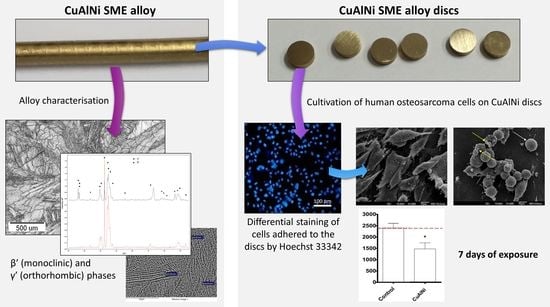
In Vitro Evaluation of the Potential Anticancer Properties of Cu-Based Shape Memory Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Determination of the Chemical Composition and Microstructure/Phase Characterisation
2.3. Hardness Measurements
- (a)
- For the hardness measurement, a WPN HPO 250 machine (Leipzig, Germany)was used, which applied the nominal value of a 49.03 N test force load for 5 s.
- (b)
- For the microhardness measurement, a Zwick-Roell ZHV10 hardness tester (Kennesaw, GA, USA) was used, and the force applied was 0.49 N for 5 s indentation time.
2.4. Biocompatibility
2.4.1. Cell Culture (MG-63 Osteosarcoma Cells)
2.4.2. Cell Adhesion and Viability of MG-63 Cells
2.4.3. Preparation of the Samples for SEM Observation
2.5. Statistical Analysis
3. Results
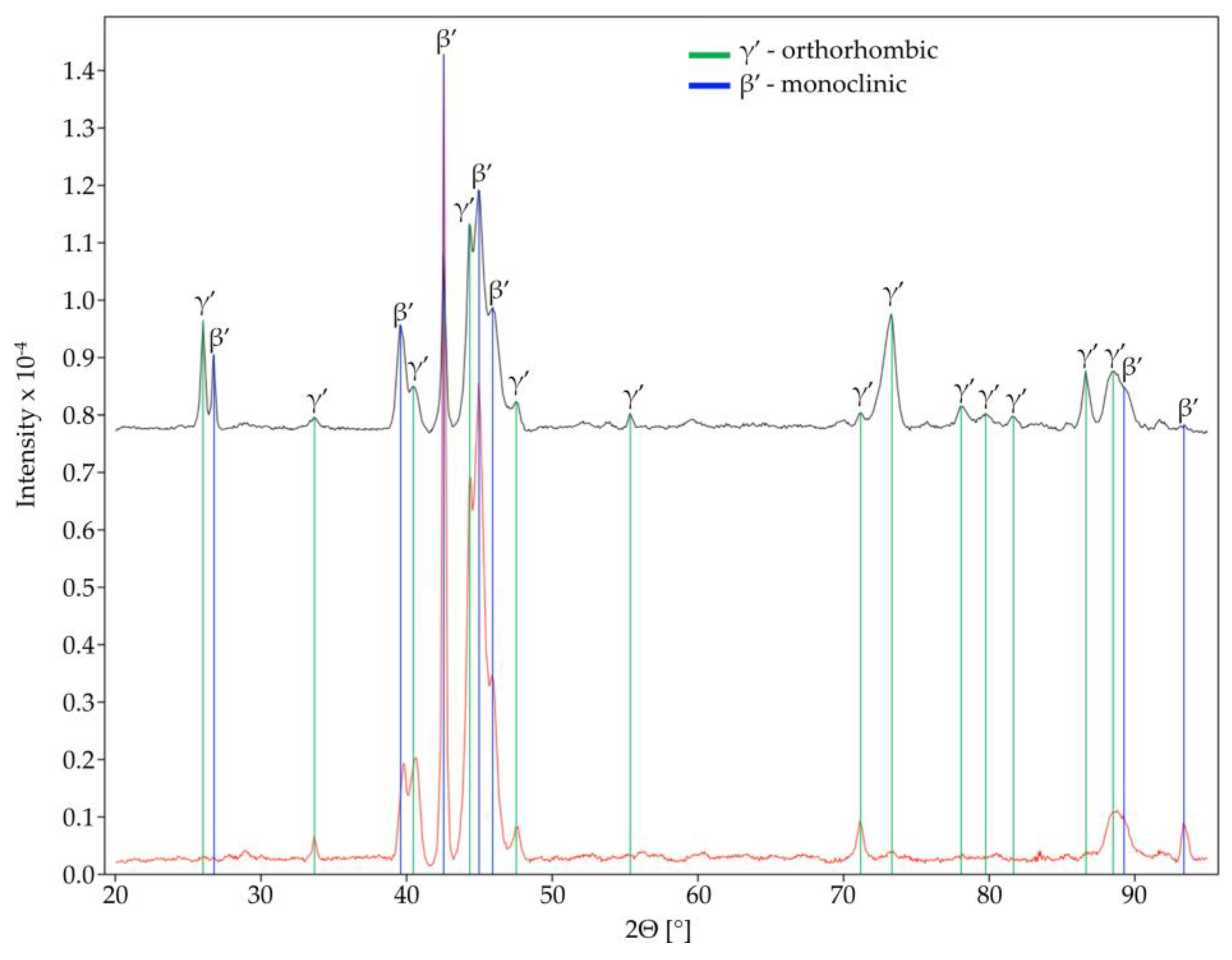
3.1. Chemical Composition and Microstructure
3.2. Hardness and Microhardness
3.3. Biocompatibility
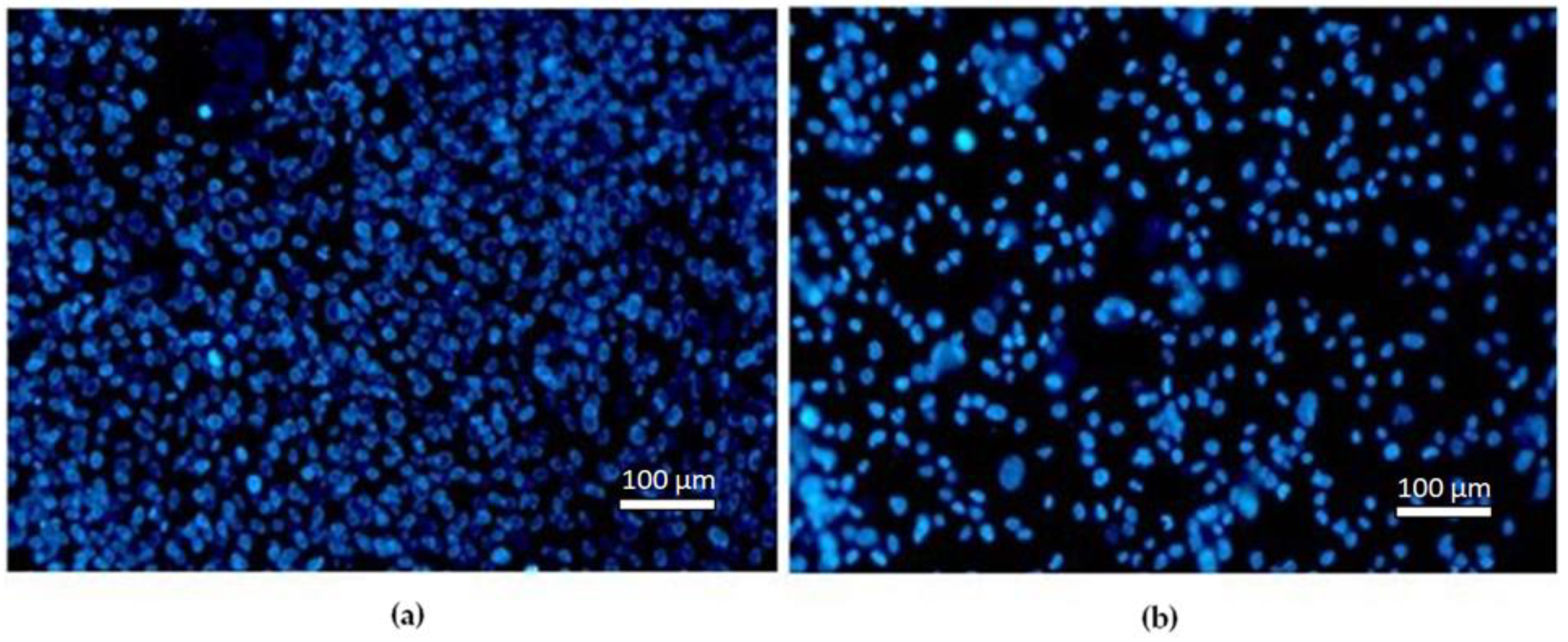
3.3.1. Fluorescence Microscopy
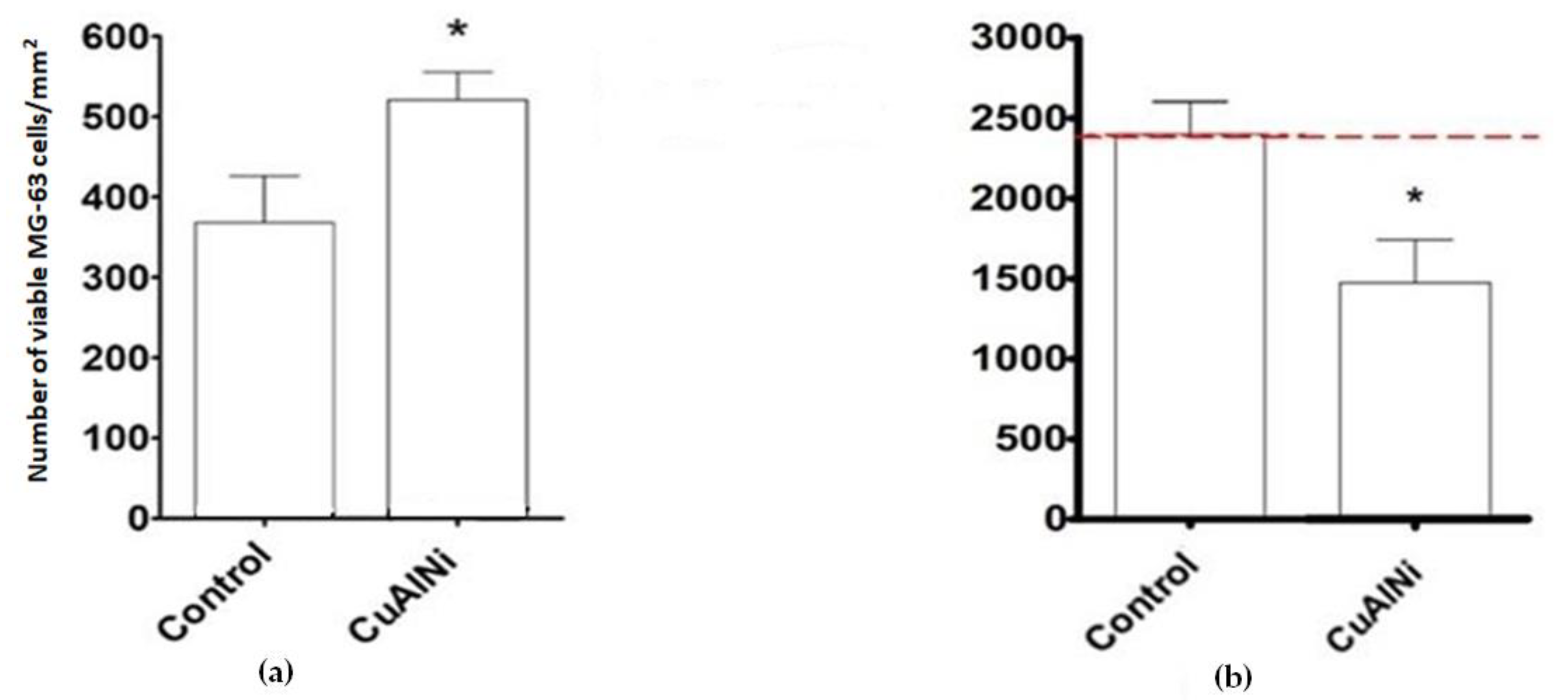
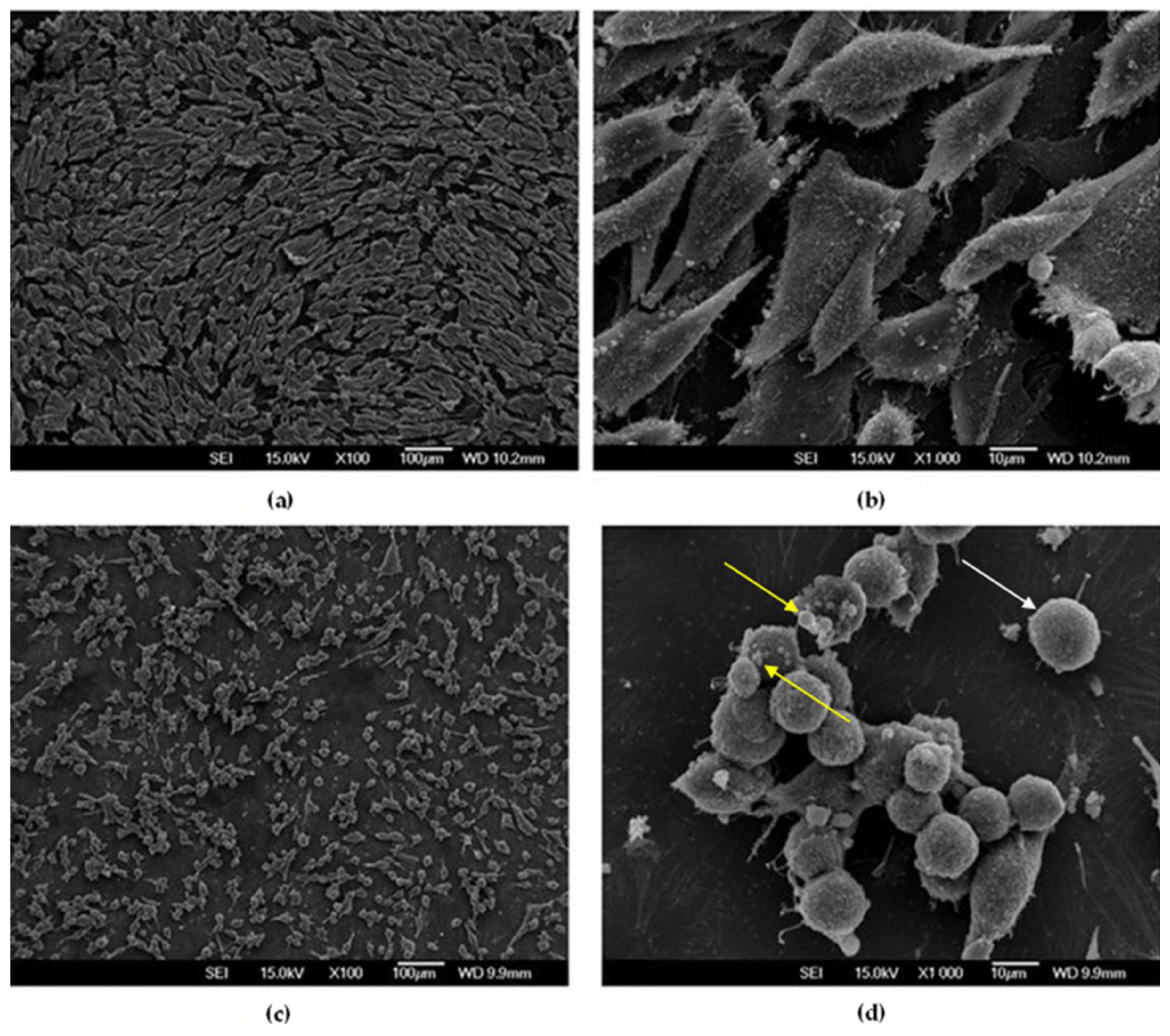
3.3.2. SEM Observations of MG-63 Cells after 24 h
3.3.3. SEM Observations of MG-63 Cells after 7 Days
4. Discussion
5. Conclusions
- The microstructure is homogeneous, with the presence of martensite lamellae 50 µm long, grain size G = 3, and the average grain size was estimated between 62.5–125 µm. This represents a coarse-grained microstructure of the Cu-Al-Ni disc.
- The chemical composition of the discs, determined by EDX analyses, differed minimally from the chemical composition obtained by XRF analysis, where an Fe content (0.03 wt.) was detected additionally. The content of Fe was negligible, and did not affect the other properties significantly.
- The XRD structural analysis showed the presence of phases: β′ (monoclinic) and γ′ (orthorhombic).
- The hardness and microhardness of HV had comparable values and were in the range of 229–290.
- Cu-Al-Ni discs can achieve an anti-neoplastic effect in the selected environment, which is clearly visible after 7 days on the SEM micrographs. The number of viable cells decreased sharply, and, contrary to cell behaviour after 24 h of culture, the morphological characteristics of the osteosarcoma cells after 7 days pointed to a high level of apoptosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABAM | Antibiotic-Antimycotic |
| BioMEMS | Biomedical Micro-electro-mechanical systems |
| Cu-Al-Ni | Copper-Aluminium-Nickel |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EDM | Electrical discharge machining |
| EDX | Energy Dispersive X-ray Analysis |
| FBS | Foetal Bovine Serum |
| HV | Hardness according to Vickers |
| PBS | Phosphate Buffered Saline |
| SEM | Scanning Electron Microscope |
| SMAs | Shape Memory Alloys |
| SME | Shape Memory Effect |
| TWSME | Two-Way Shape Memory Effect |
| XRD | X-ray Diffraction |
| XRF | X-ray Fluorescence |
References
- Kaya, E.; Kaya, İ. A Review on Machining of NiTi Shape Memory Alloys: The Process and Post Process Perspective. Int. J. Adv. Manuf. Technol. 2019, 100, 2045–2087. [Google Scholar] [CrossRef]
- Kneissl, A.C.; Unterweger, E.; Lojen, G.; Anzel, I. Microstructure and Properties of Shape Memory Alloys. Microsc. Microanal. 2005, 11, 1704–1705. [Google Scholar] [CrossRef]
- Lobo, P.S.; Almeida, J.; Guerreiro, L. Shape Memory Alloys Behaviour: A Review. Procedia Eng. 2015, 114, 776–783. [Google Scholar] [CrossRef]
- Lexcellent, C.; Leclercq, S.; Gabry, B.; Bourbon, G. The Two Way Shape Memory Effect of Shape Memory Alloys: An Experimental Study and a Phenomenological Model. Int. J. Plast. 2000, 16, 1155–1168. [Google Scholar] [CrossRef]
- Agrawal, A.; Dube, R.K. Methods of Fabricating Cu-Al-Ni Shape Memory Alloys. J. Alloys Compd. 2018, 750, 235–247. [Google Scholar] [CrossRef]
- Gustmann, T.; Neves, A.; Kühn, U.; Gargarella, P.; Kiminami, C.S.; Bolfarini, C.; Eckert, J.; Pauly, S. Influence of Processing Parameters on the Fabrication of a Cu-Al-Ni-Mn Shape-Memory Alloy by Selective Laser Melting. Addit. Manuf. 2016, 11, 23–31. [Google Scholar] [CrossRef]
- Peltier, L.; Perroud, O.; Moll, P.; Slowensky, J.; Charbonnier, P.; Eberhardt, A.; Hautcoeur, A. Production and Mechanical Properties of Cu-Al-Ni-Be Shape Memory Alloy Thin Ribbons Using a Cold Co-Rolled Process. Shape Mem. Superelast. 2021, 7, 344–352. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kim, H.S.; Chung, E.K. Effect of Continuous Casting Parameters on Microstructure and Texture of Gold Bonding Wire for Semiconductor Packaging. Adv. Mater. Res. 2007, 26–28, 589–592. [Google Scholar] [CrossRef]
- Strzępek, P.; Mamala, A.; Zasadzińska, M.; Noga, P.; Sadzikowski, M. The Influence of the Continuous Casting Conditions on the Properties of High-Strength Two-Phase CuMg Alloys. Materials 2020, 13, 4805. [Google Scholar] [CrossRef]
- Vynnycky, M. Continuous Casting. Metals 2019, 9, 643. [Google Scholar] [CrossRef]
- Molinaro, C.; Martoriati, A.; Pelinski, L.; Cailliau, K. Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.B.; Lu, F.; Fisch, M.; Beumer, J.H.; Guo, J.; Watters, R.J.; Weiss, K.R. Combination Therapy with Disulfiram, Copper, and Doxorubicin for Osteosarcoma: In Vitro Support for a Novel Drug Repurposing Strategy. Sarcoma 2019, 2019, 1320201. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Nasulewicz, A.; Mazur, A.; Opolski, A. Role of Copper in Tumour Angiogenesis—Clinical Implications. J. Trace Elem. Med. Biol. 2004, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lojen, G.; Stambolić, A.; Šetina Batič, B.; Rudolf, R. Experimental Continuous Casting of Nitinol. Metals 2020, 10, 505. [Google Scholar] [CrossRef]
- Mitchell, M.R.; Link, R.E.; Filho, P.P.R.; da Cavalcante, T.S.; de Albuquerque, V.H.C.; Tavares, J.M.R.S. Brinell and Vickers Hardness Measurement Using Image Processing and Analysis Techniques. J. Test. Eval. 2010, 38, 102220. [Google Scholar] [CrossRef]
- Hočevar, M.; Šetina Batič, B.; Godec, M.; Kononenko, V.; Drobne, D.; Gregorčič, P. The Interaction between the Osteosarcoma Cell and Stainless Steel Surface, Modified by High-Fluence, Nanosecond Laser Pulses. Surf. Coat. Technol. 2020, 394, 125878. [Google Scholar] [CrossRef]
- Ziółkowski, A. Pseudoelasticity of Shape Memory Alloys; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128016978. [Google Scholar]
- Svirid, A.E.; Pushin, V.G.; Kuranova, N.N.; Makarov, V.V.; Ustyugov, Y.M. Structural and Phase Transformations and Physical and Mechanical Properties of Cu-Al-Ni Shape Memory Alloys Subjected to Severe Plastic Deformation and Annealing. Materials 2021, 14, 4394. [Google Scholar] [CrossRef]
- Čolić, M.; Rudolf, R.; Stamenković, D.; Anžel, I.; Vučević, D.; Jenko, M.; Lazić, V.; Lojen, G. Relationship between Microstructure, Cytotoxicity and Corrosion Properties of a Cu–Al–Ni Shape Memory Alloy. Acta Biomater. 2010, 6, 308–317. [Google Scholar] [CrossRef]
- Tomić, S.Z. Biocompatibility and Immunomodulatory Properties of Nanomaterials Based on Gold Nanoparticles and Carbon Nanotubes, and NITi-and CuAINi Alloy-Based Advanced Biomaterials. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2016. Available online: https://nardus.mpn.gov.rs/handle/123456789/6331 (accessed on 24 March 2023).
- Todorović, A.; Rudolf, R.; Romčević, N.; Đorđević, I.; Milošević, N.; Trifković, B.; Veselinović, V.; Čolić, M. Biocompatibility Evaluation of Cu-Al-Ni Shape Memory Alloys. Contemp. Mater. 2014, 5, 228–238. [Google Scholar] [CrossRef]
- Kneissl, A.C.; Unterweger, E.; Bruncko, M.; Lojen, G.; Mehrabi, K.; Scherngell, H. Microstructure and Properties of NiTi and CuAlNi Shape Memory Alloys. Metalurgija 2008, 14, 89–100. [Google Scholar]
- Jayachandran, S.; Akash, K.; Mani Prabu, S.S.; Manikandan, M.; Muralidharan, M.; Brolin, A.; Palani, I.A. Investigations on Performance Viability of NiTi, NiTiCu, CuAlNi and CuAlNiMn Shape Memory Alloy/Kapton Composite Thin Film for Actuator Application. Compos. Part B Eng. 2019, 176, 107182. [Google Scholar] [CrossRef]
- Mohd Ghazali, F.A.; Hasan, M.N.; Rehman, T.; Nafea, M.; Mohamed Ali, M.S.; Takahata, K. MEMS Actuators for Biomedical Applications: A Review. J. Micromech. Microeng. 2020, 30, 073001. [Google Scholar] [CrossRef]
- Gopinath, S.; Mathew, S.; Nair, P.R. Shape Memory Actuators. In Actuators; Wiley: Hoboken, NJ, USA, 2020; pp. 139–158. ISBN 9781119662693. [Google Scholar]
- Payandeh, Y.; Mirzakhani, B.; Bakhtiari, Z.; Hautcoeur, A. Precipitation and Martensitic Transformation in Polycrystalline CuAlNi Shape Memory Alloy—Effect of Short Heat Treatment. J. Alloys Compd. 2022, 891, 162046. [Google Scholar] [CrossRef]
- Sarı, U.; Aksoy, İ. Electron Microscopy Study of 2H and 18R Martensites in Cu–11.92wt% Al–3.78wt% Ni Shape Memory Alloy. J. Alloys Compd. 2006, 417, 138–142. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q. Cu-Al-Ni-V High-Temperature Shape Memory Alloys. Intermetallics 2018, 92, 108–112. [Google Scholar] [CrossRef]
- Gao, J.J.; Thibon, I.; Castany, P.; Gloriant, T. Effect of Grain Size on the Recovery Strain in a New Ti–20Zr–12Nb–2Sn Superelastic Alloy. Mater. Sci. Eng. A 2020, 793, 139878. [Google Scholar] [CrossRef]
- Sampath, V. Improvement of Shape-Memory Characteristics and Mechanical Properties of Copper–Zinc–Aluminum Shape-Memory Alloy with Low Aluminum Content by Grain Refinement. Mater. Manuf. Process. 2006, 21, 789–795. [Google Scholar] [CrossRef]
- Montecinos, S.; Cuniberti, A.; Sepúlveda, A. Grain Size and Pseudoelastic Behaviour of a Cu–Al–Be Alloy. Mater. Charact. 2008, 59, 117–123. [Google Scholar] [CrossRef]
- Aydogdu, A.; Aydogdu, Y.; Adigüzel, O. Improvement of Hardness and Microstructures by Ageing in Shape Memory CuAlNi Alloys. J. Phys. IV 1997, 07, C5-311–C5-316. [Google Scholar] [CrossRef]
- Rajeshkumar, R.; Vigraman, T. Formation of Cu-Al-Ni Shape Memory Alloy with the Addition of Alloying Elements Mn and Si. Int. J. Sci. Eng. Res. 2014, 5, 179–182. [Google Scholar]
- Lapér, M.L.; Guimarães, R.; Barrioni, B.R.; Silva, P.A.P.; Houmard, M.; Mazzer, E.M.; Nunes, E.H.M. Fabrication of Porous Samples from a High-Temperature Cu–Al–Ni–Mn–Nb Shape Memory Alloy by Freeze-Drying and Partial Sintering. J. Mater. Res. Technol. 2020, 9, 3676–3685. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Koliha, N. Analysis of the MicroRNA Profile and Origin of Exosomes in Plasma of Melanoma Patients and Healthy Individuals. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2016. [Google Scholar]
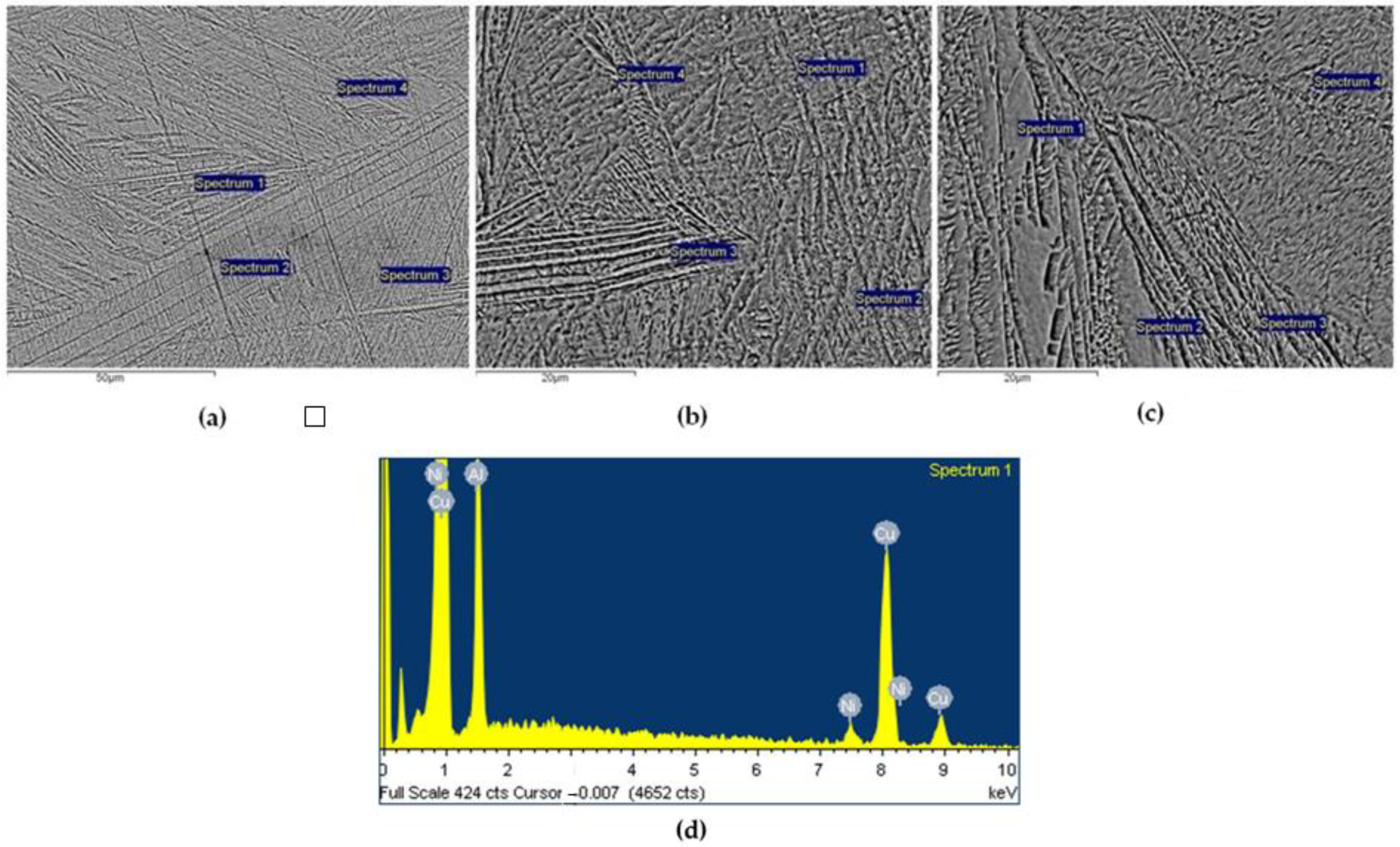


| Cu | Al | Ni | Fe | |
|---|---|---|---|---|
| Mean value (in wt.%) | Base | 12 | 3.9 | 0.03 |
| Measuring Segment | 1 | 2 | 3 | |
|---|---|---|---|---|
| Mean value | Cu | 83.71 ± 0.30 | 83.81 ± 0.29 | 84.42 ± 0.67 |
| in wt.% | Al | 12.14 ± 0.32 | 12.20 ± 1.06 | 12.01 ± 0.84 |
| ±SD | Ni | 4.15 ± 0.59 | 3.99 ± 0.91 | 3.58 ± 0.52 |
| Grain Number G | Number of Grains per mm2 | Mean Number of Intersections mm |
|---|---|---|
| 3 | 64 | 0.113 |
| Mean ± SD | Min | Max | N | |
|---|---|---|---|---|
| Hardness HV5 | 253 ± 12.43 | 229 | 266 | 8 |
| Microhardness HV0.05 | 280 ± 19.50 | 260 | 299 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazić, M.M.; Lazić, M.; Milašin, J.; Popović, D.; Majerič, P.; Rudolf, R. In Vitro Evaluation of the Potential Anticancer Properties of Cu-Based Shape Memory Alloys. Materials 2023, 16, 2851. https://doi.org/10.3390/ma16072851
Lazić MM, Lazić M, Milašin J, Popović D, Majerič P, Rudolf R. In Vitro Evaluation of the Potential Anticancer Properties of Cu-Based Shape Memory Alloys. Materials. 2023; 16(7):2851. https://doi.org/10.3390/ma16072851
Chicago/Turabian StyleLazić, Minja Miličić, Marko Lazić, Jelena Milašin, Danica Popović, Peter Majerič, and Rebeka Rudolf. 2023. "In Vitro Evaluation of the Potential Anticancer Properties of Cu-Based Shape Memory Alloys" Materials 16, no. 7: 2851. https://doi.org/10.3390/ma16072851
APA StyleLazić, M. M., Lazić, M., Milašin, J., Popović, D., Majerič, P., & Rudolf, R. (2023). In Vitro Evaluation of the Potential Anticancer Properties of Cu-Based Shape Memory Alloys. Materials, 16(7), 2851. https://doi.org/10.3390/ma16072851