Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Alloys and Chemical Compositions
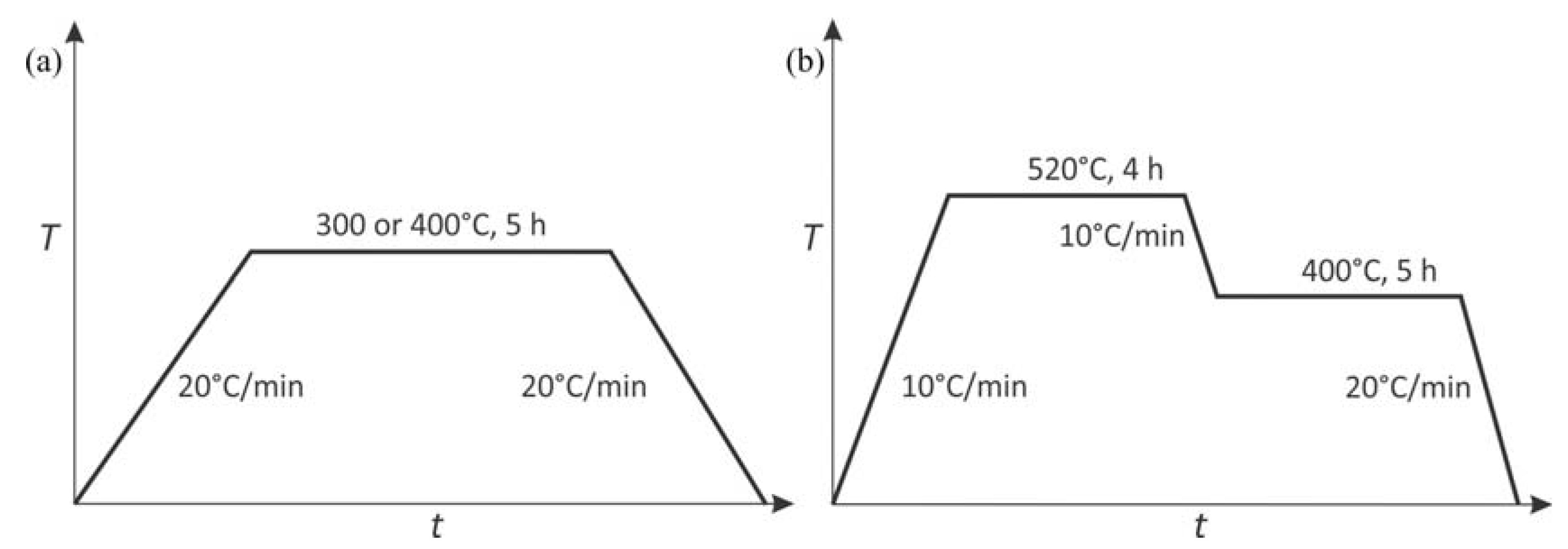
2.2. Characterization of Alloys
3. Results
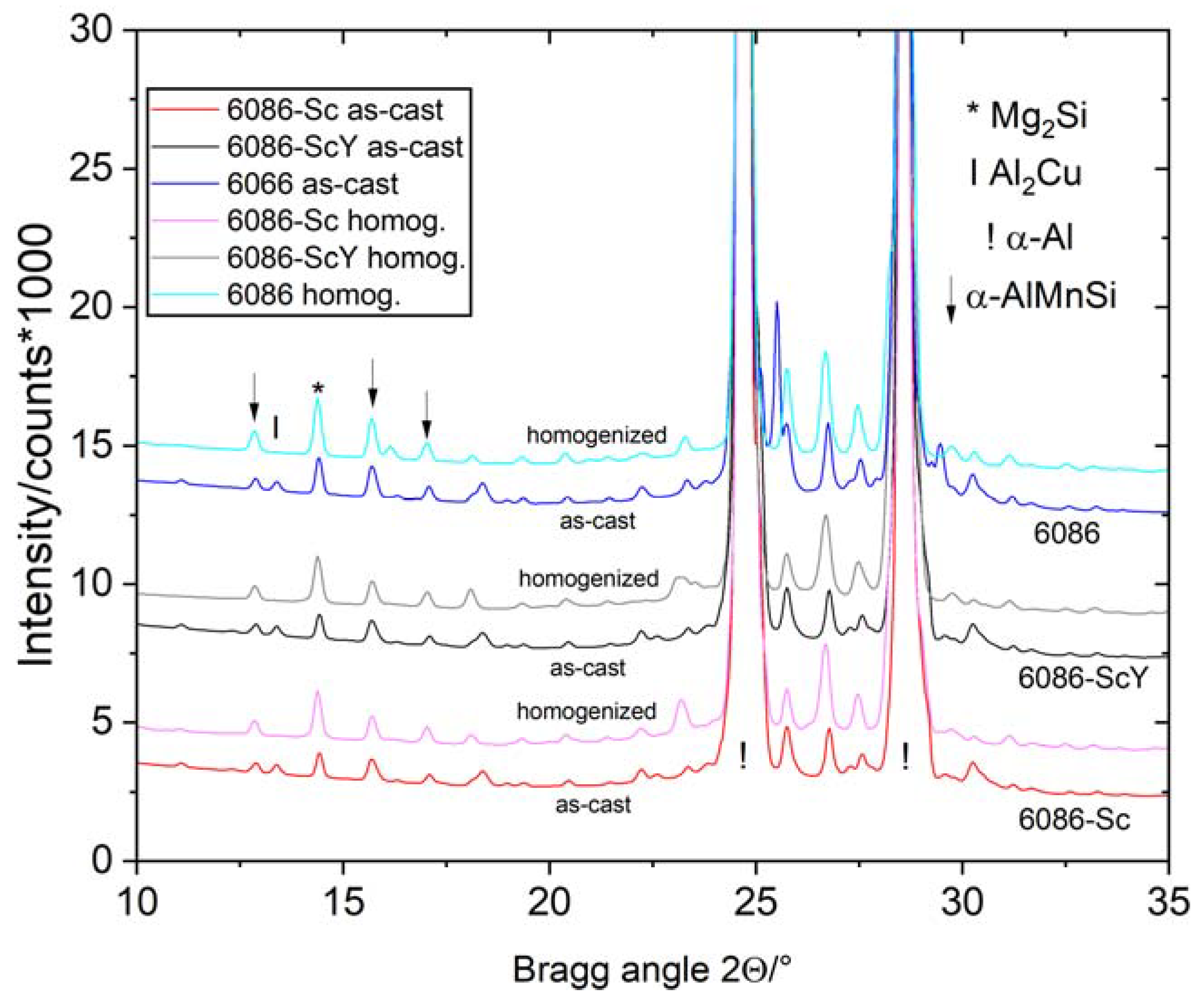
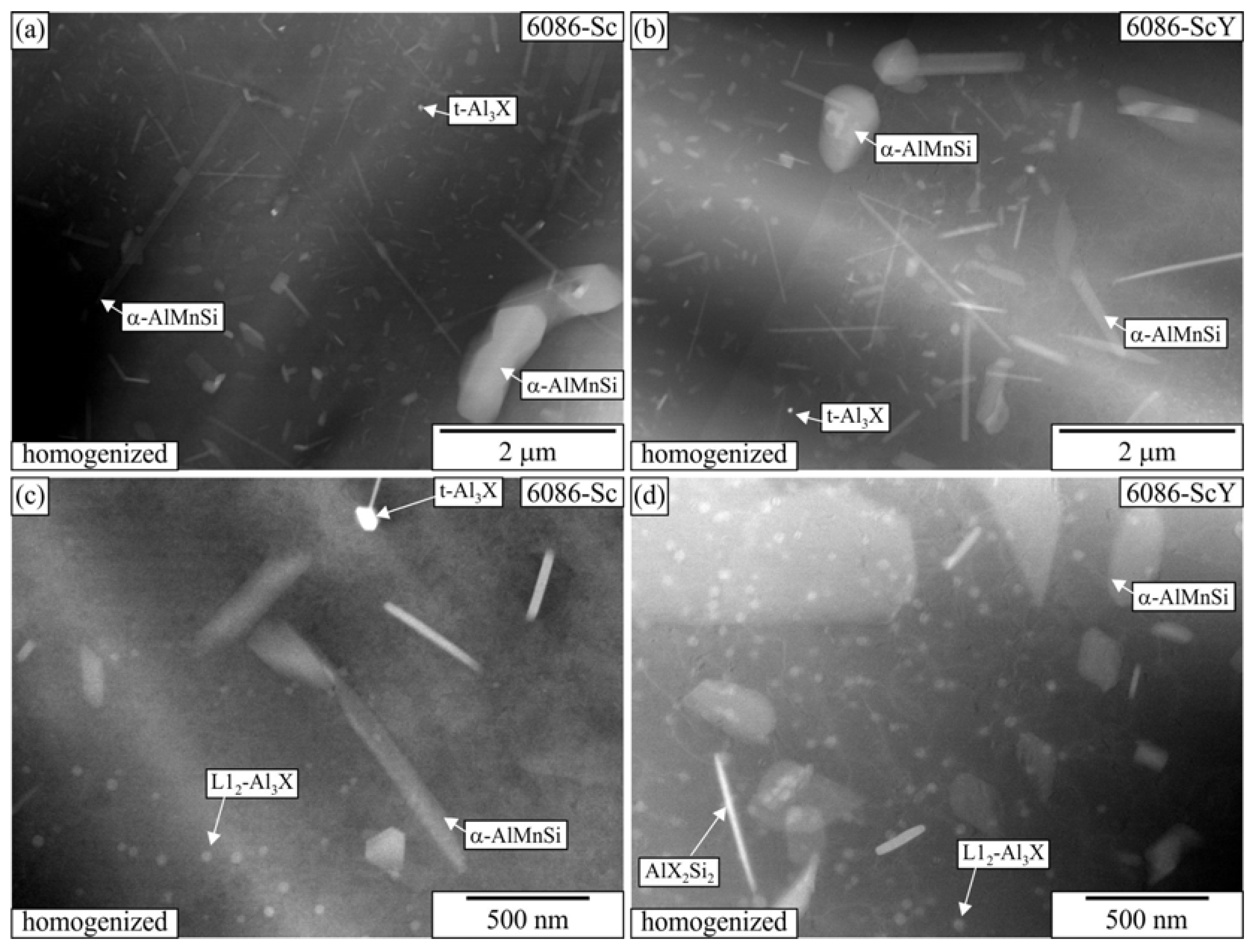
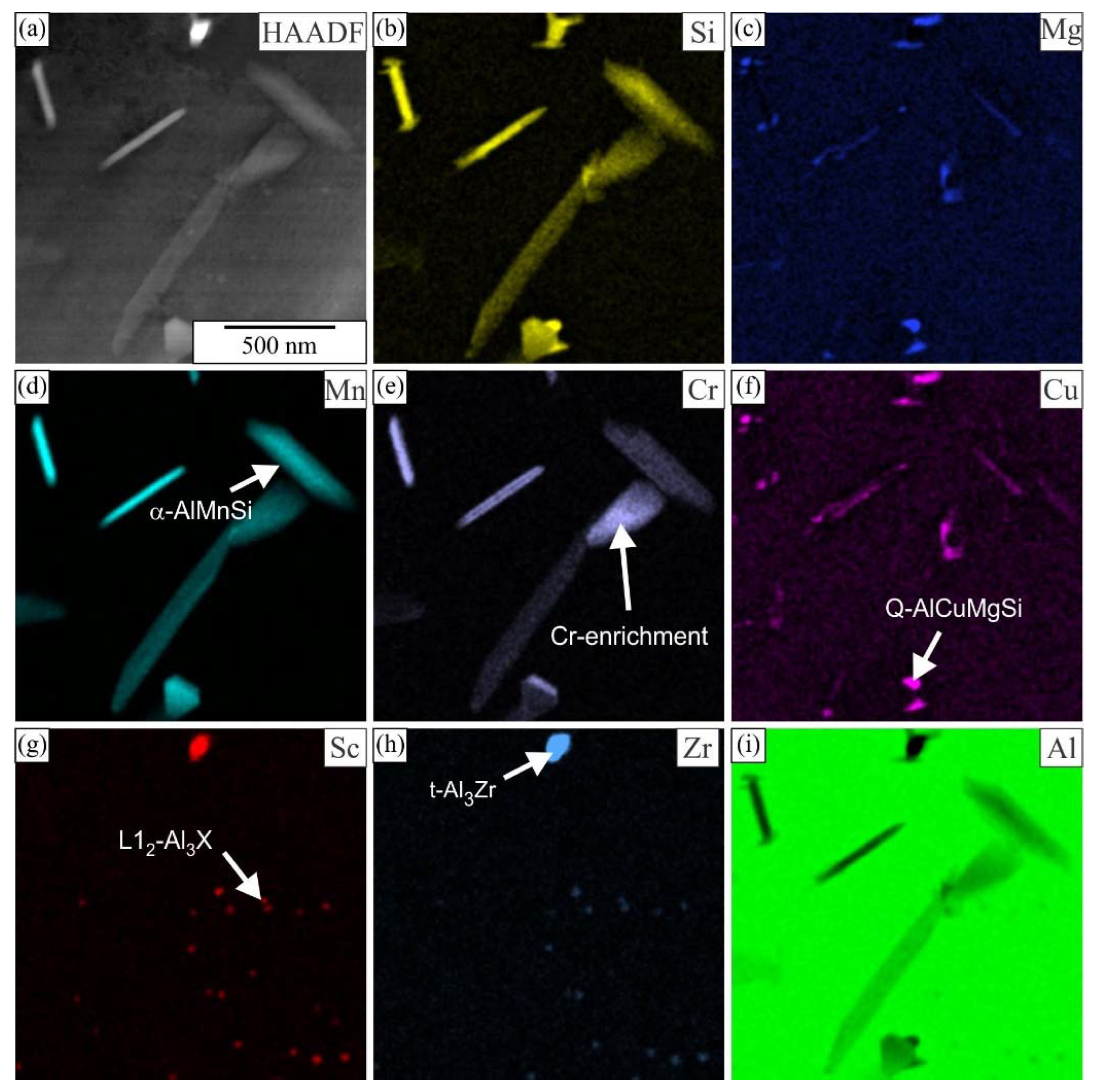
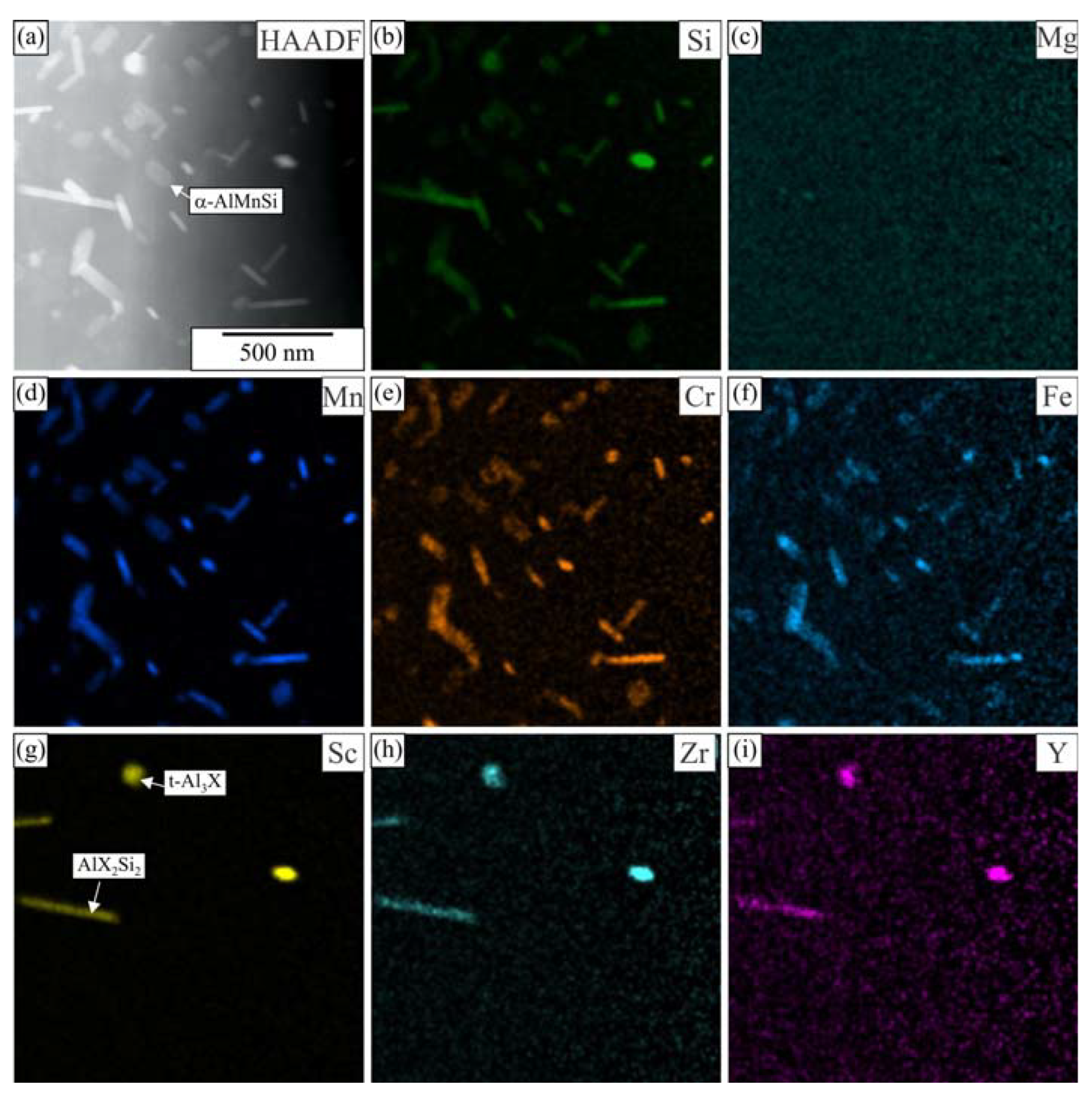
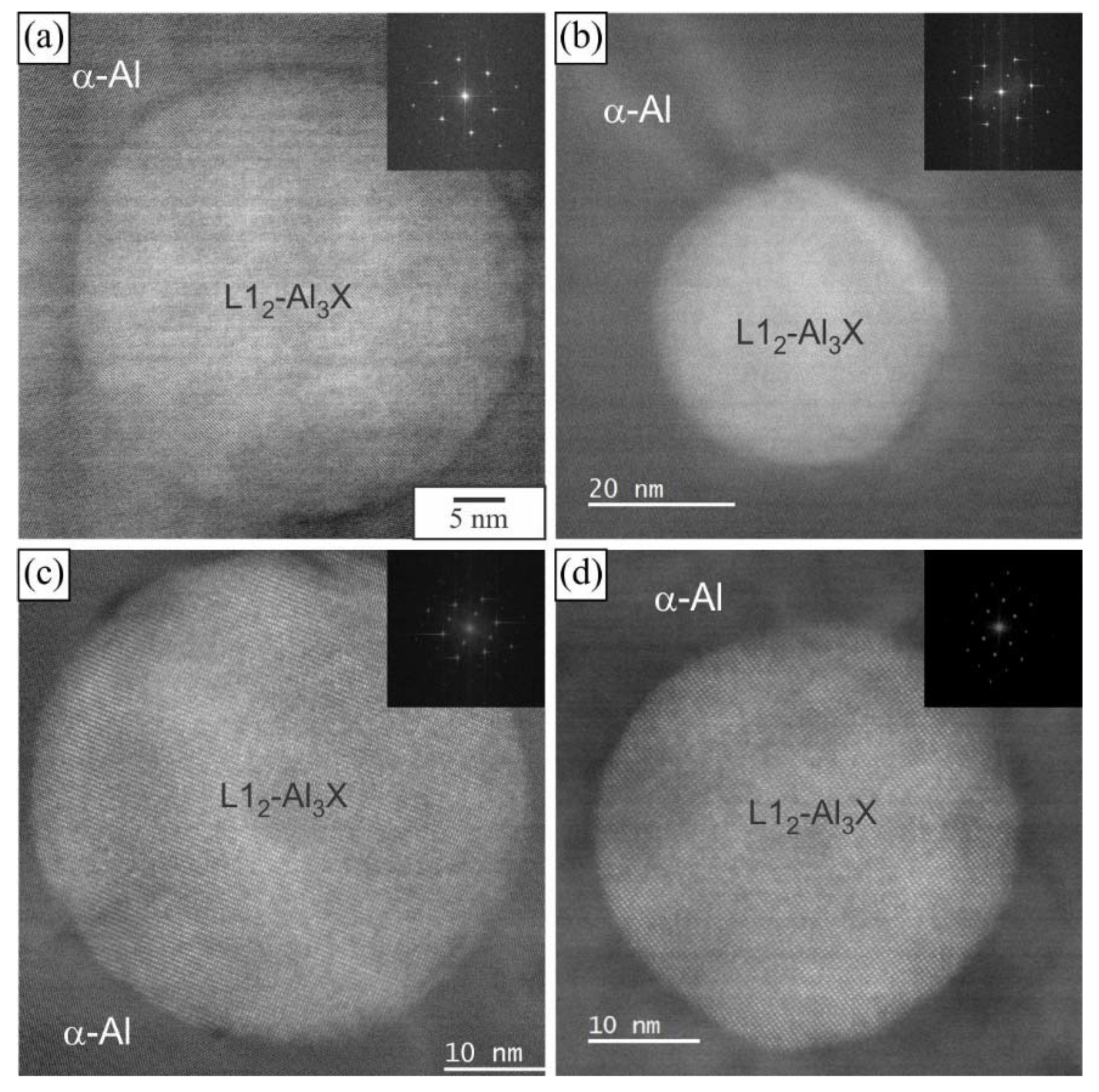
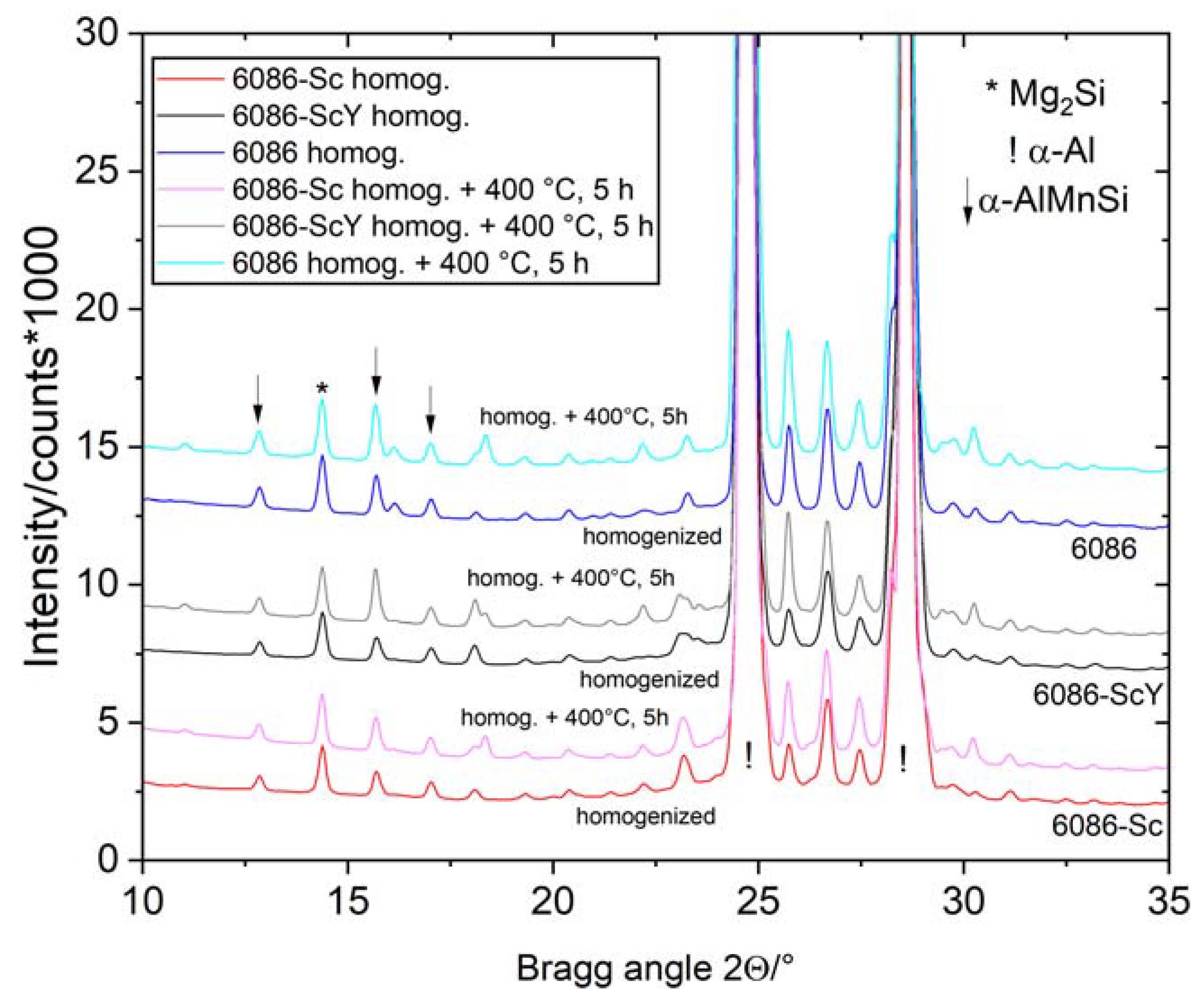
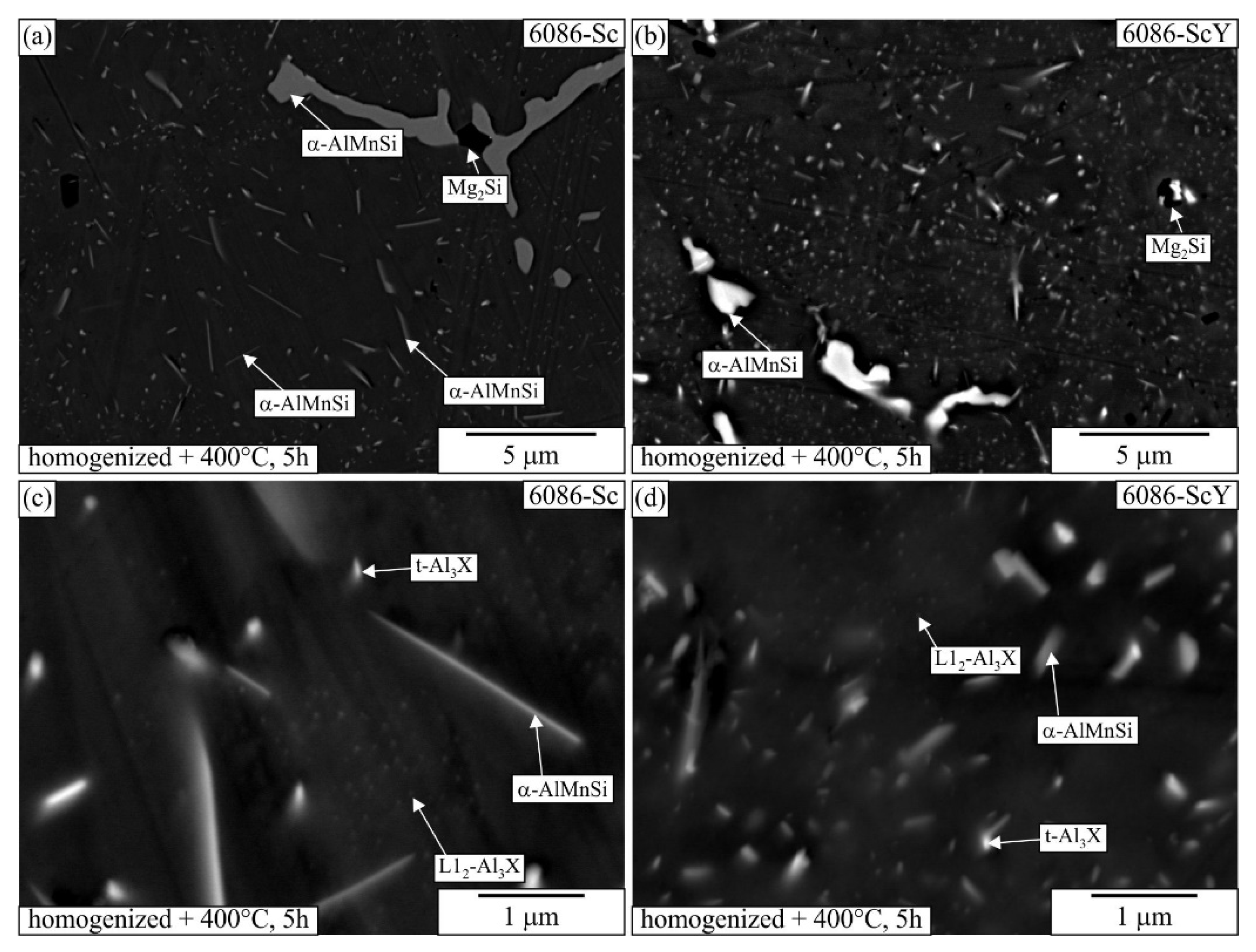
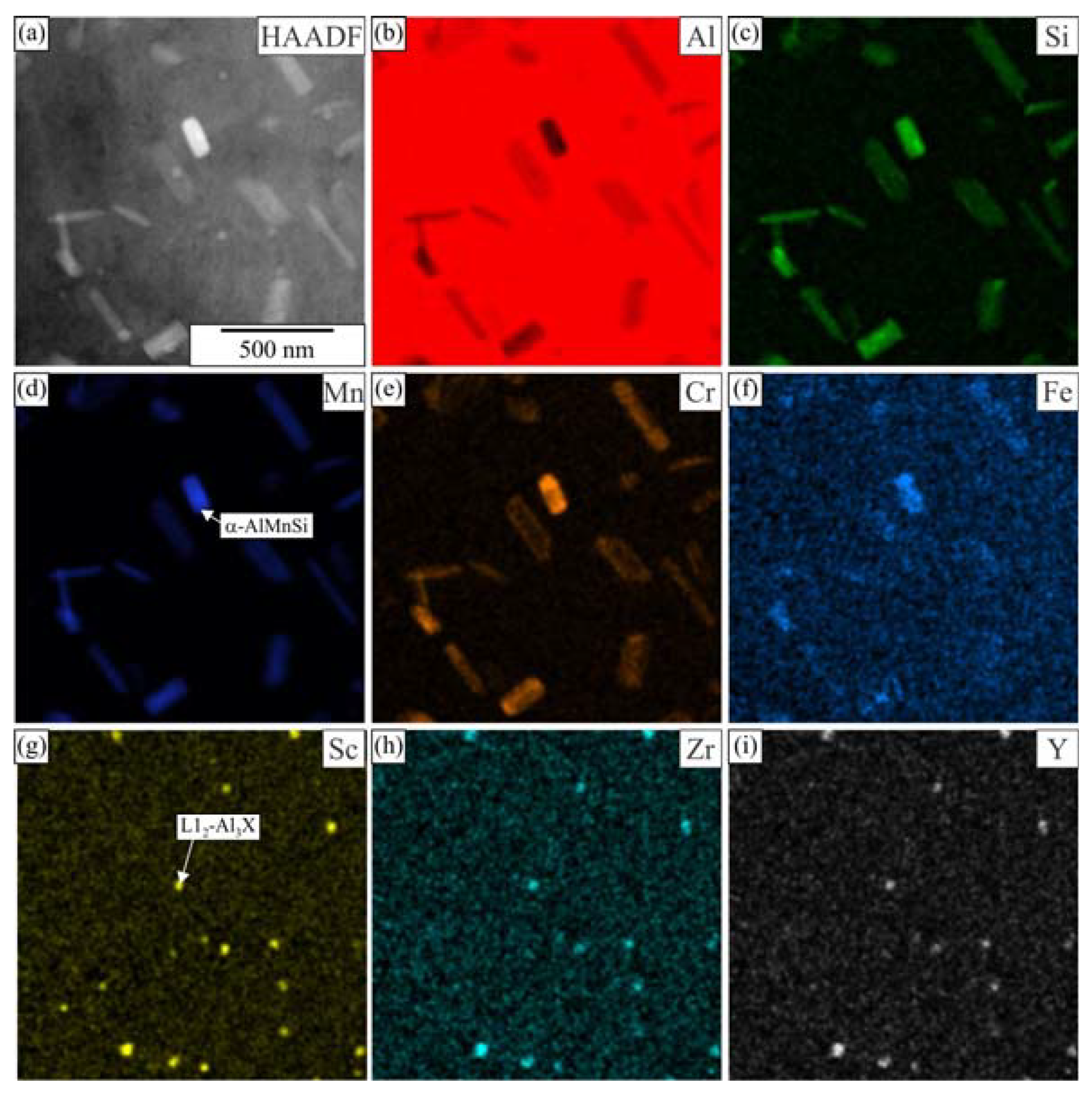
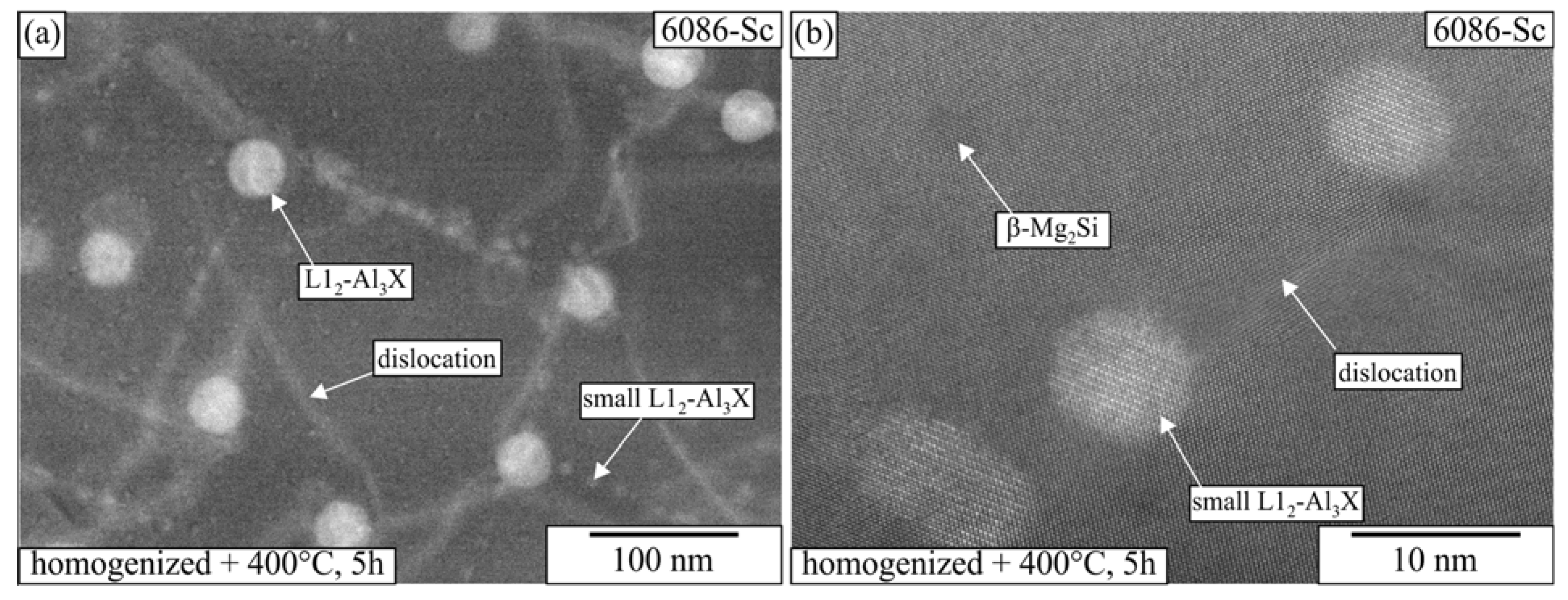
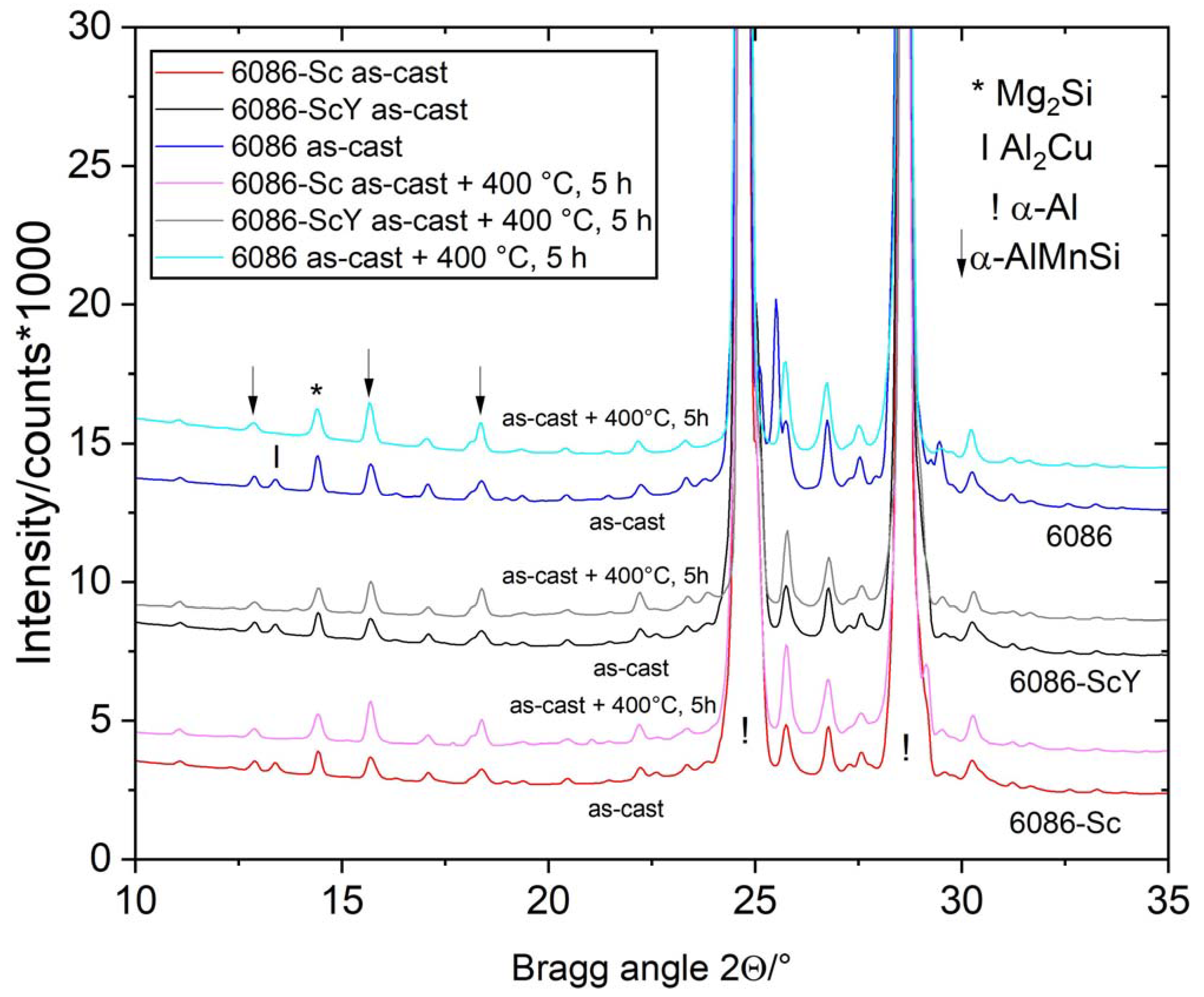
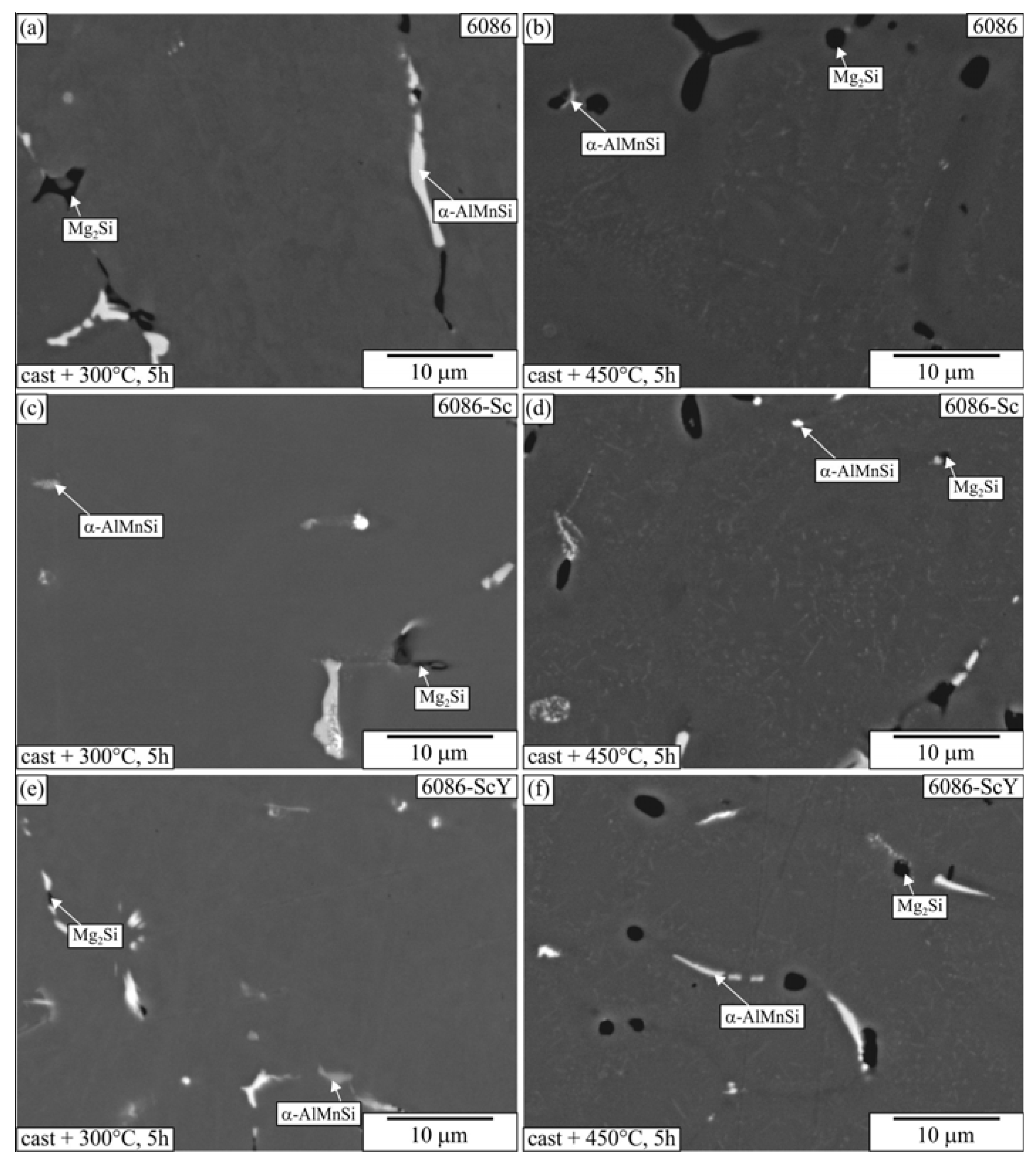
3.1. Initial as-Cast and Homogenized Conditions
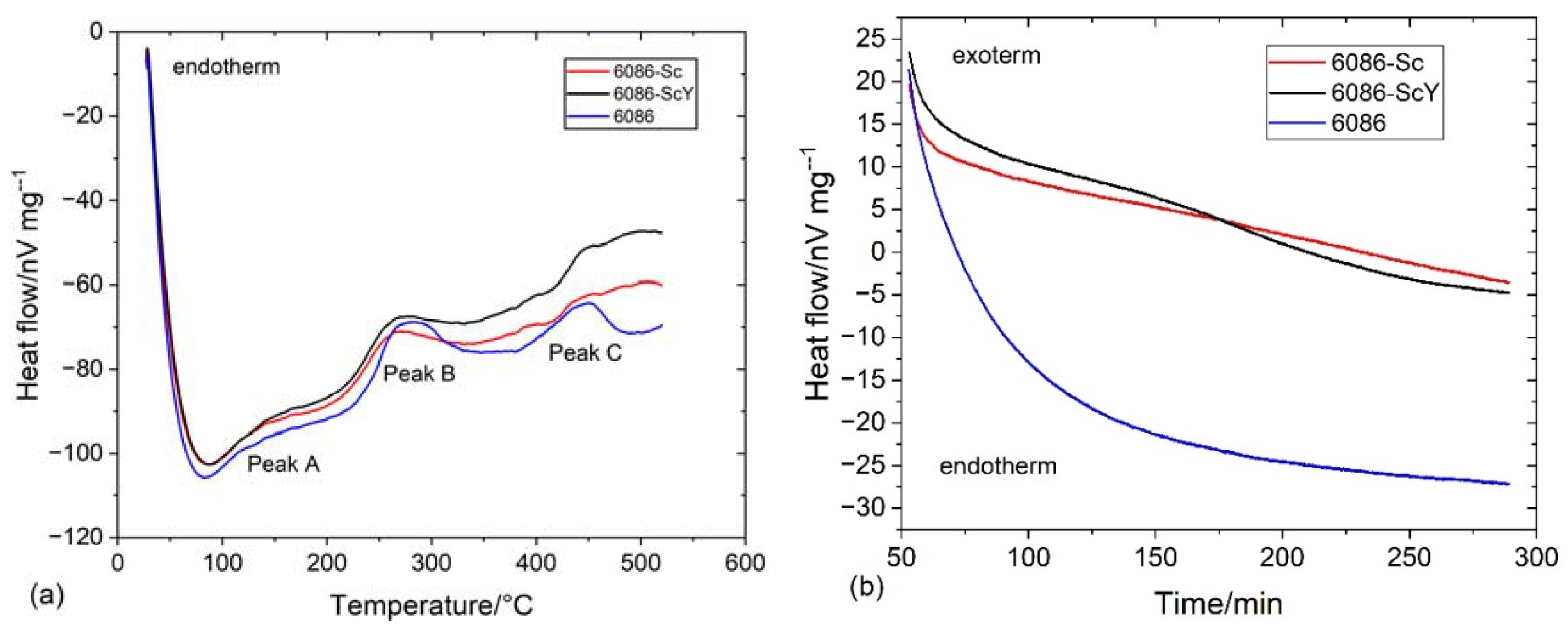
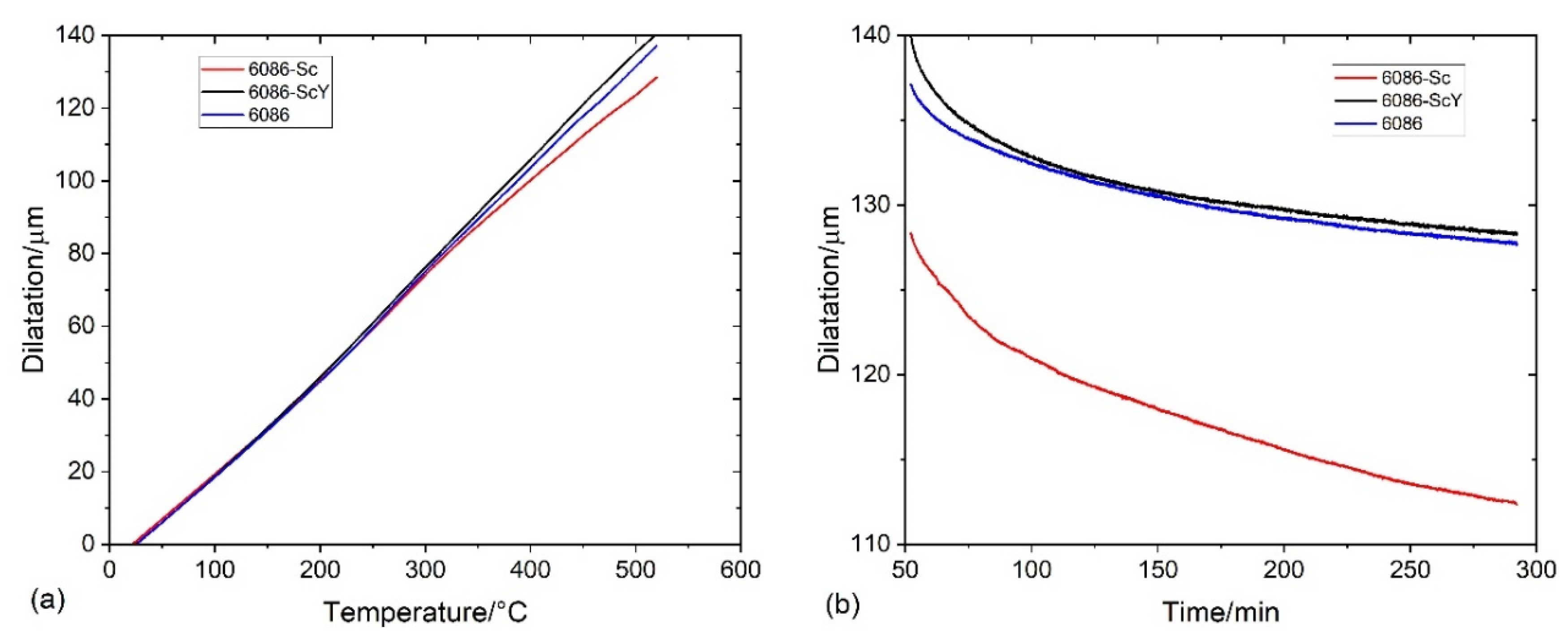
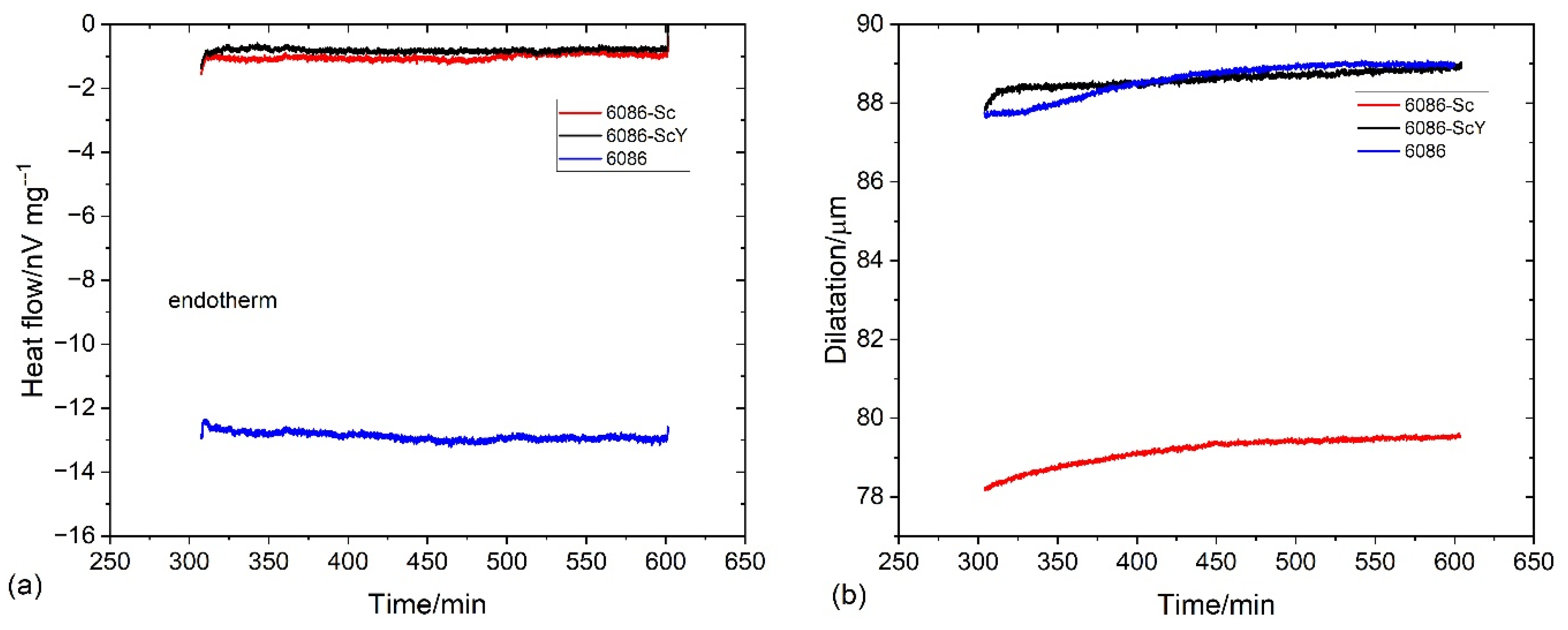
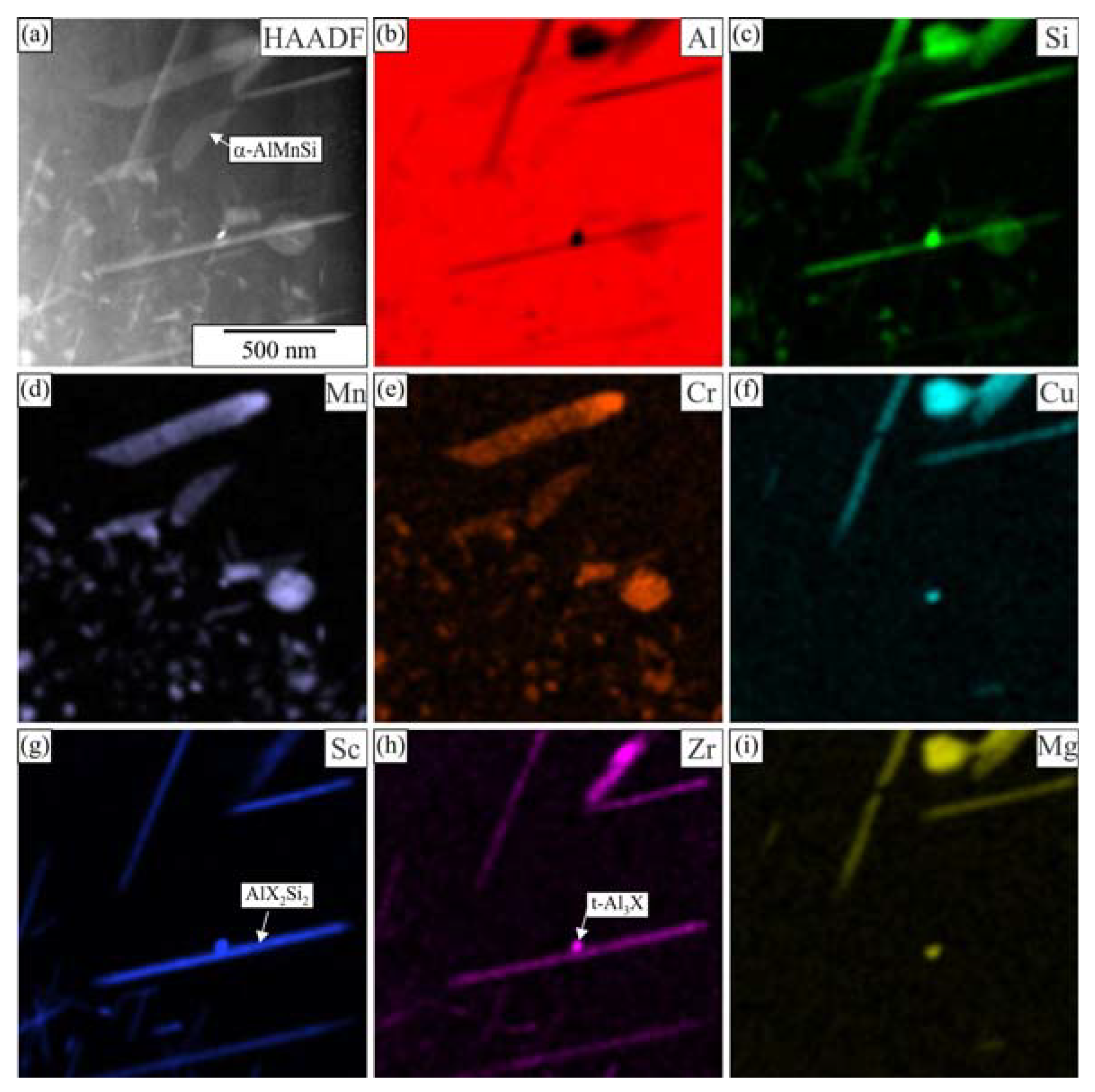
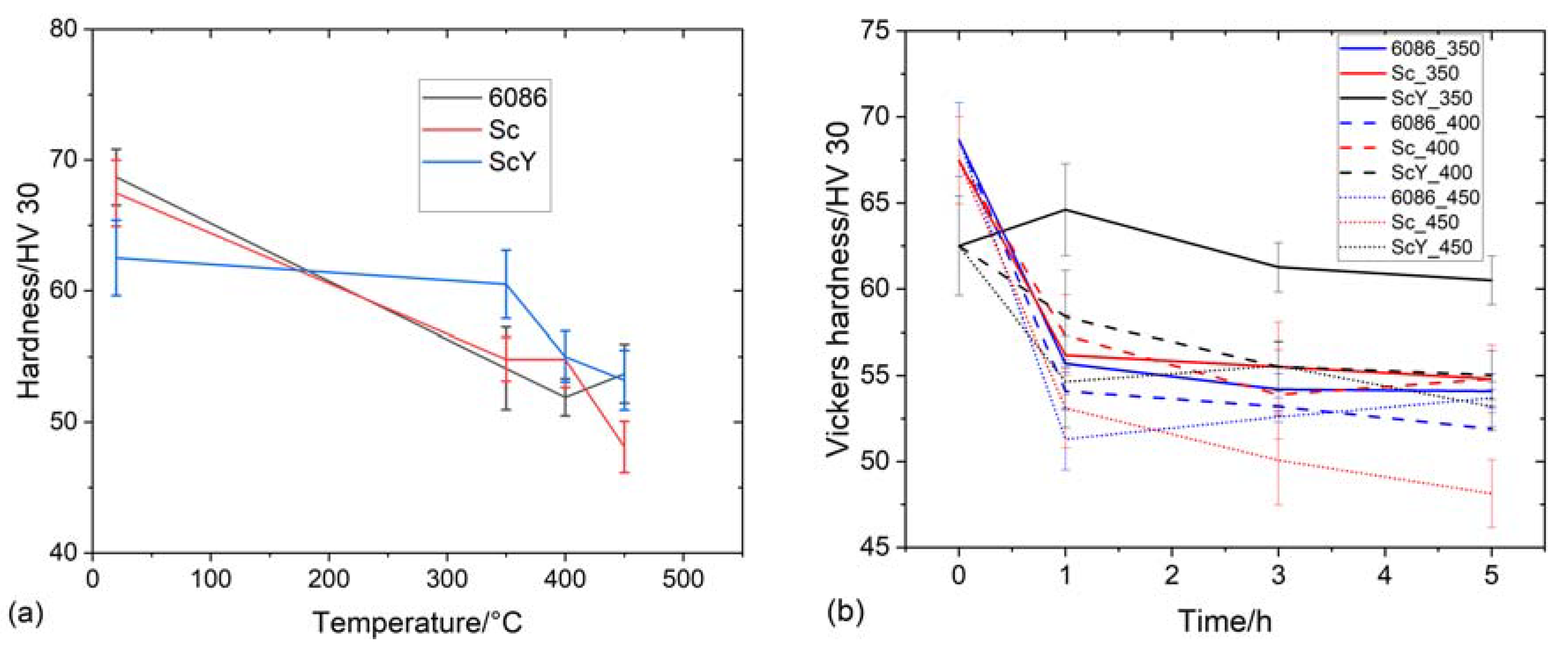
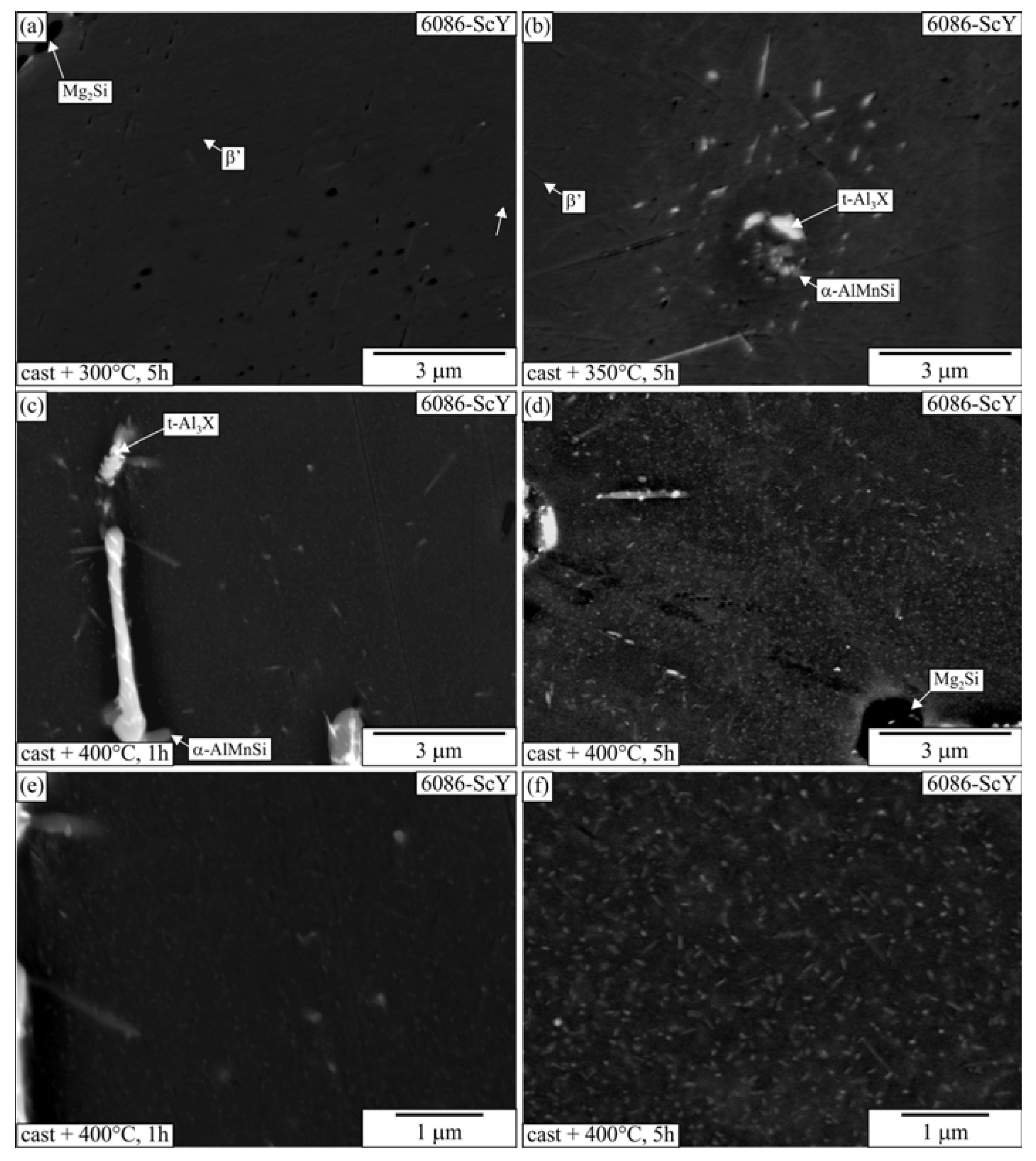
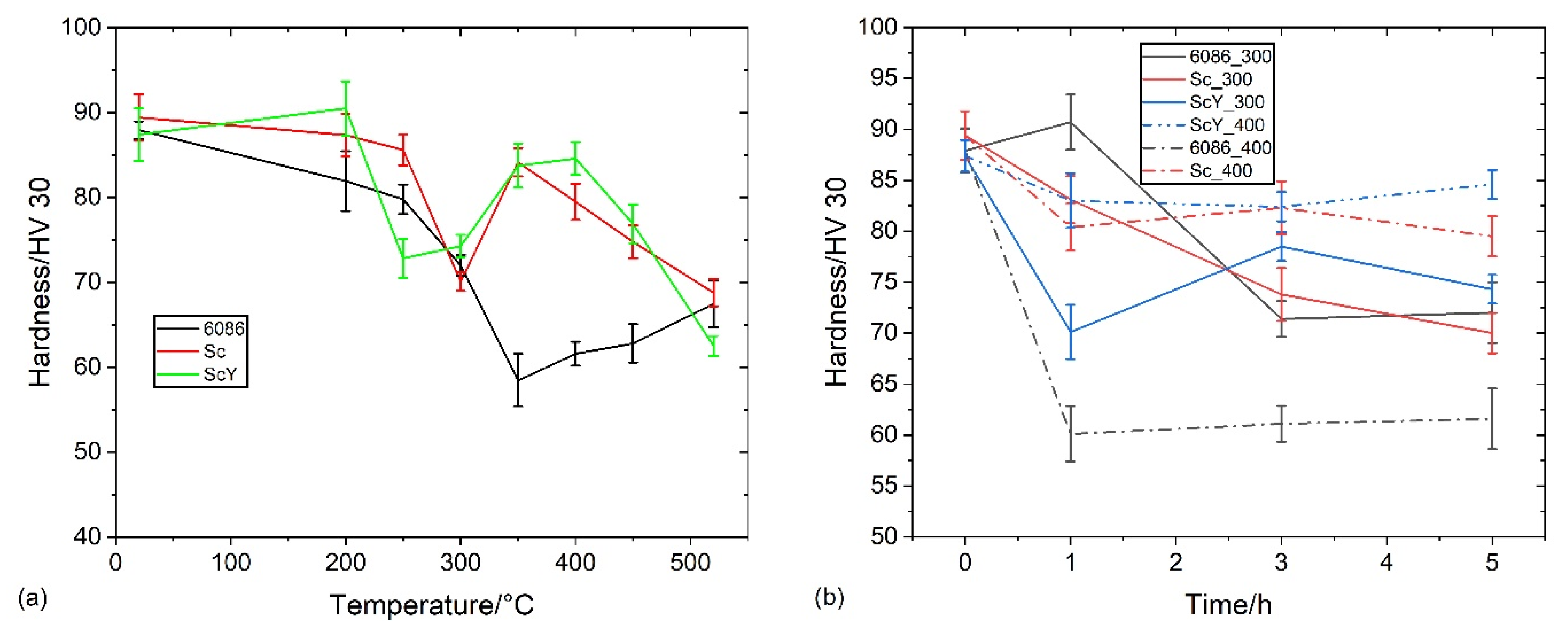
3.2. Heat Treatment of the Homogenized Alloys
3.3. Heat Treatment of the Alloys in the As-Cast Condition
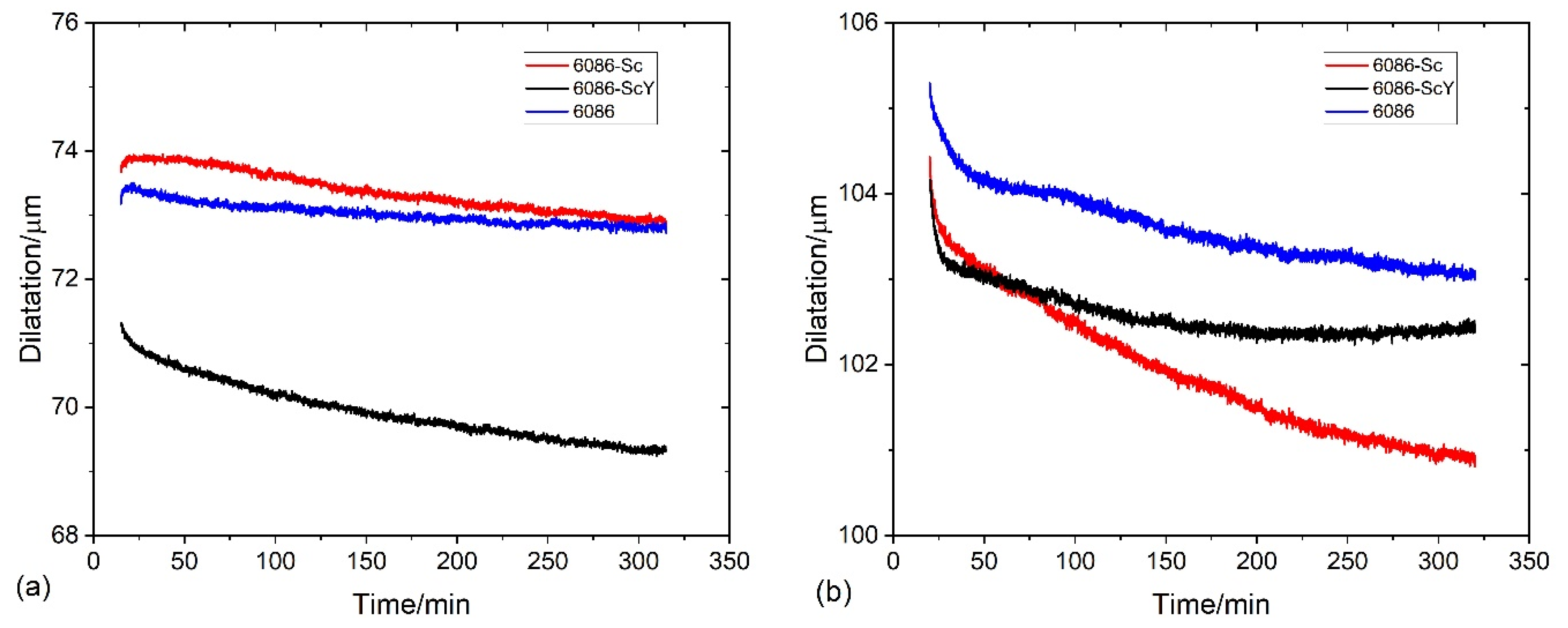
3.4. Compression Tests of the Specimens after Dilatometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, P. Alloy Designation, Processing, and Use of AA6XXX Series Aluminium Alloys. ISRN Metall. 2012, 2012, 165082. [Google Scholar] [CrossRef]
- Berneder, J.; Prillhofer, R.; Enser, J.; Grohmann, T. AMAG 6XXX Series Alloys for Chassis Application in the Automotive Industry. In Light Metals 2014; Grandfield, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; p. 177. [Google Scholar]
- Schulz, P.; Berneder, J.; Uffelmann, D.; Zelger, C.; Melzer, C. Advanced 5xxx-, 6xxx- and 7xxx- Aluminium Alloys for Applications in Automotive and Consumer Electronics. Mater. Sci. Forum 2011, 690, 451. [Google Scholar] [CrossRef]
- Yang, M.; Chen, H.; Orekhov, A.; Lu, Q.; Lan, X.; Li, K.; Zhang, S.; Song, M.; Kong, Y.; Schryvers, D.; et al. Quantified contribution of β″ and β′ precipitates to the strengthening of an aged Al–Mg–Si alloy. Mater. Sci. Eng. A 2020, 774, 138776. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437. [Google Scholar] [CrossRef]
- Mao, H.; Kong, Y.; Cai, D.; Yang, M.; Peng, Y.; Zeng, Y.; Zhang, G.; Shuai, X.; Huang, Q.; Li, K.; et al. β″ needle-shape precipitate formation in Al-Mg-Si alloy: Phase field simulation and experimental verification. Comput. Mater. Sci. 2020, 184, 109878. [Google Scholar] [CrossRef]
- Sunde, J.K.; Marioara, C.D.; Holmestad, R. The effect of low Cu additions on precipitate crystal structures in overaged Al-Mg-Si(-Cu) alloys. Mater. Charact. 2020, 160, 110087. [Google Scholar] [CrossRef]
- Meng, Y.; Cui, J.; Zhao, Z.; He, L. Effect of Zr on microstructures and mechanical properties of an AlMgSiCuCr alloy prepared by low frequency electromagnetic casting. Mater. Charact. 2014, 92, 138. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Modelling Al3Zr dispersoid precipitation in multi-component aluminium alloys. Mater. Sci. Eng. A 2003, 352, 240. [Google Scholar] [CrossRef]
- Zhang, Y.; Bettles, C.; Rometsch, P.A. Effect of recrystallisation on Al3Zr dispersoid behaviour in thick plates of aluminium alloy AA7150. J. Mater. Sci. 2014, 49, 1709. [Google Scholar] [CrossRef]
- Jia, Z.; Hu, G.; Forbord, B.; Solberg, J.K. Effect of homogenization and alloying elements on recrystallization resistance of Al–Zr–Mn alloys. Mater. Sci. Eng. A 2007, 444, 284. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Vahid, A.; Langan, T. Chapter 12—Aluminium Scandium Alloys. In Fundamentals of Aluminium Metallurgy; Lumley, R.N., Ed.; Woodhead Publishing: Cambridge, UK, 2018; p. 439. [Google Scholar]
- Kharakterova, M.L.; Eskin, D.G.; Rokhlin, L.L. Effect of scandium and zirconium on structure and age hardening of the Al-Mg-Si alloys. Russ. Metall. 1997, 123, 104–109. [Google Scholar]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multi-component alloys. Int. Mater. Rev. 2016, 61, 203. [Google Scholar] [CrossRef]
- Royset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19. [Google Scholar] [CrossRef]
- Milman, Y.V. Scandium effect on increasing mechanical properties of aluminum alloys. High Temp. Mater. Process. 2006, 25, 1. [Google Scholar] [CrossRef]
- Buttard, M.; Chehab, B.; Shahani, R.; Robaut, F.; Renou, G.; Tassin, C.; Rauch, E.; Donnadieu, P.; Deschamps, A.; Blandin, J.-J.; et al. Multi-scale microstuctural investigation of a new Al-Mn-Ni-Cu-Zr aluminium alloy processed by laser powder bed fusion. Materialia 2021, 18, 101160. [Google Scholar] [CrossRef]
- Li, J.H.; Oberdorfer, B.; Wurster, S.; Schumacher, P. Impurity effects on the nucleation and growth of primary Al3(Sc,Zr) phase in Al alloys. J. Mater. Sci. 2014, 49, 5961. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Hanada, S.; Yamagata, H.; Ma, C. The effect of scandium addition on microstructure and mechanical properties of Al–Si–Mg alloy: A multi-refinement modifier. Mater. Charact. 2015, 110, 160. [Google Scholar] [CrossRef]
- Knipling, K.E.; Karnesky, R.A.; Lee, C.P.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al-0.1Sc, Al-0.1Zr and Al-0.1Sc-0.1Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184. [Google Scholar] [CrossRef]
- Knipling, K.E.; Seidman, D.N.; Dunand, D.C. Ambient- and high-temperature mechanical properties of isochronally aged Al-0.06Sc, Al-0.06Zr and Al-0.06Sc-0.06Zr (at.%) alloys. Acta Mater. 2011, 59, 943. [Google Scholar] [CrossRef]
- Tolley, A.; Radmilovic, V.; Dahmen, U. Segregation in Al-3 (Sc,Zr) precipitates in Al-Sc-Zr alloys. Scr. Mater. 2005, 52, 621. [Google Scholar] [CrossRef]
- Zupanič, F.; Gspan, C.; Burja, J.; Bončina, T. Quasicrystalline and L12 precipitates in a microalloyed Al-Mn-Cu alloy. Mater. Today Commun. 2020, 22, 100809. [Google Scholar] [CrossRef]
- Zupanič, F.; Bončina, T. Heat-Resistant Al-Alloys with Quasicrystalline and L12- Precipitates. Solid State Phenom. 2022, 327, 26. [Google Scholar] [CrossRef]
- Booth-Morrison, C.; Mao, Z.; Diaz, M.; Dunand, D.C.; Wolverton, C.; Seidman, D.N. Role of silicon in accelerating the nucleation of Al-3(Sc,Zr) precipitates in dilute Al-Sc-Zr alloys. Acta Mater. 2012, 60, 4740. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Babaniaris, S.; Langan, T.J. Micro-segregation and precipitates in as-solidified Al-Sc-Zr-(Mg)-(Si)-(Cu) alloys. Mater. Charact. 2019, 154, 353. [Google Scholar] [CrossRef]
- Dumbre, J.; Kairy, S.K.; Anber, E.; Langan, T.; Taheri, M.L.; Dorin, T.; Birbilis, N. Understanding the formation of (Al,Si)3Sc and V-phase (AlSc2Si2) in Al-Si-Sc alloys via ex situ heat treatments and in situ transmission electron microscopy studies. J. Alloys Compd. 2021, 861, 158511. [Google Scholar] [CrossRef]
- Cavaliere, P.; Cabibbo, M. Effect of Sc and Zr additions on the microstructure and fatigue properties of AA6106 produced by equal-channel-angular-pressing. Mater. Charact. 2008, 59, 197. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.L.; Zheng, R.X.; Hanada, S.; Yamagata, H.; Ma, C.L. The synergic effects of Sc and Zr on the microstructure and mechanical properties of Al-Si-Mg alloy. Mater. Des. 2015, 88, 485. [Google Scholar] [CrossRef]
- Wan, B.; Chen, W.; Liu, L.; Cao, X.; Zhou, L.; Fu, Z. Effect of trace yttrium addition on the microstructure and tensile properties of recycled Al–7Si–0.3Mg–1.0Fe casting alloys. Mater. Sci. Eng. A 2016, 666, 165. [Google Scholar] [CrossRef]
- Xu, R.; Sun, Q.; Wang, Z.; Xu, Y.; Ren, W. A Novel Developed Grain Refiner (Al–Y–B Master Alloys) Using Yttrium and KBF4 Powders. Russ. J. Non-Ferr. Met. 2018, 59, 50. [Google Scholar] [CrossRef]
- Shuai, L.; Zou, X.; Rao, Y.; Lu, X.; Yan, H. Synergistic Effects of La and Y on the Microstructure and Mechanical Properties of Cast Al-Si-Cu Alloys. Materials 2022, 15, 7283. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Yan, H.; Zhong, S.; Huang, Z. Effect of Trace Yttrium Addition and Heat Treatment on the Microstructure and Mechanical Properties of As-Cast ADC12 Aluminum Alloy. Appl. Sci. 2019, 9, 53. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, S.; Li, B.; Zhu, Y.; Liu, J.; Liu, D.; Lan, Y.; Xia, T. Modification of multi-component Al–Si casting piston alloys by addition of rare earth yttrium. Mater. Res. Express 2019, 6, 106525. [Google Scholar] [CrossRef]
- Shen, H.; Liang, H.; Yang, W.D.; Yao, G.C.; Wang, C.S. Effect of Y on Microstructure and Mechanical Properties of Aluminium Alloy. Appl. Mech. Mater. 2013, 421, 250. [Google Scholar] [CrossRef]
- Guo, T.B.; Zhang, F.; Li, Q.; Wang, C.; Ding, W.W. Microstructure and Properties of Al-Cu-Mn Alloy with Y, Zr and (Y + Zr). Mater. Sci. Forum 2017, 898, 334. [Google Scholar] [CrossRef]
- Wang, S.; Matsuda, K.; Kawabata, T.; Zou, Y.; Yamazaki, T.; Ikeno, S. Effect of TM-Addition on the Aging Behaviour of Al-Mg-Si Alloys. Mater. Trans. 2011, 52, 229. [Google Scholar] [CrossRef]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D.; Blaou, A.B.Y.; Nuhu, A.H.; Bagaber, S.A. Effect of Yttrium on the Microstructure and Mechanical Properties of A5083 Secondary Aluminium Alloy. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 62, 168. [Google Scholar]
- Barkov, R.Y.; Pozdniakov, A.V.; Tkachuk, E.; Zolotorevskiy, V.S. Effect of Y on microstructure and mechanical properties of Al-Mg-Mn-Zr-Sc alloy with low Sc content. Mater. Lett. 2018, 217, 135. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Barkov, R.Y. Microstructure and mechanical properties of novel Al-Y-Sc alloys with high thermal stability and electrical conductivity. J. Mater. Sci. Technol. 2020, 36, 1. [Google Scholar] [CrossRef]
- Strobel, K.; Sweet, E.; Easton, M.; Nie, J.F.; Couper, M. Dispersoid Phases in 6xxx Series Aluminium Alloys. Mater. Sci. Forum 2010, 654–656, 926. [Google Scholar] [CrossRef]
- Liu, C.L.; Azizi-Alizamini, H.; Parson, N.C.; Poole, W.J.; Du, Q. Microstructure evolution during homogenization of Al-Mg-Si-Mn-Fe alloys: Modelling and experimental results. Trans. Nonferrous Met. Soc. China 2017, 27, 747. [Google Scholar] [CrossRef]
- Wang, X.G.; Qin, J.; Nagaumi, H.; Wu, R.R.; Li, Q.S. The Effect of alpha-Al(MnCr)Si Dispersoids on Activation Energy and Workability of Al-Mg-Si-Cu Alloys during Hot Deformation. Adv. Mater. Sci. Eng. 2020, 2020, 12. [Google Scholar]
- Österreicher, J.A.; Kumar, M.; Schiffl, A.; Schwarz, S.; Bourret, G.R. Secondary precipitation during homogenization of Al-Mg-Si alloys: Influence on high temperature flow stress. Mater. Sci. Eng. A 2017, 687, 175. [Google Scholar] [CrossRef]
- Hichem, F.; Rebai, G. Study of dispersoid particles in two Al-Mg-Si aluminium alloys and their effects on the recrystallization. Appl. Phys. A-Mater. Sci. Process. 2015, 119, 285. [Google Scholar] [CrossRef]
- Li, C.; Liu, K.; Chen, X.G. Improvement of elevated-temperature strength and recrystallization resistance via Mn-containing dispersoid strengthening in Al-Mg-Si 6082 alloys. J. Mater. Sci. Technol. 2020, 39, 135. [Google Scholar] [CrossRef]
- Remoe, M.S.; Westermann, I.; Marthinsen, K. Characterization of the Density and Spatial Distribution of Dispersoids in Al-Mg-Si Alloys. Metals 2019, 9, 20. [Google Scholar] [CrossRef]
- Qian, F.; Jin, S.; Sha, G.; Li, Y. Enhanced dispersoid precipitation and dispersion strengthening in an Al alloy by microalloying with Cd. Acta Mater. 2018, 157, 114. [Google Scholar] [CrossRef]
- Liu, K.; Ma, H.; Chen, X.G. Enhanced elevated-temperature properties via Mo addition in Al-Mn-Mg 3004 alloy. J. Alloys Compd. 2017, 694, 354. [Google Scholar] [CrossRef]
- De Luca, A.; Shu, S.; Seidman, D.N. Effect of microadditions of Mn and Mo on dual L12- and α-precipitation in a dilute Al-Zr-Sc-Er-Si alloy. Mater. Charact. 2020, 169, 110585. [Google Scholar] [CrossRef]
- Zupanič, F.; Steinacher, M.; Žist, S.; Bončina, T. Microstructure and Properties of a Novel Al-Mg-Si Alloy AA 6086. Metals 2021, 11, 368. [Google Scholar] [CrossRef]
- Zupanič, F.; Klemenc, J.; Steinacher, M.; Glodež, S. Microstructure, mechanical properties and fatigue behaviour of a new high-strength aluminium alloy AA 6086. J. Alloys Compd. 2023, 941, 168976. [Google Scholar] [CrossRef]
- Cann, J.L.; De Luca, A.; Dunand, D.C.; Dye, D.; Miracle, D.B.; Oh, H.S.; Olivetti, E.A.; Pollock, T.M.; Poole, W.J.; Yang, R.; et al. Sustainability through alloy design: Challenges and opportunities. Prog. Mater. Sci. 2021, 117, 100722. [Google Scholar] [CrossRef]
- Macerl, M.; Zupanič, F.; Hočuršćak, L.; Klobčar, D.; Kovács, A.; Bončina, T. Microstructure and Properties after Friction Stir Processing of Twin-Roll Cast Al-Mn-Cu-Be Alloy. Crystals 2022, 12, 630. [Google Scholar] [CrossRef]
- Žist, S.; Steinacher, M.; Bončina, T.; Albu, M.; Burja, J.; Vončina, M.; Zupanič, F. The Effect of Scandium on the Microstructure of the Aluminium Alloy AA 6086. Crystals 2022, 12, 973. [Google Scholar] [CrossRef]
- Song, M.; Kobayashi, E.; Kim, J. Clustering evolution during low temperature aging and thermal stability during two-step aging in Al-Mg-Si alloys. J. Alloys Compd. 2023, 946, 169291. [Google Scholar] [CrossRef]
- Afshar, M.; Mao, F.; Jiang, H.; Mohles, V.; Schick, M.; Hack, K.; Korte-Kerzel, S.; Barrales-Mora, L.A. Modelling of differential scanning calorimetry heating curves for precipitation and dissolution in an Al-Mg-Si. Comput. Mater. Sci. 2019, 158, 235. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Chen, D.; Xia, C.; Liu, X.; Wu, Y.; Wang, M. The Effect of Alloying Elements on the Structural Stability, and Mechanical and Electronic Properties of Al3Sc: A First-Principles Study. Materials 2019, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Simensen, C.J.; Bjørneklett, A. A Model for α-Al(Mn,Fe)Si Crystals; Ratvik, A.P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; p. 197. [Google Scholar]
- Dorin, T.; Babaniaris, S.; Jiang, L.; Cassel, A.; Race, C.P.; Eggeman, A.; Kelly, D.J.; Haigh, S.J.; Robson, J.D. Stability and stoichiometry of L12 Al3(Sc,Zr) dispersoids in Al-(Si)-Sc-Zr alloys. Acta Mater. 2021, 216, 117117. [Google Scholar] [CrossRef]
- Lypchanskyi, O.; Rigas, N.; Korpala, G.; Merklein, M.; Prahl, U. Ex-situ and in-situ investigations of the microstructural evolution of AA6082 aluminum alloy during heat treatment. Mater. Sci. Eng. A 2023, 870, 144828. [Google Scholar] [CrossRef]
- Hu, J.L.; Bo, H.; Liu, L.B.; Jin, Z.P. Thermodynamic study of the Al-Sc-Y system. Thermochim. Acta 2018, 661, 147. [Google Scholar] [CrossRef]
- Farh, H.; Djemmal, K.; Guemini, R.; Serradj, F. Nucleation of dispersoids study in some Al-Mg-Si alloys. Ann. Chim. Sci. Des. Mater. 2010, 35, 283. [Google Scholar] [CrossRef]
- Pan, S.; Qian, F.; Li, C.; Wang, Z.; Li, Y. Synergistic strengthening by nano-sized α-Al(Mn,Fe)Si and Al3Zr dispersoids in a heat-resistant Al–Mn–Fe–Si–Zr alloy. Mater. Sci. Eng. A 2021, 819, 141460. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.G. Effects of Al(MnFe)Si dispersoids with different sizes and number densities on microstructure and ambient/elevated-temperature mechanical properties of extruded Al–Mg–Si AA6082 alloys with varying Mn content. J. Alloys Compd. 2021, 861, 157937. [Google Scholar] [CrossRef]
- Qian, X.; Parson, N.; Chen, X.G. Effects of Mn addition and related Mn-containing dispersoids on the hot deformation behavior of 6082 aluminum alloys. Mater. Sci. Eng. A 2019, 764, 138253. [Google Scholar] [CrossRef]




| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Zr | Sc | Y | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6086 | 1.58 | 0.18 | 0.55 | 0.70 | 1.00 | 0.19 | 0.02 | 0.04 | 0.17 | - | - | balance |
| 6086-Sc | 1.37 | 0.17 | 0.49 | 0.72 | 0.88 | 0.17 | 0.02 | 0.04 | 0.16 | 0.22 | - | balance |
| 6086-ScY | 1.36 | 0.17 | 0.49 | 0.72 | 0.89 | 0.17 | 0.02 | 0.04 | 0.16 | 0.18 | 0.09 | balance |
| Alloy | Maximum True Stress MPa | True Strain at Maximum True Stress |
|---|---|---|
| 6086, homogenized + 400 °C, 5 h | 263.6 | 0.684 |
| 6086-Sc, homogenized + 400 °C, 5 h | 247.1 | 0.301 |
| 6086-ScY, homogenized + 400 °C, 5 h | 287.2 | 0.693 |
| 6086, as-cast + 300 °C, 5 h | 266.1 | 0.143 |
| 6086-Sc, as-cast + 300 °C, 5 h | 282.7 | 0.151 |
| 6086-ScY, as-cast + 300 °C, 5 h | 298.9 | 0.122 |
| 6086, as-cast + 400 °C, 5 h | 272.3 | 0.197 |
| 6086-Sc, as-cast + 400 °C, 5 h | 302.2 | 0.141 |
| 6086-ScY, as-cast + 400 °C, 5 h | 305.2 | 0.138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupanič, F.; Žist, S.; Albu, M.; Letofsky-Papst, I.; Burja, J.; Vončina, M.; Bončina, T. Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y. Materials 2023, 16, 2949. https://doi.org/10.3390/ma16082949
Zupanič F, Žist S, Albu M, Letofsky-Papst I, Burja J, Vončina M, Bončina T. Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y. Materials. 2023; 16(8):2949. https://doi.org/10.3390/ma16082949
Chicago/Turabian StyleZupanič, Franc, Sandi Žist, Mihaela Albu, Ilse Letofsky-Papst, Jaka Burja, Maja Vončina, and Tonica Bončina. 2023. "Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y" Materials 16, no. 8: 2949. https://doi.org/10.3390/ma16082949
APA StyleZupanič, F., Žist, S., Albu, M., Letofsky-Papst, I., Burja, J., Vončina, M., & Bončina, T. (2023). Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y. Materials, 16(8), 2949. https://doi.org/10.3390/ma16082949








