Durability and Thermal Behavior of Functional Paints Formulated with Recycled-Glass Hollow Microspheres of Different Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Production
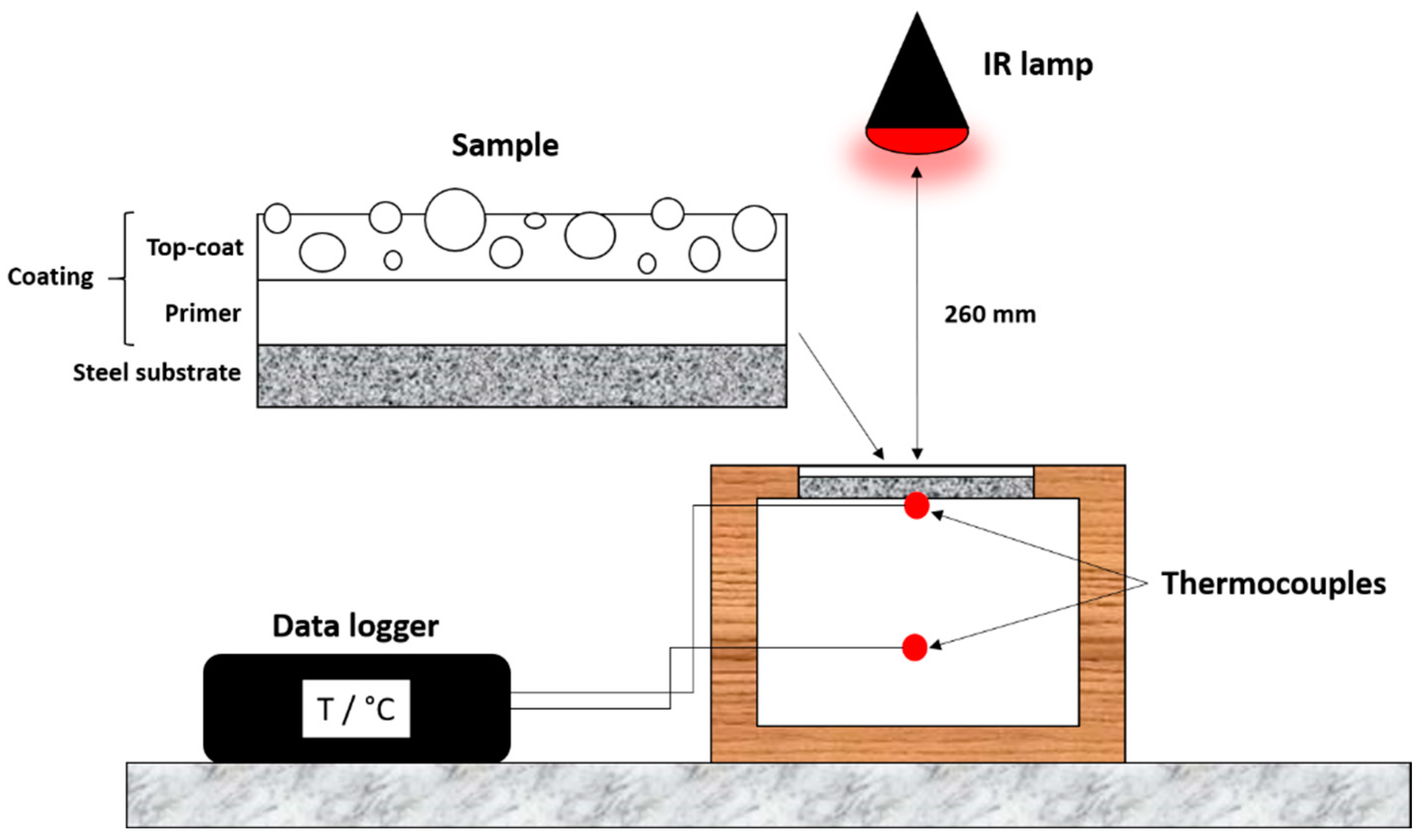
2.3. Characterization
- 72 h of exposure to UV and water in accordance with ISO 11507:1997 [50], alternating periods of 4 h exposure to UV-A (340 nm) at (60 ± 3) °C and 4 h exposure to condensation at (50 ± 3) °C, employing a UV173 Box Co.Fo.Me.Gra (Co.Fo.Me.Gra, Milan, Italy);
- 72 h of exposure to salt spray in accordance with ISO 7253:1996 [51], using a salt spray chamber (Ascott Analytical Equipment Limited, Tamworth, UK);
- 24 h at room temperature.
3. Results and Discussion
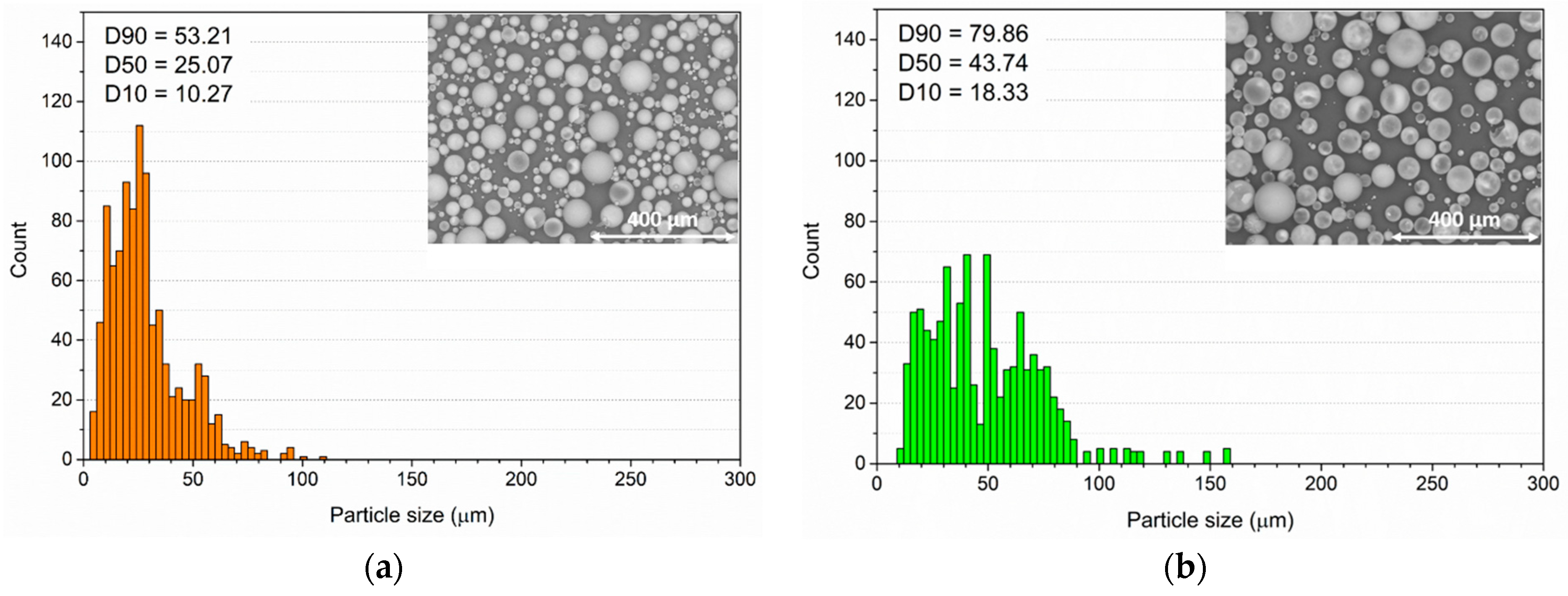
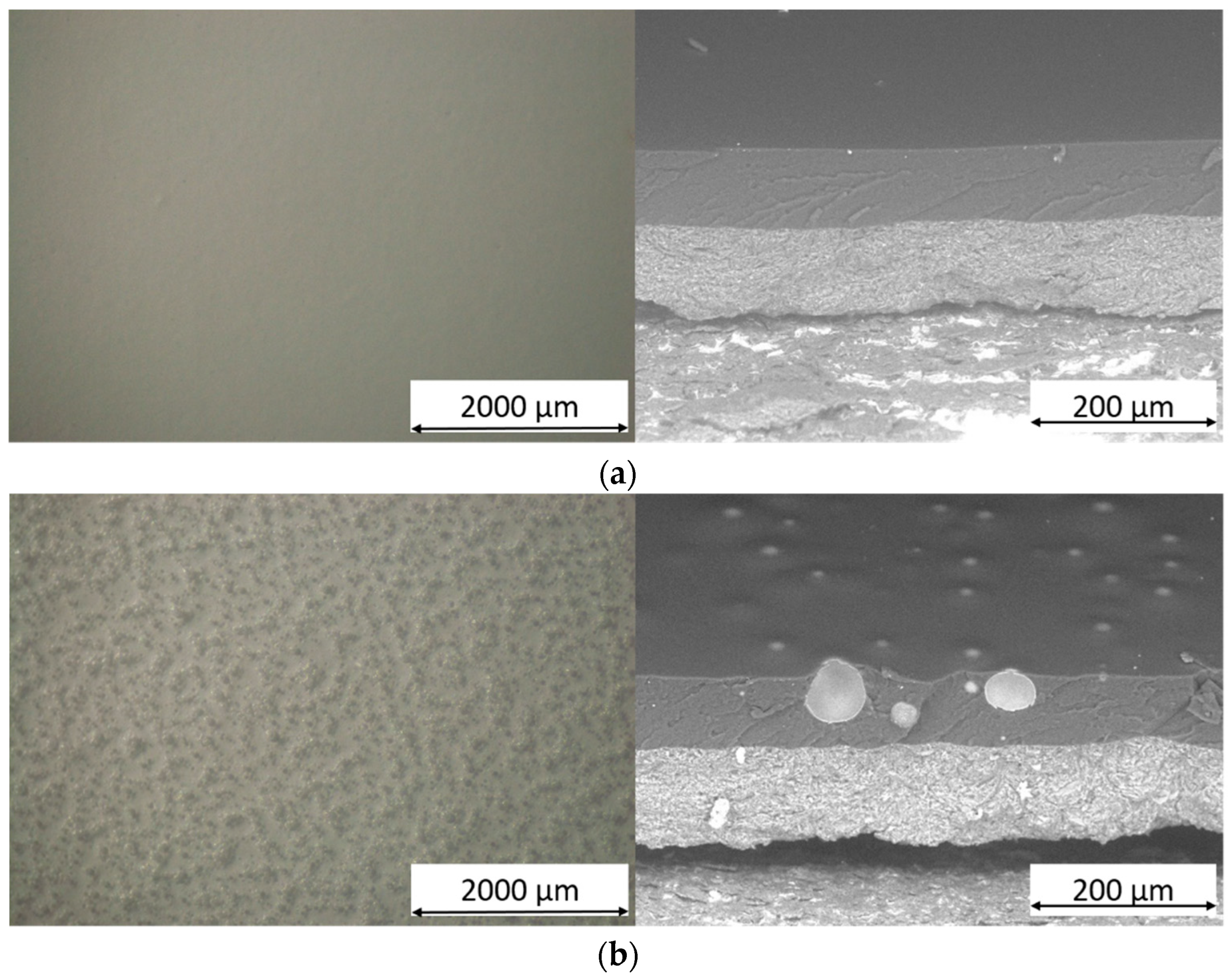
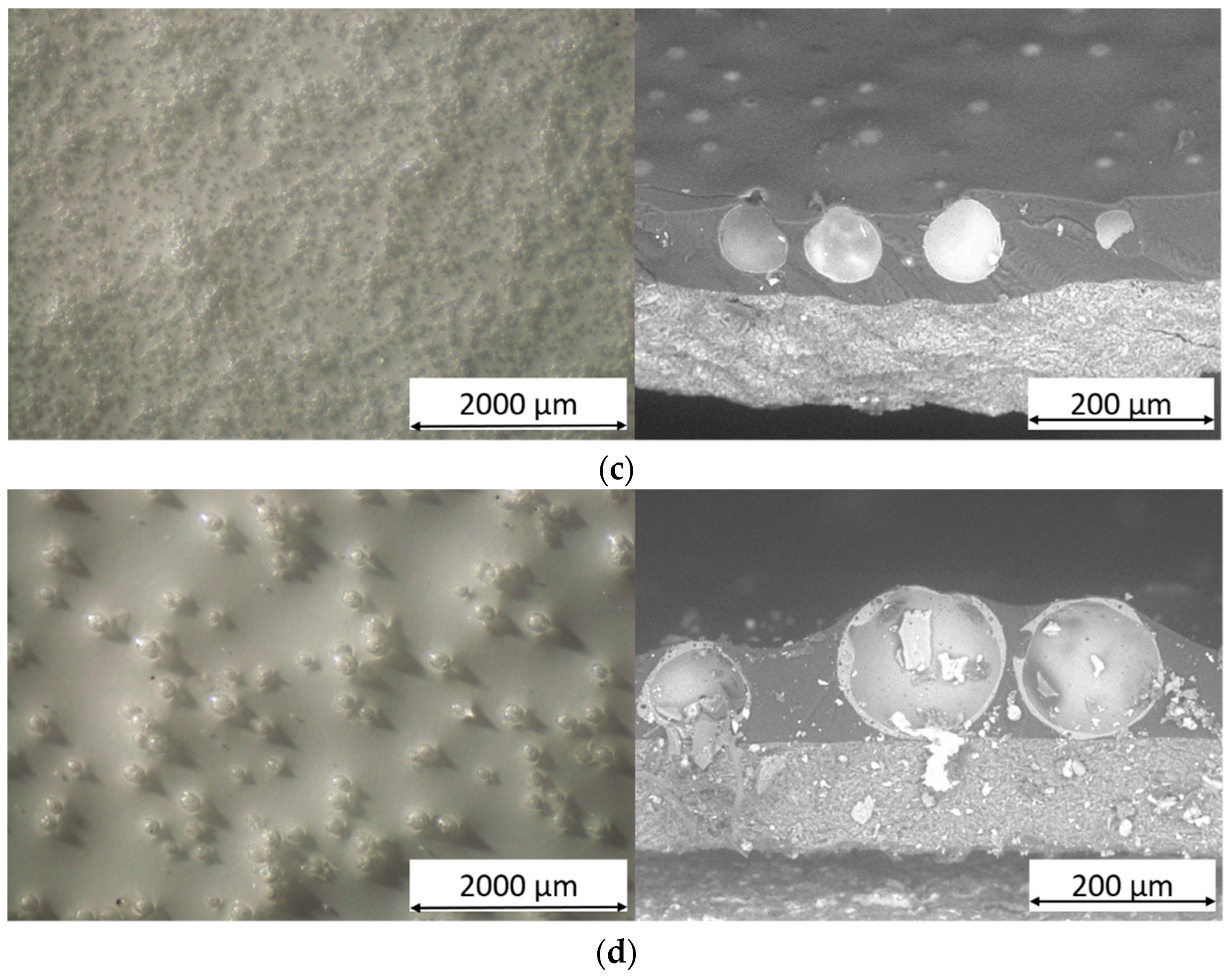
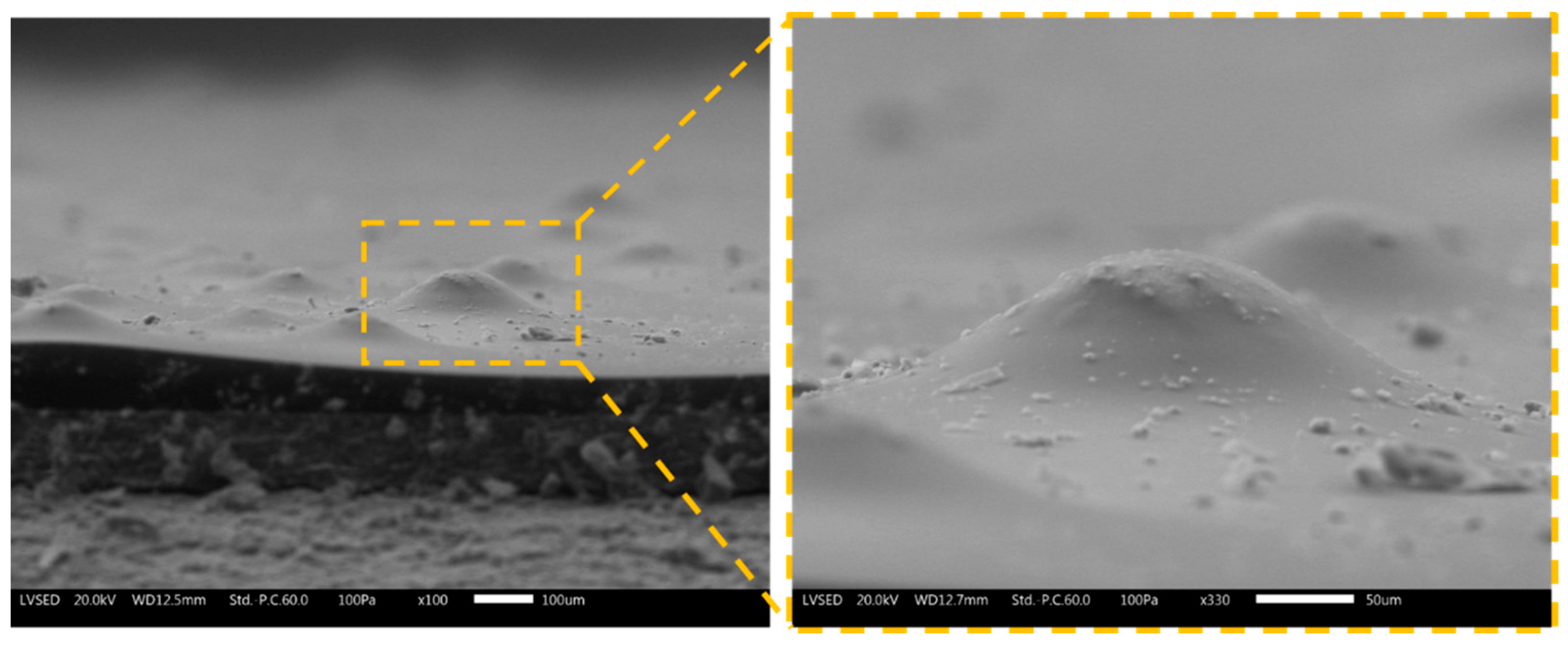
3.1. Powders and Coatings Morphology
3.2. Effect of the Glass Spheres on Paint Mechanical Features
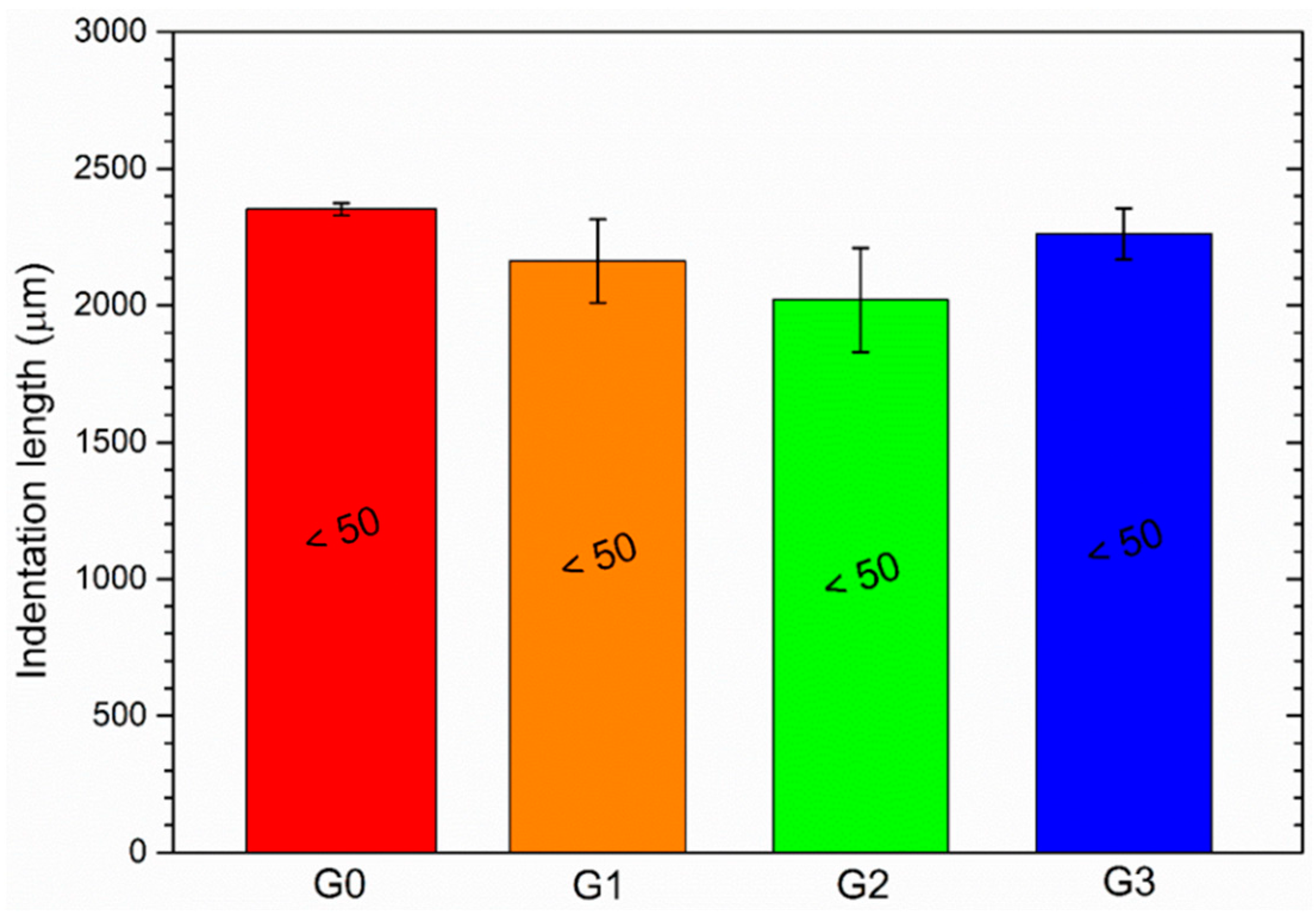
3.2.1. Buchholz Hardness
3.2.2. Scrub Test
3.3. Effect of the Glass Spheres on Paint Durability
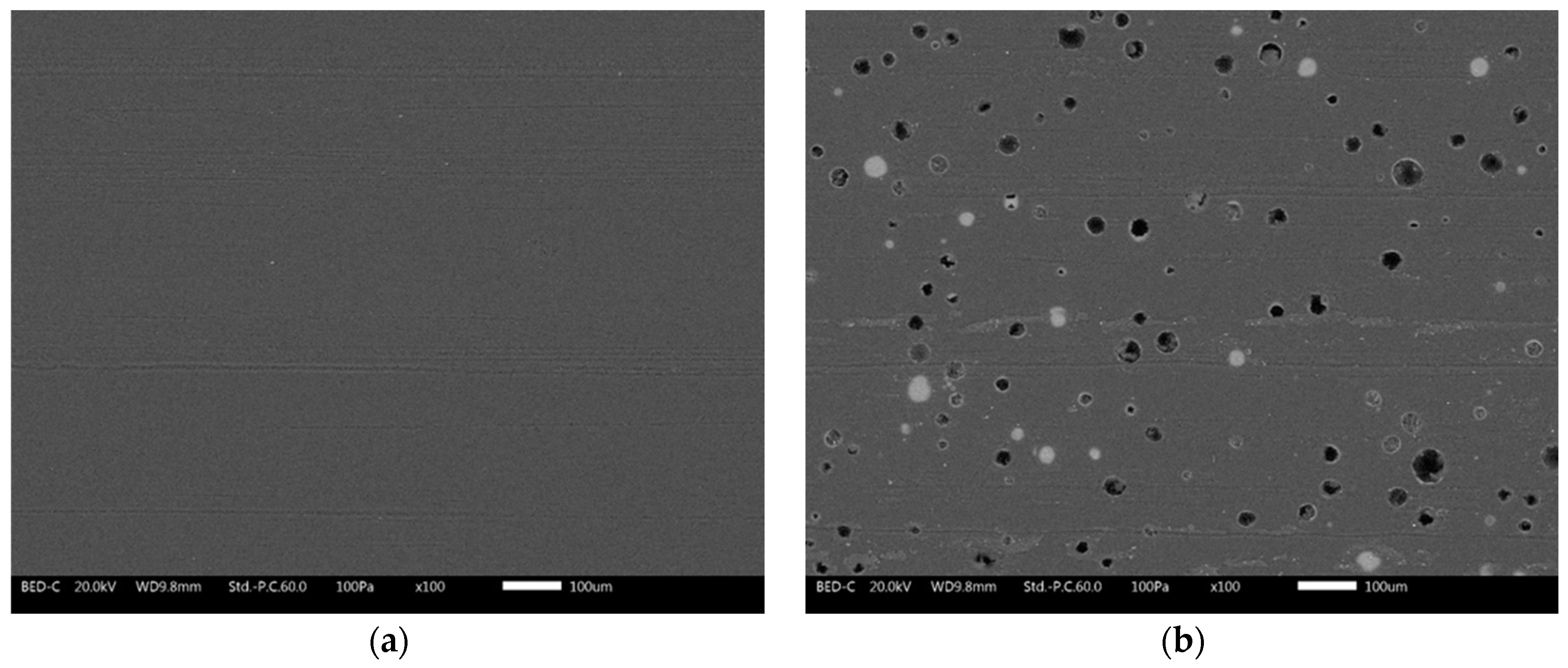
3.3.1. Salt Spray Test
3.3.2. Electrochemical Impedance Spectroscopy Measurements
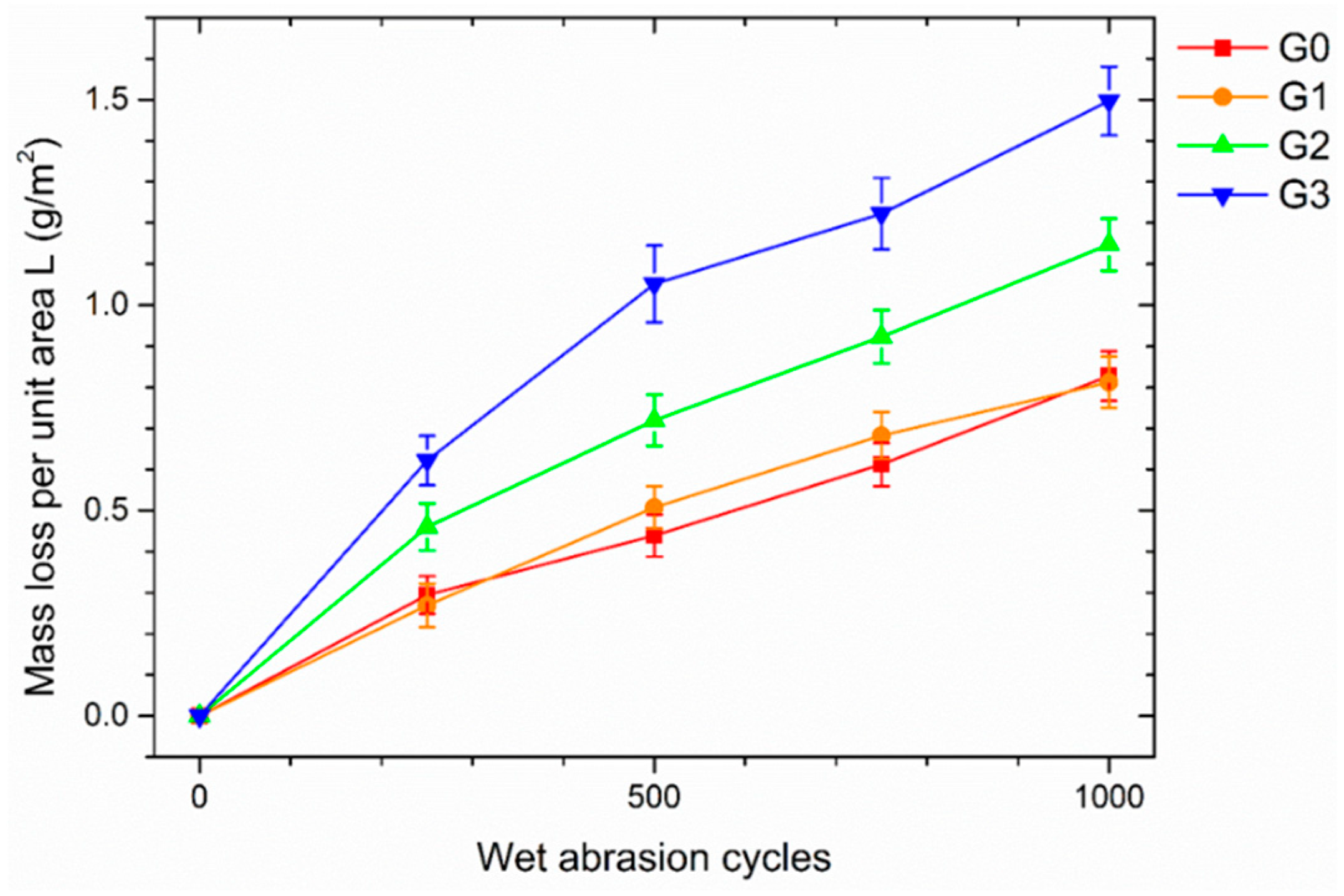
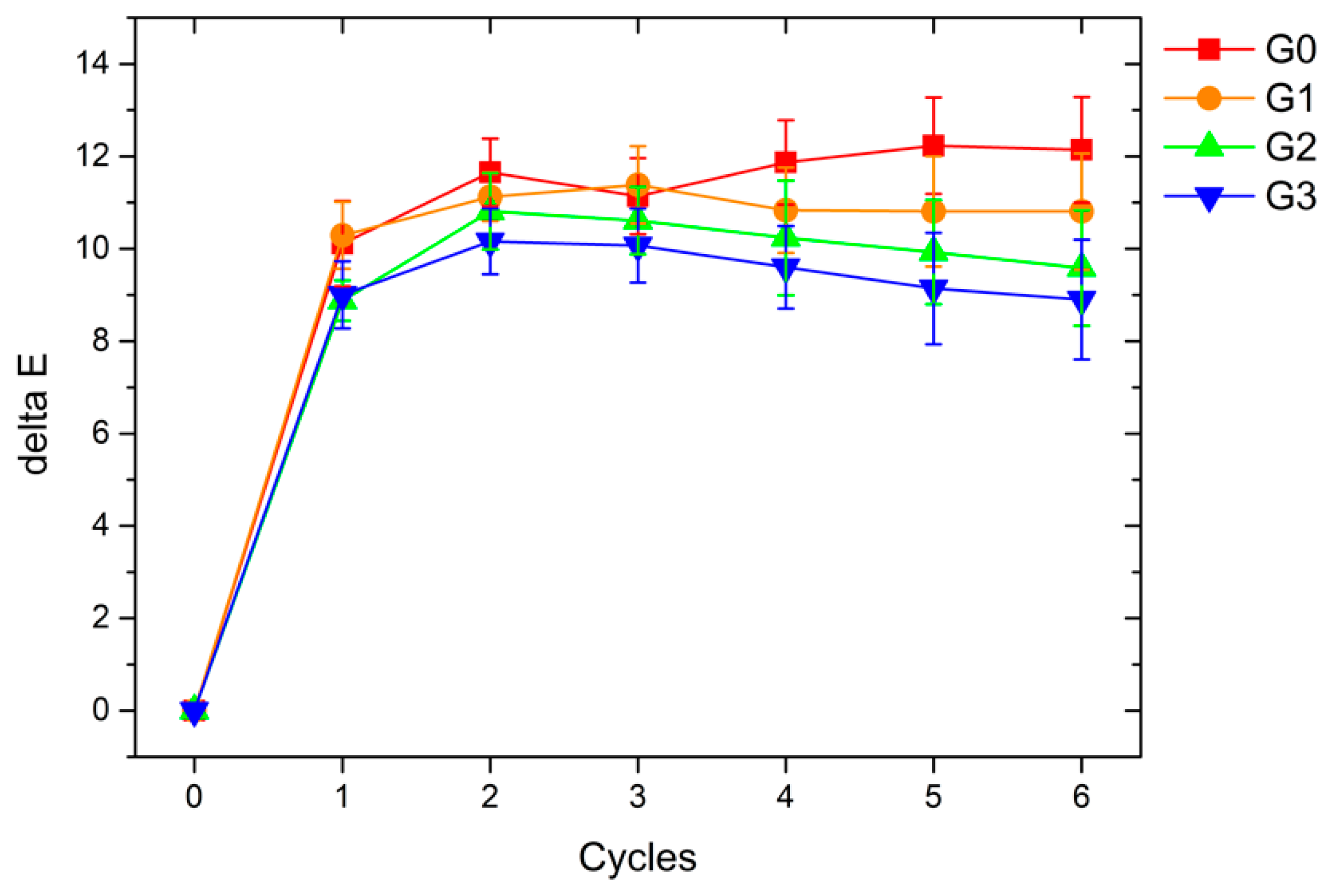
3.3.3. Accelerated Degradation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Ramírez, L.C.; Cano, M.; de Villoria, R.G. Low thermal and high electrical conductivity in hollow glass microspheres covered with carbon nanofiber–polymer composites. Compos. Sci. Technol. 2017, 151, 211–218. [Google Scholar] [CrossRef]
- Hou, J.; Shi, Y.; Li, Z.; Wu, J.; Gong, Y.; Zou, W.; Zuo, H.; Ning, D. Numerical simulation and experimental study on flexible buoyancy material of hollow glass microsphere and silicone rubber for small deep-sea soft robots. Appl. Mater. Today 2020, 21, 100875. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Zhou, Y.; Li, X.; Yang, E.-H.; Yu, T.; Yang, J. The effect of strain rate and filler volume fraction on the mechanical properties of hollow glass microsphere modified polymer. Compos. Part B Eng. 2016, 101, 53–63. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.; Zhang, B.; Ye, F.; Gao, Y. Preparation and characterization of hollow glass microsphere ceramics and silica aerogel/hollow glass microsphere ceramics having low density and low thermal conductivity. J. Alloys Compd. 2020, 831, 154737. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Liu, H.; Xie, D.; Wan, C.; Pan, H.; Jiang, S. Mechanical, thermal and fire performance of an inorganic-organic insulation material composed of hollow glass microspheres and phenolic resin. J. Colloid Interface Sci. 2018, 530, 163–170. [Google Scholar] [CrossRef]
- Wu, B.; Liu, H.; Fu, R.; Song, X.; Su, X.; Liu, X. Epoxy-matrix composite with low dielectric constant and high thermal conductivity fabricated by HGMs/Al2O3 co-continuous skeleton. J. Alloys Compd. 2021, 869, 159332. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, C. Preparation and physical properties of hollow glass microspheres-reinforced epoxy matrix resins. Mater. Sci. Eng. A 2005, 402, 335–340. [Google Scholar] [CrossRef]
- Dalai, S.; Vijayalakshmi, S.; Shrivastava, P.; Sivam, S.P.; Sharma, P. Effect of Co loading on the hydrogen storage characteristics of hollow glass microspheres (HGMs). Int. J. Hydrogen Energy 2014, 39, 3304–3312. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Zhang, Z.; Chen, S.; Li, B.; Wei, S. Production and characterization of hollow glass microspheres with high diffusivity for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 1518–1530. [Google Scholar] [CrossRef]
- Xu, N.; Dai, J.; Zhu, Z.; Huang, X.; Wu, P. Synthesis and characterization of hollow glass–ceramics microspheres. Compos. Sci. Technol. 2012, 72, 528–532. [Google Scholar] [CrossRef]
- Ren, S.; Tao, X.; Ma, X.; Liu, J.; Du, H.; Guo, A.; Xu, J.; Liang, J.; Chen, S.; Ge, J. Fabrication of fly ash cenospheres-hollow glass microspheres/borosilicate glass composites for high temperature application. Ceram. Int. 2018, 44, 1147–1155. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Gui, B.; Zhai, T.; Yang, D.a. Fabrication and properties of thermal insulating material using hollow glass microspheres bonded by aluminum–chrome–phosphate and tetraethyl orthosilicate. Ceram. Int. 2016, 42, 4886–4892. [Google Scholar] [CrossRef]
- Long, J.; Jiang, C.; Zhu, J.; Song, Q.; Hu, J. Controlled TiO2 coating on hollow glass microspheres and their reflective thermal insulation properties. Particuology 2020, 49, 33–39. [Google Scholar] [CrossRef]
- Wu, C.; Wang, W.; Ji, H. Preparation and Properties of TiO2-Coated Hollow Glass Microspheres as Thermal Insulation Materials for Energy-Saving Buildings. Trans. Tianjin Univ. 2020, 26, 283–291. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Facet-dependent photocatalytic properties of TiO2-based composites for energy conversion and environmental remediation. ChemSusChem 2014, 7, 690–719. [Google Scholar] [CrossRef]
- Lund, H. Renewable energy strategies for sustainable development. Energy 2007, 32, 912–919. [Google Scholar] [CrossRef]
- Atsonios, I.A.; Mandilaras, I.D.; Kontogeorgos, D.A.; Founti, M.A. A comparative assessment of the standardized methods for the in–situ measurement of the thermal resistance of building walls. Energy Build. 2017, 154, 198–206. [Google Scholar] [CrossRef]
- European Commision. Energy Efficiency in Buildings. Available online: https://ec.europa.eu/energy/en/topics/energy-efficiency/energy-performance-of-buildings (accessed on 19 February 2023).
- Konstantinidou, C.A.; Lang, W.; Papadopoulos, A.M.; Santamouris, M. Life cycle and life cycle cost implications of integrated phase change materials in office buildings. Int. J. Energy Res. 2019, 43, 150–166. [Google Scholar] [CrossRef]
- Mavrakou, T.; Polydoros, A.; Cartalis, C.; Santamouris, M. Recognition of thermal hot and cold spots in urban areas in support of mitigation plans to counteract overheating: Application for Athens. Climate 2018, 6, 16. [Google Scholar] [CrossRef]
- Santamouris, M.; Cartalis, C.; Synnefa, A.; Kolokotsa, D. On the impact of urban heat island and global warming on the power demand and electricity consumption of buildings—A review. Energy Build. 2015, 98, 119–124. [Google Scholar] [CrossRef]
- Hong, T.; Li, C.; Yan, D. Updates to the China Design Standard for Energy Efficiency in public buildings. Energy Policy 2015, 87, 187–198. [Google Scholar] [CrossRef]
- Kang, Q.; Bao, Y.; Li, M.; Ma, J. Effect of wall thickness of hollow TiO2 spheres on properties of polyacrylate film: Thermal insulation, UV-shielding and mechanical property. Prog. Org. Coat. 2017, 112, 153–161. [Google Scholar] [CrossRef]
- Patankar, S.; Kranov, Y. Hollow glass microsphere HDPE composites for low energy sustainability. Mater. Sci. Eng. A 2010, 527, 1361–1366. [Google Scholar] [CrossRef]
- Li, B.; Yuan, J.; An, Z.; Zhang, J. Effect of microstructure and physical parameters of hollow glass microsphere on insulation performance. Mater. Lett. 2011, 65, 1992–1994. [Google Scholar] [CrossRef]
- Vahtrus, M.; Oras, S.; Antsov, M.; Reedo, V.; Mäeorg, U.; Lõhmus, A.; Saal, K.; Lõhmus, R. Mechanical and thermal properties of epoxy composite thermal insulators filled with silica aerogel and hollow glass microspheres. Proc. Est. Acad. Sci. 2017, 66, 339–346. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Guo, A.; Zang, W.; Geng, H.; Tao, X.; Du, H. Mechanical properties and thermal conductivity of a temperature resistance hollow glass microspheres/borosilicate glass buoyance material. Mater. Sci. Eng. A 2016, 674, 604–614. [Google Scholar] [CrossRef]
- Ozkutlu, M.; Dilek, C.; Bayram, G. Effects of hollow glass microsphere density and surface modification on the mechanical and thermal properties of poly (methyl methacrylate) syntactic foams. Compos. Struct. 2018, 202, 545–550. [Google Scholar] [CrossRef]
- Pakdel, E.; Naebe, M.; Kashi, S.; Cai, Z.; Xie, W.; Yuen, A.C.Y.; Montazer, M.; Sun, L.; Wang, X. Functional cotton fabric using hollow glass microspheres: Focus on thermal insulation, flame retardancy, UV-protection and acoustic performance. Prog. Org. Coat. 2020, 141, 105553. [Google Scholar] [CrossRef]
- Yağci, Ö.; Eker Gümüş, B.; Taşdemir, M. Thermal, structural and dynamical mechanical properties of hollow glass sphere-reinforced polypropylene composites. Polym. Bull. 2021, 78, 3089–3101. [Google Scholar] [CrossRef]
- Imran, M.; Rahaman, A.; Pal, S. Effect of low concentration hollow glass microspheres on mechanical and thermomechanical properties of epoxy composites. Polym. Compos. 2019, 40, 3493–3499. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, R.; An, Z.; Zhang, J. Silicon rubber/hollow glass microsphere composites: Influence of broken hollow glass microsphere on mechanical and thermal insulation property. Compos. Sci. Technol. 2013, 79, 64–69. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Ren, S.; Hu, X.; Ren, H.; Wang, M.; Guo, A.; Liu, J.; Du, H.; Xian, L. Development of a buoyancy material of hollow glass microspheres/SiO2 for high-temperature application. J. Alloys Compd. 2017, 721, 213–219. [Google Scholar] [CrossRef]
- Adekomaya, O.; Majozi, T. Mitigating environmental impact of waste glass materials: Review of the existing reclamation options and future outlook. Environ. Sci. Pollut. Res. 2021, 28, 10488–10502. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef] [PubMed]
- Razza, F.; Briani, C.; Breton, T.; Marazza, D. Metrics for quantifying the circularity of bioplastics: The case of bio-based and biodegradable mulch films. Resour. Conserv. Recycl. 2020, 159, 104753. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Diebold, M. Optimizing the benefits of TiO2 in paints. J. Coat. Technol. Res. 2020, 17, 1–17. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R.; Guo, X.; Frankel, G.S. Effects of Graphene-Based Fillers on Cathodic Delamination and Abrasion Resistance of Cataphoretic Organic Coatings. Coatings 2020, 10, 602. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Durability of Acrylic Cataphoretic Coatings Additivated with Colloidal Silver. Coatings 2022, 12, 486. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Olive pit powder as multifunctional pigment for waterborne paint: Influence of the bio-based filler on the aesthetics, durability and mechanical features of the polymer matrix. Ind. Crops Prod. 2023, 194, 116326. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Functional Olive Pit Powders: The Role of the Bio-Based Filler in Reducing the Water Uptake Phenomena of the Waterborne Paint. Coatings 2023, 13, 442. [Google Scholar] [CrossRef]
- UNI EN ISO 2815; Dterminazione Della Durezza Con il Metodo di Penetrazione Buchholz. UNI—Ente Nazionale Italiano di Unificazione: Milan, Italy, 2000.
- BS EN ISO 11998:2006; Paints and Varnishes: Determination of Wet-Scrub Resistance and Cleanability of Coatings. BSI British Standards: London, UK, 2006; pp. 1–11.
- ASTM B117:2011; Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2011; pp. 1–12.
- Rossi, S.; Calovi, M.; Dalpiaz, D.; Fedel, M. The influence of NIR pigments on coil coatings’ thermal behaviors. Coatings 2020, 10, 514. [Google Scholar] [CrossRef]
- Rosati, A.; Fedel, M.; Rossi, S. Laboratory scale characterization of cool roof paints: Comparison among different artificial radiation sources. Prog. Org. Coat. 2021, 161, 106464. [Google Scholar] [CrossRef]
- ISO 20340:2009; Paints and Varnishes—Performance Requirements for Protective Paint Systems for Offshore and Related Structures. ISO—International Organization for Standardization: Geneva, Switzerland, 2009; pp. 1–23.
- ISO 11507:1997; Paints and Varnishes—Exposure of Coatings to Artificial Weathering—Exposure to Fluorescent UV and Water. ISO—International Organization for Standardization: Geneva, Switzerland, 1997; pp. 1–9.
- ISO 7253:1996; Paints and Varnishes—Determination of Resistance to Neutral Salt Spray (Fog). ISO—International Organization for Standardization: Geneva, Switzerland, 1996; pp. 1–10.
- Oliveira, C.; Ferreira, M. Ranking high-quality paint systems using EIS. Part I: Intact coatings. Corros. Sci. 2003, 45, 123–138. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S. An EIS study of ion diffusion through organic coatings. Electrochim. Acta 2006, 51, 1736–1744. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Calovi, M.; Russo, F.; Rossi, S. Synergic behavior of graphene-based filler and thermochromic pigments in cataphoretic coatings. Prog. Org. Coat. 2021, 150, 105978. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 383–389. [Google Scholar]
- Macdonald, J.R.; Barsoukov, E. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Kunwong, D.; Sumanochitraporn, N.; Kaewpirom, S. Curing behavior of a UV-curable coating based on urethane acrylate oligomer: The influence of reactive monomers. Sonklanakarin J. Sci. Technol. 2011, 33, 201. [Google Scholar]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR spectroscopic and thermal study of waterborne polyurethane-acrylate leather coatings using tartaric acid as an ionomer. e-Polymers 2016, 16, 463–474. [Google Scholar] [CrossRef]
- Mashouf, G.; Ebrahimi, M.; Bastani, S. UV curable urethane acrylate coatings formulation: Experimental design approach. Pigment. Resin Technol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus 2013, 2, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, W.; Wang, X.; Mu, Y. Effect of average functionality on properties of UV-curable waterborne polyurethane-acrylate. Prog. Org. Coat. 2010, 68, 201–207. [Google Scholar] [CrossRef]
- ASTM E308-18; Standard Practice for Computing the Colors of Objectives by Using the CIE System. ASTM International: West Conshohocken, PA, USA, 2018; pp. 1–45.
- Hasani, M.; Mahdavian, M.; Yari, H.; Ramezanzadeh, B. Versatile protection of exterior coatings by the aid of graphene oxide nano-sheets; comparison with conventional UV absorbers. Prog. Org. Coat. 2018, 116, 90–101. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, X.; Liu, H.; Chen, Y. Thermal conductivity of powder silica hollow spheres. Thermochim. Acta 2011, 526, 178–184. [Google Scholar] [CrossRef]
- Azemati, A.A.; Koloor, S.S.R.; Khorasanizadeh, H.; Sheikhzadeh, G.A.; Hadavand, B.S.; Eldessouki, M. Thermal evaluation of a room coated by thin urethane nanocomposite layer coating for energy-saving efficiency in building applications. Case Stud. Therm. Eng. 2022, 43, 102688. [Google Scholar] [CrossRef]
- Yu, Z.; Du, X.; Zhu, P.; Zhao, T.; Sun, R.; Chen, J.; Wang, N.; Li, W. Surface modified hollow glass microspheres-epoxy composites with enhanced thermal insulation and reduced dielectric constant. Mater. Today Commun. 2022, 32, 104046. [Google Scholar] [CrossRef]
- Magnani, G.; Filippi, F.; Cascone, M.; Ferraresi, A. Glass Microspheres Covering Film: Agronomic Evaluations on the Production of Cut Flowers. In Proceedings of the International Symposium on High Technology for Greenhouse System Management: Greensys2007, Naples, Italy, 4–6 October 2007; pp. 147–154. [Google Scholar]

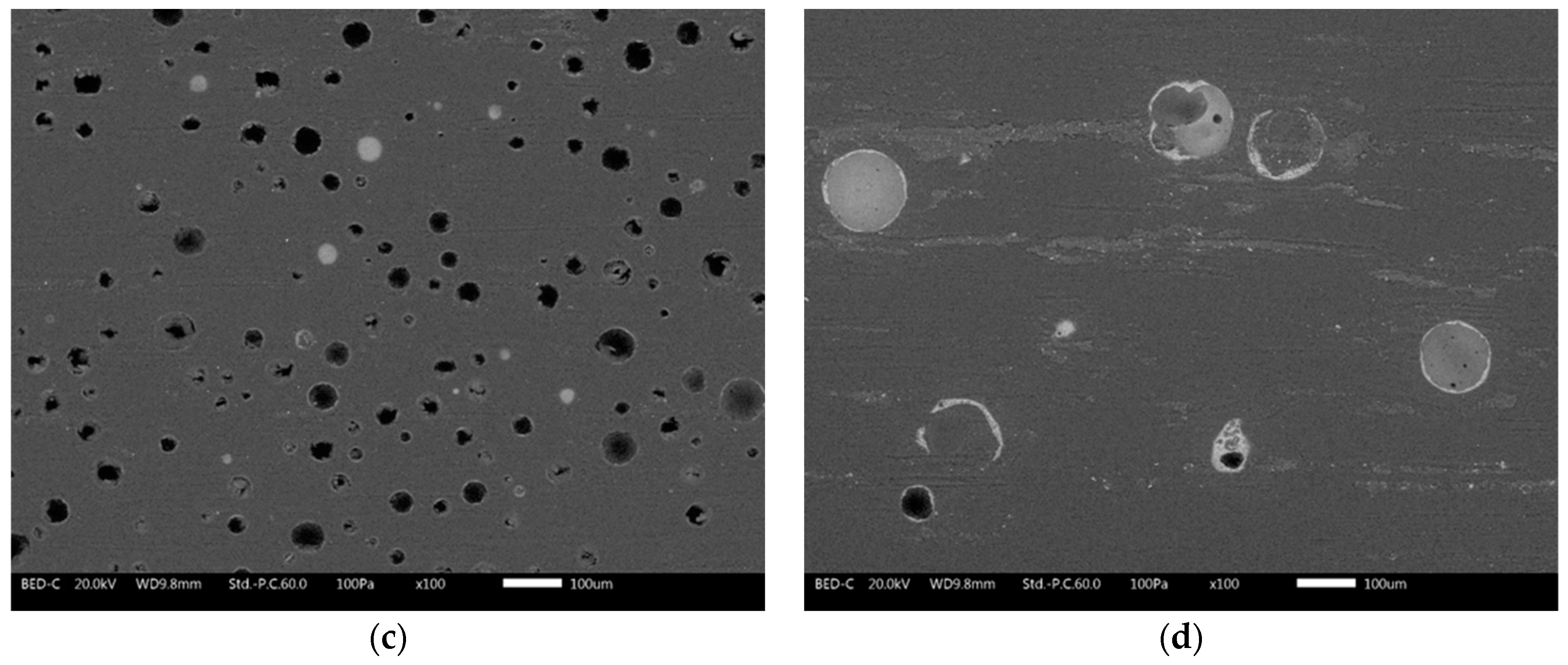

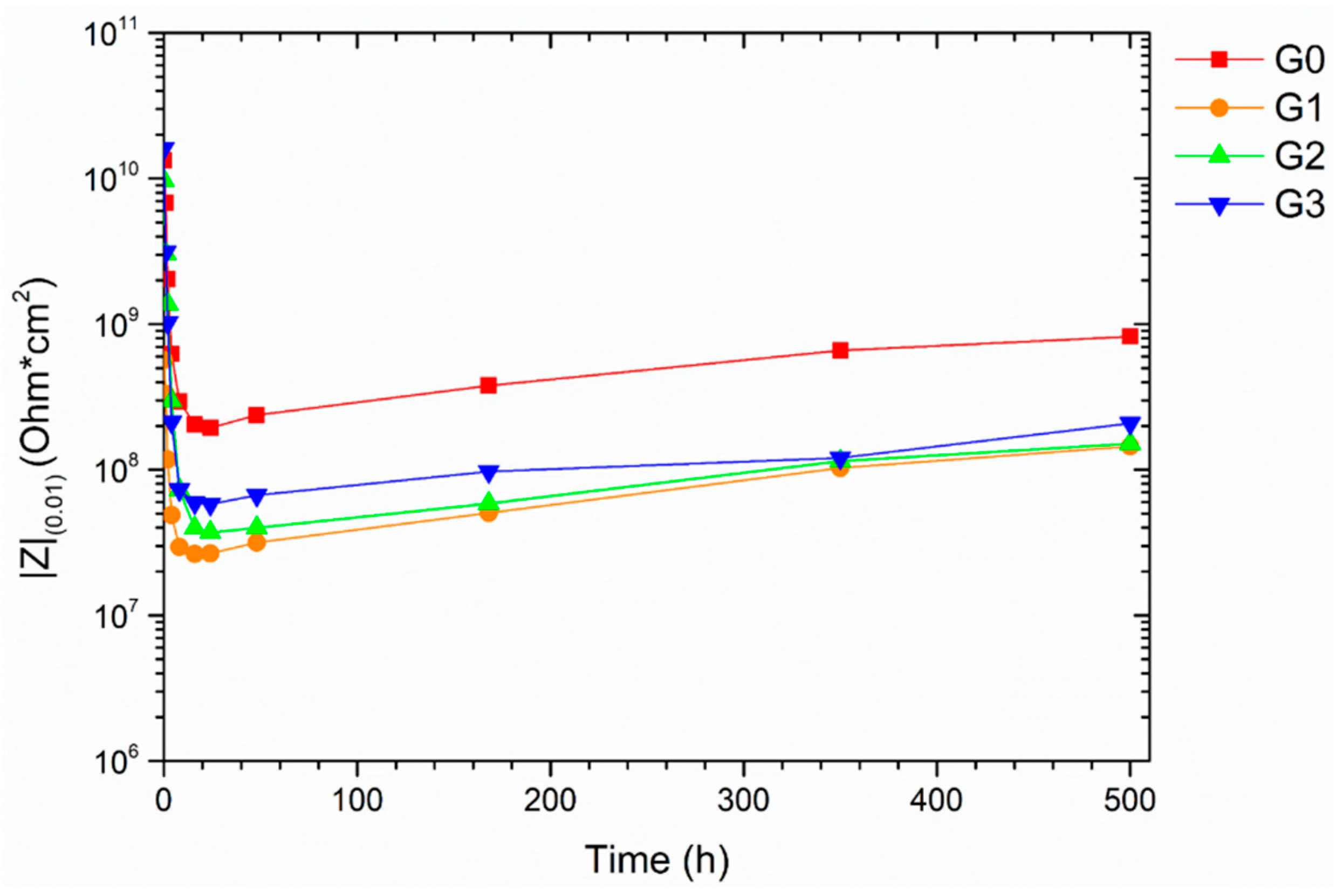
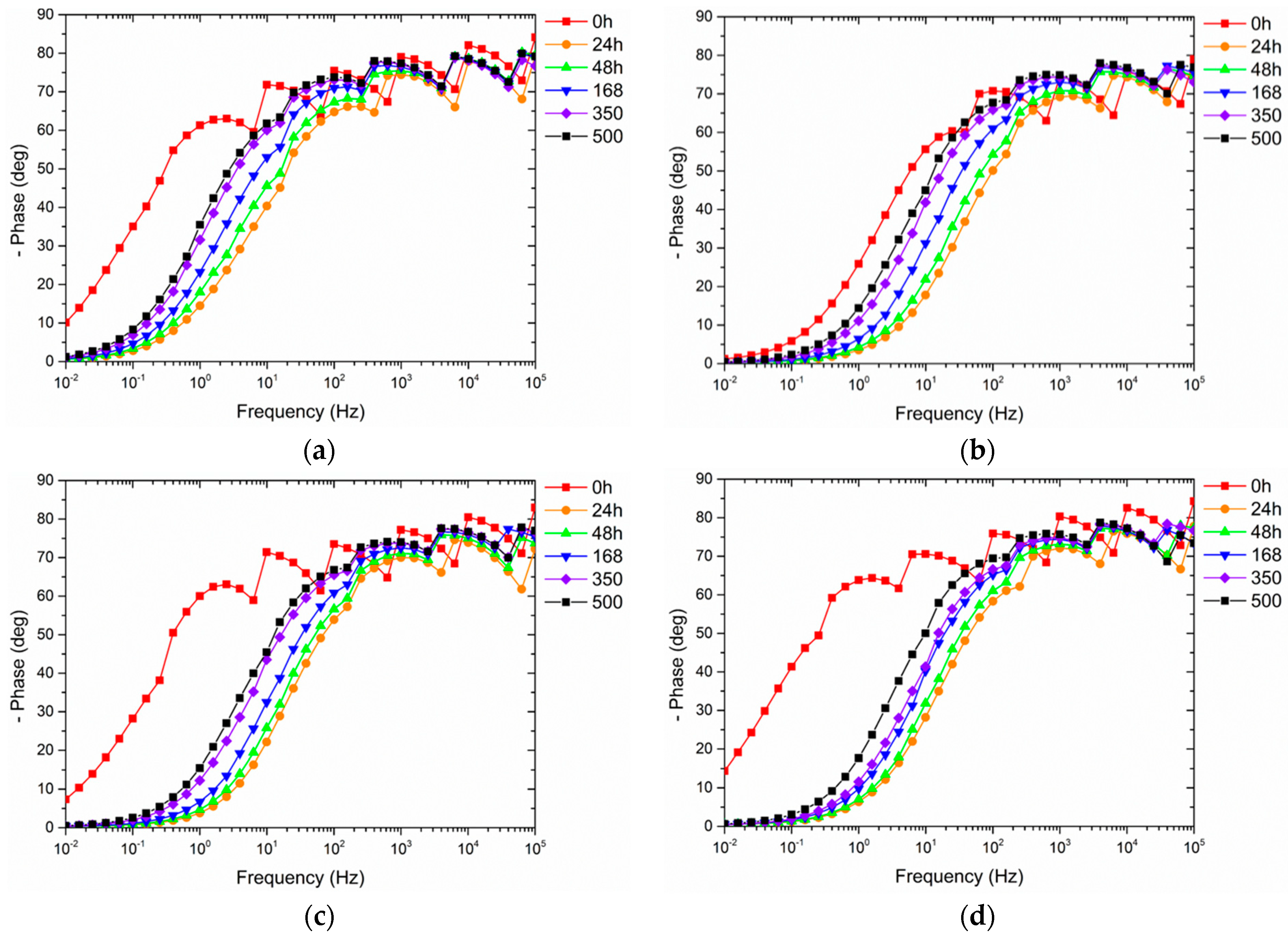
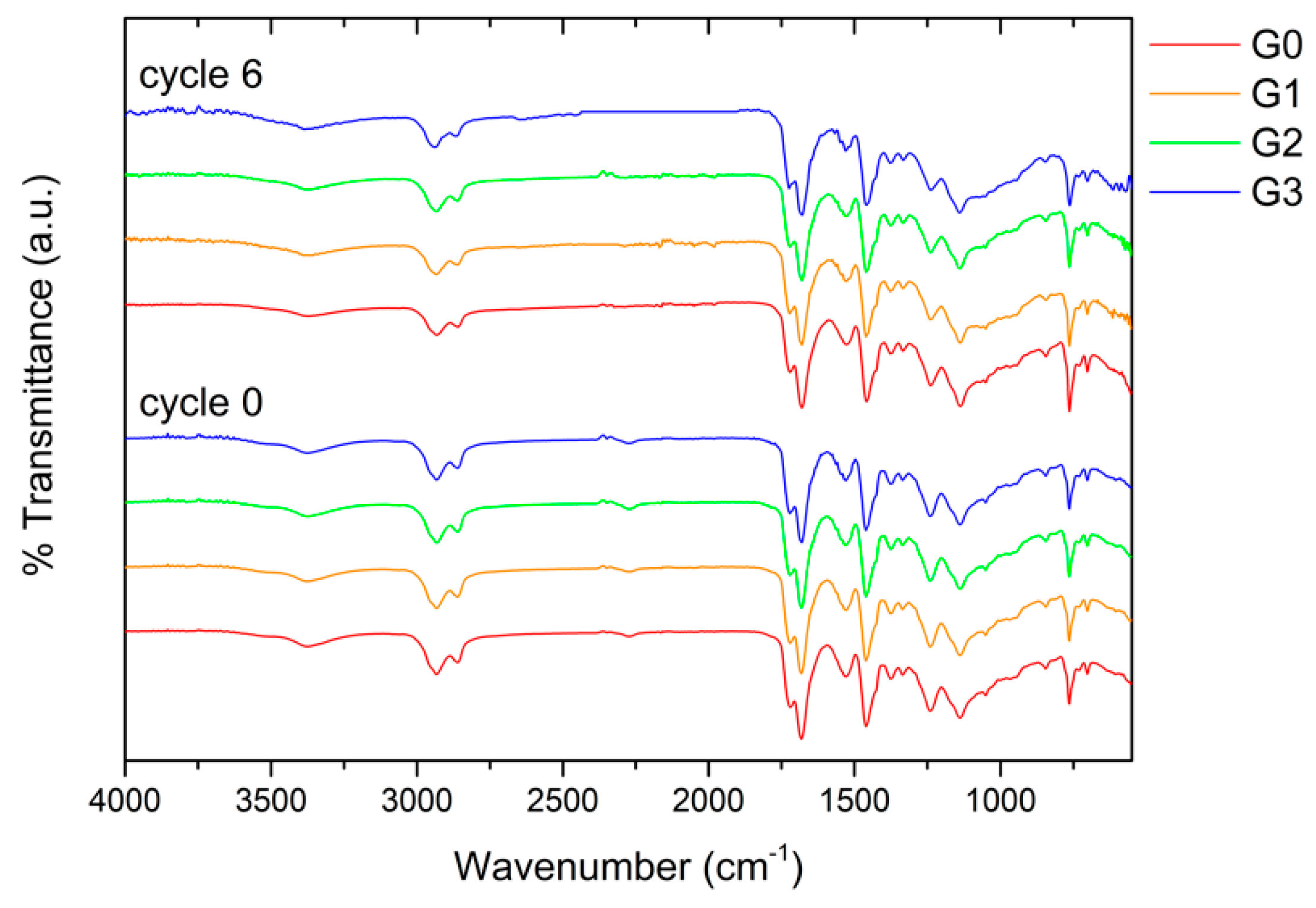


| Samples Nomenclature | Microspheres in the Top-Coat [1 wt.%] |
|---|---|
| G0 | / |
| G1 | C40 |
| G2 | C15 |
| G3 | P |
| Sample | Roughness Ra [µm] |
|---|---|
| G0 | 0.13 ± 0.01 |
| G1 | 1.09 ± 0.06 |
| G2 | 1.12 ± 0.02 |
| G3 | 2.41 ± 0.12 |
| Sample | Coating Detachment [µm] |
|---|---|
| G0 | 3692 ± 411 |
| G1 | 3342 ± 672 |
| G2 | 3742 ± 482 |
| G3 | 3142 ± 724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calovi, M.; Rossi, S. Durability and Thermal Behavior of Functional Paints Formulated with Recycled-Glass Hollow Microspheres of Different Size. Materials 2023, 16, 2678. https://doi.org/10.3390/ma16072678
Calovi M, Rossi S. Durability and Thermal Behavior of Functional Paints Formulated with Recycled-Glass Hollow Microspheres of Different Size. Materials. 2023; 16(7):2678. https://doi.org/10.3390/ma16072678
Chicago/Turabian StyleCalovi, Massimo, and Stefano Rossi. 2023. "Durability and Thermal Behavior of Functional Paints Formulated with Recycled-Glass Hollow Microspheres of Different Size" Materials 16, no. 7: 2678. https://doi.org/10.3390/ma16072678
APA StyleCalovi, M., & Rossi, S. (2023). Durability and Thermal Behavior of Functional Paints Formulated with Recycled-Glass Hollow Microspheres of Different Size. Materials, 16(7), 2678. https://doi.org/10.3390/ma16072678









