 13, 2367. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.A.; Kennedy, Z.C.; Arey, B.W.; Christ, J.F.; Schaef, H.T.; Nune, S.K.; Erikson, R.L. Chemically Active, Porous 3D-Printed Thermoplastic Composites. ACS Appl. Mater. Interfaces 2018, 10, 15112–15121. [Google Scholar] [CrossRef] [PubMed]
- Golubchikov, D.; Safronova, T.V.; Nemygina, E.; Shatalova, T.B.; Tikhomirova, I.N.; Roslyakov, I.V.; Khayrutdinova, D.; Platonov, V.; Boytsova, O.; Kaimonov, M.; et al. Powder Synthesized from Aqueous Solution of Calcium Nitrate and Mixed-Anionic Solution of Orthophosphate and Silicate Anions for Bioceramics Production. Coatings 2023, 13, 374. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of Polymer-Based Biomaterials and Medical Devices-Regulations, In Vitro Screening and Risk-Management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Zuev, D.M.; Golubchikov, D.O.; Evdokimov, P.V.; Putlyaev, V.I. Synthesis of Amorphous Calcium Phosphate Powders for Production of Bioceramics and Composites by 3D Printing. Russ. J. Inorg. Chem. 2022, 67, 940–951. [Google Scholar] [CrossRef]
- Hurle, K.; Oliveira, J.M.; Reis, R.L.; Pina, S.; Goetz-Neunhoeffer, F. Ion-Doped Brushite Cements for Bone Regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef]
- Spirandeli, B.R.; Ribas, R.G.; Amaral, S.S.; Martins, E.F.; Esposito, E.; Vasconcellos, L.M.R.; Campos, T.M.B.; Thim, G.P.; Trichês, E.S. Incorporation of 45S5 Bioglass via Sol-Gel in β-TCP Scaffolds: Bioactivity and Antimicrobial Activity Evaluation. Mater. Sci. Eng. C 2021, 131, 112453. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scafflds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent Advances in PLGA-Based Biomaterials for Bone Tissue Regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Schilling, A.F.; Filke, S.; Brink, S.; Korbmacher, H.; Amling, M.; Rueger, J.M. Osteoclasts and Biomaterials. Eur. J. Trauma 2006, 32, 107–113. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen-Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Goto, M.; Matsumine, A.; Yamaguchi, S.; Takahashi, H.; Akeda, K.; Nakamura, T.; Asanuma, K.; Matsushita, T.; Kokubo, T.; Sudo, A. Osteoconductivity of Bioactive Ti-6Al-4V Implants with Lattice-Shaped Interconnected Large Pores Fabricated by Electron Beam Melting. J. Biomater. Appl. 2020, 35, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated Degradation of HAP/PLLA Bone Scaffold by PGA Blending Facilitates Bioactivity and Osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Osteoconductive Visible Light-Crosslinkable Nanocomposite for Hard Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127761. [Google Scholar] [CrossRef]
- Sahithi, K.; Swetha, M.; Ramasamy, K.; Srinivasan, N.; Selvamurugan, N. Polymeric Composites Containing Carbon Nanotubes for Bone Tissue Engineering. Int. J. Biol. Macromol. 2010, 46, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowska, E.; Romelczyk-Baishya, B.; Chodara, A.; Koltsov, I.; Smogór, H.; Mizeracki, J.; Pakieła, Z.; Łojkowski, W. Microstructure and Mechanical Properties of Inverse Nanocomposite Made from Polylactide and Hydroxyapatite Nanoparticles. Materials 2022, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, C.A.G.; Shimomura, K.M.B.; Backes, E.H.; Harb, S.V.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Engineering Printable Composites of Poly (ε-Polycaprolactone)/β-Tricalcium Phosphate for Biomedical Applications. Polym. Compos. 2020, 42, 1198–1213. [Google Scholar] [CrossRef]
- Rabiei, M.; Raziyan, M.S.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Palevicius, A.; Janusas, G.; Vilkauskas, A. Effects of 5 Wt.% Polycaprolactone, Polyhydroxybutyrate and Polyvinyltrimethoxysilane on the Properties of Ag/Zn/Mg Alloy. Materials 2022, 15, 5421. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, M.; Parham, S.; Abbastabbar Ahangar, H.; Zargar Kharazi, A. Preparation and Evaluation of Bioactive Bilayer Composite Membrane PHB/β-TCP with Ciprofloxacin and Vitamin D3 Delivery for Regenerative Damaged Tissue in Periodontal Disease. J. Appl. Polym. Sci. 2021, 139, 51507. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Matykiewicz, D.; Skórczewska, K.; Lewandowski, K.; Andrzejewski, J.; Piasecki, A. Development of Polylactide Composites with Improved Thermomechanical Properties by Simultaneous Use of Basalt Powder and a Nucleating Agent. Polym. Compos. 2020, 41, 2947–2957. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Helú, M.A.B.; Liu, L. Fused Deposition Modeling (FDM) Based 3D Printing of Microelectrodes and Multi-Electrode Probes. Electrochim. Acta 2021, 365, 137279. [Google Scholar] [CrossRef]
- Silva, A.D.R.; Rigoli, W.R.; Osiro, D.; Mello, D.C.R.; Vasconcellos, L.M.R.; Lobo, A.O.; Pallone, E.M.J.A. Surface Modification Using the Biomimetic Method in Alumina-Zirconia Porous Ceramics Obtained by the Replica Method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2615–2624. [Google Scholar] [CrossRef]
- Liska, R.; Schwager, F.; Maier, C.; Cano-Vives, R.; Stampfl, J. Water-Soluble Photopolymers for Rapid Prototyping of Cellular Materials. J. Appl. Polym. Sci. 2005, 97, 2286–2298. [Google Scholar] [CrossRef]
- Xie, L.; Yu, H.; Deng, Y.; Yang, W.; Liao, L.; Long, Q. Preparation, Characterization and in Vitro Dissolution Behavior of Porous Biphasic α/β-Tricalcium Phosphate Bioceramics. Mater. Sci. Eng. C 2016, 59, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V.; Shatalova, T.B.; Filippov, Y.Y.; Toshev, O.U.; Knotko, A.V.; Vaimugin, L.A.; Savchenkova, D.V. Na2O-CaO-SO3 Ceramics as Promising Inorganic Porogens. Glass Ceramics. 2022, 79, 88–94. [Google Scholar] [CrossRef]
- Cui, P.; Wu, C.-R.; Chen, J.; Luo, F.-M.; Kou, S.-C. Preparation of Magnesium Oxysulfate Cement as a 3D Printing Material. Constr. Build. Mater. 2021, 282, 122677. [Google Scholar] [CrossRef]
- Li, G.; Tang, S.; Yang, L.; Qian, L.; Liu, F.; Fan, Z.; Zuo, K.; Wei, Q.; Jiang, W. Fabrication of Soluble Salt-Based Support for Suspended Ceramic Structure by Layered Extrusion Forming Method. Mater. Des. 2019, 183, 108173. [Google Scholar] [CrossRef]
- Li, W.; Ghazanfari, A.; McMillen, D.; Leu, M.C.; Hilmas, G.E.; Watts, J. Fabricating Ceramic Components with Water Dissolvable Support Structures by the Ceramic On-Demand Extrusion Process. CIRP Ann.-Manuf. Technol. 2017, 66, 225–228. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.J.; Pajares, A.; Miranda, P. A Simple Graphite-Based Support Material for Robocasting of Ceramic Parts. J. Eur. Ceram. Soc. 2018, 38, 2247–2250. [Google Scholar] [CrossRef]
- Rowe, J.J.; Morey, G.W.; Smber, C.C. The ternary system K2SO4-MgSO4-CaSO4. J. Inorg. Nucl. Chem. 1967, 29, 925–942. [Google Scholar] [CrossRef]
- Du, H. Thermodynamic Assesment of the K2SO4-Na2SO4-MgSO4-CaSO4 System. J. Phase Equilibria Diffus. 2000, 21, 6–18. [Google Scholar] [CrossRef]
- Körber, S.; Völkl, R.; Glatzel, U. 3D printed polymer positive models for the investment casting of extremely thin-walled single crystals. J. Mater. Process. Technol. 2021, 293, 117095. [Google Scholar] [CrossRef]
- Oosterbeek, R.N.; Margaronis, K.I.; Zhang, X.C.; Best, S.M.; Cameron, R.E. Non-Linear Dissolution Mechanisms of Sodium Calcium Phosphate Glasses as a Function of PH in Various Aqueous Media. J. Eur. Ceram. Soc. 2021, 41, 901–911. [Google Scholar] [CrossRef]
- Huang, J.; Sun, Y.; Zhang, Y.; Zou, G.; Yan, C.; Cong, S.; Lei, T.; Dai, X.; Guo, J.; Lu, R.; et al. A New Member of Electrocatalysts Based on Nickel Metaphosphate Nanocrystals for Efficient Water Oxidation. Adv. Mater. 2018, 30, 1705045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Jiang, M. Effect of Na Ions on Melt Structure and Viscosity of CaO-SiO2-Na2O by Molecular Dynamics Simulations. ISIJ Int. 2021, 61, 1389–1395. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid Phase Sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Qi, L.; He, S.; Chen, C.; Jiang, B.; Hao, Y.; Ye, H.; Yang, R.; Du, K. Diffusional-Displacive Transformation in a Metastable β Titanium Alloy and Its Strengthening Effect. Acta Mater. 2020, 195, 151–162. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, L.; Liang, J.; Liu, B.; Liu, J.; Chen, R.; Deng, X.; Wu, S.; Huang, M. Effect of Initial WC Particle Size on Grain Growth Behavior and Gradient Structure Formation of Bilayer Functionally Graded Cemented Carbides. Mater. Chem. Phys. 2021, 271, 124919. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of Zirconium Oxide Nanoparticles Addition on the Optical and Tensile Properties of Polymethyl Methacrylate Denture Base Material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef]
- Kim, M.I.; Kim, S.; Kim, T.; Lee, D.K.; Seo, B.; Lim, C.S. Mechanical and Thermal Properties of Epoxy Composites Containing Zirconium Oxide Impregnated Halloysite Nanotubes. Coatings 2017, 7, 231. [Google Scholar] [CrossRef]
- Mamivand, M.; Zaeem, M.A.; Kadiri, H.E.; Chen, L.Q. Phase Field Modeling of the Tetragonal-to-Monoclinic Phase Transformation in Zirconia. Acta Mater. 2013, 61, 5223–5235. [Google Scholar] [CrossRef]
- Wang, C.; Zinkevich, M.; Aldinger, F. The Zirconia-Hafnia System: DTA Measurements and Thermodynamic Calculations. J. Am. Ceram. Soc. 2006, 89, 3751–3758. [Google Scholar] [CrossRef]
- Navrotsky, A. Thermochemical Insights into Refractory Ceramic Materials Based on Oxides with Large Tetravalent Cations. J. Mater. Chem. 2005, 15, 1883–1890. [Google Scholar] [CrossRef]
- Gehensel, R.J.; Zierold, R.; Schaan, G.; Shang, G.; Petrov, A.Y.; Eich, M.; Blick, R.; Krekeler, T.; Janssen, R.; Furlan, K.P. Improved Thermal Stability of Zirconia Macroporous Structures via Homogeneous Aluminum Oxide Doping and Nanostructuring Using Atomic Layer Deposition. J. Eur. Ceram. Soc. 2021, 41, 4302–4312. [Google Scholar] [CrossRef]
- Jang, B.K.; Lee, J.H.; Fisher, C.A.J. Mechanical Properties and Phase-Transformation Behavior of Carbon Nanotube-Reinforced Yttria-Stabilized Zirconia Composites. Ceram. Int. 2021, 47, 35287–35293. [Google Scholar] [CrossRef]
- Magnani, G.; Fabbri, P.; Leoni, E.; Salernitano, E.; Mazzanti, F. New Perspectives on Zirconia Composites as Biomaterials. J. Compos. Sci. 2021, 5, 244. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; Cardoso, K.B.; Lino, L.F.O.; Campos, T.M.B.; Monteiro, K.N.; Cesar, P.F.; Genova, L.A.; Thim, G.P.; Coelho, P.G.; Bonfante, E.A. Alumina-Toughened Zirconia for Dental Applications: Physicochemical, Mechanical, Optical, and Residual Stress Characterization after Artificial Aging. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Chłędowska, J.; Wyrwa, J.; Rękas, M.; Brylewski, T. Effects of Aluminum Oxide Addition on Electrical and Mechanical Properties of 3 Mol% Yttria-Stabilized Tetragonal Zirconia Electrolyte for IT-SOFCs. Materials 2022, 15, 2101. [Google Scholar] [CrossRef]
- Jewad, R.; Bentham, C.; Hancock, B.; Bonfield, W.; Best, S.M. Dispersant Selection for Aqueous Medium Pressure Injection Moulding of Anhydrous Dicalcium Phosphate. J. Eur. Ceram. Soc. 2008, 28, 547–553. [Google Scholar] [CrossRef]
- Wang, Z.G.; Hsiao, B.S.; Stribeck, N.; Gehrke, R. Nanostructure Evolution of Isotropic High-Pressure Injection-Molded UHMWPE during Heating. Macromolecules 2002, 35, 2200–2206. [Google Scholar] [CrossRef]
- Bai, R.; Sun, Q.; He, Y.; Peng, L.; Zhang, Y.; Zhang, L.; Lu, W.; Deng, J.; Zhuang, Z.; Yu, T.; et al. Ceramic Toughening Strategies for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 840372. [Google Scholar] [CrossRef]

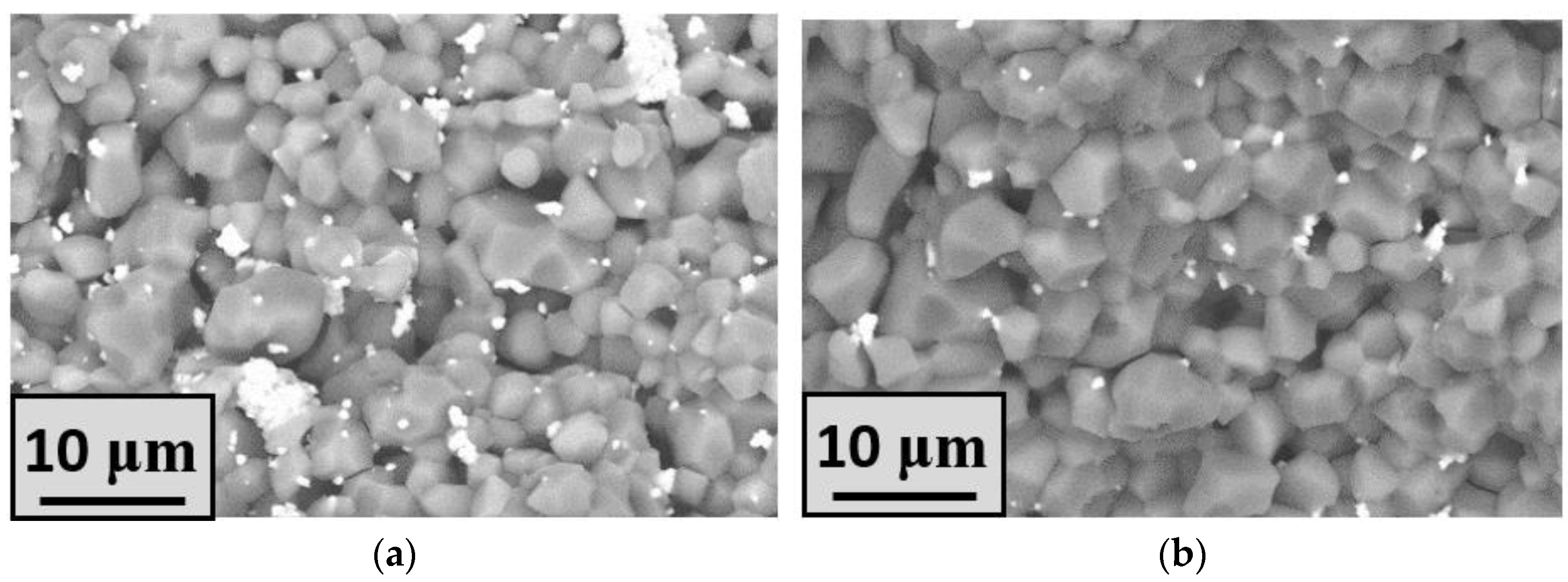
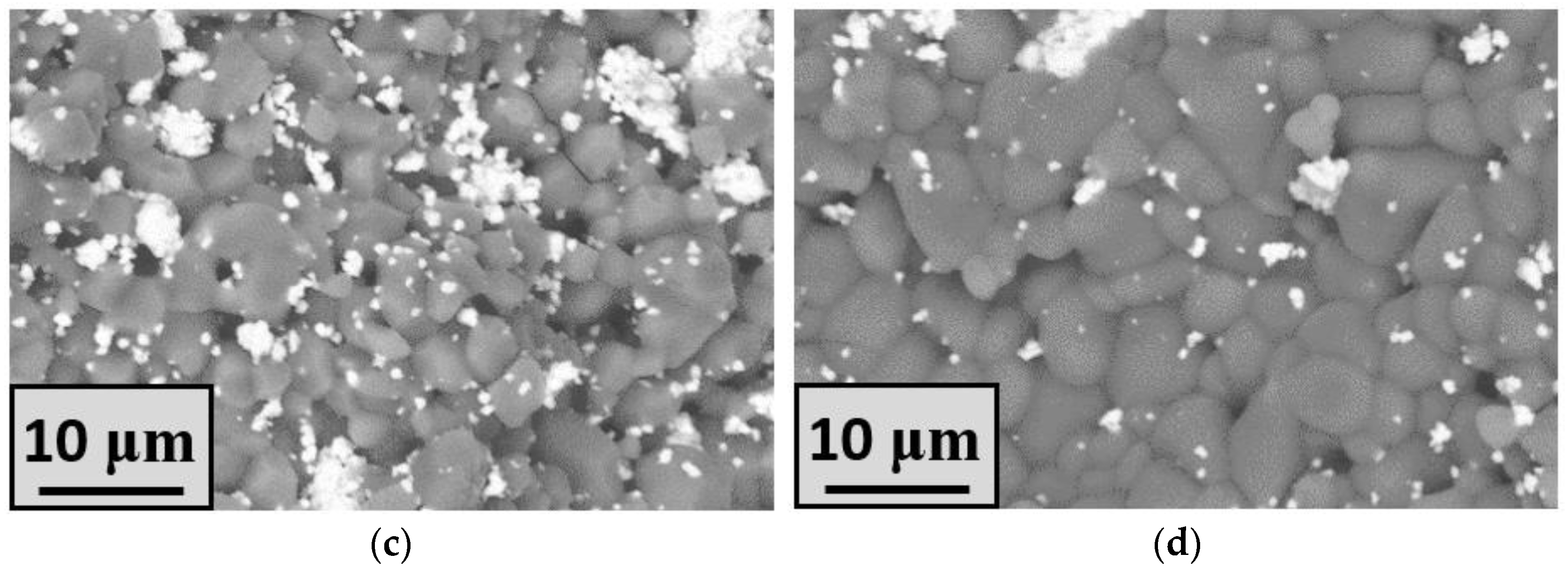



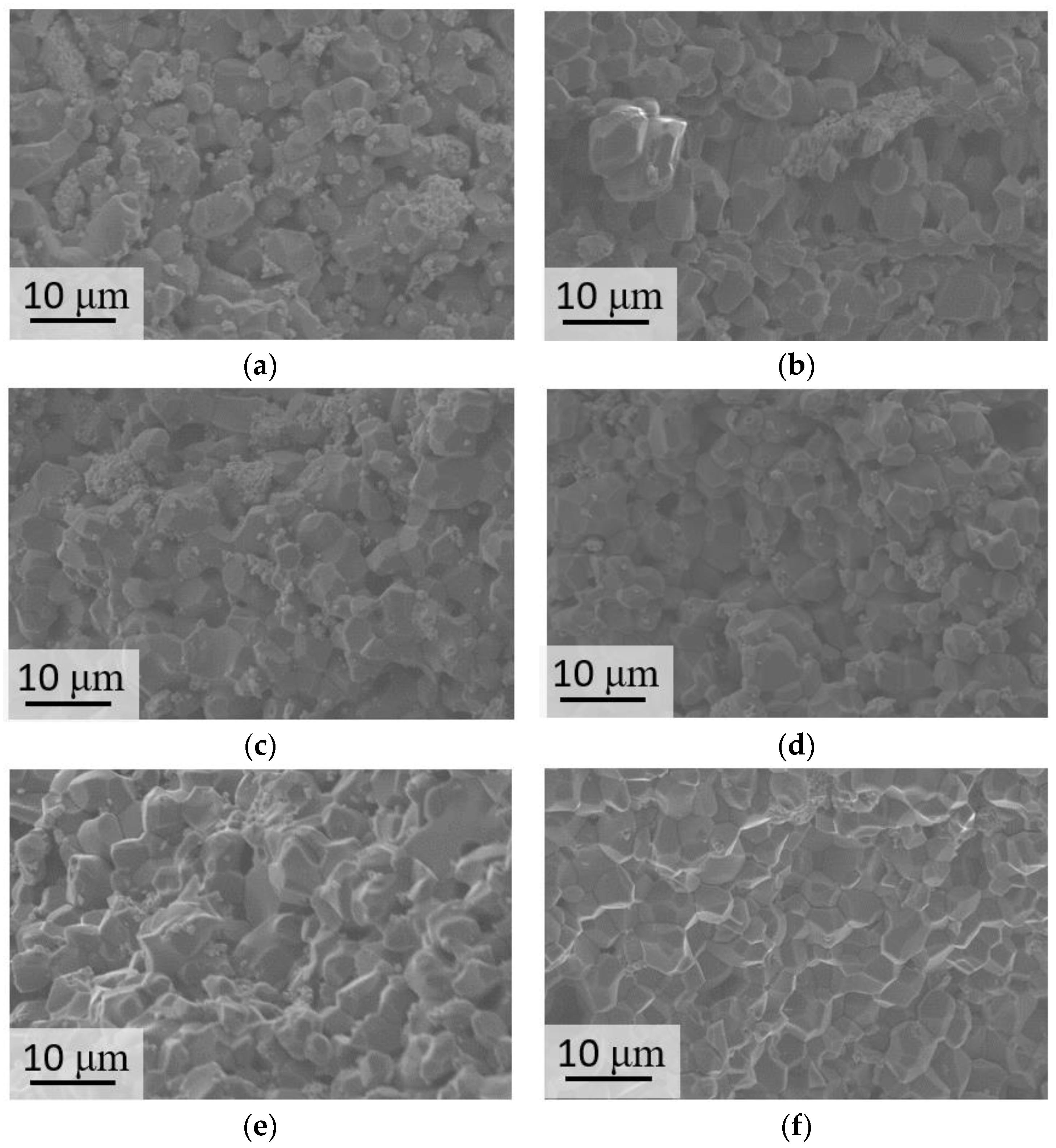




| Label | Phase Composition, mol.% | ||
|---|---|---|---|
| MgSO4 | Na2SO4 | K2SO4 | |
| Mg5Na and Mg5NaY-TZP | 95 | 5 | – |
| Mg10Na and Mg10NaY-TZP | 91 | 9 | – |
| Mg15Na and Mg15NaY-TZP | 87 | 13 | – |
| Mg20Na and Mg20NaY-TZP | 83 | 17 | – |
| Mg5K and Mg5KY-TZP | 95 | – | 5 |
| Mg10K and Mg10KY-TZP | 91 | – | 9 |
| Mg15K and Mg15KY-TZP | 87 | – | 13 |
| Mg20K and Mg20KY-TZP | 83 | – | 17 |
| Sample | Calculated Mg/(Na, K) Ratio | Found Mg/(Na, K) Ratio |
|---|---|---|
| Mg10Na | 5 | 6.5 |
| Mg20Na | 2.5 | 4.1 |
| Mg10K | 5 | 5.3 |
| Mg20K | 2.5 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubchikov, D.; Evdokimov, P.; Zuev, D.; Filippov, Y.; Shatalova, T.; Putlayev, V. Three-Dimensional-Printed Molds from Water-Soluble Sulfate Ceramics for Biocomposite Formation through Low-Pressure Injection Molding. Materials 2023, 16, 3077. https://doi.org/10.3390/ma16083077
Golubchikov D, Evdokimov P, Zuev D, Filippov Y, Shatalova T, Putlayev V. Three-Dimensional-Printed Molds from Water-Soluble Sulfate Ceramics for Biocomposite Formation through Low-Pressure Injection Molding. Materials. 2023; 16(8):3077. https://doi.org/10.3390/ma16083077
Chicago/Turabian StyleGolubchikov, Daniil, Pavel Evdokimov, Dmitry Zuev, Yaroslav Filippov, Tatiana Shatalova, and Valery Putlayev. 2023. "Three-Dimensional-Printed Molds from Water-Soluble Sulfate Ceramics for Biocomposite Formation through Low-Pressure Injection Molding" Materials 16, no. 8: 3077. https://doi.org/10.3390/ma16083077
APA StyleGolubchikov, D., Evdokimov, P., Zuev, D., Filippov, Y., Shatalova, T., & Putlayev, V. (2023). Three-Dimensional-Printed Molds from Water-Soluble Sulfate Ceramics for Biocomposite Formation through Low-Pressure Injection Molding. Materials, 16(8), 3077. https://doi.org/10.3390/ma16083077







