Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Alumina Starting Material
2.2. Mn-Assisted Conversion into α-Al2O3
2.3. Characterization Techniques
3. Results and Discussion
3.1. Properties of the Starting Material
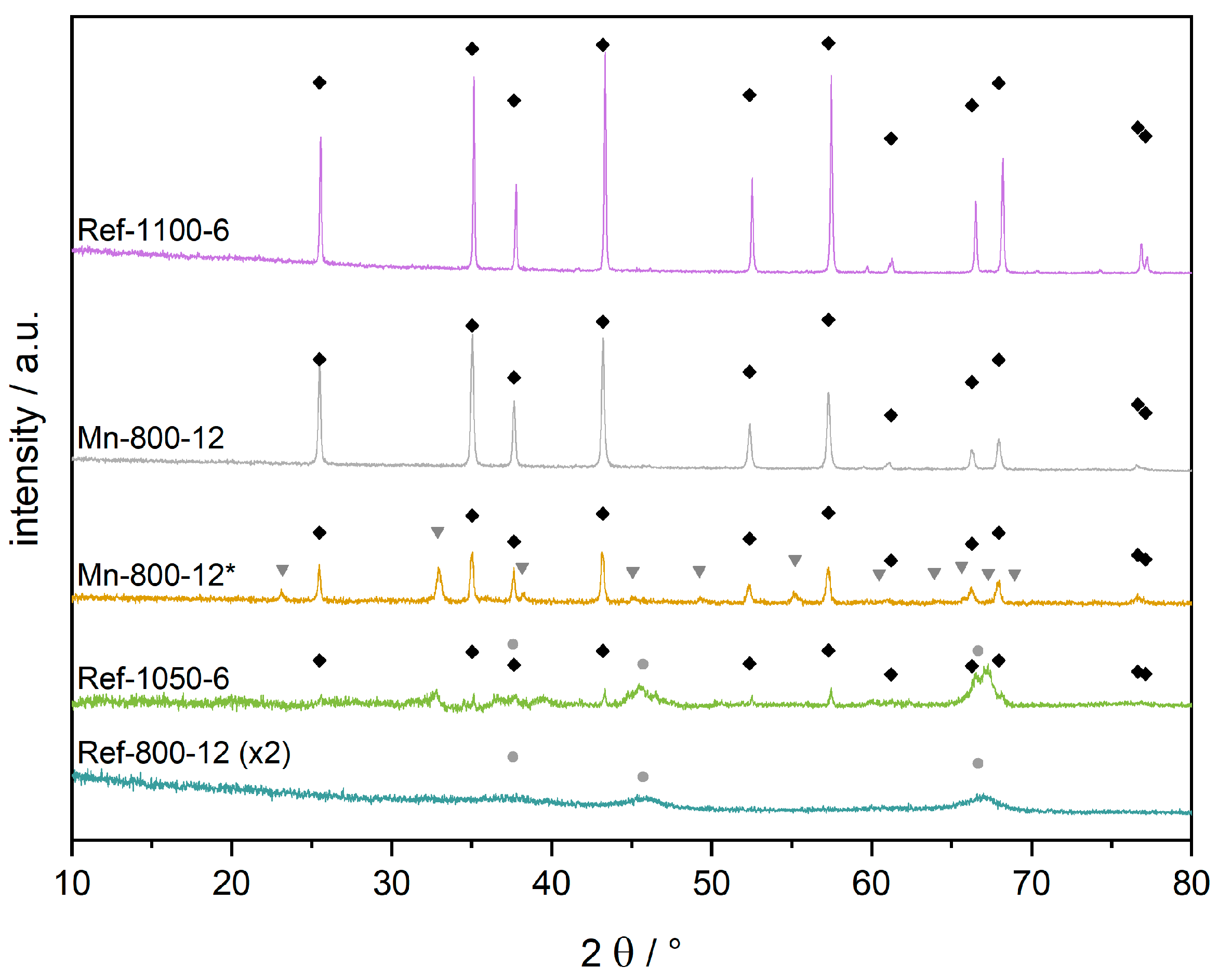
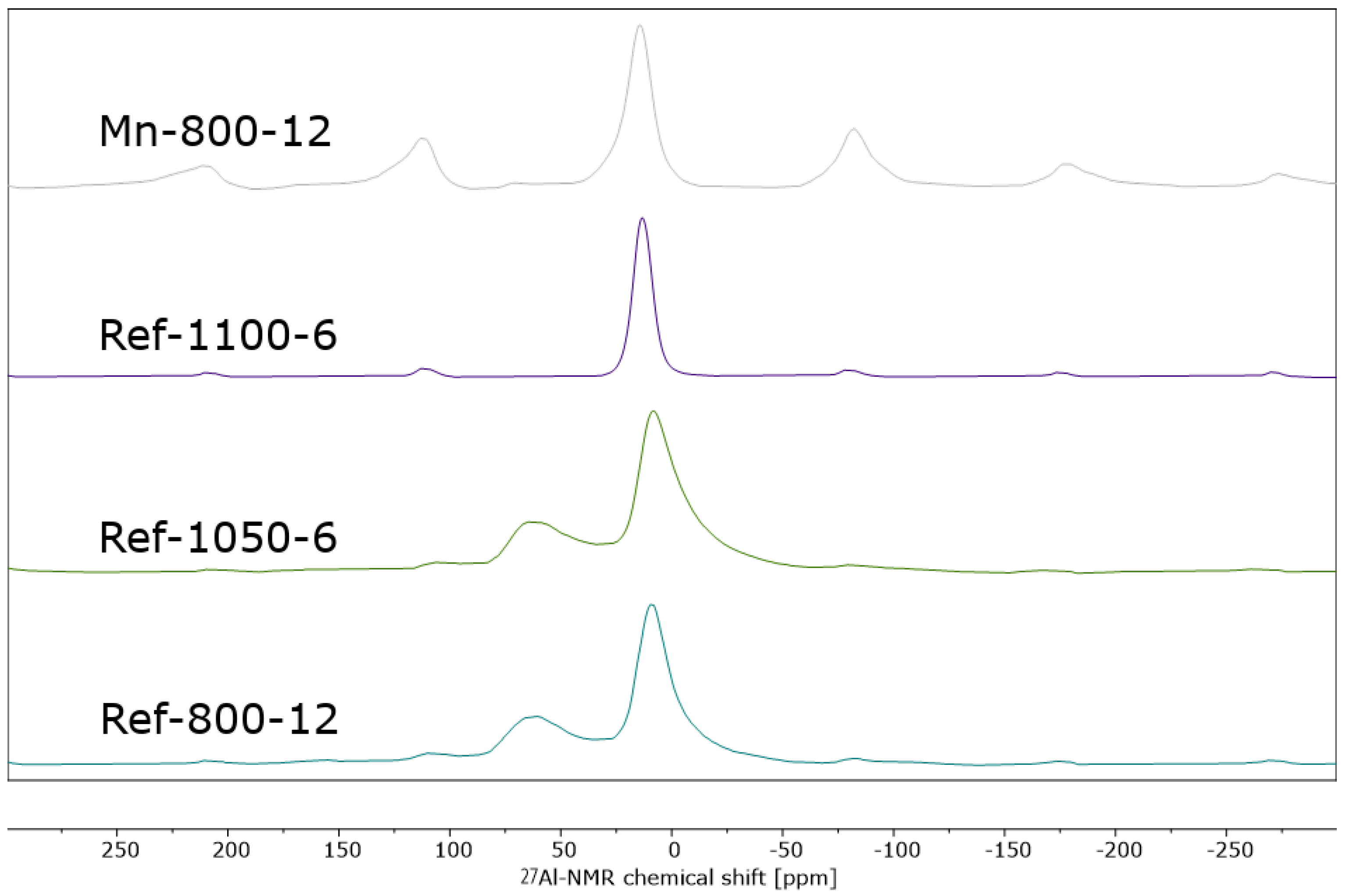
3.2. Phase Identification of α-Al2O3 by XRD and NMR
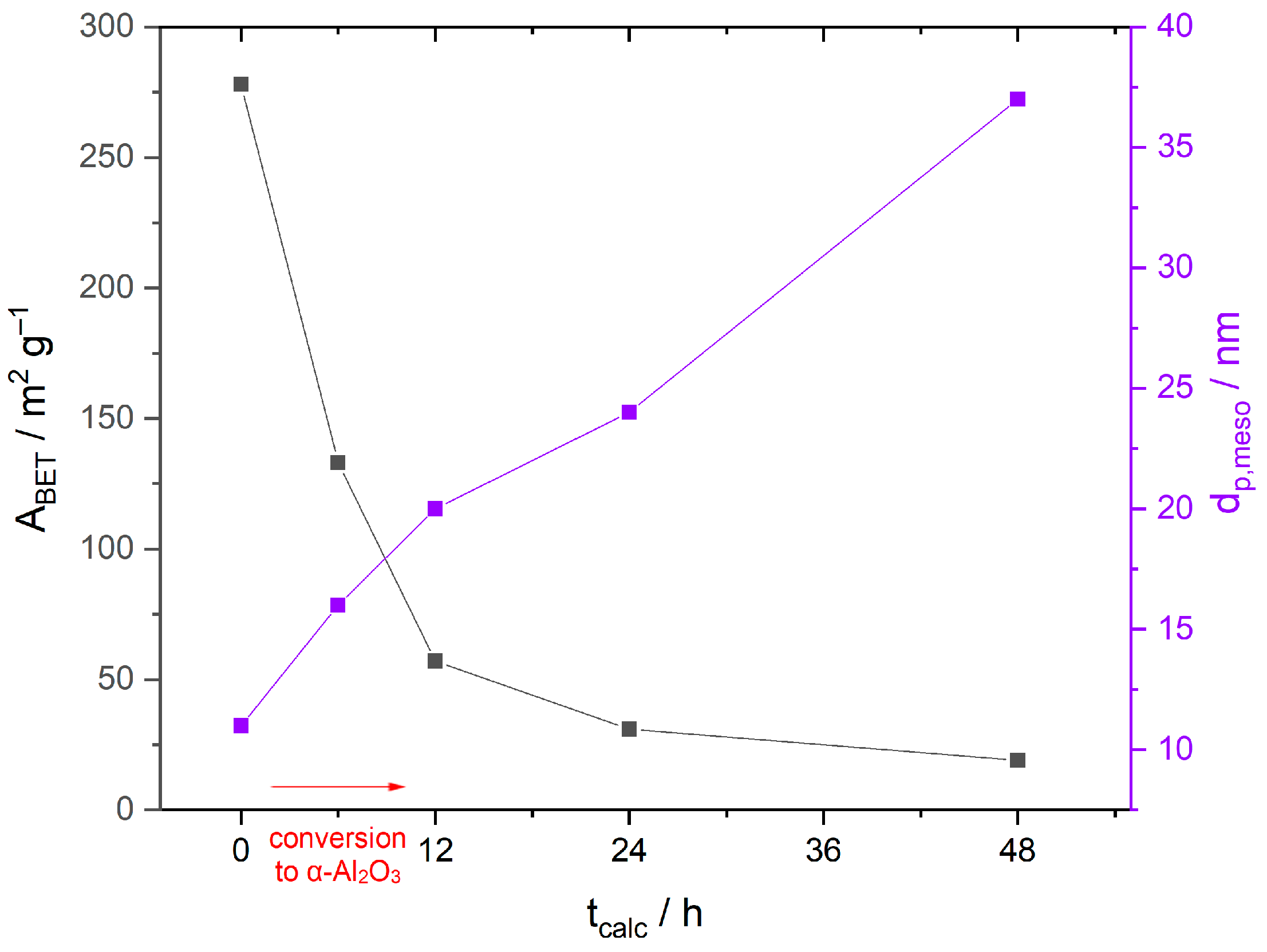
3.3. Porosity and Long-Term Stability of Al2O3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartholomew, C.H.; Farrauto, R.J. (Eds.) Fundamentals of Industrial Catalytic Processes, 2nd ed.; Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. (Eds.) Catalytic Air Pollution Control: Commercial Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Faure, R.; Rossignol, F.; Chartier, T.; Bonhomme, C.; Maître, A.; Etchegoyen, G.; Del Gallo, P.; Gary, D. Alumina foam catalyst supports for industrial steam reforming processes. J. Eur. Ceram. Soc. 2011, 31, 303–312. [Google Scholar] [CrossRef]
- Murrell, L.L.; Grenoble, D.C.; DeLuca, J.P. Process for Preparing Ultra-Stable, High Surface Area Alpha-Alumina. U.S. Patent 4,169,883, 2 October 1979. [Google Scholar]
- Hartmann, I.; Billig, E.; Bindig, R.; Carstens, S.; Liebetrau, J. Möglichkeiten, Limitierungen und Entwicklungsbedarf zur katalytischen Emissionsminderung; DBFZ: Frankfurt/Main, Germany, 2014. [Google Scholar]
- Dvoracek, D. Festkörper-, Metathese- und Thermit-Reaktion. Neue Wege zu porösen Metall-bzw. Mischmetalloxid Monolithen. Ph.D. Dissertation, Universität Leipzig, Leipzig, Germany, 2015. [Google Scholar]
- Zhang, K.; Fu, Z.; Nakayama, T.; Niihara, K. Structural evolution of hierarchically macro/mesoporous Al2O3 monoliths under heat-treatment. Microporous Mesoporous Mater. 2012, 153, 41–46. [Google Scholar] [CrossRef]
- Wefers, K.; Misra, C. (Eds.) Oxides and Hydroxides of Aluminum: Alcoa Technical Paper No. 19, Revised; Aluminum Company of America: Pittsburgh, PA, USA, 1987. [Google Scholar]
- Schaper, H.; Doesburg, E.; Dekorte, P.; Vanreijen, L. Thermal stabilization of high surface area alumina. Solid State Ion. 1985, 16, 261–265. [Google Scholar] [CrossRef]
- Carstens, S.; Meyer, R.; Enke, D. Towards Macroporous α-Al2O3-Routes, Possibilities and Limitations. Materials 2020, 13, 1787. [Google Scholar] [CrossRef]
- Tsyrulnikov, P.G.; Tsybulya, S.V.; Kryukova, G.N.; Boronin, A.I.; Koscheev, S.V.; Starostina, T.G.; Bubnov, A.V.; Kudrya, E.N. Phase transformations in the thermoactivated MnOx–Al2O3 catalytic system. J. Mol. Catal. A Chem. 2002, 179, 213–220. [Google Scholar] [CrossRef]
- Carstens, S.; Enke, D. Investigation of the formation process of highly porous α-Al2O3 via citric acid-assisted sol-gel synthesis. J. Eur. Ceram. Soc. 2019, 39, 2493–2502. [Google Scholar] [CrossRef]
- Carstens, S.; Splith, C.; Enke, D. Sol-gel synthesis of α-Al2O3 with enhanced porosity via dicarboxylic acid templating. Sci. Rep. 2019, 9, 19982. [Google Scholar] [CrossRef]
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; de Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Isotopic compositions of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 293–306. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.; Piedra, G.; Dec, S.F.; Seger, M.; Maciel, G.E. Dehydration Studies of a High-Surface-Area Alumina (Pseudo-boehmite) Using Solid-State 1 H and 27 Al NMR. J. Am. Chem. Soc. 1997, 119, 7832–7842. [Google Scholar] [CrossRef]
- Nýblová, D.; Senna, M.; Düvel, A.; Heitjans, P.; Billik, P.; Filo, J.; Šepelák, V. NMR study on reaction processes from aluminum chloride hydroxides to alpha alumina powders. J. Am. Ceram. Soc. 2019, 102, 2871–2881. [Google Scholar] [CrossRef]
- Ferreira, A.; Viana-Gomes, J.; Bludov, Y.V.; Pereira, V.; Peres, N.M.R.; Castro Neto, A.H. Faraday effect in graphene enclosed in an optical cavity and the equation of motion method for the study of magneto-optical transport in solids. Phys. Rev. B 2011, 84, 235410. [Google Scholar] [CrossRef]
- O’Dell, L.A.; Savin, S.L.P.; Chadwick, A.V.; Smith, M.E. A (27)Al MAS NMR study of a sol-gel produced alumina: Identification of the NMR parameters of the theta-Al(2)O(3) transition alumina phase. Solid State Nucl. Magn. Reson. 2007, 31, 169–173. [Google Scholar] [CrossRef]
- Wen, H.-L.; Yen, F.-S. Growth characteristics of boehmite-derived ultrafine theta and alpha-alumina particles during phase transformation. J. Cryst. Growth 2000, 208, 696–708. [Google Scholar] [CrossRef]
- Legros, C.; Carry, C.; Bowen, P.; Hofmann, H. Sintering of a transition alumina: Effects of phase transformation, powder characteristics and thermal cycle. J. Eur. Ceram. Soc. 1999, 19, 1967–1978. [Google Scholar] [CrossRef]
- Tsuchida, T. Preparation of high surface area α-Al2O3 and its surface properties. Appl. Catal. A Gen. 1993, 105, L141–L146. [Google Scholar] [CrossRef]
- Wefers, K. Über die thermische Umwandlung des Diaspors. Z. Erzbergbau Met. 1962, 15, 339–343. [Google Scholar]
- Mao, C.-F.; Vannice, M.A.s. High surface area a-alumina. I. Appl. Catal. A Gen. 1994, 111, 151–173. [Google Scholar] [CrossRef]
- Zaki, T.; Kabel, K.I.; Hassan, H. Using modified Pechini method to synthesize α-Al2O3 nanoparticles of high surface area. Ceram. Int. 2012, 38, 4861–4866. [Google Scholar] [CrossRef]
- Martín-Ruiz, M.M.; Pérez-Maqueda, L.A.; Cordero, T.; Balek, V.; Subrt, J.; Murafa, N.; Pascual-Cosp, J. High surface area α-alumina preparation by using urban waste. Ceram. Int. 2009, 35, 2111–2117. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, Y.-J.; Jun, K.-W.; Park, J.-Y.; Potdar, H.S. Synthesis of thermo-stable high surface area alumina powder from sol–gel derived boehmite. Mater. Chem. Phys. 2007, 104, 56–61. [Google Scholar] [CrossRef]
- Dekker, E.H.L.J.; Rieck, G.D. Revised phase diagram and X-ray data of The Mn3O4-Al2O3 System in air. Z. Anorg. Allg. Chem. 1975, 415, 69–80. [Google Scholar] [CrossRef]
- Roque-Ruiz, J.H.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Fabrication of α-alumina fibers by sol-gel and electrospinning of aluminum nitrate precursor solutions. Results Phys. 2019, 12, 193–204. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, C.; Cai, J.; Wang, J.; Liu, K.; Cheng, X. Synthesis and characterization of activated alumina with high thermal stability by a low-heat solid-phase precursor method. Microporous Mesoporous Mater. 2022, 337, 111921. [Google Scholar] [CrossRef]



| Sample | Mn Weight-Content Determined by… | |
|---|---|---|
| ICP-OES/wt.% | SEM-EDX/wt.% | |
| Mn-800-12 | 4.0 | 5.5 |
| Mn-800-12* | 17.6 | 50.7 |
| Mn-900-6 | 3.0 | 3.6 |
| Sample | ABET/m2 g−1 | Pore Diameter/nm | Mesopore Volume/cm3 g−1 | Cumulative Pore Volume/cm3 g−1 |
|---|---|---|---|---|
| Ref-650-6 | 276 | 12 | 0.51 | 2.87 |
| Ref-650-6@750-172 | 180 | 13 | 0.51 | 2.79 |
| Mn-800-6 | 122 | 16 | 0.11 | 0.61 |
| Mn-800-12 | 56 | 20 | 0.07 | 0.47 |
| Mn-800-12@750-172 | 29 | 31 | 0.10 | 0.54 |
| Mn-800-24 | 31 | 24 | 0.10 | 0.52 |
| Mn-800-48 | 18 | 37 | 0.08 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jähnichen, T.; Carstens, S.; Franz, M.; Laufer, O.; Wenzel, M.; Matysik, J.; Enke, D. Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation. Materials 2023, 16, 3047. https://doi.org/10.3390/ma16083047
Jähnichen T, Carstens S, Franz M, Laufer O, Wenzel M, Matysik J, Enke D. Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation. Materials. 2023; 16(8):3047. https://doi.org/10.3390/ma16083047
Chicago/Turabian StyleJähnichen, Tim, Simon Carstens, Maximilian Franz, Otto Laufer, Marianne Wenzel, Jörg Matysik, and Dirk Enke. 2023. "Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation" Materials 16, no. 8: 3047. https://doi.org/10.3390/ma16083047
APA StyleJähnichen, T., Carstens, S., Franz, M., Laufer, O., Wenzel, M., Matysik, J., & Enke, D. (2023). Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation. Materials, 16(8), 3047. https://doi.org/10.3390/ma16083047









