Investigation of the Formation, Characterization, and Oxidative Catalytic Valorization of Humins
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for Humin Formation Experiments
2.2. General Procedure for Catalytic Oxidation of Humins
2.3. Analytical Methods
- The nuclear magnetic resonance (NMR) spectra were measured using a Bruker AVANCEII 600 MHz spectrometer. Samples were prepared by mixing 0.4 mL of the filtered reaction solutions with 0.2 mL of D2O. Spectra of the reaction solutions can be found in the Supporting Information.
- Elemental analyses were carried out using the Model EA-3000 analyzer of Fa. EuroVector (Milano, Italy).
- SEM micrographs were recorded using a LEO Gemini 15505 from Zeiss (Oberkochen, Germany).
- MALDI-TOF measurements were taken with a Bruker ultrafleXtreme (Billerica, MA, USA) in positive reflector mode. A 2 mg/mL dispersion of the humic acid in water and a 20 mg/mL dihydroxybenzoic acid/TA30 (ACN/H2O 30/70 + 0.1%TFA) solution were prepared for the measurements. A total of 0.7 μL of both solutions were then mixed and left to crystallize.
3. Results and Discussion
3.1. Influence of Sugar, Acid, and Solvent on Humin Formation, Composition, and Structure
3.2. Oxidative Conversion of Humins Using Polyoxometalate Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Cheminform 2007, 38, 7164–7183. [Google Scholar] [CrossRef]
- Horvat, J.; Klaic, B.; Metelko, B.; Sunjic, V. Mechanism of Levulinic Acid Formation. Tetrahedron Lett. 1985, 26, 2111–2114. [Google Scholar] [CrossRef]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.R.H. The Biofine Process—Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. In Biorefineries-Industrial Processes and Products; Wiley: Weinheim, Germany, 2005; pp. 139–164. [Google Scholar]
- Maerten, S. Humine: Bildung Und Wertschöpfung; Rwth Publications: Aachen, Germany, 2018. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for The Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.K.R.; Lund, C.R.F. Formation and Growth of Humins Via Aldol Addition and Condensation During Acid-Catalyzed Conversion of 5-Hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of Structural Features of Humins Formed Catalytically from Glucose, Fructose, and 5-Hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. Chemsuschem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, G.; Yang, X.; Yin, H.; Fu, X.; Liao, J.; Tu, J.; Huang, X.; Qin, F.G.F.; Xu, Y. Effects of P-toluenesulfonic Acid in The Conversion of Glucose for Levulinic Acid and Sulfonated Carbon Production. Energy Fuels 2017, 31, 2847–2854. [Google Scholar] [CrossRef]
- Cheng, Z.; Goulas, K.A.; Quiroz Rodriguez, N.; Saha, B.; Vlachos, D.G. Growth Kinetics of Humins Studied via X-ray Scattering. Green Chem. 2020, 22, 2301–2309. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, X.; Lin, Q.; Zhang, X.; Meng, L.; Sun, R.-C.; Xin, F.; Ren, J. New Understandings of The Relationship and Initial Formation Mechanism for Pseudo-Lignin, Humins, and Acid-Induced Hydrothermal Carbon. J. Agric. Food Chem. 2018, 66, 11981–11989. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Orella, M.J.; Lin, Z.; Cheng, Z.; Zheng, W.; Nikolakis, V.; Vlachos, D.G. Molecular Structure, Morphology and Growth Mechanisms and Rates of 5-Hydroxymethyl Furfural (Hmf) Derived Humins. Green Chem. 2016, 18, 1983–1993. [Google Scholar] [CrossRef]
- Thapa, I.; Mullen, B.; Saleem, A.; Leibig, C.; Baker, R.T.; Giorgi, J.B. Efficient Green Catalysis for The Conversion of Fructose to Levulinic Acid. Appl. Catal. A Gen. 2017, 539, 70–79. [Google Scholar] [CrossRef]
- Velaga, B.; Parde, R.P.; Soni, J.; Peela, N.R. Synthesized Hierarchical Mordenite Zeolites for The Biomass Conversion to Levulinic Acid and The Mechanistic Insights into Humins Formation. Microporous Mesoporous Mater. 2019, 287, 18–28. [Google Scholar] [CrossRef]
- Dee, S.J.; Bell, A.T. A Study of The Acid-Catalyzed Hydrolysis of Cellulose Dissolved in Ionic Liquids and the Factors Influencing the Dehydration of Glucose and The Formation of Humins. Chemsuschem 2011, 4, 1166–1173. [Google Scholar] [CrossRef]
- Chuntanapum, A.; Matsumura, Y. Char Formation Mechanism in Supercritical Water Gasification Process: A Study of Model Compounds. Ind. Eng. Chem. Res. 2010, 49, 4055–4062. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Cen, H.; Ju, R.; He, X.; Ma, L. Formation of Humins During Degradation of Carbohydrates and Furfural Derivatives in Various Solvents. Biomass Conv. Bioref. 2020, 10, 277–287. [Google Scholar] [CrossRef]
- Hu, X.; Lievens, C.; Larcher, A.; Li, C.-Z. Reaction Pathways of Glucose During Esterification: Effects of Reaction Parameters on The Formation of Humin Type Polymers. Bioresour. Technol. 2011, 102, 10104–10113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly Efficient Preparation of Hmf from Cellulose Using Temperature-Responsive Heteropolyacid Catalysts in Cascade Reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Kinetic Study of Glucose Conversion to Levulinic Acid Over Fe/Hy Zeolite Catalyst. Chem. Eng. J. 2016, 283, 150–159. [Google Scholar] [CrossRef]
- Garcés, D.; Faba, L.; Díaz, E.; Ordóñez, S. Aqueous-Phase Transformation of Glucose into Hydroxymethylfurfural and Levulinic Acid by Combining Homogeneous and Heterogeneous Catalysis. Chemsuschem 2019, 12, 924–934. [Google Scholar]
- Hoang, T.M.C.; Lefferts, L.; Seshan, K. Valorization of Humin-Based Byproducts from Biomass Processing-A Route to Sustainable Hydrogen. Chemsuschem 2013, 6, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.M.C.; Geerdink, B.; Sturm, J.M.; Lefferts, L.; Seshan, K. Steam Reforming of Acetic Acid—A Major Component in The Volatiles Formed During Gasification of Humin. Appl. Catal. B Environ. 2015, 163, 74–82. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; Van Eck, E.R.H.; Bula, W.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Humin Based By-Products from Biomass Processing as A Potential Carbonaceous Source for Synthesis Gas Production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Tosi, P.; Van Klink, G.P.M.; Celzard, A.; Fierro, V.; Vincent, L.; De Jong, E.; Mija, A. Auto-Crosslinked Rigid Foams Derived from Biorefinery Byproducts. Chemsuschem 2018, 11, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Van Es, D.; Heeres, H.J. Catalytic Pyrolysis of Recalcitrant, Insoluble Humin Byproducts from C6 Sugar Biorefineries. J. Anal. Appl. Pyrolysis 2017, 123, 134–143. [Google Scholar] [CrossRef]
- Wang, Y.; Agarwal, S.; Heeres, H.J. Catalytic Liquefaction of Humin Substances from Sugar Biorefineries with Pt/C in 2-Propanol. Acs Sustain. Chem. Eng. 2017, 5, 469–480. [Google Scholar] [CrossRef]
- Maerten, S.G.; Voß, D.; Liauw, M.A.; Albert, J. Selective Catalytic Oxidation of Humins to Low-Chain Carboxylic Acids with Tailor-Made Polyoxometalate Catalysts. Chemistryselect 2017, 2, 7296–7302. [Google Scholar] [CrossRef]
- Shen, H.; Shan, H.; Liu, L. Evolution Process and Controlled Synthesis of Humins with 5-Hydroxymethylfurfural (Hmf) As Model Molecule. Chemsuschem 2020, 13, 513–519. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Yao, C.; Shin, Y.; Wang, L.-Q.; Windisch, C.F.; Samuels, W.D.; Arey, B.W.; Wang, C.; Risen, W.M.; Exarhos, G.J. Hydrothermal Dehydration of Aqueous Fructose Solutions in A Closed System. J. Phys. Chem. C 2007, 111, 15141–15145. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Xie, H.; Zhao, Z.K. An Unexpected Reaction Between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures. Molecules 2011, 16, 8463–8474. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Hiraga, Y.; Aida, T.M.; Smith, R.L. Efficient Conversion of Fructose into 5-Ethoxymethylfurfural with Hydrogen Sulfate Ionic Liquids as Co-Solvent and Catalyst. Chem. Eng. J. 2017, 314, 508–514. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. Hydrophobic Precipitation of Carbonaceous Spheres from Fructose by A Hydrothermal Process. Carbon 2012, 50, 2155–2161. [Google Scholar] [CrossRef]
- Voß, D.; Kahl, M.; Albert, J. Continuous Production of Formic Acid from Biomass in A Three-Phase Liquid–Liquid–Gas Reaction Process. ACS Sustain. Chem. Eng. 2020, 8, 10444–10453. [Google Scholar] [CrossRef]
- Reichert, J.; Brunner, B.; Jess, A.; Wasserscheid, P.; Albert, J. Biomass Oxidation to Formic Acid in Aqueous Media Using Polyoxometalate Catalysts—Boosting Fa Selectivity by In-Situ Extraction. Energy Environ. Sci. 2015, 8, 2985–2990. [Google Scholar] [CrossRef]
- Albert, J.; Lüders, D.; Bösmann, A.; Guldi, D.M.; Wasserscheid, P. Spectroscopic and Electrochemical Characterization of Heteropoly Acids for Their Optimized Application in Selective Biomass Oxidation to Formic Acid. Green Chem. 2014, 16, 226–237. [Google Scholar] [CrossRef]
- Odyakov, V.F.; Zhizhina, E.G. A Novel Method of The Synthesis of Molybdovanadophosphoric Heteropoly Acid Solutions. React. Kinet. Catal. Lett. 2008, 95, 21–28. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Hu, P.; Li, L.; Wu, D.; Xue, Z.; Zhu, L.; Hu, C. Probing the Effects of Fructose Concentration on The Evolution of Humins During Fructose Dehydration. React. Chem. Eng. 2022, 8, 175–183. [Google Scholar] [CrossRef]
- Albert, J.; Wölfel, R.; Bösmann, A.; Wasserscheid, P. Selective Oxidation of Complex, Water-Insoluble Biomass to Formic Acid Using Additives as Reaction Accelerators. Energy Environ. Sci. 2012, 5, 7956. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable Production of Levulinic Acid and Its Derivatives for Fuel Additives and Chemicals: Progress, Challenges, and Prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Divya, P.S.; Nair, S.; Kunnikuruvan, S. Identification of Crucial Intermediates in The Formation of Humins from Cellulose-Derived Platform Chemicals Under Brønsted Acid Catalyzed Reaction Conditions. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2022, 23, E202200057. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fu, J.; Zhang, G. from Lignocellulosic Biomass to Levulinic Acid: A Review on Acid-Catalyzed Hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Jung, D.; Körner, P.; Kruse, A. Kinetic Study on The Impact of Acidity and Acid Concentration on The Formation of 5-Hydroxymethylfurfural (Hmf), Humins, and Levulinic Acid in The Hydrothermal Conversion of Fructose. Biomass Conv. Bioref. 2021, 11, 1155–1170. [Google Scholar] [CrossRef]
- Girisuta, B.; Janssen, L.P.B.M.; Heeres, H.J. A Kinetic Study on The Decomposition of 5-Hydroxymethylfurfural into Levulinic Acid. Green Chem. 2006, 8, 701. [Google Scholar] [CrossRef]
- Köchermann, J.; Schreiber, J.; Klemm, M. Conversion of D-Xylose and Hemicellulose in Water/Ethanol Mixtures. Acs Sustain. Chem. Eng. 2019, 7, 12323–12330. [Google Scholar] [CrossRef]
- Sumerskii, I.V.; Krutov, S.M.; Zarubin, M.Y. Humin-Like Substances Formed Under the Conditions of Industrial Hydrolysis of Wood. Russ. J. Appl. Chem. 2010, 83, 320–327. [Google Scholar] [CrossRef]
- Chen, X.; Guigo, N.; Pizzi, A.; Sbirrazzuoli, N.; Li, B.; Fredon, E.; Gerardin, C. Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers 2020, 12, 2732. [Google Scholar] [CrossRef]
- Wesinger, S.; Mendt, M.; Albert, J. Alcohol-Activated Vanadium-Containing Polyoxometalate Complexes in Homogeneous Glucose Oxidation Identified with 51 V-Nmr and Epr Spectroscopy. Chemcatchem 2021, 13, 3662–3670. [Google Scholar] [CrossRef]
- Raabe, J.-C.; Aceituno Cruz, J.; Albert, J.; Poller, M.J. Comparative Spectroscopic and Electrochemical Study of V(V)-Substituted Keggin-Type Phosphomolybdates and -Tungstates. Inorganics 2023, 11, 138. [Google Scholar] [CrossRef]
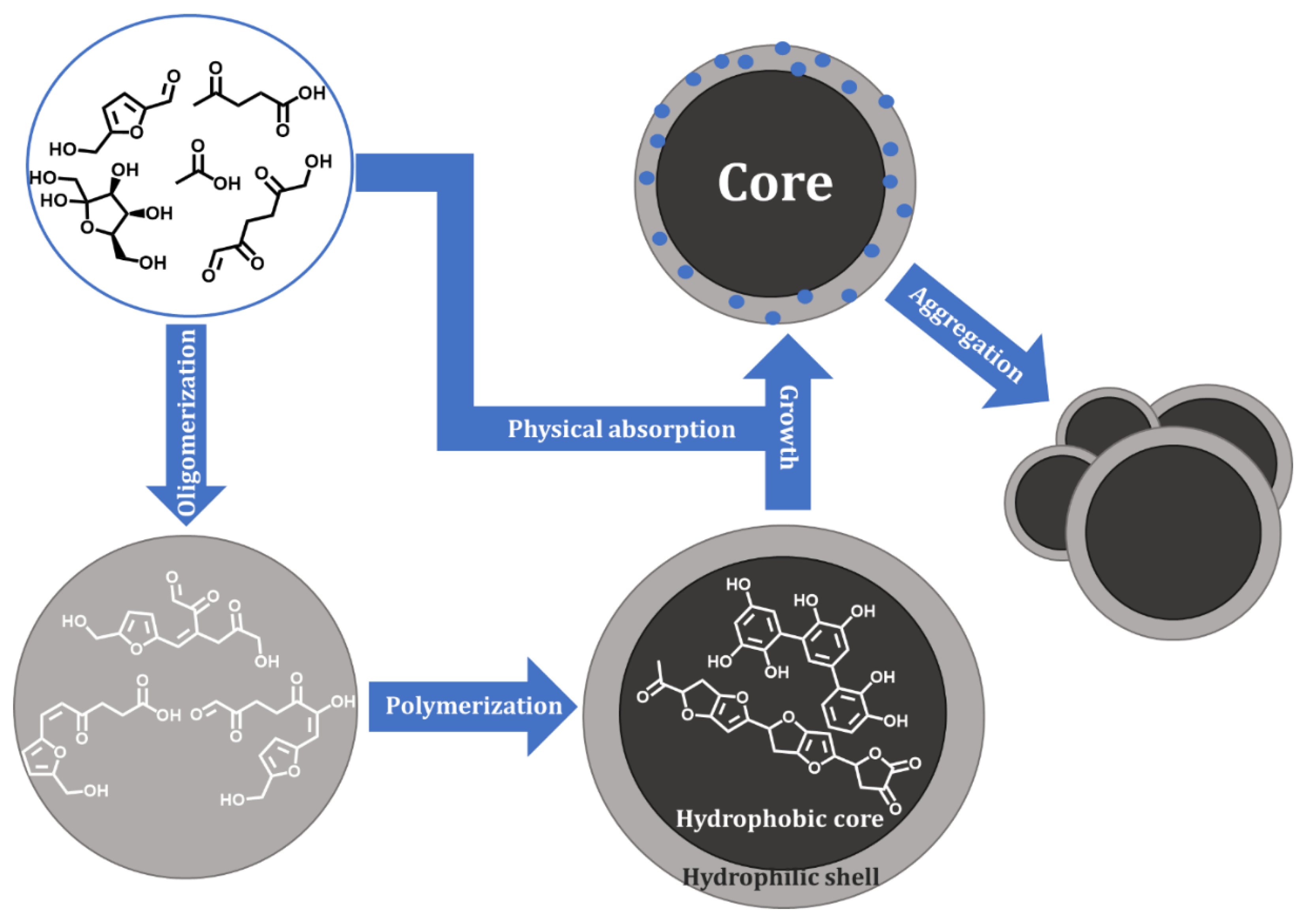

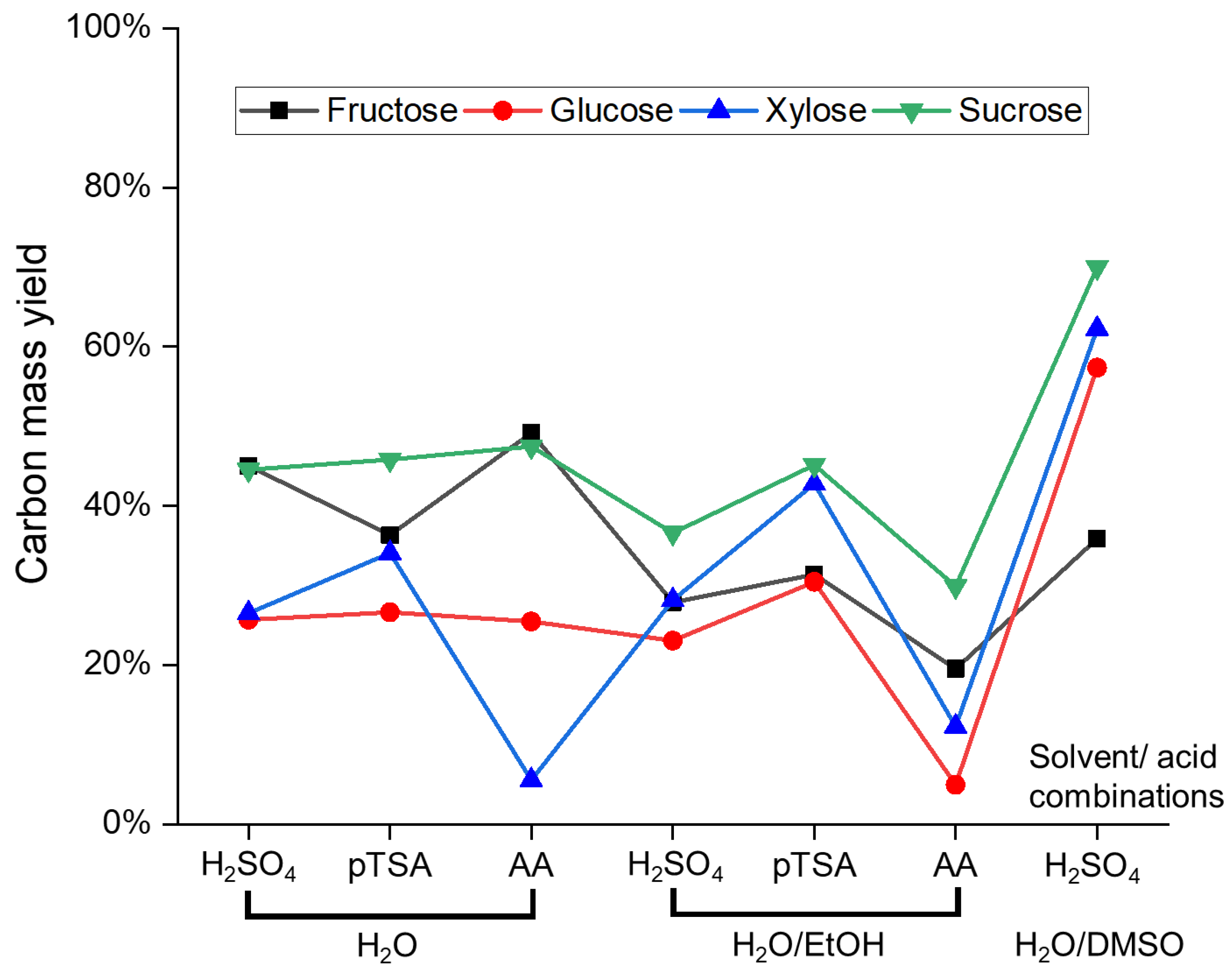
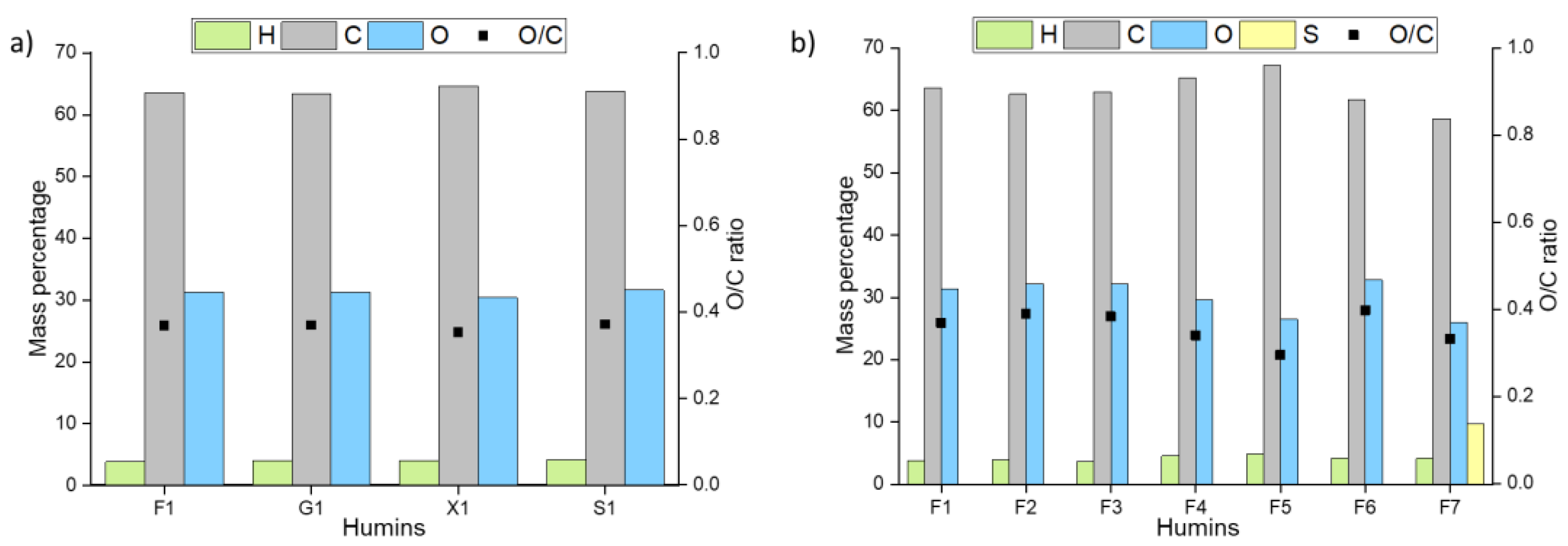
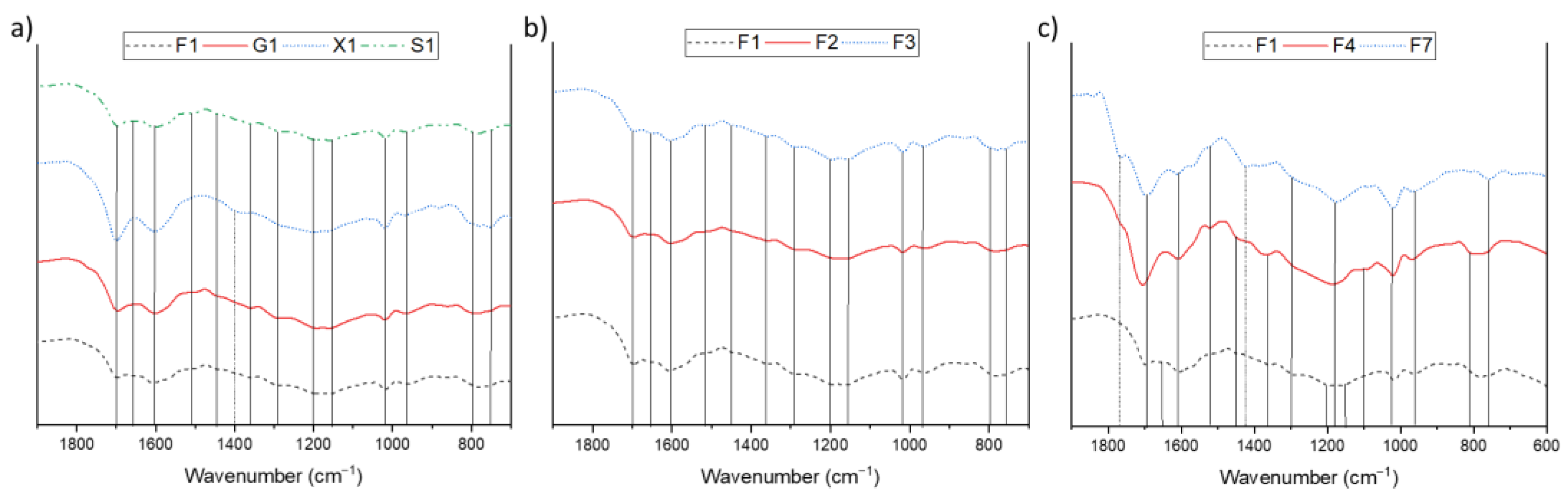
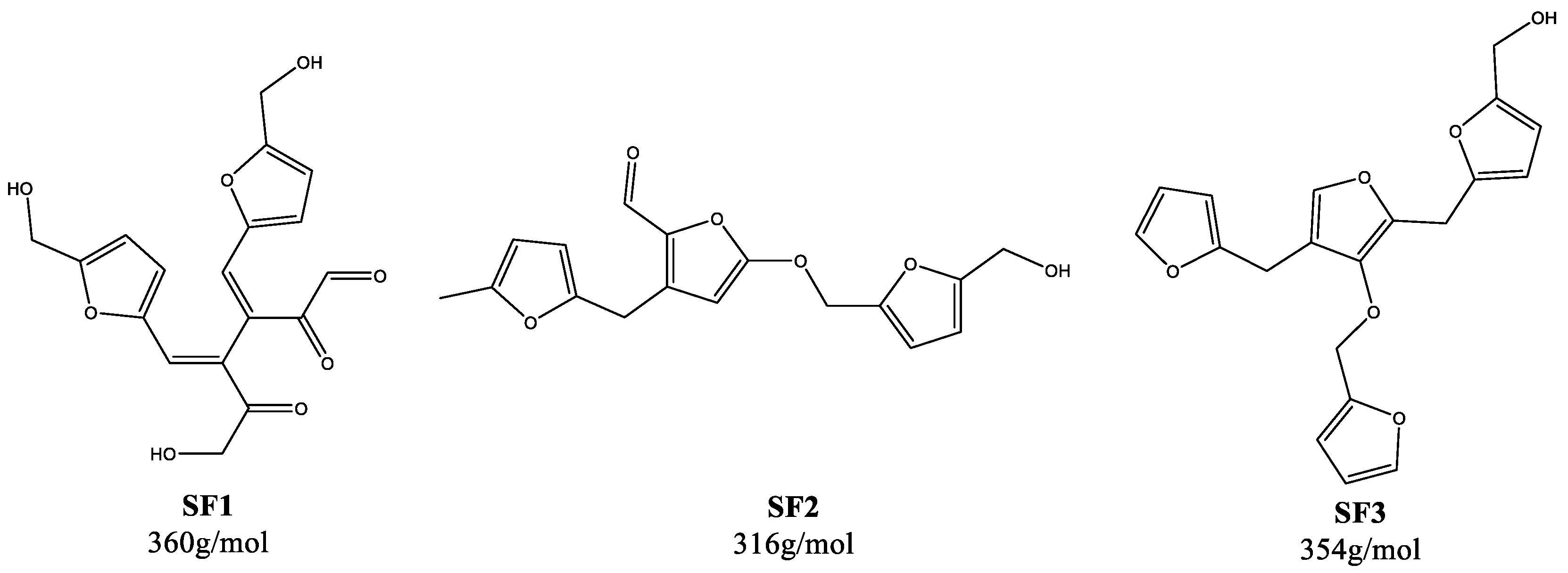
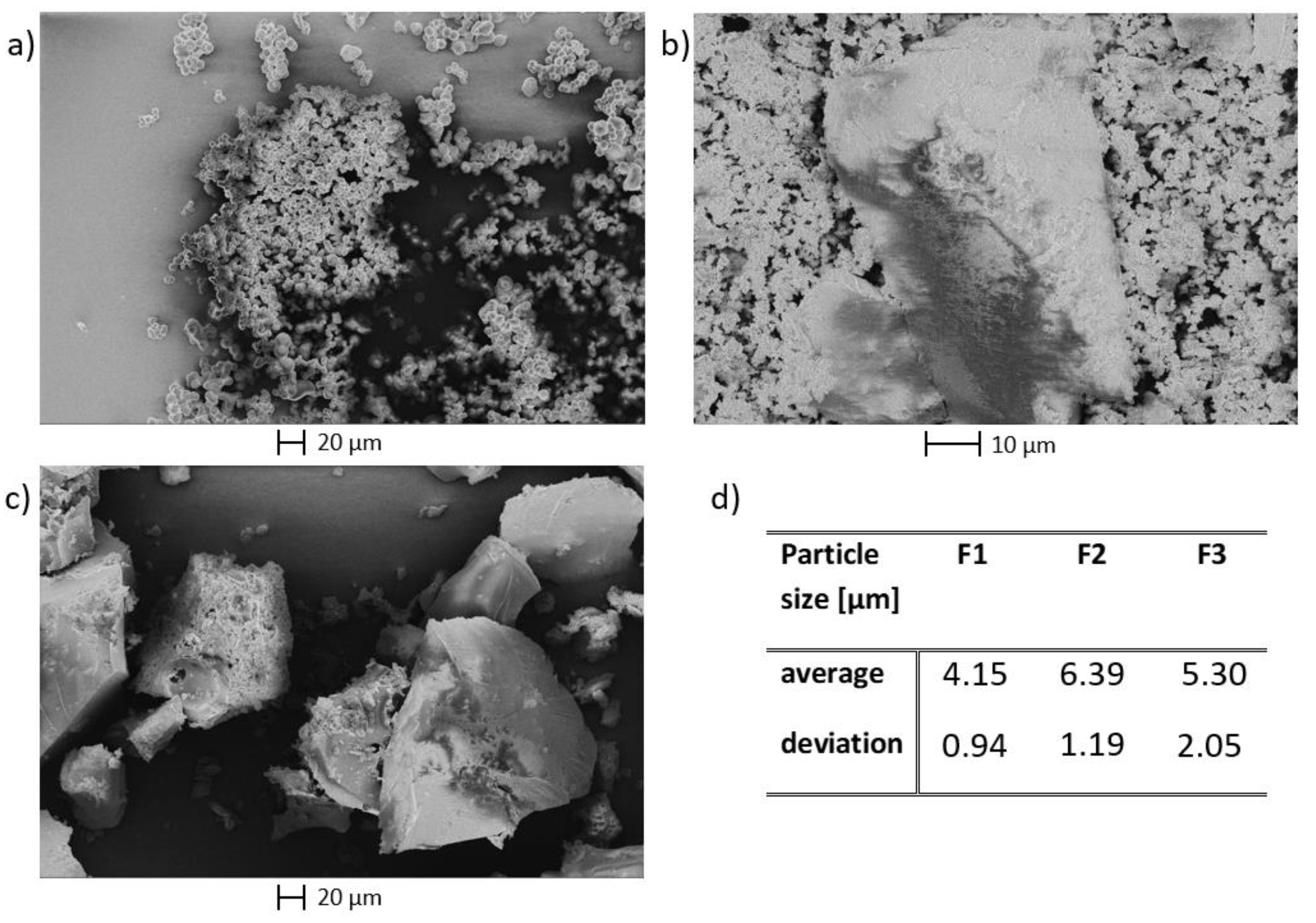

| Humin | H2O | EtOH/H2O | DMSO/H2O | ||||
|---|---|---|---|---|---|---|---|
| Designation | H2SO4 | p-TSA | AA | H2SO4 | p-TSA | AA | H2SO4 |
| Fructose | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
| Glucose | G1 | G2 | G3 | G4 | G5 | G6 | G7 |
| Xylose | X1 | X2 | X3 | X4 | X5 | X6 | X7 |
| Sucrose | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
| H2O | EtOH/H2O | DMSO/H2O | |||||
|---|---|---|---|---|---|---|---|
| X (%) | H2SO4 a | p-TSA a | AA a | H2SO4 b | p-TSA b | AA b | H2SO4 b |
| Fructose | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Glucose | 90 | 74 | 69 | 100 | 100 | 100 | 100 |
| Xylose | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sucrose | 90.5 | 94 | 84 | 100 | 100 | 100 | 100 |
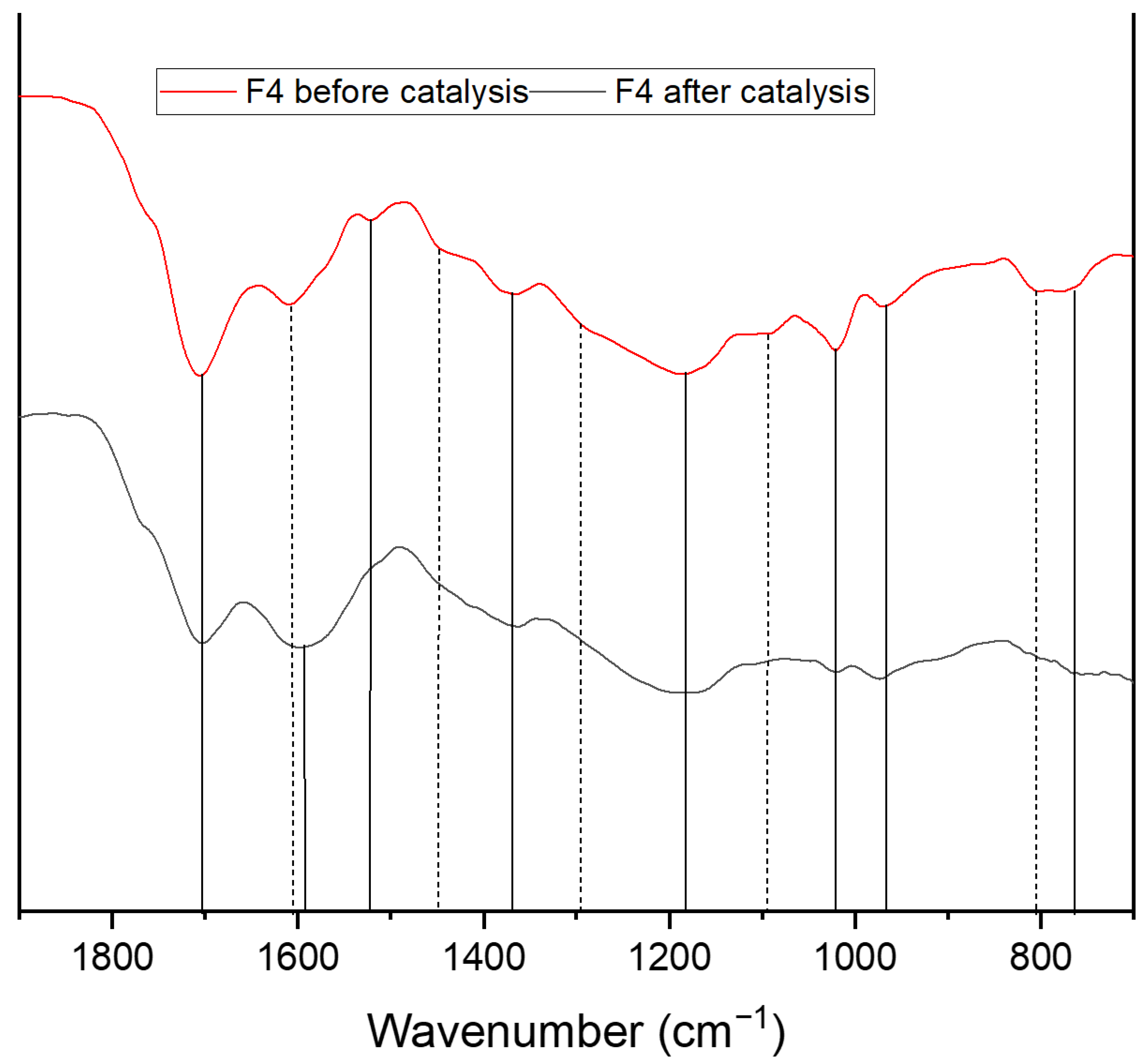
| Wavenumber [cm−1] | Assignment |
| 750 + 795 965 | C-H Out of plane vibration substituted furan ring C-H vibration furan ring |
| 1020 | C=C stretch vibration |
| 1090 | C-O-C ether vibration |
| 1160 + 1200 | C-O-C deformation vibration furan ring |
| 1295 | C-H rocking vibration |
| 1360 | C-C framework vibration (furan) C6 sugars |
| 1395 | C-C framework vibration (furan) C5 sugars |
| 1420 | C=S stretch |
| 1460 | C-H aliphatic chain vibration |
| 1510 | C=C vibration aromatic double bonds of poly substituted furans |
| 1600 | C=C stretch vibration conjugated with carbonyl |
| 1670 | C=O carbyonyl, aldehyde vibrations |
| 1700 | C=O stretch of acids, aldehydes and ketons |
| 1775 | C-S Thioester |
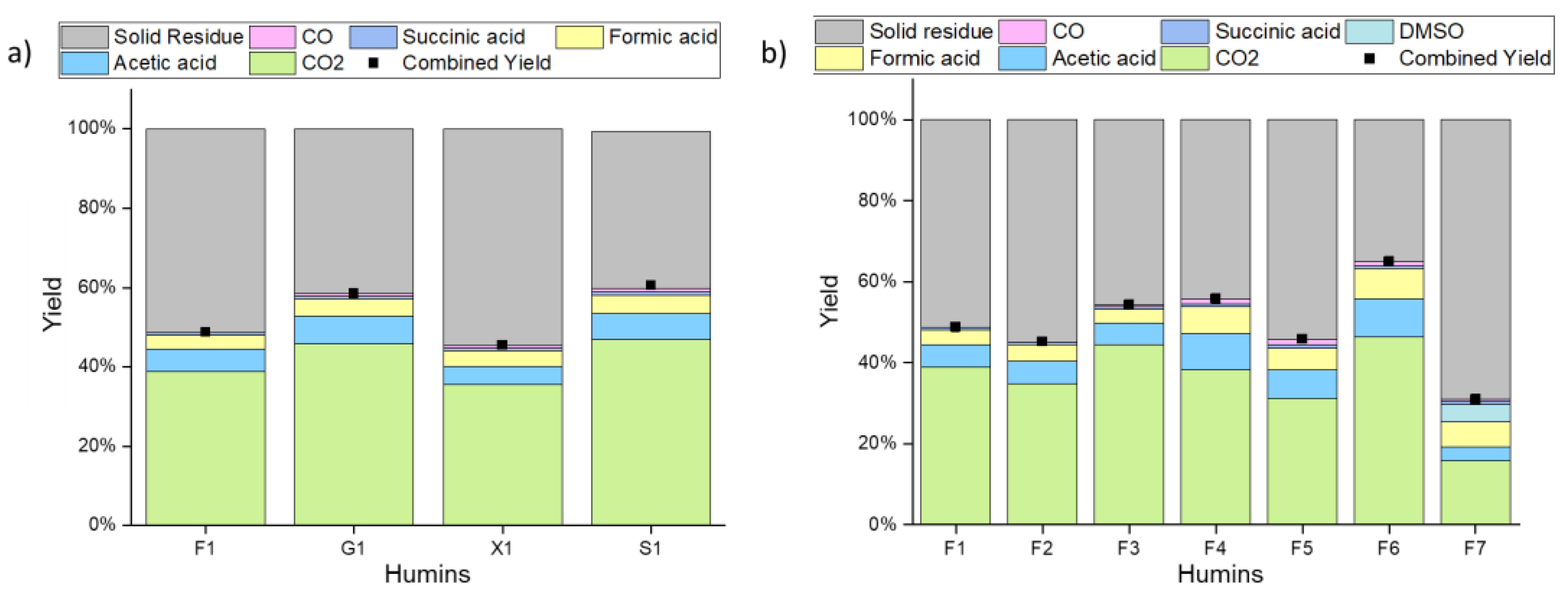
| (a) Product Yields | F1 | G1 | X1 | S1 |
|---|---|---|---|---|
| CO2 | 39% | 46% | 36% | 47% |
| Acetic acid | 6% | 7% | 5% | 7% |
| Formic acid | 4% | 4% | 4% | 5% |
| Succinic acid | 1% | 1% | 1% | 1% |
| Combined Yield | 49% | 59% | 45% | 61% |
| (a) Product Yields | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| CO2 | 39% | 35% | 44% | 38% | 31% | 46% | 16% |
| Acetic acid | 6% | 6% | 5% | 9% | 7% | 9% | 3% |
| Formic acid | 4% | 4% | 3% | 7% | 5% | 7% | 6% |
| Succinic acid | 1% | 1% | 1% | 1% | 1% | 1% | 1% |
| Combined Yield | 52% | 61% | 65% | 74% | 53% | 82% | 73% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassenberg, A.; Esser, T.; Poller, M.J.; Albert, J. Investigation of the Formation, Characterization, and Oxidative Catalytic Valorization of Humins. Materials 2023, 16, 2864. https://doi.org/10.3390/ma16072864
Wassenberg A, Esser T, Poller MJ, Albert J. Investigation of the Formation, Characterization, and Oxidative Catalytic Valorization of Humins. Materials. 2023; 16(7):2864. https://doi.org/10.3390/ma16072864
Chicago/Turabian StyleWassenberg, André, Tobias Esser, Maximilian J. Poller, and Jakob Albert. 2023. "Investigation of the Formation, Characterization, and Oxidative Catalytic Valorization of Humins" Materials 16, no. 7: 2864. https://doi.org/10.3390/ma16072864
APA StyleWassenberg, A., Esser, T., Poller, M. J., & Albert, J. (2023). Investigation of the Formation, Characterization, and Oxidative Catalytic Valorization of Humins. Materials, 16(7), 2864. https://doi.org/10.3390/ma16072864





